Abstract
Considerable research indicates that long-term synaptic plasticity in the amygdala underlies the acquisition of emotional memories, including those learned during Pavlovian fear conditioning. Much less is known about the synaptic mechanisms involved in other forms of associative learning, including extinction, that update fear memories. Extinction learning might reverse conditioning-related changes (e.g., depotentiation) or induce plasticity at inhibitory synapses (e.g., long-term potentiation) to suppress conditioned fear responses. Either mechanism must account for fear recovery phenomena after extinction, as well as savings of extinction after fear recovery.
Keywords: Long-term potentiation, depotentiation, fear conditioning, extinction, interneuron, learning, memory
Pavlovian fear conditioning is a form of associative learning that is an instrumental model for understanding the molecular and synaptic basis of memory formation (Johansen et al., 2011; Maren, 2005; Pape and Pare, 2010; Sah et al., 2008). Among its many advantages, Pavlovian fear conditioning is rapidly acquired, requiring only a single aversive experience to generate a long-term memory. In this procedure, pairing an innocuous conditioned stimulus (CS), such as a tone, with an aversive footshock unconditioned stimulus (US) produces a robust and enduring conditioned fear response (CR) to the CS. The neural substrates underlying this form of learning are well-characterized, and considerable evidence indicates that neurons in the amygdaloid complex, particularly those in the basolateral amygdala (BLA), play an essential role in encoding the CS-US associations that underlie long-term fear memories (Davis and Whalen, 2001; Fanselow and Poulos, 2005; LeDoux, 2000; Maren, 2001; Maren and Quirk, 2004).
Within the amygdala, an abundance of research indicates that long-term potentiation (LTP) is a mechanism for fear conditioning (Blair et al., 2001; Davis and Whalen, 2001; Fanselow and Poulos, 2005; Goosens and Maren, 2002; LeDoux, 2000; Maren, 2001; 1999; Maren and Quirk, 2004; Sigurdsson et al., 2007). Amygdaloid LTP was first described in extracellular field recordings in vivo (Clugnet and LeDoux, 1990; Racine et al., 1983) and subsequently confirmed by intracellular recordings of synaptic currents in lateral nucleus (LA) neurons in vitro (Chapman and Bellavance, 1992; Chapman et al., 1990). Soon after the discovery of LTP, it was appreciated that its Hebbian nature would allow strong aversive stimuli, such as footshock USs, to associatively potentiate weak CS inputs thereby enabling the CS to drive fear CRs (Maren and Fanselow, 1996). Some key findings consistent with this view are that both fear conditioning and amygdaloid LTP are prevented by N-methyl-D-aspartate (NMDA) receptor antagonists (Chapman and Bellavance, 1992; H. Lee and Kim, 1998; Maren et al., 1996; Maren and Fanselow, 1995; Miserendino et al., 1990), the induction of amygdalar LTP is sensitive to stimulus contingencies that support fear conditioning (Bauer et al., 2001), fear conditioning induces LTP-like changes at amygdala synapses (McKernan and Shinnick-Gallagher, 1997; Rogan et al., 1997; Tsvetkov et al., 2002) including AMPA receptor insertion (Rumpel, 2005), and single-unit correlates of fear conditioning in the amygdala are eliminated by NMDA receptor antagonists (Goosens and Maren, 2004). Indeed, recent evidence indicates that optogenetic induction of LTP in auditory thalamic afferents in the amygdala is sufficient to support fear memory and that long-term depression (LTD) of these inputs after learning yields retention impairments (Nabavi et al., 2014).
Once fear conditioning has been acquired, however, it is much less clear how new learning about an aversive CS is encoded by amygdalar synapses that have already been modified by conditioning. One form of post-conditioning learning that has attracted considerable attention is extinction, a form of learning that occurs when a CS is arranged to no longer predict the US (i.e., the CS is presented alone) (Jovanovic and Ressler, 2010; Maren, 2011; Milad and Quirk, 2012; Orsini and Maren, 2012; Pape and Pare, 2010). After fear conditioning, extinction procedures lead to a reduction in the magnitude and frequency of conditioned responses, including freezing behavior. A parsimonious view of extinction learning is that extinction results from a loss of associative strength accrued during conditioning (Rescorla and Wagner, 1972). By this view, the loss of conditional responding after extinction might be expected to result from the reversal of conditioning-induced plasticity. Consistent with this mechanism, there do appear to be conditions under which extinction training results in non-recoverable loss of the fear memory. In synaptic terms, therefore, extinction might be viewed as a depotentiation of learning-induced LTP in the amygdala (Kim et al., 2007).
