Abstract
Objectives
To investigate the effects of the urinary metabolite profiles of background exposure to the atmospheric pollutants polycyclic aromatic hydrocarbons (PAHs), and Framingham risk score (FRS), which assesses an individual’s cardiovascular disease risk, on heart rate variability (HRV).
Methods
The study, conducted from April to May 2011 in Wuhan, China, included 1,978 adult residents with completed questionnaires, physical examinations, blood and urine samples, and 5-min HRV indices [including standard deviation of NN intervals (SDNN), root mean square successive difference (rMSSD), low frequency (LF), high frequency (HF) and their ratio (LF/HF), and total power (TP)] obtained from 3-channel Holter monitor. 12 urinary PAH metabolites were measured by gas chromatography–mass spectrometry. FRS was calculated based on age, sex, lipid profiles, blood pressure, diabetes, and smoking status. Linear regression models were constructed after adjusting for potential confounders.
Results
Elevated ΣOHNa was significantly associated, in a dose-responsive manner, with decreased SDNN and LF/HF (Ptrend = 0.014 and 0.007, respectively); elevated ΣOHFlu was significantly associated with reduced SDNN, LF, and LF/HF (Ptrend = 0.027, 0.003, and < 0.0001, respectively); and elevated ΣOH-PAHs was associated with decreased LF and LF/HF (Ptrend = 0.005 and < 0.0001, respectively). Moreover, increasing quartiles of FRS were significantly associated with decreased HRV indices, except LF/HF (Ptrend <0.001). Interestingly, individuals in low-risk subgroups had greater decreases in SDNN, LF, and LF/HF in relation to ΣOH-PAHs, ΣOHNa, and ΣOHFlu than those in high-risk subgroups (all P <0.05).
Conclusions
Environmental PAHs exposure may differentially affect HRV based on individual coronary risk profiles.
Keywords: Framingham risk score, heart rate variability, PAH
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are ubiquitous air pollutants generated mainly from incomplete combustion of organic material, as in automobile exhaust, tobacco smoking, coal burning, home cooking, and industrial production; they represent an important public health concern.1–2 Humans are exposed to PAHs by inhalation, ingestion, and dermal contact.3 PAHs absorbed into the human body are metabolized as monohydroxylated PAH (OH-PAH) and excreted through urine. The half-life of urinary 1-hydroxypyrene (1-OHP) excretion ranges from 4 to 35 h.4 Information on the half-lives of the other OH-PAHs is scarce; however, because the similar metabolites are formed under similar biological pathways, other OH-PAHs are assumed to have similar half-lives.5 Due to the short half-lives of PAHs, the information provided by biomonitoring of urinary OH-PAHs is limited to recent exposure.4–5 Recent studies, including those from our lab,6–7 found that occupational exposure to PAHs is associated with decreased cardiac autonomic function, as assessed by heart rate variability (HRV), which is one of the major mechanistic pathways for adverse cardiac events. However, very little is known about adverse effects of background, enduring environmental exposure to PAH on HRV in the general population.
Individuals with cardiovascular risk factors are more vulnerable to the autonomic effects of pollution, particularly older individuals8 and those with diabetes,9 obesity,10 heart and pulmonary disease,11 and elevated systemic inflammation.12 However, most studies have focused on the effect modification of a single risk factor. To date, only one study has reported that individual coronary artery disease (CAD) risk profiles modify autonomic nervous system responses to occupational metal particulate exposure.13 Global cardiac risk and prediction of CAD development can be calculated using the Framingham Risk Score (FRS), which incorporates seven cardiovascular risk factors,14 but little evidence is available about the relationship between FRS and HRV,6 and no prior studies have examined the differential autonomic cardiac response to PAHs in low- versus high-FRS individuals. In the present study, we sought to investigate the association of environmental PAH exposure and FRS with HRV, as well as the potential effect of urinary OH-PAH on HRV in adults.
METHODS
Study population
The cross-sectional survey covering two communities was conducted in the general population from April to May 2011 in Wuhan, the capital of Hubei Province in Central China. Approximately 6 million people live in the center city within an area of 201 km2 where air pollution levels are higher and pollution ranges are wider than in the majority of cities described in the literature. The major sources of air pollution in the city are motor vehicles and the use of coal for domestic cooking, heating, and industrial processes.15–16
Individuals >18 years of age were invited to participate in the survey. Recruitment encompassed 3,092 volunteers, 18 to 90 years of age. Medical histories and physical examinations were performed on all participants. Structured questionnaires were used by trained interviewers to collect information on demographic variables, occupational history, medications, history of disease, and lifestyle habits including smoking, passive smoking, alcohol consumption, physical activity, and diet. Smoking was defined as having at least one cigarette per day over the previous 6 months (never smoker, current smoker, and ever smoker); alcohol consumption was considered as consuming a drink containing alcohol at least once a week over the previous 6 months (never, past, and current); physical activity was defined as regularly doing at least 20 minutes of physical activity during leisure time over the previous 6 months (yes or no). A fasting blood sample was drawn for examination of cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL), low-density lipoprotein cholesterol (LDL), and blood glucose. One morning urine sample (20 mL) was collected from each subject into sterile conical tubes, and all urine samples were divided into 4 aliquots and stored at −20 °C until laboratory analysis. The study was approved by the Institutional Review Board of Tongji Medical College and informed written consent was obtained from each subject.
Participants were excluded if they were unable to attend clinic visits, had not lived in the city for at least 5 years, and had serious medical conditions such as cancer, life-threatening arrhythmias, cardiomyopathy, heart failure, angina, myocardial infarction, and other non-classified heart problems, severe asthma, stoke, renal failure, medication use such as beta blockers, ACE inhibitors, ARBs affecting HRV, and those with missing data on FRS. After exclusion, 1,978 participants who were 20 to 74 years of age remained eligible.
Framingham Risk Score and other covariates
Sex-specific FRS values were calculated on the basis of age, sex, LDL, HDL, blood pressure, diabetes, and smoking status, as described previously.14 Blood pressure was measured twice on the left upper arm while participants were seated and the average of the two measures was taken. Diabetes was defined as fasting blood glucose of ≥ 7.0 mmol/L, or taking insulin or oral hypoglycemic agents. Body height and weight were measured to compute body mass index (BMI). Other covariates—passive smoking, physical activity, alcohol consumption, diet, and heart rate—that potentially acted as confounders were carefully selected based on evidence from literature.
