Abstract
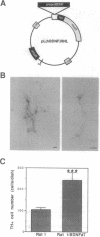
The trophism of brain-derived neurotrophic factor (BDNF) for dopaminergic cells in culture has led to significant interest in the role of BDNF in the etiology and potential treatment of Parkinson disease. Previous in vivo investigation of BDNF delivery to axotomized substantia nigra dopaminergic neurons in the adult rat has shown no protective effect. In this study, we produced nigral degeneration by infusing 1-methyl-4-phenylpyridinium (MPP+), a mitochondrial complex I inhibitor and the active metabolite of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP), into the rat striatum. The subsequent loss of nigral neurons was presumably due to mitochondrial toxicity after MPP+ uptake and retrograde transport to the substantia nigra. We engineered immortalized rat fibroblasts to secrete human BDNF and implanted these cells near the substantia nigra 7 days before striatal MPP+ infusion. We found that BDNF-secreting fibroblasts markedly increased nigral dopaminergic neuronal survival when compared to control fibroblast implants. The observation that BDNF prevents MPTP-induced dopaminergic neuronal degeneration in the adult brain has significance for the treatment of neurodegenerative disorders, which may involve mitochondrial dysfunction, such as Parkinson disease.
Full text
PDF
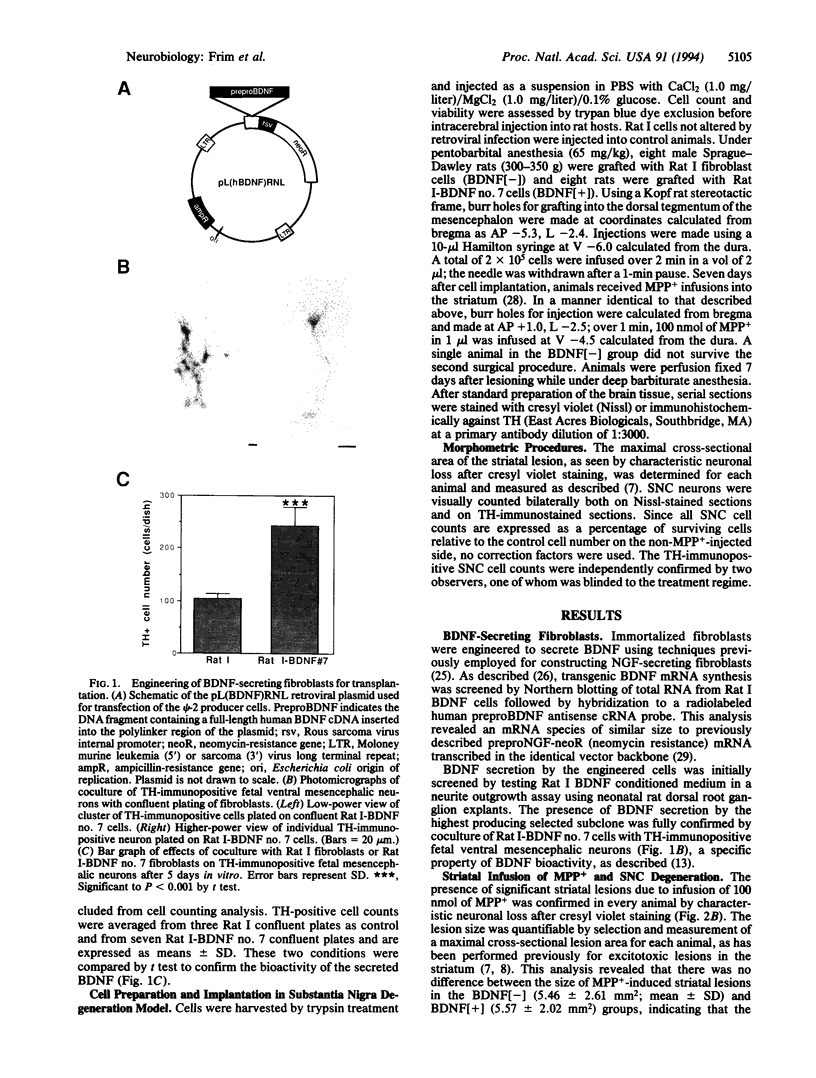
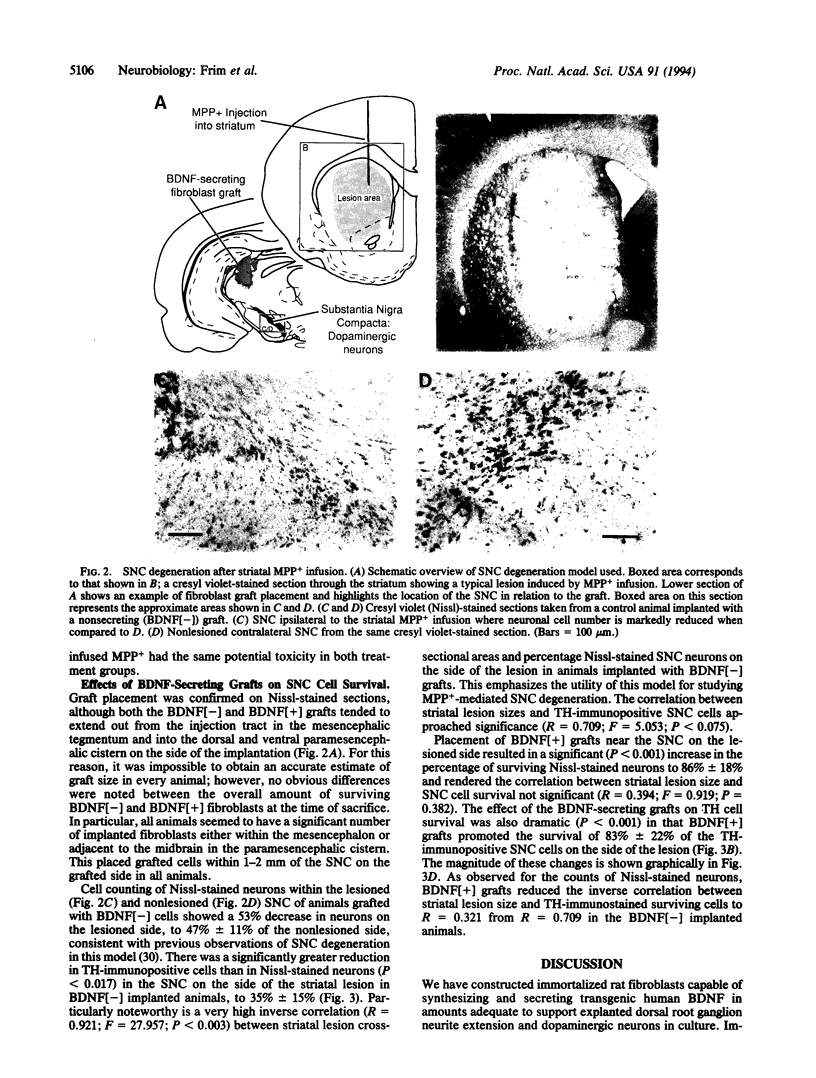
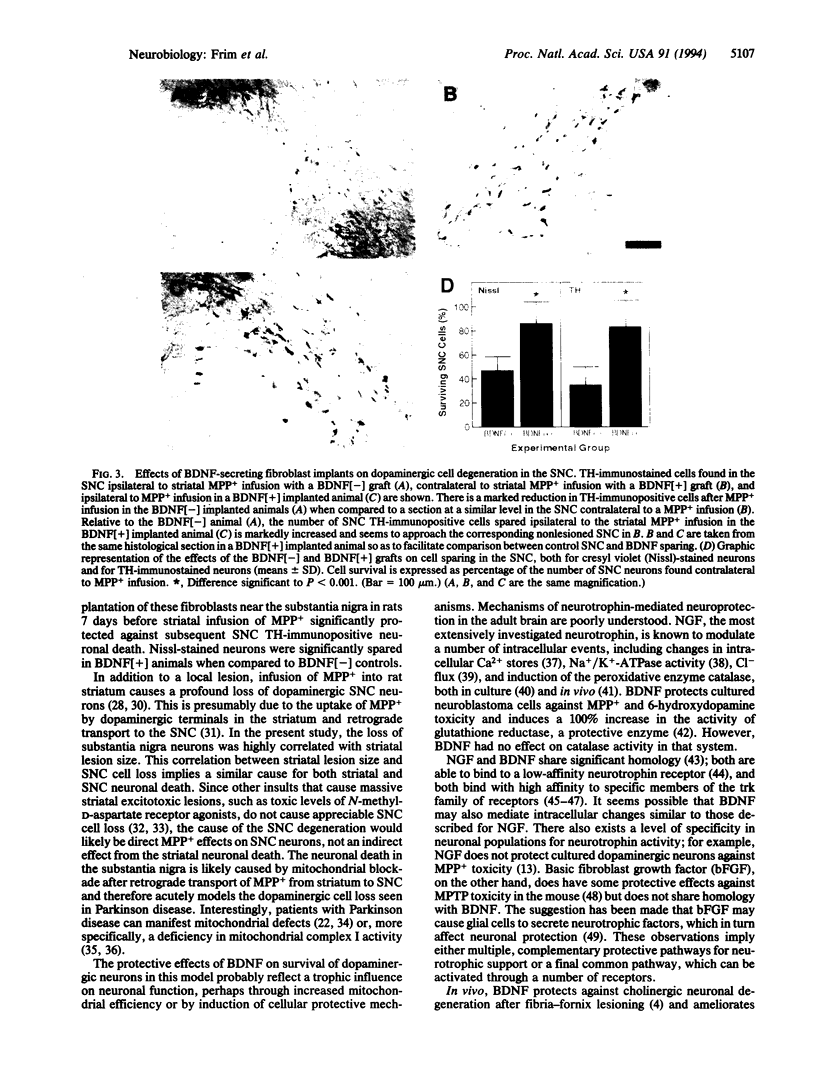

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. J., Dam D., Lee S., Cotman C. W. Basic fibroblast growth factor prevents death of lesioned cholinergic neurons in vivo. Nature. 1988 Mar 24;332(6162):360–361. doi: 10.1038/332360a0. [DOI] [PubMed] [Google Scholar]
- Barde Y. A., Edgar D., Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M. F. Does impairment of energy metabolism result in excitotoxic neuronal death in neurodegenerative illnesses? Ann Neurol. 1992 Feb;31(2):119–130. doi: 10.1002/ana.410310202. [DOI] [PubMed] [Google Scholar]
- Bloem B. R., Irwin I., Buruma O. J., Haan J., Roos R. A., Tetrud J. W., Langston J. W. The MPTP model: versatile contributions to the treatment of idiopathic Parkinson's disease. J Neurol Sci. 1990 Jul;97(2-3):273–293. doi: 10.1016/0022-510x(90)90225-c. [DOI] [PubMed] [Google Scholar]
- Brennan W. A., Jr, Bird E. D., Aprille J. R. Regional mitochondrial respiratory activity in Huntington's disease brain. J Neurochem. 1985 Jun;44(6):1948–1950. doi: 10.1111/j.1471-4159.1985.tb07192.x. [DOI] [PubMed] [Google Scholar]