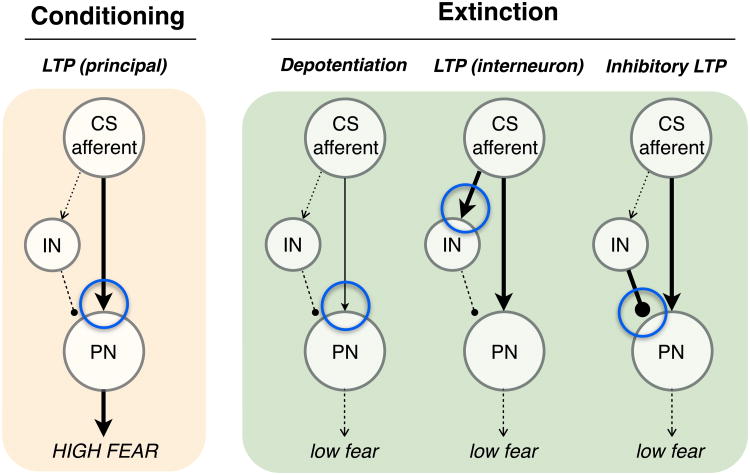
Although this account of extinction is parsimonious, it cannot explain the recovery of extinguished CRs under a number of circumstances. Decades of behavioral work have established that extinction does not eliminate, overwrite, or erase memories acquired during conditioning (Bouton and Bolles, 1979; Bouton, 2004; Bouton et al., 2006; Harris et al., 2000; Konorski, 1967; McConnell and Miller, 2014), because extinguished CRs return with the passage of time (spontaneous recovery), changes in context (renewal), and after aversive presentation of the US (reinstatement). The fact that extinction procedures spare the conditioning memory suggests that extinction results in new learning (e.g., an inhibitory CS-‘no US’ association) that interferes with the existing conditioning memory (CS-US association) to suppress conditional responding (Bouton, 2004; McConnell and Miller, 2014). By this view, extinction must be encoded, at least in part, by synaptic changes that preserve the content of conditioning. Here we will consider synaptic plasticity mechanisms in the amygdala (Figure 1) that might mediate the non-recoverable loss of conditional responding on the one hand and encode new extinction memories on the other.
Figure 1. Synaptic mechanisms of conditioning and extinction.
Conditioning (tan panel) is associated with long-term potentiation (LTP) at excitatory synapses from afferents carrying conditioned stimulus (CS) information that terminate on principal neurons (PN) in the basolateral amygdala [LTP (principal)]. Extinction (green panel) might result from a number of synaptic plasticity mechanisms including 1) depotentiation or long-term depression of previously potentiated PN synapses, 2) induction of LTP at CS afferents on interneurons (LTP-IN), or 3) LTP of inhibitory synaptic transmission (‘inhibitory’ LTP). Blue circles indicate the target synapses undergoing the forms of plasticity indicated above each microcircuit.
Out With the Old
Neuronal correlates of fear conditioning in the amygdala, including increases in CS-evoked single-unit firing (McEchron et al., 1995; Muramoto et al., 1993; Quirk et al., 1995) and synaptic potentiation (Nabavi et al., 2014; Rogan et al., 1997), are dampened after extinction. Extinction-related declines in amygdala activity may be mediated by depotentiation: a weakening of LTP at principal neuron synapses that were modified during conditioning (Figure 1). This mechanism is supported by a number of studies reporting that extinction reverses conditioning-related changes in the physiology and biochemistry of BLA neurons.
Extinction and depotentiation drive calcineurin-mediated Akt dephosphorylation in BLA
In a series of ex vivo biochemical experiments, Gean and colleagues (2003) have shown in a fear-potentiated startle paradigm that extinction reverses some of the biochemical changes induced by fear conditioning (Lin et al., 2003b). Specifically, fear-potentiated startle training increased levels of phosphorylated Akt (p-Akt) in the BLA, and subsequent extinction trials reduced p-Akt levels to those found in unpaired controls. This effect of extinction was prevented by a variety of calcineurin inhibitors, suggesting that phosphatase activity is important in reversing conditioning-related changes in protein kinases, such as Akt, in the amygdala. Consistent with this, the authors showed that extinction training was associated with an increase in calcineurin activity in the BLA (Lin et al., 2003b).
