Abstract
Background
The auditory P3 event-related potential (ERP) is thought to index cognitive processing relevant to attention and working memory processes. Drug challenge studies suggest that glutamate neurotransmission plays an important role in modulating P3 ERP. However, while direct links between glutamate activity and P3 ERP response in humans are suspected, mechanistic details remain largely unknown. We investigated here the relationships between P3 ERP and indices of glutamatergic processing measured in vivo with proton magnetic resonance spectroscopy (1H MRS). We hypothesized that a higher index of glutamatergic processing (Glutamine/Glutamate ratio; abbreviated Gln/Glu) in the anterior cingulate (ACC) and in the parietal-occipital (POC) cortices would associate with larger frontal P3a and parietal P3b amplitudes, respectively.
Methods
Frontal P3a (Fz) and parietal P3b (Pz) were collected from 32 healthy participants who performed an auditory oddball task. Resting glutamate (Glu), glutamine (Gln), and Gln/Glu (an index of glutamatergic processing) measures were obtained on a 4 Tesla MR scanner using J-resolved MR spectroscopy. Linear regression and partial correlations were used for statistical analysis.
Results
Significant positive correlations were found between frontal P3a amplitude and ACC Gln/Glu ratio (partial R=0.57; P=0.001) and between frontal P3a amplitude and ACC Gln concentration (partial R=0.43; P=0.02). Relationships between parietal P3b and the glutamate indices in the POC were not significant.
Conclusions
These results indicate a specific connection between an index of glutamate neurotransmitter function in ACC and frontal P3 ERP, providing a novel insight into the neurochemistry underlying scalp recorded EEG response. Abnormalities in glutamate neurotransmission have been observed in schizophrenia and other psychiatric conditions and may underlie illness related deficits of P3 ERP.
Keywords: P3, Event-Related Potential, magnetic resonance spectroscopy (MRS), glutamate
Introduction
Event-related potentials (ERPs) are the brain’s response to specific sensory or cognitive events. The P3 ERP has two main components: an earlier, more frontal P3a and a later, more parietal P3b (Polich, 2007). The frontal P3a is associated with the involuntary capture of attention or “bottom-up” orienting processes (Friedman et al., 2001). One of the primary generators of the scalp-recorded component comes from the anterior cingulate cortex (ACC) (Dien et al., 2003), although other regions, including the medial prefrontal cortex and lateral prefrontal cortex, are also involved (Knight et al., 1989). The parietal P3b is associated with the updating of working memory (Donchin and Coles, 1988). Evidence suggests that several regions are involved in generating the P3b, including the temporal-parietal, ventral temporo-frontal, and hippocampus areas (Halgren et al., 1998, Polich and Criado, 2006).
Deficits in both P3a and P3b ERP components have been proposed as biomarkers for drug discovery in psychiatric disorders (Mathalon et al., 2000, Javitt et al., 2008, Hall et al., 2009). However, the neurotransmitter systems underlying P3 generation remain elusive. Although studies have found that P3a and P3b are partially mediated by dopaminergic (Polich and Criado, 2006) and noradrenergic activity (Nieuwenhuis et al., 2005), respectively, evidence suggests that the glutamatergic system is also involved. For example, the noncompetitive N-methyl-D-aspartate (NMDA) glutamate receptor antagonist ketamine attenuates P3a and P3b amplitudes (Oranje et al., 2000), and N-acetylcysteine (NAC), a stimulator of the cystine-glutamate exchanger, increases P3 amplitude (Gunduz-Bruce et al., 2012). While these studies suggest glutamatergic processing plays an important role in mediating the P3 ERP, the link between changes in glutamate neurotransmission and the P3 ERP in humans has not been examined.