Determination of urinary PAH metabolites
We measured 12 urinary PAH metabolites [pyrene metabolite: 1-OHP; naphthalene metabolites: 1-hydroxynaphthalene (1-OHNa), 2-OHNa; fluorene metabolites: 2-hydroxyfluorene (2-OHFlu), 9-OHFlu; phenanthrene metabolites: 1-hydroxyphenanthrene (1-OHPh), 2-OHPh, 3-OHPh, 4-OHPh, 9-OHPh; chrysene metabolite: 6-hydroxychrysene (6-OHChr); and benzo[a]pyrene metabolite: 3-hydroxybenzo[a]pyrene (3-OHBaP)] by gas chromatography–mass spectrometry (GC/MS, Agilent 6890N+5975B, Agilent Technologies Inc., Santa Clara, CA, USA) as previously described.7 Briefly, each 3.0 mL of urine were extracted with 20 µL β-Glucuronidase/sulphatase (type H-2 from Helix pomatia; β-glucuronidase activity ≥85,000 units/mL and sulphatase activity 2400 U/mL, aqueous solution type, Sigma-Aldrich, Milan, Italy), 20 µL pure deuterated compounds as internal standards (IS) solution containing 1-OHPYRd9 (Toronto Research Chemicals, Toronto, Canada) and 1-OHNAPd7 (C/D/N isotopes inc., Beijing, China) at a concentration of 5 mg/L and 50 mg/L in acetonitrile. The set of the standard curve was operated about every 100 samples, and about 10% of the total samples were used as quality control. The identification and quantification of urinary PAH metabolites were based on retention time, mass-to-charge ratio, and peak area using a linear regression curve obtained from separate internal standard solutions. The limits of detection (LOD) for urinary PAH metabolites were in the range 0.1–0.9 µg/L; default values were replaced with 50% of the LOD. Valid urinary PAH metabolite concentrations were calibrated by levels of urinary creatinine and calculated as nmol/mmol creatinine. Because 6-OHChr and 3-OHBaP were below the limits of quantification, we only analyzed 10 metabolites of PAH.
HRV measurement
HRV measurement methods have been described previously.7 Briefly, after at least a 5-min rest, each participant was seated comfortably on a chair and fitted with a 3-channel digital Holter monitor (Lifecard CF; Del Mar Reynolds Medical, Inc., Whitney, Irvine, USA) with a 1024 samples/second sampling rate for 10 minutes. The scanner collected data automatically, and all of the HRV indices were calculated on 5-min epoch in the entire recording. Only heart rates between 40 and 100 beats per minute were submitted to HRV analyses. We selected 5 consecutive minutes of ECG reading in the statistical analysis without atria and ventricular premature beats and flutter. The HRV spectrum was computed with a fast Fourier transform method. The HRV was analyzed in both time and frequency domains. The measured time domain parameters included standard deviation of NN intervals (SDNN) and root mean square successive difference (rMSSD). The frequency-domain variables included low-frequency (LF), high-frequency (HF), and their ratio (LF/HF), and total power (TP).
Statistical analyses
Baseline characteristics are presented as means (SD) for continuous variables and as percentages for categorical data. Because urinary PAH metabolites and HRV indices were not normally distributed, they are expressed as medians and interquartile ranges and data were natural logarithmic transformation before linear regression analysis. To assess exposure from each kind of PAH, we summed metabolites from the same parent PAH to give total concentration for that PAH, including total concentrations of hydroxynaphthalene (ΣOHNa) = 1-OHNa + 2-OHNa; total concentrations of hydroxy fluorine (ΣOHFlu) = 2-OHFlu + 9-OHFlu; total concentrations of hydroxyphenanthrene (ΣOHPh) = 1-OHPh + 2-OHPh + 3-OHPh + 4-OHPh + 9-OHPh; and total concentrations of hydroxypyrene = 1-OHP since 1-OHP is the only monohydroxylated pyrene metabolite measured. Spearman correlation coefficients were computed to examine the associations among PAH metabolites. Linear regression models were used to assess the relationship between PAH metabolites and HRV indices, with appropriate adjustments including BMI, passive smoking, physical activity, alcohol consumption, diet, and FRS; the effects of FRS on HRV were also analyzed using linear regression models, adjusted for BMI, passive smoking, physical activity, alcohol consumption, and diet. Moreover, to analyze the potential interactions between PAH metabolites and FRS, an interaction product term of PAH metabolites-FRS was included in the models. We also conducted sensitivity analysis by additionally adjusting for heart rate in the models. All P values presented are 2-tailed, and P values < 0.05 are considered statistically significant. Analyses were performed using SPSS 12.0 software (SPSS Inc.).
RESULTS
Characteristics of study population
General characteristics of the study population are presented in Table 1. Mean age was 50.4 ± 12.2 years, and the majority was female (64.5%). Of the 1,978 participants, 7.8% had diabetes and 21.7% were smokers. Mean FRS value was 7.3 ± 6.8%, which is considered intermediate risk (FRS 6% to 19%) by the third report of the Adult Treatment Panel of the National Cholesterol Education Program (ATP III).17
Table 1.
Characteristics of the Study Population*
| Characteristics | Total population (n=1,978) |
|---|---|
| Age (years) | 50.4 (12.2) |
| Sex (female, %) | 64.5 |
| Current smoker (%) | 21.7 |
| Current alcohol drinker (%) | 17.6 |
| Physical activity (yes, %) | 41 |
| BMI (kg/m2) | 24.0 (3.4) |
| Waist circumference (mm) | 816.2 (97.0) |
| Systolic blood pressure (mmHg) | 128.8 (19.7) |
| Diastolic blood pressure (mmHg) | 78.3 (11.2) |
| Fasting glucose (mmol/L) | 5.1 (1.4) |
| Total cholesterol (mmol/L) | 5.0 (1.1) |
| Triglycerides (mmol/L) | 1.5 (1.3) |
| HDL cholesterol (mmol/L) | 1.6 (0.4) |
| LDL cholesterol (mmol/L) | 3.3 (0.9) |
| Diabetes (%) | 7.8 |
| FRS, 10-year % | 7.3 (6.8) |
| ΣOH-PAHs (nmol/mmol creatinine) | 36.5 (25.9, 54.0) |
| HRV indices | |
| SDNN (msec) | 35.2 (27.6, 44.6) |
| RMSSD (msec) | 22.8 (17.7, 29.2) |
| TP (msec2) | 863.4 (498.5, 1470.0) |
| LF (msec2) | 242.1 (119.5, 456.0) |
| HF (msec2) | 131.2 (63.6, 264.6) |
| LF/HF | 1.8 (1.0, 3.1) |
Abbreviations: BMI, body mass index; FRS, Framingham risk score; HDL, high-density lipoprotein; HF, high frequency; LDL, low-density lipoprotein; LF, low frequency; LF/HF, LF to HF ratio; ΣOH-PAHs, total concentration of all PAH metabolites; rMSSD, root mean square of successive differences in adjacent NN intervals; SD, standard deviation; SDNN, standard deviation of NN intervals; TP, total power.
