Abstract
The CBA/CaJ mouse strain's auditory function is normal during the early phases of life and gradually declines over its lifespan, much like human age-related hearing loss (ARHL), but on a mouse life cycle “time frame”. This pattern of ARHL is relatively similar to that of most humans: difficult to clinically diagnose at its onset, and currently not treatable medically. To address the challenge of early diagnosis, CBA mice were used for the present study to analyze the beginning stages and functional onset biomarkers of ARHL. The results from Auditory Brainstem Response (ABR) audiogram and Gap-in-noise (GIN) ABR tests were compared for two groups of mice of different ages, young adult and middle age. ABR peak components from the middle age group displayed minor changes in audibility, but had a significantly higher prolonged peak latency and decreased peak amplitude in response to temporal gaps in comparison to the young adult group. The results for the younger subjects revealed gap thresholds and recovery rates that were comparable to previous studies of auditory neural gap coding. Our findings suggest that age-linked degeneration of the peripheral and brainstem auditory system is already beginning in middle age, allowing for the possibility of preventative biomedical or hearing protection measures to be implemented as a possibility for attenuating further damage to the auditory system due to ARHL.
Keywords: Presbycusis, Temporal Processing, Auditory Brainstem Response (ABR), Mouse, Brain Plasticity
Introduction
Age-related hearing loss (ARHL) comprises the most prevalent neurodegenerative disorder and communications problem of our aging population; and is in the highest prevalence category of chronic medical conditions of the aged, along with arthritis and cardiovascular diseases (Cruikshanks et al., 1998; Gopinath et al., 2009). In previous investigations of ARHL- clinically termed presbycusis, the gap detection paradigm has been employed to measure temporal processing in both animals and human subjects. Auditory temporal processing refers to the ability of auditory neurons to code stimulus temporal features with high temporal resolution, so that this temporal information can be processed accurately in the parts of the brain used for hearing and complex sound feature perception. Traditionally, temporal processing deficiencies within the auditory system were attributed to deficits within the inner ear, commonly defined as peripheral sensorineural hearing loss (SNHL) (Fitzgibbons & Wightman, 1982; Tyler et al., 1982; Florentine and Buus, 1984; Buus and Florentine, 1985). For instance, Florentine and Buus (1984) found that subjects with SNHL displayed poorer temporal gap detection even at high sound levels when compared to listeners with normal hearing. Analogously, Fitzgibbon and Wightman (1982) assessed gap detection thresholds for various octave-band conditions and found that hearing impaired listeners consistently had higher thresholds than normal-hearing listeners at equivalent sound pressure levels (SPL). The difference was attributed to “processing distortions imposed by cochlear damage” (Fitzgibbon & Wightman, 1982).
Prevailing evidence links age-related difficulties with temporal coding to neural deficits s in the central auditory system (CAS), in addition to peripheral processing problems. For example, Walton and colleagues found significant age-changes in behavioral temporal responses and single-neuron temporal processing in the brainstem in aging CBA/CaJ mice with relatively good peripheral hearing sensitivity (Walton et al., 1997; 1998; Frisina, 2001). Additionally, Moore et al. (1992) reported differences between young and old subjects for suprathreshold gap detection measured from 400 to 2000 Hz. Similarly, Lutman (1991) observed age-related differences in dynamic frequency resolution abilities of subjects ranging from 17 to 80 years of age. He et al. (1999) reported that aged subjects had considerably higher gap detection thresholds than young adults, especially when the gap was presented randomly and placed at 5% or 95% time points in the overall noise stimulus. Likewise, Schneider and Hamastra (1999) presented findings indicating that older adults had impaired temporal processing abilities in comparison to their younger counterparts, notably for shorter gap durations utilizing two 2 kHz tone pips.
Additional animal model experiments have also shed light on the conflicting theories concerning peripheral vs. central age-related effects on auditory temporal processing (Poth et al., 2001; Simon et al., 2004; Walton et al., 2008; Gleich & Strutz, 2011). For example, Walton et al. (2008) reported that 6 to10 month old C57 mice began to show signs of high frequency SNHL, but also had temporal processing abilities parallel to those of 6 to 10 month old CBA mice with no significant peripheral hearing loss (Walton et al., 1998). Also, Gleich & Strutz (2011) reported gap thresholds that were significantly higher in aged gerbils than in young adult gerbils before and after gabapentin treatment was administered. More of a difference could be seen between the groups when the sound intensity was decreased from 30 dB SPL to 15 dB SPL (Gleich & Strutz, 2011). Overall, these results indicated that the aging central auditory system was a major contributor to auditory temporal processing deficiencies characteristic of ARHL.
So, although age changes in auditory temporal processing are becoming better understood, there are still aspects that remain unclear. For instance, at what age or stage in life does temporal processing of sound features start to noticeably decline? In most previous studies, comparisons made have only been between young adult and old subjects. The ages at which temporal processing declines start to take place remain somewhat elusive. In light of this, the present study aimed to compare the temporal processing abilities of young adult and middle age CBA/CaJ mice, using auditory brainstem response (ABR) Gap-in-noise (GIN) paradigm. ABR GIN is a novel approach for measuring temporal processing in aging animals, whereas most previous studies have used single- or multi-unit gap detection paradigms, or behavioral methodologies (Walton et al., 1997). The ABR GIN paradigm provides information on the ability of the auditory periphery and brainstem to process temporal gaps in ongoing sound by measuring various waveform components of the ABR. For the present investigation, Peak I (P1) and Peak IV (P4) were assessed to determine the minimum gap thresholds (MGT), the peak latency, the peak amplitude, and the recovery function of P1 and P4 for mice of different ages. The results indicate that prominent signs of ARHL-linked temporal coding deficits, with higher response latency values and lower amplitude levels strikingly emerge in middle age.
Materials and Methods
Subjects
Sixteen CBA/CaJ mice, bred in-house, were classified into two groups, young adult (Y, N=8, 3 to 4 months old) and middle age (MA, N=8, 15 to 18 months old). Using the same procedures as in our previous reports, distortion product otoacoustic emissions (DPOAEs) were used to characterize the functionality of the outer hair cell system (Zhu et al., 2007; Frisina et al., 2011; Borkholder et al., 2013). No medications or other potentially ototoxic procedures were administered. The subjects were anesthetized using a ketamine/xylazine mixture (120 and 10 kg/mg body weight) intraperitoneal injection, prior to all experimental sessions. All of the animal protocols were approved by the University of South Florida Institutional Animal Care and Use Committee (IACUC).