To determine whether extinction-induced changes in calcineurin activity and Akt phosphorylation were related to synaptic depotentiation, a subsequent experiment examined whether depotentiation in BLA afferents engaged calcineurin activity and promoted behavioral extinction of fear (Lin et al., 2003a). Long-term potentiation in the BLA was reversed by trains of low-frequency stimulation (LFS; 5 Hz for 3 min) applied to the external capsule in vivo, and both NMDA receptor antagonists and calcineurin inhibitors blocked LFS-induced depotentiation. Interestingly, LFS also reduced the expression of fear-potentiated startle, suggesting that it induced behavioral extinction. LFS also increased calcineurin activity and reduced levels of p-Akt in the BLA when it was applied 1-hour after a post-conditioning retention test. These results suggest that NMDA receptor-dependent depotentiation in the BLA may be involved in the calcineurin-mediated reversal of conditioning-induced changes after extinction training.
However, it is important to note that in the majority of these experiments pre-extinction tests of CS-induced startle enhancement were performed 10 min to 1 hr prior to either the extinction or LFS procedures (Lin et al., 2003a; 2003b). These pre-extinction tests would be expected to re-activate the conditioning memory and trigger reconsolidation processes that might themselves be sensitive to subsequent extinction training (Auber et al., 2013; Bauer et al., 2001; Chapman and Bellavance, 1992; H. Lee and Kim, 1998; Maren et al., 1996; Maren and Fanselow, 1995; McKernan and Shinnick-Gallagher, 1997; Miserendino et al., 1990; Monfils et al., 2009; Rogan et al., 1997; Tsvetkov et al., 2002). For example, reductions in BLA p-Akt levels might be due to a failure to reconsolidate the reactivated memory when memory reactivation is followed by post-retrieval extinction trials (Nabavi et al., 2014; Rumpel, 2005). Thus, it is not clear whether extinction trials or trains of LFS that have not been preceded by pre-extinction or pre-LFS reminders would be sufficient to produce the calcineurin-dependent changes observed in these studies.
Extinction depotentiates thalamic afferents in lateral amygdala and reduces GluA surface expression
In a more recent series of studies, Choi and colleagues (2007) used an ex vivo slice electrophysiology approach to examine the synaptic correlates of extinction of conditioned fear in rats (Kim et al., 2007). In their experiments, rats received a standard auditory fear conditioning protocol, which was followed 24 hours later by an extinction procedure. One day after extinction, the animals were sacrificed for an electrophysiological analysis of synaptic plasticity in LA neurons. These experiments revealed that fear conditioning increases internal capsule-evoked synaptic currents in the LA (indexed by whole-cell recordings), reflecting the induction of LTP at thalamo-amygdala synapses after conditioning (McKernan and Shinnick-Gallagher, 1997; Tsvetkov et al., 2002). Interestingly, extinction training eliminated these changes insofar as synaptic currents in LA neurons from extinguished rats were similar to those in cells from untrained or unpaired controls. This suggests that extinction training depotentiated conditioning-induced increases in synaptic transmission in LA neurons.
The possibility that extinction depotentiates conditioning-induced LTP at thalamic synapses in LA is supported by experiments indicating that extinction training occludes depotentiation in the amygdala (Kim et al., 2007). Extracellular field recordings in LA revealed that paired-pulse LFS (1 Hz for 15 min; 50 ms ISI) reduced synaptic responses in rats that had undergone fear conditioning, but not in naïve, unpaired, or context-exposed control rats. Depotentiation was blocked by both NMDA receptor and metabotropic glutamate receptor (mGluR1) antagonists and could be induced in conditioned rats by an mGluR1 agonist (Kim et al., 2007). Biochemical experiments revealed that extinction was associated with a reduction in the expression of AMPA receptors (GluA1 and GluA2) on the membrane surface of LA synaptosomes. In addition, ex vivo synaptic depotentiation and extinction could be prevented by a synthetic GluR2-derived peptide that blocks AMPA receptor endocytosis (Kim et al., 2007). Depotentiation at cortical synapses in BLA may also be involved in extinction, although regulation of presynaptic plasticity appears to play a greater role in that pathway (Hong et al., 2009). Together, these studies suggest that extinction may reduce conditioned fear by depotentiating thalamo-amygdala synapses critical for driving CS-evoked fear responses.