Variables are presented as mean (SD), percentage, or median (25th, 75th quartile).
Distributions and correlations of PAH metabolites
The number of participants who were tested for 10 urinary PAH metabolites ranged from 1,962 to 1,978, and the percentage of detectable values was over 99% for these PAH metabolites. Among the 4 main categories of PAH metabolites, the most abundant was ΣOHNa: 1-OHNa (median 4.28 nmol/mmol creatinine) and 2-OHNa (median 8.71 nmol/mmol creatinine) accounted for 12.7% and 27.0%, respectively, of ΣOH-PAHs, for a sum of 39.7%. ΣOHFlu and ΣOHPh contributed less, about 25% each, while 1-OHP contributed the lowest percentage (9.8%) (Table 2). All correlations between PAH metabolites were significant, with correlation coefficients ranging from 0.32 to 0.92, but ΣOHNa, ΣOHFlu, and ΣOHPh were more strongly correlated with ΣOH-PAHs (r=0.84, 0.78 and 0.81, respectively) than was 1-OHP (r=0.63) (Supplementary Table 1).
Table 2.
Sample Size (N), Limits of Detection (LOD), and Distributions of Urinary PAH Metabolites (nmol/mmol creatinine)
| Percentile | ||||||||
|---|---|---|---|---|---|---|---|---|
| PAH metabolites | N | LOD | Percent detection (%) | 5th | 25th | 50th | 75th | 95th |
| Pyrene metabolite | ||||||||
| 1-OHP | 1976 | 0.50 | 99.90 | 1.05 | 2.15 | 3.42 | 5.49 | 12.01 |
| Naphthalene metabolites | ||||||||
| 1-OHNa | 1977 | 0.90 | 99.95 | 1.66 | 2.85 | 4.28 | 6.88 | 15.96 |
| 2-OHNa | 1978 | 0.90 | 100.00 | 3.68 | 5.78 | 8.71 | 14.30 | 31.77 |
| ΣOHNa | 1978 | 1.80 | 100.00 | 5.67 | 9.04 | 13.75 | 21.86 | 48.76 |
| Fluorene metabolites | ||||||||
| 2-OHFlu | 1978 | 0.10 | 100.00 | 0.71 | 1.13 | 1.62 | 2.40 | 5.03 |
| 9-OHFlu | 1978 | 0.20 | 100.00 | 1.52 | 3.10 | 5.26 | 10.20 | 30.18 |
| ΣOHFlu | 1978 | 0.30 | 100.00 | 2.52 | 4.57 | 7.13 | 12.70 | 33.90 |
| Phenanthrene metabolites | ||||||||
| 1-OHPh | 1975 | 0.30 | 99.85 | 0.55 | 0.87 | 1.22 | 1.84 | 4.13 |
| 2-OHPh | 1962 | 0.10 | 99.19 | 0.38 | 0.61 | 0.89 | 1.40 | 3.20 |
| 3-OHPh | 1978 | 0.20 | 100.00 | 0.65 | 1.17 | 1.70 | 2.68 | 6.06 |
| 4-OHPh | 1977 | 0.10 | 99.95 | 0.62 | 1.09 | 1.60 | 2.48 | 5.33 |
| 9-OHPh | 1978 | 0.20 | 100.00 | 1.40 | 2.43 | 3.55 | 5.47 | 11.77 |
| ΣOHPh | 1978 | 0.90 | 100.00 | 3.93 | 6.50 | 9.13 | 13.85 | 29.88 |
| ΣOH-PAHs* | 1978 | 3.50 | 100.00 | 15.68 | 25.89 | 36.50 | 54.02 | 116.02 |
Abbreviations: 2-OHFlu, 2-hydroxyfluorene; 9-OHFlu, 9-hydroxyfluorene; ΣOHFlu, total concentration of hydroxyfluorene; 1-OHNa, 1-hydroxynaphthalene; 2-OHNa, 2-hydroxynaphthalene; ΣOHNa, total concentration of hydroxynaphthalene; 1-OHP, 1-hydroxypyrene; ΣOH-PAHs, total concentration of all PAH metabolites; 1-OHPh, 1-hydroxyphenanthrene; 2-OHPh, 2-hydroxyphenanthrene; 3-OHPh, 3-hydroxyphenanthrene; 4-OHPh, 4-hydroxyphenanthrene; 9-OHPh, 9-hydroxyphenanthrene; ΣOHPh, total concentration of hydroxyphenanthrene; PAHs, polycyclic aromatic hydrocarbons.
ΣOH-PAHs = 1-OHP + ΣOHNa + ΣOHFlu + ΣOHPh.