Glutamatergic processing can be quantified using glutamine (Gln), glutamate (Glu), the sum of glutamate and glutamine (Glx), or the glutamine /glutamate (Gln/Glu) ratio (Posse et al., 2007, Jensen et al., 2009). Following its release to the synaptic cleft, Glu is taken up into astrocytes where it is converted to Gln, released, and accumulated by neurons. Recycled Gln is the precursor for synaptic Glu (Pellerin and Magistretti, 2004, Schousboe and Waagepetersen, 2005). In addition to the glutamine-glutamate cycling rate, activity-dependent changes in glutamatergic neurotransmission also alter tissue levels of Gln and Glu and these parameters are readily measured using proton-magnetic resonance spectroscopy (1H-MRS). The MRS signal arises from all free Gln and Glu, not only those at the synapse. Because they also play roles in intermediary metabolism, Gln and Glu measurements alone are not a particularly useful index of synaptic glutamatergic measure. On the other hand, the Gln/Glu ratio is more specific as a synaptic measure because it reflects the relative amounts of metabolites. Given Gln synthesis in glial cells and Glu synthesis in neurons, the Gln/Glu ratio is a potentially useful index for quantifying neuronal-glial interactions and the balance of glutamatergic metabolites. In this context, Gln/Glu elevations and reductions may reflect increased and decreased Glu neurotransmission, respectively.
In the present study, we tested the hypothesis that glutamate neurotransmission measured using 1H-MRS is associated with the magnitude of the P3 ERP. We hypothesized that frontal P3a amplitude (at Fz) would be positively correlated with glutamatergic function in the ACC and parietal P3b amplitude (at Pz) would be positively correlated with glutamatergic function in parietal-occipital cortex (POC).
Methods
Participants
Thirty-two healthy subjects (11 men, 21 women, mean age, 27 yr [SD 6.5]) participated in the study. Subjects were recruited through local advertisements. Participants were assessed using the Structured Clinical Interview for Axis I disorders (SCID-I) (First et al., 1997) and were included only if they met the following criteria: no DSM-IV Axis I disorder, no history of substance dependence, no known neurological disorder, no prior head injury with loss of consciousness, and no first-degree relative with a history of psychosis or bipolar disorder. All participants in the study were non-smokers and had normal intellectual ability based on the North American Adult Reading Test (NAART). This study was approved by the McLean Hospital Institutional Review Board. EEG and MRS data were acquired on separate days (mean number of days between EEG and MRS = 34 [SD 22.6], range 0–95 days).
EEG Methods
The EEG was recorded (DC-100 Hz bandpass filter, 512 Hz digitization rate) with an Active-Two system (Biosemi B.V.) using active electrodes in an electrode cap at 18 scalp sites. During data acquisition the DC offsets were kept below 25 mV and all channels were referred to the system’s internal loop (CMS/DRL electrodes). Blinks and eye movements were monitored through electrodes placed on the left temple and above and below the left eye for horizontal and vertical EOGs. The EEG data were re-referenced offline to the averaged mastoid. Epoching and filtering of continuous EEG data were performed with BrainVision Analyzer 2.0.1 (Brain Products GmbH).
P3a and P3b ERPs were elicited from an auditory Oddball paradigm (400 binaural tones; 50-msec duration, 5 ms rise/fall times; 15% 1500 Hz target tones; 85% 1000 Hz standard tones). Inter-stimulus Interval (ISI) was variable between 1.8 and 2.2 s. All participants had >95% accuracy counting the target tones. Signal processing was performed off-line using Brain Vision Analyzer software (Brain Products, Inc, 2000). EEG data were segmented (−100 to 1000 ms), filtered 0.1–8.5Hz), baseline corrected, eye-blink corrected using (Gratton et al., 1983), and artifact rejected if activity exceeding >100 μV. P3a and P3b amplitudes were automatically detected from the average waveforms for target tones between 280 and 650 ms at the Fz and Pz sites, respectively (Dien et al., 2003, Hall et al., 2009).