Stimulus Presentation and Physiology Recordings
Acoustic stimuli were synthesized digitally using the System III Tucker-Davis Technology (TDT, Alachua FL) signal-processing platform. The stimuli were then attenuated and filtered (low pass cutoff at 5 kHz). Stimulus sounds were presented through an electrostatic speaker (TDT EC1) connected to the external ear canal by 4 cm tubes, so via a calibrated, closed system. A ¼″ B&K microphone (Type 4938, Bruel & Kjaer, Naerum, Denmark) attached to a 0.1 cm3 coupler was used to calibrate the TDT system daily. The mice were placed on a heating pad inside a soundproof booth. Three sub-cutaneous needle electrodes were inserted at the vertex (non-inverted) and in the mastoid area muscle of the ipsilateral (testing) side (inverted), with a ground inserted in the muscle posterior to the contralateral pinna to record the ABR responses of each mouse. These electrodes were connected to a bioamp headstage (HS4 Fiber Optic, TDT). For ABR threshold experiments, the subjects were presented with tonal stimuli in the frequency range of 3 to 48 kHz at various sound levels (5 dB intensity steps) to determine thresholds. A wide band noise (WBN) stimulus, having a bandwidth from 0 to 48 kHz, was also used. The duration for each ABR stimulus was 5 msec, presented at a repetition rate of 21/s. For ABR GIN, a silent gap was inserted in the center of two WBN bursts, NB1 and NB2, at 80 dB SPL, each with a repetition rate of 21/sec, duration of 25 msec and rise-fall times of 0.5 msec, and each averaged response was obtained from 100 stimulus presentations and then duplicated with another 100 repetitions. Gap durations were 0, 1, 2, 4, 8, 16, 32, and 64 msec. Responses were recorded using BioSig (TDT) and duplicated for each gap duration tested, an example of which is given in Figure 1. The time span for each ABR GIN audiogram response window was 150 msec.
Fig. 1.
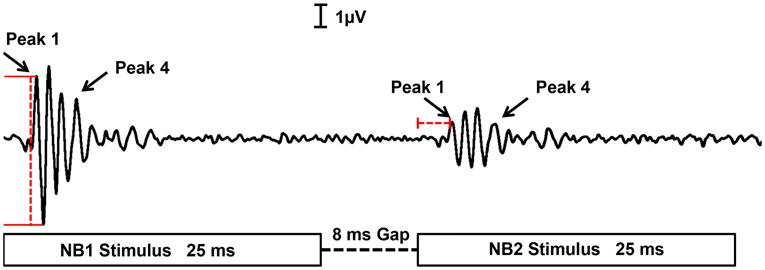
A representative of an ABR waveform from a young adult mouse elicited by the GIN stimulus. It should be noted that the following waveform is the averaged response for a gap duration of 8 msec that was replicated twice. The amplitude for each ABR wave peak was measured using the peak-to-trough technique. Meanwhile, the latency was calculated by subtracting the time of the ABR peak response to the stimulus from the starting time of NB2, for the following study.
Data Analysis: Auditory Brainstem Response (ABR) Thresholds and Gap-in-noise (GIN) ABR
For ABR testing, thresholds were determined at each frequency by different experimenters who tested both age groups, blinded to the animal's age; and the threshold was defined as the lowest sound level at which a distinct ABR wave could be identified. ABR recordings for all 9 tonal stimuli (3-48 kHz), along with WBN stimuli, were evaluated and compared for the two different subject groups, young adult and middle age.
ABR GIN analysis consisted of first identifying the smallest gap duration with a response to the second noise burst (NB2), i.e., the minimal gap threshold (MGT) (Florentine & Buus, 1985; Nelson & Thomas, 1997). Responses to the first noise burst (NB1) consist of a series of 4 to 5 ABR peak waves. Meanwhile, an ABR peak response for NB2 was elicited depending on the gap duration being presented; at shorter gap durations, either very small or no ABR peaks would be generated, and larger gap durations produced clearer ABR peak waves. The time and magnitude of these wave peaks were used to formulate latency and amplitude statistics. Latency values were measured by subtracting the time of the P1 NB2 response from the ending time of the gap duration (refer to Fig. 1). This value signified the time at which the onset response to the termination of the gap occurred, or the delayed response to NB2. Latency shift values were calculated by subtracting the time at which the NB1 response occurred from the time the NB2 response occurred. The latency shift indicates how quickly the system recovered from NB1. Furthermore, the wave amplitudes were obtained by subtracting the trough from the peak levels for P1 and P4 (Fig. 1). Control amplitude values for P1 and P4 were identified for each mouse using NB1. A comparison was then made to the NB2 amplitude values. Specifically, recovery ratio values were determined by dividing the NB2 amplitude by the NB1 amplitude for both P1 and P4.
A two-way analysis of variance (ANOVA) was used to statistically analyze the amplitude levels, recovery ratios and latency values for the young adult and middle age groups. Bonferonni's multiple comparisons post-hoc tests (MCT) were used to assess pairwise comparisons between conditions when the ANOVA main effects were statistically significant. All error bars in the figures are standard error of the mean (SEM).
Results
Hearing Sensitivity in Middle Age: ABR Thresholds
Individual ABR audiograms presented in Figure 2a show that although most of the MA group has higher thresholds than the Y group, some of the thresholds do overlap for the two age groups. This indicates that ARHL is just beginning in this strain of mice at about 15 months. A clearer depiction of the differences between the two groups can be seen in the averaged threshold values. According to Figure 2b, the MA group has a threshold that is approximately 10 dB higher than that of the Y group across the stimulus frequency range used. This analysis confirmed that there were modest differences in the hearing levels between the two groups of mice, Y and MA.
Fig. 2.