Effects of PAH metabolites on HRV
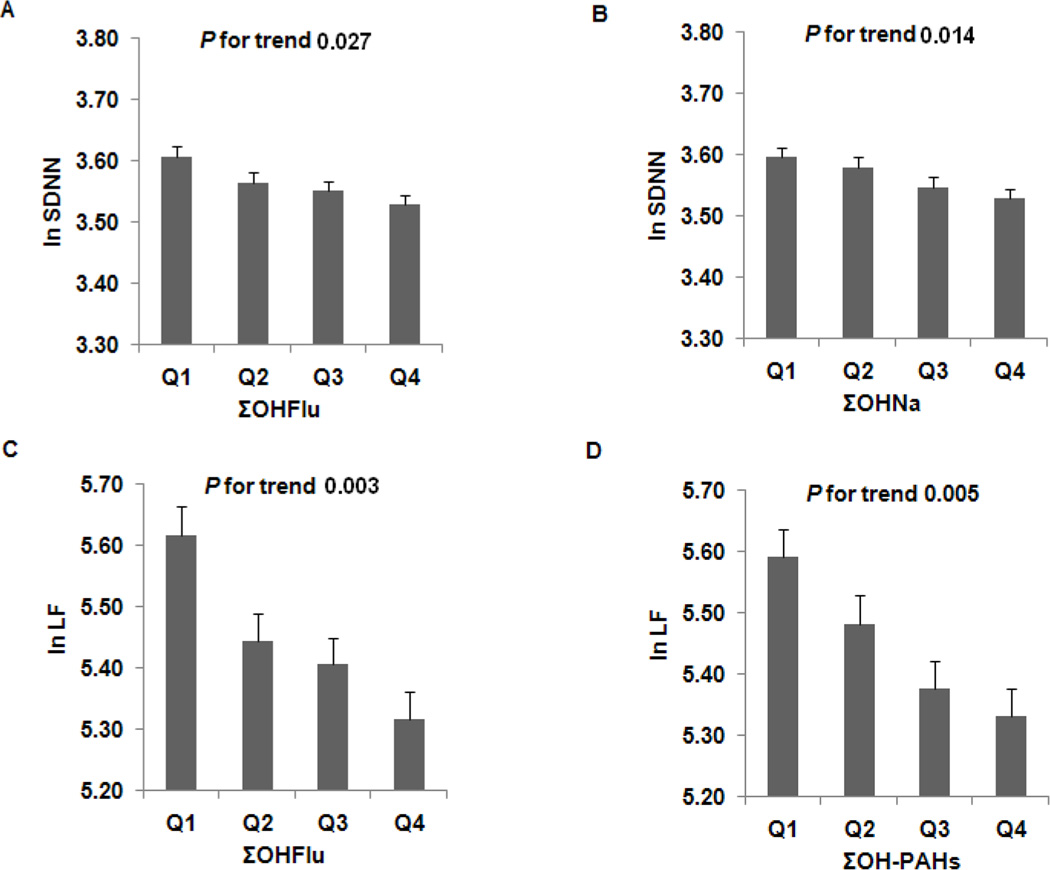
For each urinary PAH metabolite, only 2-OHNa had a predominant contribution to the reductions in SDNN, LF, and LF/HF. Increasing quartiles of 2-OHNa were significantly associated with decreased SDNN, LF, and LF/HF (Ptrend <0.05); no significant association between other PAH metabolites and HRV indices was found (data not shown). But when PAH metabolites were analyzed jointly, an individual’s ΣOHFlu, ΣOHNa, and ΣOH-PAHs measures had a consistent effect on HRV measures (Supplementary Table 2). As shown in Figure 1, after adjustment for BMI, passive smoking, physical activity, alcohol consumption, diet, and FRS, increasing quartiles of ΣOHNa were significantly associated, in a dose-responsive manner, with decreased SDNN and LF/HF (Ptrend = 0.014 and 0.007, respectively); increasing quartiles of ΣOHFlu were significantly associated with reduced SDNN, LF, and LF/HF (Ptrend = 0.027, 0.003, and < 0.0001, respectively); and increasing quartiles of ΣOH-PAHs were significantly associated with decreased LF and LF/HF (Ptrend = 0.005 and < 0.0001, respectively). No significant associations were found between 1-OHP or ΣOHPh and HRV indices. In addition, further adjustment for heart rate did not appreciably change the results (data not shown).
Figure 1.
Effects of ΣOHFlu (A and C), ΣOHNa (B), and ΣOH-PAHs (D) on HRV. Values calculated from the regression models, adjusting for BMI, passive smoking, physical activity, alcohol consumption, diet, and FRS. LF, low frequency; LF/HF, LF to HF ratio; ΣOHFlu, total concentration of hydroxyfluorene; ΣOHNa, total concentration of hydroxynaphthalene; ΣOH-PAHs, total concentration of all PAH metabolites; SDNN, standard deviation of NN intervals.
Effects of FRS on HRV
After adjustment for potential confounders (BMI, passive smoking, physical activity, alcohol consumption, and diet), increasing quartiles of FRS were significantly associated with decreases in five HRV indices (SDNN, RMSSD, TP, LF, and HF), but not the 6th (LF/HF) (all Ptrend < 0.001). Compared to those in the lowest quartile, individuals in the highest quartile of FRS had significant associations, with a 0.24–0.95 reduction for those five HRV indices (Table 3). Sensitivity analysis by adding heart rate did not change the results.
Table 3.
Effects of FRS on HRV by FRS Quartiles*
| FRS | |||||
|---|---|---|---|---|---|
| HRV indices | Q1 | Q2 | Q3 | Q4 | Ptrend† |
| SDNN | Ref | −0.16 −0.20, −0.11 |
−0.25 −0.29, −0.20 |
−0.33 −0.37, −0.28 |
<0.0001 |
| RMSSD | Ref | −0.14 −0.19, −0.09 |
−0.21 −0.26, −0.16 |
−0.24 −0.29, −0.19 |
<0.0001 |
| TP | Ref | −0.32 −0.42, −0.22 |
−0.54 −0.64, −0.44 |
−0.73 −0.83, −0.63 |
<0.0001 |
| LF | Ref | −0.35 −0.47, −0.23 |
−0.66 −0.78, −0.53 |
−0.95 −1.07, −0.82 |
<0.0001 |
| HF | Ref | −0.44 −0.57, −0.32 |
−0.68 −0.81, −0.54 |
−0.84 −0.98, −0.71 |
<0.0001 |
| LF/HF | Ref | 0.10 −0.01, 0.20 |
0.02 −0.09, 0.13 |
−0.10 −0.21, 0.01 |
0.126 |
Abbreviations: FRS, Framingham risk score; HF, high frequency; HRV, heart rate variability; LF, low frequency; LF/HF, LF to HF ratio; rMSSD, root mean square of successive differences in adjacent NN intervals; SDNN, standard deviation of NN intervals; TP, total power.
Variables are presented as beta coefficients (β) and 95% confidence intervals (CIs). Q1: FRS, 10-year % < 2; Q2: 2 ≤ FRS, 10-year % < 7.3; Q3: 7.3 ≤ FRS, 10-year % < 9; Q4: FRS, 10-year % ≥ 9.
P value for the multivariable linear regression analysis adjusting for BMI, passive smoking, physical activity, alcohol consumption, and diet.