MRS Methods
We focused on the anterior cingulate (ACC) and the parietal occipital cortex (POC) regions because they are implicated in the pathophysiology of schizophrenia (Carter et al., 1997, Turner et al., 2013). Also, ACC is one of the primary neuronal sources of the frontal P3a (Dien et al., 2003) and POC is implicated in generating P3b ERPs (Halgren et al., 1998, Polich and Criado, 2006). The Gln/Glu ratio is somewhat more specific as a synaptic measure than a combined glutamate-glutamine (Glx) level because it reflects the relative amounts of metabolites (Xu et al., 2005, Iltis et al., 2009). A growing literature confirms that Gln/Glu is modulated by glutamatergic activity: elevations are seen during somatosensory stimulation in rodents (Xu et al., 2005) and experimentally induced ischemia (Igarashi et al., 2001, Mlynarik et al., 2008), with infusion of NMDA-receptor blockers phencyclidine in rodents (Iltis et al., 2009). An inhibitor of glutamine synthetase reduced Gln/Glu by 41–48% in an animal model of amyotrophic lateral sclerosis (Ghoddoussi et al., 2010). Finally, several groups, including us, have used Gln/Glu ratio extensively to characterize neurotransmission abnormalities in patients with schizophrenia (Bustillo et al., 2014), bipolar mania (Ongur et al., 2008) and in antidepressant treatment of bipolar depression (Brennan et al., 2010). Thus, we focused on the Gln/Glu ratio as our primary measure of glutamatergic processing but we also measured the Glu, Gln, and Glx.
MR Imaging
Imaging and spectroscopy was performed on a whole body 4-Tesla MR scanner (DirectDrive™, Agilent Technologies Inc, Santa Clara, CA) at McLean Hospital in Belmont, MA. Data collection utilized a birdcage-design, radio-frequency (RF) head coil operating at 170.3MHz for proton (XLR Imaging, London, Canada). Scout images confirmed optimal positioning, and unsuppressed water signal was shimmed to a global water linewidth of less than or equal to 25Hz. The water linewidths in the ACC voxel ranged from 8–14Hz and 7–11Hz for the POC voxel. High-contrast T1-weighted anatomical images were taken in the sagittal and axial planes for voxel positioning and image-based voxel tissue segmentation analysis.
Proton MRS
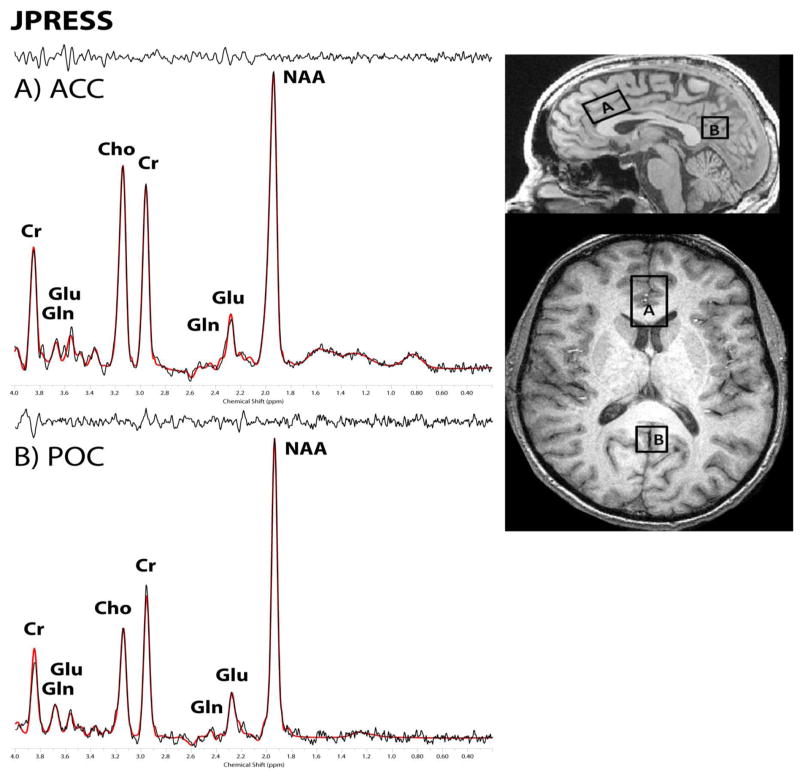
Single voxels in the bilateral dorsal ACC (35×25×20 mm - obliqued), and bilateral POC (20×20×20 mm - planar) (Figure 1) were placed using the anatomical image sets. Data quality is typically higher in the POC, permitting J-resolved data collection in a somewhat smaller voxel than in the ACC. Proton spectroscopy employed a modified PRESS (Bottomley, 1987) sequence which increments the TE time in 20ms steps ranging from 30–490ms in 24 steps (Jensen et al, 2009). The parameters for the J-resolved acquisition were: TR=2s, TE=30–490ms (20ms steps), NEX/step=16, spectral bandwidth=2kHz, duration = 13min.