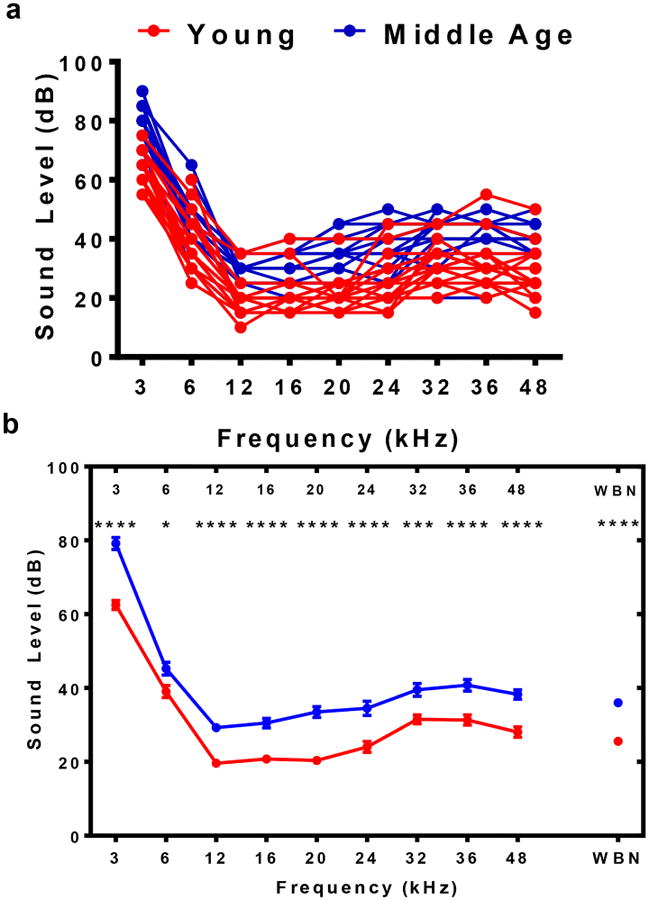
ABR threshold at frequencies ranging from 3 kHz to 48 kHz for individual mice and mean data. Wide band noise (WBN) thresholds were determined as well. a) Thresholds for each mouse in both groups Y and MA show that although the majority of the MA values are higher than Y, some of the thresholds still overlap between the two groups. b) Averaged threshold values clearly show that the Y group has thresholds that are approximately 10 dB lower than the MA group, especially at 12, 16, 20 and 24 kHz. Statistical tests: ANOVA followed by Bonferroni; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
Minimum gap thresholds (MGT) were determined as the lowest gap duration at which a response appeared for NB2, as presented in Figure 3. All 8 Y mice had an NB2 response for gaps of 2 msec and greater. However, only about half (5 out of 8) of the mice in the MA group generated a response for NB2 at 2 msec gaps; whereas, all 8 MA mice had an NB2 response for gaps of 4 msec and greater. So, as predicted from previous studies showing central auditory temporal coding deficits with age, the MA group had a higher mean MGT than that of Y. These findings were confirmed by computing the ABR GIN grand average at 2, 4 and 8 msec for both groups. As seen in Figure 4, MA's response at 4 and 8 msec can be clearly depicted; however, at 2 msec, the NB2 response is not present. The Y group, on the other hand, elicited a response that was clearly distinct for all 3 of the shortest gap durations.
Fig. 3.
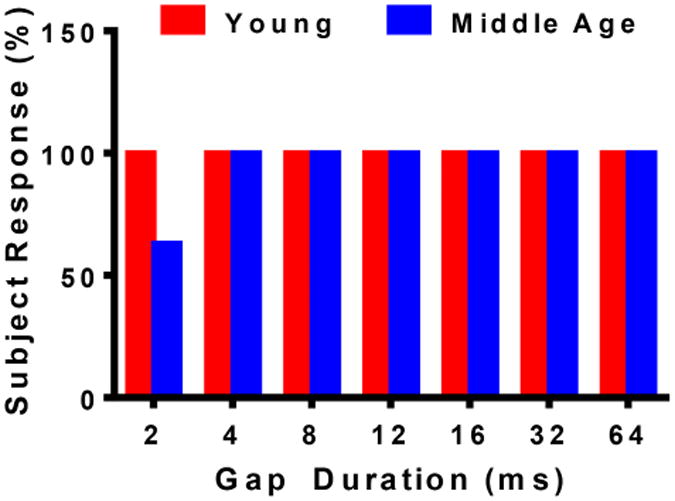
The subject response shows the percentage of mice that elicited a NB2 response. MA displayed percentage values lower than Y at 2 msec; however, as the gap duration increased, more mice responded to the stimulus. By 4 msec, all of the MA mice responded to the NB2 stimulus. The Y groups consistently showed a 100% group response for all of the gap duration.
Fig. 4.
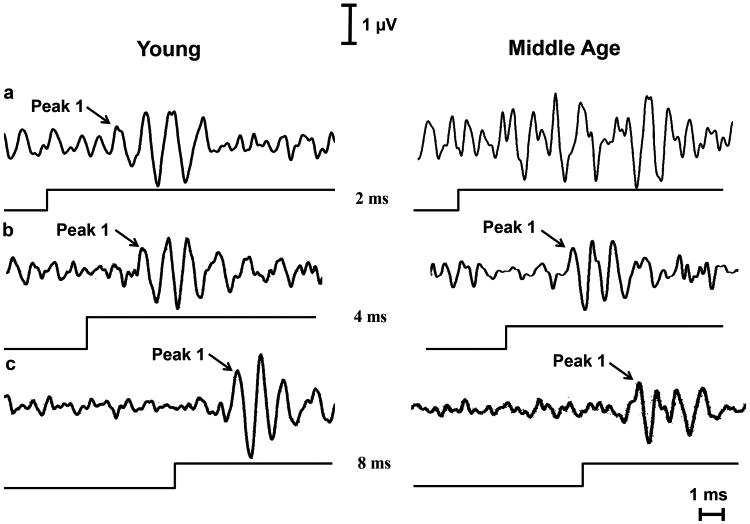
A representative of a waveform analysis between Y and MA that was used to determine the minimum gap threshold (MGT). MGT is defined as the lowest gap duration at which there is a NB2 response. a) At 2 msec, MA doesn't have a NB2 response; however, Y clearly displays ABR waves. Therefore, the MGT for group Y is 2 msec. b) By 4 msec, MA starts to show signs of P1 waveform. As a result, the MA group has a MGT of 4 msec. c) At 8 msec, both groups also show P1 waveforms. However, the Y peaks are notably larger than MA's. The line underneath each audiogram indicates the point in time at which the NB2 stimulus was given.
Temporal Processing: ABR GIN Amplitudes
The average ABR GIN amplitude values for Peak I (P1) and Peak IV (P4) are displayed in Figure 5. A t-test analysis verified that MA has significantly lower amplitudes than Y for both peaks (P1, t=5.46, p<0.0001; P4, t=2.14, p=0.041). From Figure 5a, it can be seen that the control amplitude levels for P1 were 6.17 mV for Y and 2.79 mV for MA. Meanwhile, P4 amplitudes were 2.72 mV and 1.39 mV for Y and MA, respectively. Note that one common observation was that P1 amplitudes are larger than P4 for both groups.
Fig. 5.