- Campbell K. J., Takada M., Hattori T. Evidence for retrograde axonal transport of MPP+ in the rat. Neurosci Lett. 1990 Oct 16;118(2):151–154. doi: 10.1016/0304-3940(90)90614-f. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988 Oct;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Engele J., Bohn M. C. The neurotrophic effects of fibroblast growth factors on dopaminergic neurons in vitro are mediated by mesencephalic glia. J Neurosci. 1991 Oct;11(10):3070–3078. doi: 10.1523/JNEUROSCI.11-10-03070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frim D. M., Short M. P., Rosenberg W. S., Simpson J., Breakefield X. O., Isacson O. Local protective effects of nerve growth factor-secreting fibroblasts against excitotoxic lesions in the rat striatum. J Neurosurg. 1993 Feb;78(2):267–273. doi: 10.3171/jns.1993.78.2.0267. [DOI] [PubMed] [Google Scholar]
- Frim D. M., Uhler T. A., Short M. P., Ezzedine Z. D., Klagsbrun M., Breakefield X. O., Isacson O. Effects of biologically delivered NGF, BDNF and bFGF on striatal excitotoxic lesions. Neuroreport. 1993 Apr;4(4):367–370. doi: 10.1097/00001756-199304000-00006. [DOI] [PubMed] [Google Scholar]
- Hagg T., Varon S. Ciliary neurotrophic factor prevents degeneration of adult rat substantia nigra dopaminergic neurons in vivo. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6315–6319. doi: 10.1073/pnas.90.13.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M. M., Barde Y. A. Brain-derived neurotrophic factor prevents neuronal death in vivo. Nature. 1988 Jan 21;331(6153):261–262. doi: 10.1038/331261a0. [DOI] [PubMed] [Google Scholar]
- Hyman C., Hofer M., Barde Y. A., Juhasz M., Yancopoulos G. D., Squinto S. P., Lindsay R. M. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991 Mar 21;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- Isacson O., Brundin P., Gage F. H., Björklund A. Neural grafting in a rat model of Huntington's disease: progressive neurochemical changes after neostriatal ibotenate lesions and striatal tissue grafting. Neuroscience. 1985 Dec;16(4):799–817. doi: 10.1016/0306-4522(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Isacson O. On neuronal health. Trends Neurosci. 1993 Aug;16(8):306–308. doi: 10.1016/0166-2236(93)90104-t. [DOI] [PubMed] [Google Scholar]