Figure 1.
J-resolved (JPRESS) from a single participant in A) the dorsal anterior cingulate (ACC) and B) the parietal-occipital cortex (POC). All spectra are displayed with 1Hz exponential time-domain filtering, LCModel fit and residual.]
Proton MRS processing
The 24 TE-stepped free-induction decay series were zero-filled out to 64 points, Gaussian-filtered to minimize residual ringing from NAA and Cr signals, and Fourier-transformed in the TE dimension. This resulted in a set of 64 J-resolved spectra over 50 Hz. Using GAMMA-simulated J-resolved basis sets, every J-resolved spectral extraction throughout a bandwidth of 50 Hz was fit with its theoretically correct LCModel template (Provencher, 2001). The integrated area under the entire 2D surface for each metabolite then was calculated by summing the raw peak areas across all 64 J-resolved extractions for each metabolite.
Image segmentation
To ascertain gray and white matter contribution to each voxel, the axial T1-weighted images were segmented into gray matter, white matter, and cerebrospinal fluid compartments using FSL version 4.1 (FMRIB Software Library; Analysis Group, FMRIB; Oxford, UK) in combination with an in-house automated voxel co-registration and partial-volume analysis program.
Statistical Analysis
Linear regression and partial correlations having adjusted for the effects of covariance were used for assess the relationships between ERP measures and glutamate neurotransmitter levels using STATA (STATA version12; Stata Corp., College Station, TX). Our primary interests were the relationships between P3 ERPs and Gln/Glu ratio in the ACC and POC regions. In the exploratory analyses we examined the relationships between P3 ERPs and the Gln, Glu, or Glx measure. The effect of age, time difference between EEG and MRS testing dates (<100 days), and gray matter volume were included as covariates. Two a priori relationships