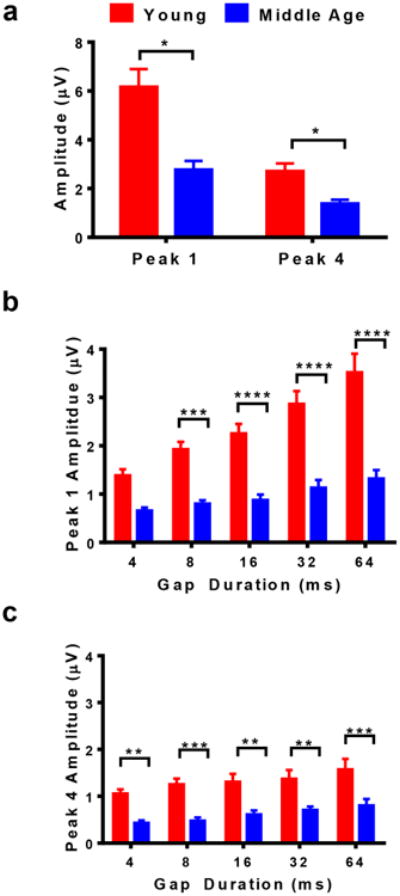
ABR GIN amplitude values for P1 and P4 for both groups show that the Y group consistently had higher amplitude values than MA for NB1 and NB2, respectively. a) The control amplitude for NB1 for P1 and P4. b) As the gap duration increases, the amplitude at NB2 for P1 gets closer to its control value. Significant differences can be seen between the Y and MA group at 4, 8, and 16 msec. c) The amplitude levels at NB2 for P4 are much smaller than P1. P4 amplitude values are notably higher for Y than MA at 4ms. Statistical tests: a) t test; b and c) ANOVA followed by Bonferroni; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001
The experimental amplitude levels of NB2 systematically increased as the gap durations increased. In Figure 5b, the amplitude levels for MA initially were 0.66 mV for 4 msec for P1 and gradually rose to 1.32 mV at 64 msec. Meanwhile, group Y had amplitude levels ranging from 1.38 mV to 3.52 mV for 4 and 64 msec gap durations, respectively. Significant differences were observed between the two groups for gap durations 12, 16, 32, and 64 msec (Fage(1,70)=136; p<0.0001). There was a 3.39 mV difference between Y and MA for P1 control values. Note that P4 had relatively smaller amplitude values than P1; however, a positive trend can still be seen in Figure 5c as the gap duration increased. Specifically at 4 msec, the amplitudes for Y and MA were 1.06 mV and 0.43 mV, respectively. By the time the gap interval increased to 64 msec, the values increased to 1.58 mV for Y and 0.81 for MA. The amplitude differences between the two groups increased as the gap duration increased for P4. For instance, the smallest difference between Y and MA for P4 took place at 4 msec (Bonferonni's MCT, p=0.006); meanwhile, at 64 msec there was a large difference in amplitude levels (Bonferonni's MCT, p=0.0005).
Significant recovery ratio values can be seen in both groups of mice for P1 and P4. However, even at longer gap durations, MA amplitude levels still did not reach those of Y, but they became increasingly similar to the control values seen in Figure 5a. P4 amplitude levels for MA seemed to become more parallel to Y with time. Both groups also had values that were striking similar to control amplitude levels at 64 msec. With longer gap durations than the ones used for this experiment, the mice might have reached levels close to the control amplitudes in Figure 5a.
ABR GIN Recovery Percentages
Figure 6 displays the same positive relations between gap durations and recovery percentages similar to the trends just seen for the amplitude levels, in that they both show positive trends. However, the recovery percentages do not show the notable differences seen in the amplitude levels, comparing Y and MA. In Figure 6a, the Y group has a recovery percentage analogous to that of MA for smaller gap durations; however, as the gap duration increased there was approximately a 10% difference in recovery function (Fage(1,70)=8.96, p=0.0038). For instance, both Y and MA had a recovery of approximately 25% at 4 msec for P1. As gap durations increased to 64 msec, the recovery ratios improved to 58% for Y and 47% for MA (Bonferroni MCT, p=0.0339). On the contrary, Figure 6b shows that the two groups of mice recovered at different rates before their values become similar for longer durations for P4 (i.e., a significant difference can be seen at 8 msec; but for the 64 msec gap, both groups have an NB2 response that is similar to NB1 at 56%).
Fig. 6.
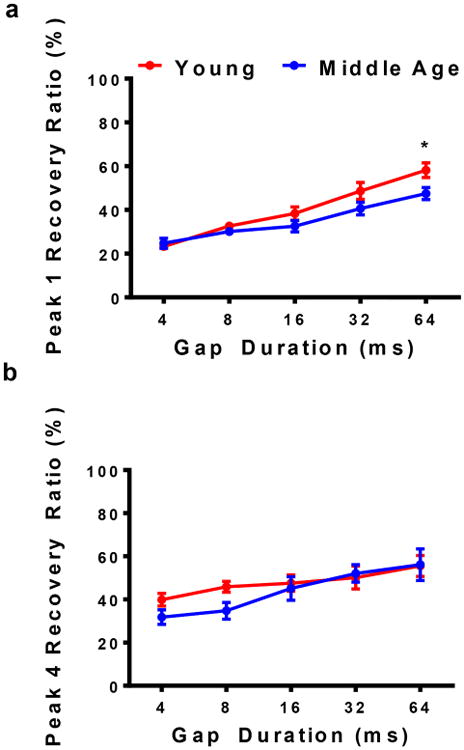
a) Recovery ratios for P1 were analogous for Y and MA at 4msec. However, ratios for the Y group began to improve as gap durations became longer. Significant differences can be seen between the groups at 16 and 64 msec. b) Conversely, recovery ratios for P4 vary greatly at 4 msec. By 32 msec, the MA group starts to recover almost as well as the Y group. The Y group displayed higher percentage values for wider GIN durations for P1. For P4, higher Y percentages could be seen for smaller GIN durations. Statistical tests: ANOVA followed by Bonferroni; *p<0.05.