- Jackson G. R., Apffel L., Werrbach-Perez K., Perez-Polo J. R. Role of nerve growth factor in oxidant-antioxidant balance and neuronal injury. I. Stimulation of hydrogen peroxide resistance. J Neurosci Res. 1990 Mar;25(3):360–368. doi: 10.1002/jnr.490250313. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Koike T., Franklin J. A "calcium set-point hypothesis" of neuronal dependence on neurotrophic factor. Exp Neurol. 1992 Jan;115(1):163–166. doi: 10.1016/0014-4886(92)90242-i. [DOI] [PubMed] [Google Scholar]
- Knüsel B., Beck K. D., Winslow J. W., Rosenthal A., Burton L. E., Widmer H. R., Nikolics K., Hefti F. Brain-derived neurotrophic factor administration protects basal forebrain cholinergic but not nigral dopaminergic neurons from degenerative changes after axotomy in the adult rat brain. J Neurosci. 1992 Nov;12(11):4391–4402. doi: 10.1523/JNEUROSCI.12-11-04391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kromer L. F. Nerve growth factor treatment after brain injury prevents neuronal death. Science. 1987 Jan 9;235(4785):214–216. doi: 10.1126/science.3798108. [DOI] [PubMed] [Google Scholar]
- Lams B. E., Isacson O., Sofroniew M. V. Loss of transmitter-associated enzyme staining following axotomy does not indicate death of brainstem cholinergic neurons. Brain Res. 1988 Dec 20;475(2):401–406. doi: 10.1016/0006-8993(88)90635-x. [DOI] [PubMed] [Google Scholar]
- Leibrock J., Lottspeich F., Hohn A., Hofer M., Hengerer B., Masiakowski P., Thoenen H., Barde Y. A. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989 Sep 14;341(6238):149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- Mizuno Y., Ohta S., Tanaka M., Takamiya S., Suzuki K., Sato T., Oya H., Ozawa T., Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson's disease. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1450–1455. doi: 10.1016/0006-291x(89)91141-8. [DOI] [PubMed] [Google Scholar]
- Morgan D. G. Considerations in the treatment of neurological disorders with trophic factors. Neurobiol Aging. 1989 Sep-Oct;10(5):547–553. doi: 10.1016/0197-4580(89)90126-7. [DOI] [PubMed] [Google Scholar]
- Otto D., Unsicker K. Basic FGF reverses chemical and morphological deficits in the nigrostriatal system of MPTP-treated mice. J Neurosci. 1990 Jun;10(6):1912–1921. doi: 10.1523/JNEUROSCI.10-06-01912.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker W. D., Jr, Boyson S. J., Parks J. K. Abnormalities of the electron transport chain in idiopathic Parkinson's disease. Ann Neurol. 1989 Dec;26(6):719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tébar A., Dechant G., Barde Y. A. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990 Apr;4(4):487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Rothman S. M. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J Neurosci. 1985 Jun;5(6):1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Clark J. B., Jenner P., Marsden C. D. Mitochondrial complex I deficiency in Parkinson's disease. J Neurochem. 1990 Mar;54(3):823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schumacher J. M., Short M. P., Hyman B. T., Breakefield X. O., Isacson O. Intracerebral implantation of nerve growth factor-producing fibroblasts protects striatum against neurotoxic levels of excitatory amino acids. Neuroscience. 1991;45(3):561–570. doi: 10.1016/0306-4522(91)90271-o. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Gnahn H., Wakade A., Thoenen H. Is activation of the Na+K+ pump necessary for NGF-mediated neuronal survival? J Neurosci. 1988 Feb;8(2):458–462. doi: 10.1523/JNEUROSCI.08-02-00458.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendtner M., Holtmann B., Kolbeck R., Thoenen H., Barde Y. A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992 Dec 24;360(6406):757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Schmalbruch H., Stöckli K. A., Carroll P., Kreutzberg G. W., Thoenen H. Ciliary neurotrophic factor prevents degeneration of motor neurons in mouse mutant progressive motor neuronopathy. Nature. 1992 Aug 6;358(6386):502–504. doi: 10.1038/358502a0. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Watts R. L., Juncos J. L., Torroni A., Wallace D. C. Mitochondrial oxidative phosphorylation defects in Parkinson's disease. Ann Neurol. 1991 Sep;30(3):332–339. doi: 10.1002/ana.410300304. [DOI] [PubMed] [Google Scholar]
- Simpson J. R., Isacson O. Mitochondrial impairment reduces the threshold for in vivo NMDA-mediated neuronal death in the striatum. Exp Neurol. 1993 May;121(1):57–64. doi: 10.1006/exnr.1993.1071. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Galletly N. P., Isacson O., Svendsen C. N. Survival of adult basal forebrain cholinergic neurons after loss of target neurons. Science. 1990 Jan 19;247(4940):338–342. doi: 10.1126/science.1688664. [DOI] [PubMed] [Google Scholar]
- Sofroniew M. V., Isacson O. Distribution of degeneration of cholinergic neurons in the septum following axotomy in different portions of the fimbria-fornix: a correlation between degree of cell loss and proximity of neuronal somata to the lesion. J Chem Neuroanat. 1988 Nov-Dec;1(6):327–337. [PubMed] [Google Scholar]