tested were P3a and ACC Gln/GLu, as well as P3b and POC Gln/Glu. Significance level was set to be P< 0.025 (corrected for two hypotheses). In exploratory analyses we tested correlations between P3 ERPs and alternative glutamate measures (Gln, Glu, and Glx). They were not independent assessments to the primary analysis and they basically served to understand what is driving the relationship found. Significance level in these exploratory analyses was set to be p<0.05.
Results
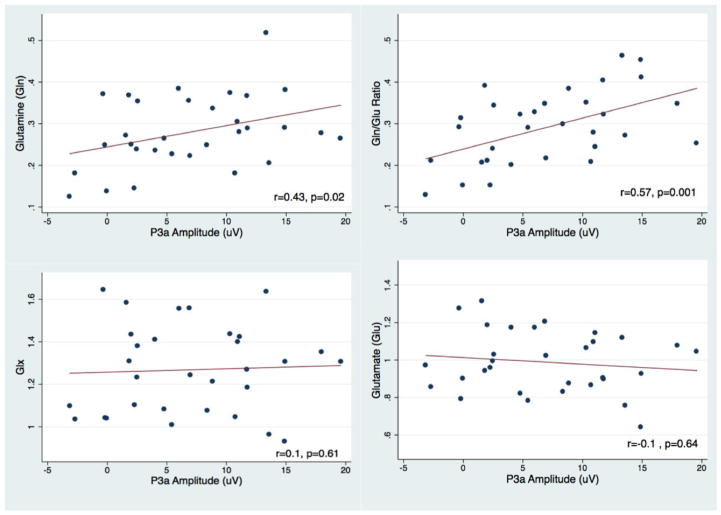
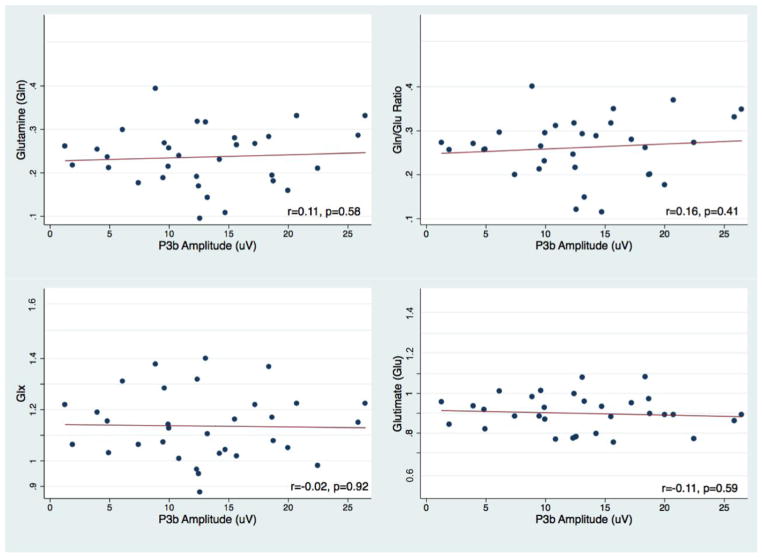
We present the MRS data characteristics of the sample in Supplementary Table 1. Data quality was high and consistent with previous reports. Figure 2 shows the averaged P3a and P3b ERPs. We excluded two outliers from the analyses, one in the ACC Gln and one in the POC Gln, because of technical artifacts during the MRS data acquisition. Thus results were based on a total of N=31. We found significant positive correlations between frontal P3a amplitude and Gln/Glu ratio (partial R=0.57; P=0.001) and between frontal P3a amplitude and Gln (partial R=0.43; P=0.02) in the ACC (see Figure 3). None of the confounding covariates were significantly correlated with P3 amplitudes. Correlations between parietal P3b and the Gln/Glu ratio in the POC were not statistically significant (Figure 4).
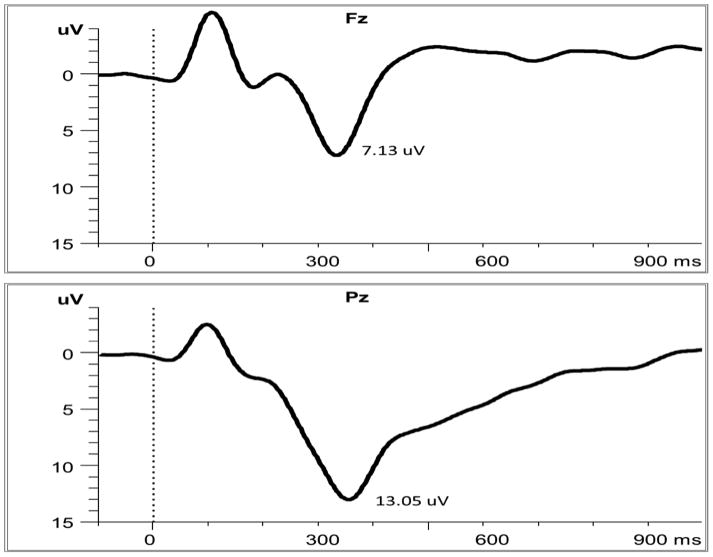
Figure 2.
Group averages of P3a at Fz (top) and P3b at Pz to target stimuli (bottom)
Figure 3.
Scatterplots of subjects excluding outliers. Frontal P3a amplitude and Anterior cingulate (ACC) glutamine (Gln) (Top left), Gln/Glu ratio (Top right), Glx (bottom left), and Glutamate (Glu) (bottom right).
Figure 4.
Scatterplots of subjects excluding outliers. Parietal P3b amplitude and posterior-occipital (POC) glutamine (Gln) (Top left), Gln/Glu ratio (Top right), Glx (bottom left), and Glutamate (Glu) (bottom right).