Response Latency
An inverse relationship can be seen in Figure 7 between the latency values and gap durations (Fage(1,84)=17, p<0.0001). Initially, the Y and MA groups had control, NB1 latency values of 2.56 msec and 2.71 msec, respectively (not shown). In response to the NB2 stimulus, P1 latency increased to 2.79 msec for Y and 2.93 msec for MA, for a gap interval of 4 msec. However, the latency decreased as the gap duration became longer. As the gaps increased, the auditory neurons had more time to recover from NB1 and could respond more efficiently to NB2. For example, group the Y latency dropped by 0.17 msec, while the MA latency declined by 0.15 msec at 64 msec. The final latency values taken at 64 msec were somewhat congruent to the control latencies, mentioned above, for both subject groups. Figure 7a shows that differences between the groups increased as the gap durations became longer. Hence, the largest difference between Y and MA was observed at 64 msec. It should be noted that latency values for NB1 and NB2 are parallel between the Y and MA groups, with MA having consistently higher values. This indicates that auditory temporal processing abilities have some common features for the two age groups; however, the initial signs of ARHL are occurring significantly in middle age.
Fig. 7.
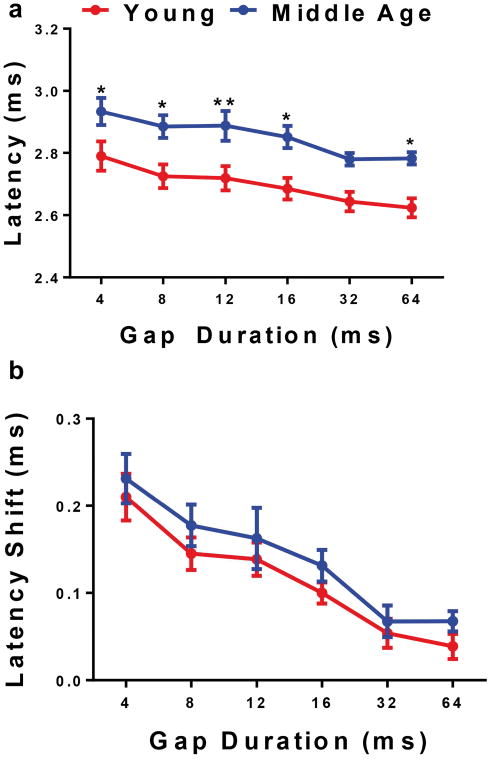
a) Plotted average latency values distinctly show that the Y latency values were consistently shorter than MA. Also, significant differences for latency can be seen at 4 and 8 msec between the two age groups. b) Mean latency shift values show that Y and MA vary slightly at 4 msec; however, by 8 msec the two age groups become increasingly similar. This latency-shift age difference was not statistically significant. Statistical tests: ANOVA followed by Bonferroni; *p<0.05, **p<0.01.
Latency shifts were also analyzed for this study. As expected, the latency shift decreased considerably as the gap duration increased. Figure 7b shows the MA group with a latency shift of 0.33 msec for the 4 msec gap. For the same gap interval, Y had a slightly lower latency shift of 0.24 msec. As the neurons had more time to recover from NB1, the latency shift gradually declined to about 0.09 msec for Y and MA. The latency shift difference between the two groups for the 4 msec gap suggests that the MA group still has the capability to respond to noise burst stimuli but not as accurately as that of Y. Figure 7b also shows how the latency shift for MA asymptotes for the two longest gap durations. Specifically, the latency shift is 0.09 msec for the 32 and 64 msec gaps. Meanwhile, the latency shift for Y continues to decrease for the longest gaps. There were no significant differences between Y and MA at any of the gap durations. Similar to the latency response, the latency shift values for MA were parallel to those for Y (Fig. 7b), but with MA being consistently higher for the absolute latencies (Fig. 7a).
Discussion
In this study, we demonstrated that middle age mice start to show temporal processing deficits characteristics of ARHL at 15 months of age, focusing on the ABR GIN response for P1 and P4, which were chosen due to their similarities to wave I and wave V ABR peaks in humans. Previous studies of wave I and wave V suggest that they comprise a very useful, robust response in humans (Boettcher, 2002), with the peaks being generated from different parts of the cochlea and brainstem auditory system. For instance, P1 (wave I for humans) is produced by the auditory nerve; while P4 (mice) and wave V (human) are generated from the lateral lemniscus/inferior colliculus (IC) of the brainstem (Hashimoto, 1989; Hashimoto et al., 1981; Møller & Janetta, 1982, 1983). With age, the degeneration of auditory brainstem neural circuitry contributes to the reduction in ABR peak responses. In the present investigation, these age-related changes were seen by comparing the amplitude levels and latency values for the Y and MA groups. For instance, referring to Figures 5 and 7, the Y group consistently displayed better gap processing relative to the MA group. Although the ABR peak amplitude and latency values were significantly different, similarities could be seen in the shape of the NB2 recovery functions for both age groups, as displayed in Figure 6. So, a major finding of this experiment: initial signs of neurophysiological changes that degrade auditory temporal processing can be detected with ABR gap measures; which probably reflects neuron and neuroglia loss, decline in myelin sheaths, and reduction in brainstem volume/weight, which start to occur in middle age (Khullar & Babbar, 2011); along with declines in auditory brainstem neuronal connectivity (Frisina & Walton, 2006).
Hearing Sensitivity: ABR Thresholds
Although there was overlap between individual ABR threshold functions (Fig. 2a), there was a mean difference of about 10 dB for the two subject groups of the present investigation (Fig. 2b). So, it is possible that absolute sensitivity differences might explain some of the temporal processing declines in middle age. However, Buus and Florentine (1985) conducted a study where they found that human subjects with high-frequency hearing loss had MGTs similar to subjects with normal hearing at high sound levels, such as those used in the present investigation (i.e. 80 dB SPL). Nelson and Thomas (1997) used the same logic to interpret their findings while testing normal and hearing-impaired subjects, who were found to have similar MGTs at 3 msec. Similarly, Walton and colleagues (2008) compared neural MGTs from IC neurons from hearing-impaired C57 mice to old CBA mice and found that neural MGTs were not significantly different from one another. So, although there may be a minor influence of sound level, aging and other factors play a more prominent role to explain the temporal processing deficits observed in the middle age mice of the present investigation (Fig. 3).