- Soppet D., Escandon E., Maragos J., Middlemas D. S., Reid S. W., Blair J., Burton L. E., Stanton B. R., Kaplan D. R., Hunter T. The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell. 1991 May 31;65(5):895–903. doi: 10.1016/0092-8674(91)90396-g. [DOI] [PubMed] [Google Scholar]
- Spina M. B., Squinto S. P., Miller J., Lindsay R. M., Hyman C. Brain-derived neurotrophic factor protects dopamine neurons against 6-hydroxydopamine and N-methyl-4-phenylpyridinium ion toxicity: involvement of the glutathione system. J Neurochem. 1992 Jul;59(1):99–106. doi: 10.1111/j.1471-4159.1992.tb08880.x. [DOI] [PubMed] [Google Scholar]
- Squinto S. P., Stitt T. N., Aldrich T. H., Davis S., Bianco S. M., Radziejewski C., Glass D. J., Masiakowski P., Furth M. E., Valenzuela D. M. trkB encodes a functional receptor for brain-derived neurotrophic factor and neurotrophin-3 but not nerve growth factor. Cell. 1991 May 31;65(5):885–893. doi: 10.1016/0092-8674(91)90395-F. [DOI] [PubMed] [Google Scholar]
- Storey E., Hyman B. T., Jenkins B., Brouillet E., Miller J. M., Rosen B. R., Beal M. F. 1-Methyl-4-phenylpyridinium produces excitotoxic lesions in rat striatum as a result of impairment of oxidative metabolism. J Neurochem. 1992 May;58(5):1975–1978. doi: 10.1111/j.1471-4159.1992.tb10080.x. [DOI] [PubMed] [Google Scholar]
- Sutter A., Riopelle R. J., Harris-Warrick R. M., Shooter E. M. Nerve growth factor receptors. Characterization of two distinct classes of binding sites on chick embryo sensory ganglia cells. J Biol Chem. 1979 Jul 10;254(13):5972–5982. [PubMed] [Google Scholar]
- Tuszynski M. H., U H. S., Amaral D. G., Gage F. H. Nerve growth factor infusion in the primate brain reduces lesion-induced cholinergic neuronal degeneration. J Neurosci. 1990 Nov;10(11):3604–3614. doi: 10.1523/JNEUROSCI.10-11-03604.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D. C. Mitochondrial genetics: a paradigm for aging and degenerative diseases? Science. 1992 May 1;256(5057):628–632. doi: 10.1126/science.1533953. [DOI] [PubMed] [Google Scholar]
- Wolf D., Richter-Landsberg C., Short M. P., Cepko C., Breakefield X. O. Retrovirus-mediated gene transfer of beta-nerve growth factor into mouse pituitary line AtT-20. Mol Biol Med. 1988 Feb;5(1):43–59. [PubMed] [Google Scholar]
- Yan Q., Elliott J., Snider W. D. Brain-derived neurotrophic factor rescues spinal motor neurons from axotomy-induced cell death. Nature. 1992 Dec 24;360(6406):753–755. doi: 10.1038/360753a0. [DOI] [PubMed] [Google Scholar]