Discussion
In this study, we found, across participants, that ACC Gln/Glu was positively correlated with the frontal P3a amplitude elicited from by auditory oddball stimuli. This result suggests that P3a activity, in part, is mediated by the glutamatergic neurotransmitter system. This result is consistent with drug challenge studies showing strong NMDA receptor influence upon P3a amplitude (Oranje et al., 2000, Watson et al., 2009, Gunduz-Bruce et al., 2012). This result is also consistent with the study of Gallinat and colleagues who reported a significant positive correlation between glutamate concentration in the hippocampal region and scalp-recorded theta oscillations, a major component of the frontal P3a ERP (Gallinat et al., 2006). In our data we observed a significant correlation between P3a and evoked theta power (r=0.42, P=0.03), suggesting overlapping between the two, and a significant positive correlation between ACC Gln/Glu and evoked theta power at Fz site (Partial r=0.41, p=0.03), replicating Gallinat and colleagues’ finding. Glutamate is the most abundant neurotransmitter in the brain and glutamatergic neurotransmission plays a key role in brain function. Our results suggest that increased frontal synaptic glutamatergic neurotransmission in the healthy brain is associated with larger EEG responses.
Reports of MRS studies have used alternative measures to quantify glutamate concentration including glutamate, glutamine, or Glx (Marsman et al., 2013). In our sample we found that P3a amplitude is significantly correlated with glutamine concentration (partial R=0.43, p=0.02) but not with either glutamate or Glx. Our result thus provides evidence supporting the sensitivity of Gln/Glu as an index associated with cognitive processing.
In this study we did not observe a significant relationship between parietal P3b and glutamate level in the POC. Also, there were no significant associations between frontal P3a and any of the POC glutamate indices, suggesting some specificity of the P3a /ACC correlation. Although previous drug challenge studies reporting involvement of both the NMDA-glutamatergic and GABAergic receptor systems in modulating the P3b amplitude (Fowler and Mitchell, 1997, Watson et al., 2009), a visual oddball task was used in the these studies. Difference in experimental paradigms used between our study and others may account for the variable results.
The significant correlations we report rests upon between-subject differences that are conserved over considerable periods of time (many days). This suggests that individuals possess traits that are manifest in terms of background glutamatergic neurotransmission and electrophysiological responses to oddball stimuli. This is potentially important because it means that the MRS and EEG markers used in this study could, potentially, be used to endophenotype people with schizophrenia or related conditions. Note that we would not have observed any correlations had glutamate neurotransmission and P300s fluctuated within-subject in a state dependent fashion.
We used the auditory oddball task, which involves presentation of standard tones and infrequent target tones to elicit P3a. Principal component analysis studies have suggested that P3a and P3b are both elicited to varying degrees by infrequent novel and target stimuli (Spencer et al., 2001, Dien et al., 2004) and that the target P3 ERP measured at frontal Fz site include not only the P3a but also some P3b components (Kayser and Tenke, 2006). Unfortunately the auditory oddball task used in this study limited our ability to disentangle these overlapping sub-components. However, our findings that glutamatergic neurotransmitters partially contribute to the frontal P3a ERP still hold out. A more sensitive experimental paradigm, including infrequent target and novel stimuli, should be used in the future to separate the P3b and P3a subcomponents. Also, studies of MRS and EEG measures are typically collected separately. A desirable future direction is to acquire EEG-MRS data concurrently inside the MRI scanner as reported by Lally et al 2014 (Lally et al., 2014).
The significant relationship between frontal P3a and ACC glutamatergic processing may be an important consideration in studies of schizophrenia where glutamate abnormalities are thought to play an important role (Coyle, 2012, Moghaddam and Javitt, 2012). The link between glutamate-mediated frontal P3a activity suggests that the measurement of frontal P3a ERP may be useful within the context of pharmacological manipulations in clinical populations.
Supplementary Material
Supplementary Table: MRS Characteristics of the Sample
Highlights.