Aging Effects on Temporal Processing: ABR GIN Amplitude Levels
For NB1, MA control amplitude levels are reduced by nearly 50% of that of Y for P1 and P4, as seen in Figure 5a. Likewise, NB2 amplitude levels for MA are consistently lower than that for Y for both peaks (Figs. 5b and 5c). As the gap durations increased, the differences between the age groups receded. Similar results were seen when Boettcher and colleagues (1996) compared the temporal processing abilities of young adult and aged gerbils. P4 NB1 amplitudes for aged gerbils were from 1.5 to 0.5 mV, while the young adults had greater amplitudes, from 3 to 1.5 mV (Boettcher et al., 1996), much like the same two-fold variance that was seen in P4 comparing Y and MA in the present investigation. As for NB2 amplitude levels, the differences between the age groups escalated when the gap duration became longer for both P2 (the gerbil equivalent to P1) and P4 (Boettcher et al., 1996). For instance, at a gap duration of 2 msec both young and aged gerbils had amplitude levels that were not significantly different from the noise floor at approximately 0.25 mV (Boettcher et al. 1996). However, as the gap durations increased, the initial P1 and P4 values progressively improved to 3 mV for the young adult gerbils and 1.5 mV for the old gerbils. Thus, Boettcher's study demonstrated that even with longer gaps, aged gerbils still had significantly lower amplitude ABR waves than their young counterparts. For the present investigation, it was confirmed that these same differences could be seen as early as middle age in CBA/CaJ mice, reinforcing the usefulness of the ABR GIN paradigm as a physiological early diagnostic biomarker for ARHL temporal processing deficits.
Prior studies have attributed the age-linked reduction in amplitude levels to reduced temporal synchronization in auditory neurons, a decrease in the number of neurons that elicit a response to the stimulus, and/or a reduction in the endocochlear potential (EP) (Boettcher, 2002; Khullar & Babbar, 2011). Primary neurons in the auditory system include spiral ganglion (SG) nerve cells whose cell bodies are in the modiolus, and whose axons carry sound information to the auditory brainstem. These auditory nerve fibers provide excitatory inputs to the central auditory system, initially to cochlear nucleus neurons, where excitatory inputs interact with inhibitory neurons, including those with glycine or gamma-amino butyric acid (GABA), as inhibitory neurotransmitters (Lee et al., 2002; Walton et al., 1998; Khullar & Babbar, 2011). Age-linked degeneration of SG neurons reduces the number of nerve fibers that can respond to a stimulus effectively, and results in a decline of normal inputs to the cochlear nucleus. This disrupts the normal balance of excitation and inhibition, decreasing neural coding capabilities in the central auditory system with age. Inhibition is critical to temporal processing, including masking and binaural coding, in that the auditory system needs to recover from NB1 in order to process NB2 effectively. By 15 months, CBA/CaJ mice are undergoing the initial stages of aging changes in hair cell/SG neuron synapses and declines in hair cell and neuron numbers. Therefore, ABR peak amplitude and latency have been degraded, as seen in the response changes in the MA group of the present investigation.
Some studies found that P4 amplitude levels are less likely to show age-related changes, relative to P1 (Sand, 1991; Helfert et al., 1999; Boettcher, 2002). Sand (1991) compared the square-root transformed amplitude (mean values of right and left ear) level differences for young adult and aged human subjects, and found a 32.7 nV and 5.6 nV disparity for wave I and wave V (the mouse equivalent to P4), respectively. Helfert and colleagues (1999) findings suggested that there is a distribution of the excitation/inhibition equilibrium as GABA+ and GABA- synapses decline in the auditory midbrain (IC) with age. These excitatory and inhibitory changes interact with the effects of the diminishing size of IC volume seen in old age. Data from both of these studies are consistent with the theory that the cell count and the packing density in the IC undergo declines with age (Allen et al., 2003). Other studies emphasize that Wave V/P4 amplitudes decrease with age (Psatta & Matei, 1988; Boettcher et al., 1996; Banay-Schwartz et al., 1999). For example, Psatta and Matei (1988) reported mean Wave I amplitude variations of 0.35 nV and 0.17 mV for 30 and 50 year old human subjects, respectively, and mean Wave V amplitude levels declined from 0.68 nV to 0.50 nV for the same subject groups. Both peaks displayed a 0.18 mV difference between the two age groups. Comparable results could be seen for the present study for P1 and P4, age-related differences, e.g. MA had mean amplitude values of approximately 3 μV and 1 μV for P1 and P4, respectively; whereas, P1 and P4 mean amplitude values for Y were at approximately 6 μV and 2 μV. So, MA amplitude levels were reduced nearly by half compared to Y for both P1 and P4. Also, significant differences were seen at 64 msec for both peaks between the age groups (refer to Figs. 5b and 5c). This supports the idea that P4 is just as much an indicator of the early onset of presbycusis as P1. This is also consistent with the fact that P4 is generated by the lateral lemniscus/IC, and previous studies have shown that these areas of the central auditory system lose neuron volumes and input/output connections with age (Konigsmark & Murphy, 1972; Willott et al., 1994; Frisina & Walton, 2001a,b; Frisina & Walton, 2006).
Age-related Effects on ABR GIN Recovery Ratios
Similar to ABR GIN amplitude levels, the recovery ratios increased as the gap duration became longer. It was hypothesized that the recovery ratios for Y would be notably higher than MA at shorter gap durations. And that gradually, the differences between the ratio values subside as the gap durations increased. However, this trend was seen only for P4 ratios. P1 ratios start with the two groups overlapping one another until Y gradually surpasses MA (see Fig. 6a). By 64 msec, there is approximately a 10% difference between the age groups. P4 exhibited recovery ratios that become increasingly similar with longer gap durations, coinciding with the initial supposition. By 12 msec, values for Y and MA overlie each other. Boettcher and colleagues (1996) displayed analogous trends for P1 and P4 for recovery ratios, comparing young adult and aged gerbils. For instance, the recovery ratio for P2 was about 0.40 at 2 msec for both gerbil age groups; and this value increased to approximately 1.2 and 0.8 for young adult and aged gerbils, respectively, at 32 msec. Meanwhile for P4 recovery ratios, the two groups varied by nearly 0.10 at 2 msec, but the ratio values overlap one another by gap durations of 8 and 16 msec.
For the present report, analysis of the P1 and P4 recovery ratios demonstrated that there are still similarities in the way that Y and MA adapt to the WBN stimulus within the auditory system. Although Y ABR amplitudes remained consistently higher than MA, the values among the groups showed some overlap as a function of gap duration, with no more than a 10% difference between Y and MA. On the contrary, the consistently decreased amplitude levels and increased latency values support the theory that the first signs of age-related temporal processing deficiencies emerge during the midlife phase. Boettcher et al. (1996) came to a similar conclusion in that their amplitude recovery ratios show less of an age difference for young adult and aged gerbils compared to the absolute amplitude levels. The logic behind this could be that while amplitude levels are considerably declining with age, MA still has enough functional hair cells, auditory nerve fibers, and brainstem auditory neurons to generate a physiologically useful NB2 response.