Effects of PAH metabolites on HRV by FRS
Individuals were divided into two subgroups (low vs. high risk) based on the FRS values (Table 4). The low-risk subgroup demonstrated a greater effect on SDNN, LF, and LF/HF in response to ΣOH-PAHs, ΣOHNa, and ΣOHFlu than the high-risk subgroup (all P < 0.05). For instance, each quartile increase in ΣOHFlu was associated with 0.030 reduction in SDNN, 0.087 reduction in LF, and 0.102 reduction in LF/HF among low-risk individuals, whereas the corresponding responses were 0.009, 0.058, and 0.058 for high-risk subjects. After further adjustment for heart rate, the results did not change (data not shown).
Table 4.
Effects of PAH Metabolites on HRV by FRS
| Low* | High† | |||||
|---|---|---|---|---|---|---|
| β‡ | 95% CI | P Value§ | β | 95% CI | P Value | |
| ΣOH-PAHs | ||||||
| SDNN | −0.021 | −0.038, −0.005 | 0.012 | −0.012 | −0.036, 0.013 | 0.350 |
| LF | −0.078 | −0.125, −0.031 | 0.001 | −0.044 | −0.110, 0.022 | 0.191 |
| LF/HF | −0.101 | −0.144, −0.058 | <0.0001 | −0.051 | −0.108, 0.007 | 0.084 |
| ΣOHNa | ||||||
| SDNN | −0.023 | −0.040, −0.006 | 0.008 | −0.014 | −0.039, 0.011 | 0.264 |
| LF | −0.062 | −0.109, −0.015 | 0.010 | −0.021 | −0.087, 0.046 | 0.541 |
| LF/HF | −0.055 | −0.098, −0.012 | 0.012 | −0.048 | −0.106, 0.009 | 0.098 |
| ΣOHFlu | ||||||
| SDNN | −0.030 | −0.047, −0.014 | 0.000 | −0.009 | −0.033, 0.016 | 0.481 |
| LF | −0.087 | −0.135, −0.040 | 0.000 | −0.058 | −0.123, 0.007 | 0.081 |
| LF/HF | −0.102 | −0.145, −0.059 | <0.0001 | −0.058 | −0.115, 0.002 | 0.063 |
Abbreviations: FRS, Framingham risk score; HRV, heart rate variability; LF, low frequency; LF/HF, LF to HF ratio; ΣOHFlu, total concentration of hydroxyfluorene; ΣOHNa, total concentration of hydroxynaphthalene; OH-PAH, urinary PAH metabolites; ΣOH-PAHs, total concentration of all PAH metabolites; SDNN, standard deviation of NN intervals.
Low: FRS, 10-year % < 7.3.
High: FRS, 10-year % ≥ 7.3.
β represents change in SDNN, LF, and LF/HF for OH-PAH.
P value was adjusted for BMI, passive smoking, physical activity, alcohol consumption, diet, and FRS.
DISCUSSION
To date, evidence demonstrating an association between environmental PAH exposure and HRV based on an individual’s CAD risk profile remains scarce. The present study demonstrates, first, that increased PAH metabolites and elevated FRS were independently related, in a dose-responsive manner, to decreased HRV in community-resident adults aged 20 years and older. More specifically, individuals in the low-risk FRS subgroup had a greater reduction in HRV response to PAH metabolites than those in the high-risk FRS subgroup.
Our results agree with epidemiological evidence from previous occupational studies, which found that occupational PAH exposure was linked to a significant dose-dependent decrease in HRV indices.7 However, our finds showing significantly stronger and consistent pattern for SDNN, LF and LF/HF relative to PAH metabolites suggested that the 3 HRV indices might be a better indicator to capture the cardiac effects of PAH metabolites in general population. SDNN is thought to reflect sympathetic influence, while interpreting LF and LF/HF is controversial. LF refers to vagal and sympathetic influences, but LF may to some extent reflect sympathetic influence, whereas LF/HF reflects sympathovagal balance with higher ratios indicating more sympathetic than vagal modulation of heart rhythm.18 We may speculate that the sympathetic influence on SDNN, LF and LF/HF was more important as a predictor of PAH metabolites than vagal modulation as reflected by RMSSD and HF.
For general populations, air pollutants, cigarette smoke, diet, and some occupational settings are the main sources of PAH exposures, thus urinary PAH metabolites can be good biomarkers for assessing multiple routes of external exposure. The concentrations of individual OH-PAHs and ΣOH-PAHs determined in our study were higher than the reference levels established for the U.S. general adult population,19 control subjects in Central Europe,20 and the general population in seven Asian countries including China (these levels should be interpreted with caution because of the small sample size available for each country).21 Importantly, reported concentrations were not creatinine-adjusted for the seven Asian countries, thus the comparison between our results and results described for those countries is not direct. Studies have shown that the concentrations of OH-PAHs in urine are influenced by people’s lifestyles as well as environmental factors and sampling time. It was not possible to directly analyze PAH exposure routes in this study because we did not measure PAH in air or food. However, PAH exposure through food intake is thought to be consistent within the same geographic area.22 Also, we found no significant associations between PAH metabolites and the consumption of tea, coffee, or meat and fish based on the dietary questionnaire. Overall, as mentioned by Li et al.,19 exposure to one PAH generally leads to the formation and excretion of multiple OH-PAH metabolites. Therefore, total PAH exposure can be better reflected by studying the total concentration of the same analogue metabolites rather than individual OH-PAHs.