There is a specific connection between glutamate neurotransmitter function in the anterior cingulate cortex and frontal P3 ERP
Frontal P3 activity, in part, is mediated by glutamatergic neurotransmitters systems
Glutamine to glutamate ratio of proton-magnetic resonance spectroscopy (1H-MRS) is an index of glutamate function
Acknowledgments
This work was supported by the National Institute of Mental Health [1K01MH086714] and Brain and Behavior Research Foundation, National Alliance for Research on Schizophrenia and Depression (NARSAD) Sidney R. Baer, Jr. Foundation to M-HH, [K24MH094614] to JWS, [R21MH096107] and [R01MH094594] to DO, and US Dept. of Veterans Affairs CX000154 and [R01MH093450] to KMS.
Footnotes
Conflict of interest:
Dr. Ongur reported being on a Scientific Advisory Board for Lilly Inc. in 2013
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Annals of the New York Academy of Sciences. 1987;508:333–348. doi: 10.1111/j.1749-6632.1987.tb32915.x. [DOI] [PubMed] [Google Scholar]
- Brennan BP, Hudson JI, Jensen JE, McCarthy J, Roberts JL, Prescot AP, Cohen BM, Pope HG, Jr, Renshaw PF, Ongur D. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustillo JR, Chen H, Jones T, Lemke N, Abbott C, Qualls C, Canive J, Gasparovic C. Increased glutamine in patients undergoing long-term treatment for schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. JAMA Psychiatry. 2014;71:265–272. doi: 10.1001/jamapsychiatry.2013.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Mintun M, Nichols T, Cohen JD. Anterior cingulate gyrus dysfunction and selective attention deficits in schizophrenia: [15O]H2O PET study during single-trial Stroop task performance. Am J Psychiatry. 1997;154:1670–1675. doi: 10.1176/ajp.154.12.1670. [DOI] [PubMed] [Google Scholar]
- Coyle JT. NMDA receptor and schizophrenia: a brief history. Schizophr Bull. 2012;38:920–926. doi: 10.1093/schbul/sbs076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Localization of the event-related potential novelty response as defined by principal components analysis. Brain research Cognitive brain research. 2003;17:637–650. doi: 10.1016/s0926-6410(03)00188-5. [DOI] [PubMed] [Google Scholar]
- Dien J, Spencer KM, Donchin E. Parsing the late positive complex: mental chronometry and the ERP components that inhabit the neighborhood of the P300. Psychophysiology. 2004;41:665–678. doi: 10.1111/j.1469-8986.2004.00193.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 Component a Manifestation of Context Updating. Behavioral and Brain Sciences. 1988;11:357–374. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) New York: New York State Psychiatric Institute, Biometrics Research; 1997. [Google Scholar]
- Fowler B, Mitchell I. Biological determinants of P300: the effects of a barbiturate on latency and amplitude. Biological psychology. 1997;46:113–124. doi: 10.1016/s0301-0511(97)05253-8. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and biobehavioral reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Kunz D, Senkowski D, Kienast T, Seifert F, Schubert F, Heinz A. Hippocampal glutamate concentration predicts cerebral theta oscillations during cognitive processing. Psychopharmacology. 2006;187:103–111. doi: 10.1007/s00213-006-0397-0. [DOI] [PubMed] [Google Scholar]
- Ghoddoussi F, Galloway MP, Jambekar A, Bame M, Needleman R, Brusilow WS. Methionine sulfoximine, an inhibitor of glutamine synthetase, lowers brain glutamine and glutamate in a mouse model of ALS. Journal of the neurological sciences. 2010;290:41–47. doi: 10.1016/j.jns.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and clinical neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Reinhart RM, Roach BJ, Gueorguieva R, Oliver S, D’Souza DC, Ford JM, Krystal JH, Mathalon DH. Glutamatergic modulation of auditory information processing in the human brain. Biol Psychiatry. 2012;71:969–977. doi: 10.1016/j.biopsych.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Marinkovic K, Chauvel P. Generators of the late cognitive potentials in auditory and visual oddball tasks. Electroencephalography and clinical neurophysiology. 1998;106:156–164. doi: 10.1016/s0013-4694(97)00119-3. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, Murray RM, Sham P. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009;39:1277–1287. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Kwee IL, Nakada T, Katayama Y, Terashi A. 1H magnetic resonance spectroscopic imaging of permanent focal cerebral ischemia in rat: longitudinal metabolic changes in ischemic core and rim. Brain research. 2001;907:208–221. doi: 10.1016/s0006-8993(01)02579-3. [DOI] [PubMed] [Google Scholar]