Latency Values and the Onset of Presbycusis
Latency values in Figure 6a comprise additional support for the idea that the first signs of ARHL occur in middle age, as previously mentioned for the ABR amplitude temporal coding measures. Specifically, the MA group displayed latency quantities that are longer than Y for all of the gap durations. Jerger and Hall (1980) revealed similar results when comparing latencies for human subjects with ages spanning from 10 to 79 years. Over a period of 25 to 55 years of age, for example, subjects had latency values that increased about 0.2 msec. Chu (1985) measured the peak latency in humans ranging from 18 -76 years of age. P1 mean latency values were reported to be 1.57 msec at ages 20-29 and gradually rose to 1.67 msec by the ages of 50-59. Thus, Chu's study showed that between the ages of 20 and 59 there is a 0.10 msec increase in latency. Similarly, Boettcher et al. (1996) observed longer latency values in aged gerbils. For 8 msec gaps, the mean latency value for aged gerbils was higher than the young adults by more than 0.5 msec. Increasing gap durations reduced this difference to 0.2 msec. The same 0.2 msec variation can be seen in Y and MA mean latency values at shorter gap durations for our current report. Conversely, some previous studies suggested that longer latencies in older subjects are linked to higher hearing thresholds as opposed to age (Beagley & Sheldrake, 1978; Harkins, 1981; Otto & McCandless, 1982; Ottaviani et al. 1991). Since these studies generally did not cleanly separate age from audibility variables, whether latency is influenced by sensorineural hearing loss or is a function of age, is still debatable. Lastly, ABR latency changes have been reported to stem from degradations of the synchrony of auditory nerve fiber and brainstem neural firing as well as abnormal changes in the movement of the basilar membrane (Boettcher, 2002; Khullar & Babbar, 2011).
Summary
Overall, the present study demonstrates that CBA/CaJ mice start to show the first signs of temporal processing deficits characteristics of ARHL at 15 to 18 months of age. Significant changes were seen in the MA amplitude levels and latency values for various temporal gap durations. Although these two ABR response measures declined with age, the recovery ratios between Y and MA did show differences, but had some notable parallel features as well. These neurophysiological results are consistent with age changes in the structure of the cochlea and auditory brainstem that occur in mammals; for instance, these functional changes may in part be due to the neuron degeneration that takes place in the cochlea and the brainstem during middle age. In conclusion, the findings of the present report support the use of the ABR GIN responses as a diagnostic, physiological biomarker for ARHL, paving the way for novel future development of acoustical, biomedical or pharmacological treatments to moderate ARHL that could be administered starting in middle age.
Acknowledgments
The authors gratefully acknowledge that this research was supported by an NIA-NIH grant to the Global Center for Hearing and Speech Research at the University of South Florida: P01 AG009524. We thank Shannon Salvog for project support.
References
- Allen PD, Burkard R, Ison JR, Walton JP. Impaired gap coding in aged mouse inferior colliculus at moderate but not high stimulus levels. Hearing Res. 2003;186:17–29. doi: 10.1016/s0378-5955(03)00300-9. [DOI] [PubMed] [Google Scholar]
- Beagley HA, Sheldrake JB. Differences in brainstem response latency with age and sex. Brit J Audiol. 1978;12:69–77. doi: 10.3109/03005367809078858. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Swerdolff JL, Holley BL. Auditory evoked potentials in aged gerbils: responses elicited by noises separated by a silent gap. Hearing Res. 1996;102:167–178. doi: 10.1016/s0378-5955(96)90016-7. [DOI] [PubMed] [Google Scholar]
- Boettcher FA. Presbycusis and the auditory brainstem response. J Speech Lang Hear Res. 2002;45:1249–1261. doi: 10.1044/1092-4388(2002/100). [DOI] [PubMed] [Google Scholar]
- Borkholder DA, Zhu X, Frisina RD. Round window membrane intracochlear drug delivery enhanced by induced advection. J Controlled Release. 2013;174(28):171–176. doi: 10.1016/j.jconrel.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S, Florentine M. Gap detection in normal and hearing impaired listeners: The effect of level and frequency. In: Michelsen A, editor. Time Resolution in Auditory Systems. Springer; Berlin Heidelberg New York: 1985. pp. 159–179. [Google Scholar]
- Chu N. Age related latency changes in the brainstem auditory evoked potentials. Electroen Clin Neuro. 1985;62:431–436. doi: 10.1016/0168-5597(85)90053-x. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed TS, Klein BK, Klein R, Mares-Perlman JA, Nondahl DM. Prevalence of Hearing Loss in Older Adults in Beaver Dam, Wisconsin: The Epidemiology of Hearing Loss Study. Am J Epidemiol. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Fitzgibbons PJ, Wightman FL. Gap detection in normal and hearing-impaired listeners. J Acoust Soc Am. 1982;72:761–765. doi: 10.1121/1.388256. [DOI] [PubMed] [Google Scholar]
- Florentine M, Buus S. Temporal gap detection in sensorineural and simulated hearing impairment. J Speech Hear Res. 1984;27:449–455. doi: 10.1044/jshr.2703.449. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hearing Res. 2001;158:1–27. doi: 10.1016/s0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- Frisina RD, Singh A, Bak M, Bozorg S, Seth R, Zhu X. F1 (CBA × C57) mice show superior hearing in old age relative to their parental strains: Hybrid vigor or a new animal model for “Golden Ears”? Neurobiol Aging. 2011;32:1716–1724. doi: 10.1016/j.neurobiolaging.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisina RD, Walton JP. Neuroanatomy of the central auditory system. In: Willott JF, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. Chapter 18. Boca Raton, FL: CRC Press; 2001a. pp. 243–277. [Google Scholar]
- Frisina RD, Walton JP. Aging of the mouse central auditory system. In: Willott JF, editor. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. Chapter 24. Boca Raton, FL: CRC Press; 2001b. pp. 339–379. [Google Scholar]