In the present study, we found no significant association between urinary 1-OHP and HRV, even though urinary 1-OHP has been widely used as a representative biomarker of occupational PAH exposures. Lee et al.6 reported a negative association between PAH exposure as measured by urinary 1-OHP and HRV in boilermakers, but 1-OHP was at least 100-fold higher in occupational workers than in non-occupationally exposed subjects.23 Further, 1-OHP is the hydroxylated metabolite for pyrene, and it is not a good biomarker to comprehensively assess total PAH exposure. Using 1-OHP as the sole biomarker for PAH exposure could lead to uncertainties for assessing human exposure and the loss of other important information on PAH exposure. In this study, 1-OHP was in the lowest concentration among the 4 kinds of PAH metabolites, which was similar to those reported for the U.S. population and Asian populations,19,21,24 and the correlation between 1-OHP and ΣOH-PAHs was lower in magnitude than between ΣOHNa, ΣOHFlu, and ΣOHPh and ΣOH-PAHs. The lowest concentration and the lower correlation with ΣOH-PAHs may be the reason why 1-OHP does not show significant association with HRV. Hence, especially for non-occupationally exposed populations, the measurement of all PAH metabolites will provide better overall assessment of PAH exposure and reduce the bias associated with the traditional measurement of urinary1-OHP as the single biomarker. Our data support the previously reported observations that, at low levels of exposure, 1-OHP does not represent total PAH concentration.25
Naphthalene is the most volatile PAH and, in addition to its emission by all sorts of incomplete combustion processes, it is also widespread as a basic substance in the chemical industry and in consumer products.26 Airborne naphthalene has been reported to be 3- to 5-fold higher than fluorene and phenanthrene and 8-fold higher than pyrene.27 Urinary 2-OHNa was the major metabolite detected in the urine samples and has been shown to be a sensitive low-level marker of PAH exposures such as smoking and environmental exposure,28–29 and the sum of both urinary 1- and 2-OHNa was a useful measure of naphthalene exposure in volunteer office employees who were not exposed occupationally to PAH.30 Consistent with the published values in both occupational and ambient settings,19,31 we found that 2-OHNa was the most abundant metabolite, followed by ΣOHFlu, ΣOHPh, and 1-OHP. Moreover, only 2-OHNa contributes to the reduction in HRV at environmental PAH exposure when calculating the association of each PAH metabolite with HRV. Importantly, naphthalene has neurotoxicity, which may directly cause damage to the autonomic nervous system, leading to an imbalance in cardiac autonomic control,32 and most PAHs and their metabolites can be detected in rat brain tissue exposed to PAHs.33 Therefore, it is not wholly surprising to find a significant association between urinary ΣOHNa and HRV in this study.
Additionally, we found a significant association between urinary ΣOHFlu and HRV, but not between ΣOHPh and HRV. Both fluorine and phenanthrene are commonly used to make dyes, plastics, pesticides, etc. The general population is generally exposed to them through breathing contaminated air and skin contact.34–35 Li et al.19 postulated that 2-OHFlu was a better surrogate of total environmental PAH exposure than the other OH-PAH metabolites when a multi-analyte method was unavailable. However, evidence on the health effects of fluorine and phenanthrene monohydroxy metabolite is very limited. Xu et al.1,36 found that the metabolites of fluorine and phenanthrene were more likely associated with self-reported cardiovascular diseases and peripheral arterial disease using NHANES, but it is far from clear what role ΣOHFlu and ΣOHPh play in contributing to reduction in HRV. The direct neurocardiac toxicity of PAH on cardiac ion channels, blood and lung receptors, and indirect effects mediated through oxidative stress or inflammation may be other potential mechanisms.37–39
The FRS is useful for assessing global cardiac risks,14 and HRV can help predict the risk of cardiovascular disease development,40 but little evidence is available about the relationship between FRS and HRV. Yoo et al. first reported that FRS in Korean men (n=55) was inversely correlated with HRV indices including SDNN, RMSSD, LF, and HF, but no significant relationship was identified between FRS and HRV in women (n=30).6 More recently, Jelinek et al.41 only observed a significant relationship between FRS and SDNN using time and frequency domain analysis in 170 patients. Because small sample sizes may reduce statistical power, the relationship between FRS and HRV has not been documented clearly. Our study, conducted on a large sample, found that increasing quartiles of FRS were associated with significant dose-dependent decreases in five HRV indices, which confirmed and extended these previous results. Our findings indicate a good agreement between the FRS and HRV indices, but the causal pathway between FRS and HRV cannot be determined using these data. It is likely that reduced HRV may be a simple marker similar to the FRS.6 However, determination of FRS requires blood sampling, which is an invasive procedure; thus the non-invasive HRV measurement, which is significantly associated with FRS, may provide an alternative for identifying cardiovascular risk.
Chen et al.13 first illustrated the use of the FRS as a multiple-risk-factor equation for risk stratification of autonomic nervous system responses to air pollution. They found that, in subjects with an increased CAD risk (FRS of 5–6), PM2.5 exposure was associated with reductions in HRV that were several folds greater than the responses observed among individuals with a low risk profile (FRS of 1–3). However, external validity or generalization of their results may be limited by the small sample size of 10 male boilermakers. In contrast, we found that individuals in the low-risk FRS subgroups (n=989) had a greater reduction in HRV in response to PAH metabolites than in the high-risk FRS (n=989) subgroup. These findings indicate that a clustering of CAD risk factors or severe comorbid conditions might mask the effect of PAH metabolites on HRV in high-risk individuals, and PAH metabolites only had obvious effects on HRV in relatively healthy subjects (low-risk). Our study did not support previous evidence that high CAD risk profiles may confer susceptibility to metal particle-induced autonomic dysfunction.13 However, we could not directly compare our results with those of Chen et al.13 because of inherent differences in population characteristics, exposure composition and levels, and sample size. When subpopulations susceptible to HRV responses to air pollution have been identified, most previous epidemiologic studies only considered a single CAD risk factor, such as smoking, age, obesity, diabetes, and hypertension, and the results were varied.8–10 Meanwhile, when Chen et al.13 analyzed component risk factors of FRS independently, no single risk factor had a consistent pattern of associations with different HRV measures, but when the overall risk profile was analyzed, individual FRS showed a consistent effect on HRV measures. Therefore, the use of FRS to assess susceptibility is appealing: individual physiopathological characteristics more likely jointly, rather than independently, define the degree of susceptibility contributed by differential cardiovascular effect responses to PAH exposure with different CAD risk profiles. However, the precise molecular mechanisms underlying the observed effects are unclear.
The present study did have several limitations. First, this is a cross-sectional study, which limits the ability to demonstrate a causal relationship between exposure and outcome. Second, we restricted the analysis to participants with complete data, which may lead to bias if the missing values are not completely random. However, baseline characteristics were similar among subjects included in the analysis and those excluded. Third, we did not adjust for multiple testing because of a high correlation between multiple PAH parameters and multiple HRV measures. Over-adjustment for multiple comparisons may increase the type II error and reduce power to detect significant differences. Furthermore, monitoring of the 5-min HRV represents only short-term cardiac autonomic nerve modulation and the definitive clinical significance should be elucidated. Finally, although we have considered some important confounders in our analysis, other unmeasured or unselected covariates such as genetic factors and home cooking exposures were not included in our study.
CONCLUSIONS
In this study, we observed that environmental PAH metabolites and FRS adversely and independently affect HRV. In particular, a prominent effect of PAH exposure on HRV was found in the low-risk FRS subgroup, while the effect was not obvious in the high-risk subgroup. This provides new insight into the mechanism of the health effects caused by exposure to PAH based on an individual’s CAD risk profiles.