- Iltis I, Koski DM, Eberly LE, Nelson CD, Deelchand DK, Valette J, Ugurbil K, Lim KO, Henry PG. Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized (1)H MRS study. NMR in biomedicine. 2009;22:737–744. doi: 10.1002/nbm.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Spencer KM, Thaker GK, Winterer G, Hajos M. Neurophysiological biomarkers for drug development in schizophrenia. Nat Rev Drug Discov. 2008;7:68–83. doi: 10.1038/nrd2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JE, Licata SC, Ongur D, Friedman SD, Prescot AP, Henry ME, Renshaw PF. Quantification of J-resolved proton spectra in two-dimensions with LCModel using GAMMA-simulated basis sets at 4 Tesla. NMR in biomedicine. 2009;22:762–769. doi: 10.1002/nbm.1390. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: II. Adequacy of low-density estimates. Clin Neurophysiol. 2006;117:369–380. doi: 10.1016/j.clinph.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D, Woods DL, Clayworth CC. Contributions of temporal-parietal junction to the human auditory P3. Brain research. 1989;502:109–116. doi: 10.1016/0006-8993(89)90466-6. [DOI] [PubMed] [Google Scholar]
- Lally N, Mullins PG, Roberts MV, Price D, Gruber T, Haenschel C. Glutamatergic correlates of gamma-band oscillatory activity during cognition: a concurrent ER-MRS and EEG study. Neuroimage. 2014;85(Pt 2):823–833. doi: 10.1016/j.neuroimage.2013.07.049. [DOI] [PubMed] [Google Scholar]
- Marsman A, van den Heuvel MP, Klomp DW, Kahn RS, Luijten PR, Hulshoff Pol HE. Glutamate in schizophrenia: a focused review and meta-analysis of (1)H-MRS studies. Schizophr Bull. 2013;39:120–129. doi: 10.1093/schbul/sbr069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathalon DH, Ford JM, Pfefferbaum A. Trait and state aspects of P300 amplitude reduction in schizophrenia: a retrospective longitudinal study. Biol Psychiatry. 2000;47:434–449. doi: 10.1016/s0006-3223(99)00277-2. [DOI] [PubMed] [Google Scholar]
- Mlynarik V, Kohler I, Gambarota G, Vaslin A, Clarke PG, Gruetter R. Quantitative proton spectroscopic imaging of the neurochemical profile in rat brain with microliter resolution at ultra-short echo times. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2008;59:52–58. doi: 10.1002/mrm.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychological bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen BM, Renshaw PF. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG, Marjanska M, Gasparovic C, Zuo C, Magnotta V, Mueller B, Mullins P, Renshaw P, Ugurbil K, Lim KO, Alger JR. Proton echo-planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 Tesla. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007;58:236–244. doi: 10.1002/mrm.21287. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in biomedicine. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Waagepetersen HS. Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotoxicity research. 2005;8:221–225. doi: 10.1007/BF03033975. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Turner JA, Damaraju E, van Erp TG, Mathalon DH, Ford JM, Voyvodic J, Mueller BA, Belger A, Bustillo J, McEwen S, Potkin SG, Fbirn, Calhoun VD. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Frontiers in neuroscience. 2013;7:137. doi: 10.3389/fnins.2013.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson TD, Petrakis IL, Edgecombe J, Perrino A, Krystal JH, Mathalon DH. Modulation of the cortical processing of novel and target stimuli by drugs affecting glutamate and GABA neurotransmission. Int J Neuropsychopharmacol. 2009;12:357–370. doi: 10.1017/S1461145708009334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Yang J, Li CQ, Zhu W, Shen J. Metabolic alterations in focally activated primary somatosensory cortex of alpha-chloralose-anesthetized rats measured by 1H MRS at 11.7 T. Neuroimage. 2005;28:401–409. doi: 10.1016/j.neuroimage.2005.06.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table: MRS Characteristics of the Sample