- Frisina RD, Walton JP. Age related structural and functional changes in the cochlear nucleus. Hearing Res. 2006:216–217. 184–193. doi: 10.1016/j.heares.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Gleich O, Strutz J. The effect of gabapentin on gap detection and forward masking in young and old gerbils. Ear Hearing. 2011;32(6):741–749. doi: 10.1097/AUD.0b013e318222289f. [DOI] [PubMed] [Google Scholar]
- Gopinath B, Rochtchina E, Wang JJ, Schneider J, Leeder SR, Mitchell P. Prevalence of Age-Related Hearing Loss in Older Adults: Blue Mountains Study. Arch Intern Med. 2009;169:415–418. doi: 10.1001/archinternmed.2008.597. [DOI] [PubMed] [Google Scholar]
- Harkins SW. Effects of age and interstimulus interval on the brainstem auditory evoked potential. Int J of Neurosci. 1981;15:107–118. doi: 10.3109/00207458108985851. [DOI] [PubMed] [Google Scholar]
- Hashimoto I. Critical analysis of short latency auditory evoked potentials recording techniques. In: Leuders H, editor. Advanced evoked potentials. Boston, MA: Kleuwar Academic; 1989. pp. 105–142. [Google Scholar]
- Hashimoto I, Ishiyama Y, Yoshimoto T, Nemoto T. Brain-stem auditory evoked potentials recorded directly from human brain-stem and thalamus. Brain. 1981;104:841–859. doi: 10.1093/brain/104.4.841. [DOI] [PubMed] [Google Scholar]
- He N, Horwitz AR, Dubno JR, Mills JH. Psychometric functions for gap detection in noise measured from young and aged subjects. J Acoust Soc Am. 1999;106(2):966–978. doi: 10.1121/1.427109. [DOI] [PubMed] [Google Scholar]
- Helfert RH, Sommer TJ, Meek J, Hofstetter P, Hughes LF. Age-related synaptic changes in the central nucleus of the inferior colliculus of Fischer-344 rats. J Comp Neurol. 1999;406:285–298. [PubMed] [Google Scholar]
- Jerger J, Hall J. Effects of age and sex on auditory brainstem response. Arch Otolaryngol. 1980;106:387–391. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- Khullar S, Babbar R. Presbycusis and auditory brainstem responses: a review. Asian Pac J Trop Dis. 2011:150–157. [Google Scholar]
- Konigsmark BW, Murphy EA. Volume of the ventral cochlear nucleus in man: it's relationship to neuronal population and age. J Neuropath Exp Neurol. 1972;31(2):304–316. doi: 10.1097/00005072-197204000-00006. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Wallani T, Mendelson JR. Temporal processing speed in the inferior colliculus of young and aged rats. Hearing Res. 2002;174:64–74. doi: 10.1016/s0378-5955(02)00639-1. [DOI] [PubMed] [Google Scholar]
- Lutman ME. Degradations in frequency and temporal resolution with age and their impact on speech identification. Acta Otolaryngol. 1991;111(Suppl. 476):120–126. doi: 10.3109/00016489109127265. [DOI] [PubMed] [Google Scholar]
- Møller AR, Janetta PJ. Auditory evoked potentials recorded intracranially from the brain-stem in man. Exp Neurol. 1982;18:144–157. doi: 10.1016/0014-4886(82)90196-0. [DOI] [PubMed] [Google Scholar]
- Møller AR, Janetta PJ. Auditory evoked potentials recorded from the cochlear nucleus and its vicinity in man. J Neurosurg. 1983;59:1013–1018. doi: 10.3171/jns.1983.59.6.1013. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Peters RW, Glasberg BR. Detection of temporal gaps in sinusoids by elderly subjects with and without hearing loss. J Acoust Soc Am. 1992;92(4):1923–1932. doi: 10.1121/1.405240. [DOI] [PubMed] [Google Scholar]
- Nelson PB, Thomas SD. Gap detection as a function of stimulus loudness for listeners with and without hearing loss. J Speech Lang Hear R. 1997;40:1387–1394. doi: 10.1044/jslhr.4006.1387. [DOI] [PubMed] [Google Scholar]
- Ottaviani F, Maurizi M, D'Alantri L, Almadori G. Auditory brainstem response in the aged. Acta Otolaryngol. 1991;(Suppl. 476):110–113. doi: 10.3109/00016489109127263. [DOI] [PubMed] [Google Scholar]
- Otto WC, McCandless GA. Aging and the auditory brainstem response. Audiology. 1982;21:466–473. doi: 10.3109/00206098209072759. [DOI] [PubMed] [Google Scholar]
- Poth EA, Boettcher FA, Mills JH, Dubno JR. Auditory brainstem responses in younger and older adults for broadband noises separated by a silent gap. Hearing Res. 2001;161:81–86. doi: 10.1016/s0378-5955(01)00352-5. [DOI] [PubMed] [Google Scholar]
- Psatta DM, Matei M. Age-dependent amplitude variation of brain-stem auditory evoked potentials. Electroen Clin Neuro. 1998;71:27–32. doi: 10.1016/0168-5597(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Sand T. BAEP amplitudes and amplitude ratios: relation to click polarity, rate, age and sex. Electroen Clin Neuro. 1991;78:291–296. doi: 10.1016/0013-4694(91)90183-5. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Hamastra SJ. Gap detection thresholds as a function of tonal duration for younger and older listeners. J Acoust Soc Am. 1999;106(1):371–380. doi: 10.1121/1.427062. [DOI] [PubMed] [Google Scholar]
- Simon H, Walton JP, Frisina RD. Age reduces response latency of mouse inferior colliculus neurons to AM sounds. J Acoust Soc Am. 2004;116(1):469–477. doi: 10.1121/1.1760796. [DOI] [PubMed] [Google Scholar]
- Tyler RS, Summerfield Q, Wood EJ, Fernandes MA. Psychoacoustic and phonetic temporal processing in normal and hearing-impaired listeners. J Acoust Am. 1982;72:740–752. doi: 10.1121/1.388254. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, Ison JR, O'Neill WE. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J Comp Physiol A. 1997;181:161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, O'Neill WE. Age-related alteration in processing of temporal sound features in the auditory midbrain of the CBA mouse. J Neurosci. 1998;18:2764–2776. doi: 10.1523/JNEUROSCI.18-07-02764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton JP, Barsz K, Wilson WW. Sensorineural hearing loss and neural correlates of temporal acuity in the inferior colliculus of the C57bl/6 mouse. J Assoc Res Otolaryngol. 2008;9:90–101. doi: 10.1007/s10162-007-0101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott JF, Bross LS, McFadden SL. Morphology of the inferior colliculus in C57BL/6J and CBA/J mice across the life span. Neurobiol Aging. 1994;12(2):175–183. doi: 10.1016/0197-4580(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Zhu X, Vasilyeva ON, Kim SH, Jacobson M, Romney J, Waterman MS, Tuttle D, Frisina RD. Auditory efferent system declines precede age-related hearing loss: Contralateral suppression of otoacoustic emissions in mice. J Comp Neurol. 2007;503:593–604. doi: 10.1002/cne.21402. [DOI] [PubMed] [Google Scholar]