Supplementary Material
What this paper adds.
-
➢
Occupational polycyclic aromatic hydrocarbon (PAH) exposure has been associated with altered heart rate variability (HRV).
-
➢
However, the association between background PAH exposure and HRV has not been well studied in the general population.
-
➢
Increased PAH metabolites and FRS were both dose-responsive related to decreased HRV.
-
➢
Subjects in the low-risk FRS group had a greater reduced HRV response to PAH metabolites.
Acknowledgements
We are particularly grateful to all volunteers for participating in the present study.
Funding XZ is supported by Program for New Century Excellent Talents in University (NCET-10-0420) and the National Natural Science Foundation of China (NNSFC 81373093); TW is funded by the National Key Basic Research and Development Program (973 project, grant no. 2011CB503806 and 2011CB512102). DC was supported by NIH(NIEHS) grant ES00002.
Abbreviations
- BMI
body mass index
- CAD
coronary artery disease
- FRS
Framingham risk score
- HDL
high-density lipoprotein
- HF
high frequency
- HRV
heart rate variability
- LDL
low-density lipoprotein
- LF
low frequency
- LF/HF
LF to HF ratio
- LOD
limits of detection
- 3-OHBaP
3-hydroxybenzo[a]pyrene
- 6-OHChr
6-hydroxychrysene
- 2-OHFlu
2-hydroxyfluorene
- 9-OHFlu
9-hydroxyfluorene
- ΣOHFlu
total concentration of hydroxyfluorene
- 1-OHNa
1-hydroxynaphthalene
- 2-OHNa
2-hydroxynaphthalene
- ΣOHNa
total concentration of hydroxynaphthalene
- ΣOH-PAHs
total concentration of all PAHs metabolites
- 1-OHPh
1-hydroxyphenanthrene
- 2-OHPh
2-hydroxyphenanthrene
- 3-OHPh
3-hydroxyphenanthrene
- 4-OHPh
4-hydroxyphenanthrene
- 9-OHPh
9-hydroxyphenanthrene
- ΣOHPh
total concentration of hydroxyphenanthrene
- OH-PAH
monohydroxylated PAH
- PAH
polycyclic aromatic hydrocarbons
- rMSSD
root mean square of successive differences in adjacent NN intervals
- SDNN
standard deviation of NN intervals
- TP
total power.
Footnotes
Contributors YF, HS, and YS contributed equally to this work. YF, HS, YS, and XZ designed the study. YF, HS, and YS analyzed the data and wrote the first draft. JB, XH, and JY collected data and participated in data interpretation. JY, WC, DCC, TW, and XZ contributed to manuscript revision.
Competing interests None.
REFERENCES
- 1.Xu X, Cook RL, Ilacqua VA, Kan H, Talbott EO, Kearney G. Studying associations between urinary metabolites of polycyclic aromatic hydrocarbons (PAHs) and cardiovascular diseases in the United States. Sci Total Environ. 2010;408:4943–4948. doi: 10.1016/j.scitotenv.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 2.International Programme on Chemical Safety (IPCS) Selected non-heterocyclic polycyclic aromatic hydrocarbons. Geneva: WHO; 1998. Environmental health criteria 202. [Google Scholar]
- 3.Suzuki K, Yoshinaga J. Inhalation and dietary exposure to polycyclic aromatic hydrocarbons and urinary 1-hydroxypyrene in non-smoking university students. Int Arch Occup Environ Health. 2007;81:115–121. doi: 10.1007/s00420-007-0188-x. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Romanoff LC, Lewin MD, et al. Variability of urinary concentrations of polycyclic aromatic hydrocarbon metabolite in general population and comparison of spot, first-morning, and 24-h void sampling. J Expo Sci Environ Epidemiol. 2010;20:526–535. doi: 10.1038/jes.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riojas-Rodriguez H, Schilmann A, Marron-Mares AT, et al. Impact of the improved patsari biomass stove on urinary polycyclic aromatic hydrocarbon biomarkers and carbon monoxide exposures in rural Mexican women. Environ Health Perspect. 2011;119:1301–1307. doi: 10.1289/ehp.1002927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo CS, Lee K, Yi SH, Kim JS, Kim HC. Association of heart rate variability with the framingham risk score in healthy adults. Korean J Fam Med. 2011;32:334–340. doi: 10.4082/kjfm.2011.32.6.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Feng Y, Deng H, et al. The dose-response decrease in heart rate variability: any association with the metabolites of polycyclic aromatic hydrocarbons in coke oven workers? PLoS One. 2012;7:e44562. doi: 10.1371/journal.pone.0044562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jia X, Song X, Shima M, Tamura K, Deng F, Guo X. Effects of fine particulate on heart rate variability in Beijing: a panel study of healthy elderly subjects. Int Arch Occup Environ Health. 2012;85:97–107. doi: 10.1007/s00420-011-0646-3. [DOI] [PubMed] [Google Scholar]
- 9.Whitsel EA, Quibrera PM, Christ SL, et al. Heart rate variability, ambient particulate matter air pollution, and glucose homeostasis: the environmental epidemiology of arrhythmogenesis in the women's health initiative. Am J Epidemiol. 2009;169:693–703. doi: 10.1093/aje/kwn400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JC, Cavallari JM, Stone PH, Christiani DC. Obesity is a modifier of autonomic cardiac responses to fine metal particulates. Environ Health Perspect. 2007;115:1002–1006. doi: 10.1289/ehp.9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wheeler A, Zanobetti A, Gold DR, Schwartz J, Stone P, Suh HH. The relationship between ambient air pollution and heart rate variability differs for individuals with heart and pulmonary disease. Environ Health Perspect. 2006;114:560–566. doi: 10.1289/ehp.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luttmann-Gibson H, Suh HH, Coull BA, et al. Systemic inflammation, heart rate variability and air pollution in a cohort of senior adults. Occup Environ Med. 2010;67:625–630. doi: 10.1136/oem.2009.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen JC, Stone PH, Verrier RL, et al. Personal coronary risk profiles modify autonomic nervous system responses to air pollution. J Occup Environ Med. 2006;48:1133–1142. doi: 10.1097/01.jom.0000245675.85924.7e. [DOI] [PubMed] [Google Scholar]
- 14.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 15.Qian Z, He Q, Lin HM, et al. Association of daily cause-specific mortality with ambient particle air pollution in Wuhan, China. Environ Res. 2007;105:380–389. doi: 10.1016/j.envres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Xiang H, Mertz KJ, Arena VC, et al. Estimation of short-term effects of air pollution on stroke hospital admissions in Wuhan, China. PLoS One. 2013;8:e61168. doi: 10.1371/journal.pone.0061168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 18.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 19.Li Z, Sandau CD, Romanoff LC, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ Res. 2008;107:320–331. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Onyemauwa F, Rappaport SM, Sobus JR, Gajdosova D, Wu R, Waidyanatha S. Using liquid chromatography-tandem mass spectrometry to quantify monohydroxylated metabolites of polycyclic aromatic hydrocarbons in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1117–1125. doi: 10.1016/j.jchromb.2009.02.067. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Senthilkumar K, Alomirah H, et al. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol. 2013;47:2932–2938. doi: 10.1021/es3052262. [DOI] [PubMed] [Google Scholar]
- 22.Fiala Z, Vyskocil A, Krajak V, et al. Environmental exposure of small children to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health. 2001;74:411–420. doi: 10.1007/s004200100239. [DOI] [PubMed] [Google Scholar]
- 23.Hansen AM, Mathiesen L, Pedersen M, Knudsen LE. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies--a review. Int J Hyg Environ Health. 2008;211:471–503. doi: 10.1016/j.ijheh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Grainger J, Huang W, Patterson DG, Jr, et al. Reference range levels of polycyclic aromatic hydrocarbons in the US population by measurement of urinary monohydroxy metabolites. Environ Res. 2006;100:394–423. doi: 10.1016/j.envres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 25.Kuusimaki L, Peltonen Y, Mutanen P, Peltonen K, Savela K. Urinary hydroxy-metabolites of naphthalene, phenanthrene and pyrene as markers of exposure to diesel exhaust. Int Arch Occup Environ Health. 2004;77:23–30. doi: 10.1007/s00420-003-0477-y. [DOI] [PubMed] [Google Scholar]
- 26.Jia C, Batterman S. A critical review of naphthalene sources and exposures relevant to indoor and outdoor air. Int J Environ Res Public Health. 2010;7:2903–2939. doi: 10.3390/ijerph7072903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strunk P, Ortlepp K, Heinz H, Rossbach B, Angerer J. Ambient and biological monitoring of coke plant workers -- determination of exposure to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health. 2002;75:354–358. doi: 10.1007/s00420-001-0305-1. [DOI] [PubMed] [Google Scholar]
- 28.Lee CY, Lee JY, Kang JW, Kim H. Effects of genetic polymorphisms of CYP1A1, CYP2E1, GSTM1, and GSTT1 on the urinary levels of 1-hydroxypyrene and 2-naphthol in aircraft maintenance workers. Toxicol Lett. 2001;123:115–124. doi: 10.1016/s0378-4274(01)00374-5. [DOI] [PubMed] [Google Scholar]
- 29.Nan HM, Kim H, Lim HS, et al. Effects of occupation, lifestyle and genetic polymorphisms of CYP1A1, CYP2E1, GSTM1 and GSTT1 on urinary 1-hydroxypyrene and 2-naphthol concentrations. Carcinogenesis. 2001;22:787–793. doi: 10.1093/carcin/22.5.787. [DOI] [PubMed] [Google Scholar]
- 30.Preuss R, Angerer J. Simultaneous determination of 1- and 2-naphthol in human urine using on-line clean-up column-switching liquid chromatography-fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;801:307–316. doi: 10.1016/j.jchromb.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 31.Rossella F, Campo L, Pavanello S, Kapka L, Siwinska E, Fustinoni S. Urinary polycyclic aromatic hydrocarbons and monohydroxy metabolites as biomarkers of exposure in coke oven workers. Occup Environ Med. 2009;66:509–516. doi: 10.1136/oem.2008.042796. [DOI] [PubMed] [Google Scholar]
- 32.Registry AfTSaD. Toxicological profile for naphthalene, 1-methylnaphthalene, and 2-methylnaphthalene. [Accessed 2013 June 12];2005 Available: http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=240&tid=43.
- 33.Grova N, Salquebre G, Schroeder H, Appenzeller BM. Determination of PAHs and OH-PAHs in rat brain by gas chromatography tandem (triple quadrupole) mass spectrometry. Chem Res Toxicol. 2011;24:1653–1667. doi: 10.1021/tx2003596. [DOI] [PubMed] [Google Scholar]
- 34.EPA. Atlanta, GA: 2012. Fluorene. http://www.epa.gov/osw/hazard/wastemin/minimize/factshts/flourene.pdf. [Google Scholar]
- 35.EPA. Atlanta, GA: 2012. Phenanthrene. http://www.epa.gov/osw/hazard/wastemin/minimize/factshts/phenanth.pdf. [Google Scholar]
- 36.Xu X, Hu H, Kearney GD, Kan H, Sheps DS. Studying the effects of polycyclic aromatic hydrocarbons on peripheral arterial disease in the United States. Sci Total Environ. 2013:461–462. 341–347. doi: 10.1016/j.scitotenv.2013.04.089. [DOI] [PubMed] [Google Scholar]
- 37.Lee MS, Magari S, Christiani DC. Cardiac autonomic dysfunction from occupational exposure to polycyclic aromatic hydrocarbons. Occup Environ Med. 2011;68:474–478. doi: 10.1136/oem.2010.055681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito Y, Son M, Sato S, et al. Effects of fluoranthene, a polycyclic aromatic hydrocarbon, on cAMP-dependent anion secretion in human airway epithelia. J Pharmacol Exp Ther. 2004;308:651–657. doi: 10.1124/jpet.103.059089. [DOI] [PubMed] [Google Scholar]
- 39.Everett CJ, King DE, Player MS, Matheson EM, Post RE, Mainous AG., 3rd Association of urinary polycyclic aromatic hydrocarbons and serum C-reactive protein. Environ Res. 2010;110:79–82. doi: 10.1016/j.envres.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Tsuji H, Larson MG, Venditti FJ, Jr, et al. Impact of reduced heart rate variability on risk for cardiac events. The Framingham Heart Study. Circulation. 1996;94:2850–2855. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 41.Jelinek HF, Md Imam H, Al-Aubaidy H, Khandoker AH. Association of cardiovascular risk using non-linear heart rate variability measures with the framingham risk score in a rural population. Front Physiol. 2013;4:186. doi: 10.3389/fphys.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.