In With the New
Dampening synaptic transmission at synapses that have undergone LTP during fear conditioning is an attractive and parsimonious mechanism for extinction learning. However, there is ample evidence that the conditioning memory survives extinction procedures insofar as CRs can return under a number of circumstances including after the mere passage of time (spontaneous recovery), encountering an extinguished CS outside the extinction context (renewal), and after exposure to a noxious (reinstatement) or novel (external disinhibition) stimulus (Bouton and Bolles, 1979; Bouton, 2004; Bouton et al., 2006; Delamater, 2004; Harris et al., 2000; Jovanovic and Ressler, 2010; Kim et al., 2007; Konorski, 1967; Maren, 2011; McConnell and Miller, 2014; Milad and Quirk, 2012; Orsini and Maren, 2012; Pape and Pare, 2010; Pavlov, 1927; Rescorla and Wagner, 1972).
Collectively, these recovery phenomena suggest that extinction does not erase the conditioning memory (i.e., the CS-US association encode in BLA synaptic plasticity), but lays down a new inhibitory memory that competes with and dampens the expression of the original fear memory (Bouton, 2004; Bouton et al., 2006; Maren, 2011; McConnell and Miller, 2014). The synaptic mechanisms that encode inhibitory extinction memories are only now beginning to be understood, and appear to involve plasticity among inhibitory interneurons in the BLA (Duvarci and Paré, 2014; Ehrlich et al., 2009; McEchron et al., 1995; Muramoto et al., 1993; Pape and Pare, 2010; Paré et al., 2004; Quirk et al., 1995).
LTP at excitatory synapses on inhibitory interneurons in the amygdale
If extinction leads to increased inhibition in the amygdala, then there are at least two possible mechanisms to consider. The first possibility is that extinction generates LTP at excitatory synapses among CS afferents that terminate on inhibitory interneurons [i.e., “LTP (interneuron)” in Figure 1]. Mahanty and Sah (1998) first described this type of plasticity at cortical afferents terminating on LA interneurons, and Bauer and colleagues (2004) subsequently reported LTP at thalamic afferents on this same cell type (Bauer and LeDoux, 2004; Mahanty and Sah, 1998). However, there appear to be important differences in the nature and mechanisms of LTP in these two afferent pathways. LTP at cortical synapses does not depend on NMDA receptors, requires calcium-permeable AMPA receptors in the postsynaptic neurons, is input-specific (cortical tetanization does not potentiate unstimulated thalamic afferents) and is not associated with a change in paired-pulse facilitation (suggesting a postsynaptic locus of expression) (Lin et al., 2003b; Mahanty and Sah, 1998). In contrast, LTP at thalamic synapses terminating on interneurons is NMDA receptor-dependent, is not input-specific (thalamic tetanization produced a heterosynaptic potentiation of cortical inputs), and is associated with a change in paired-pulse facilitation (suggesting a presynaptic locus of expression) (Bauer and LeDoux, 2004).
Second, in addition to plasticity at excitatory afferents on LA interneurons, it has been suggested that synaptic plasticity among islands of inhibitory intercalated cells (ITC) may be essential for extinction. These neurons limit excitatory transmission between the BLA and central amygdala (CEA) thereby suppressing the generation of learned fear responses (Lin et al., 2003a; 2003b; Royer et al., 1999). In support of this possibility, Paré and colleagues have shown that BLA afferents terminating on ITC neurons exhibit both NMDA receptor-dependent LTP and LTD (Royer and Paré, 2002). LTP (but not LTD) was mediated in part by alterations in presynaptic transmitter release, and these changes in presynaptic release survived low frequency stimulation trains that induced depotentiation. Subsequent ex vivo experiments have shown that extinction training increases synaptic transmission at BLA afferents terminating on inhibitory ITC cells (Amano et al., 2010). ITC neurons in turn inhibit BLA-mediated depolarization of CEA cells involved in generating learned fear responses (Amano et al., 2010). Interestingly, the potentiation of BLA inputs in ITC depended on inputs to the amygdala from the infralimbic division of the ventromedial prefrontal cortex, a brain region that plays an essential role in the acquisition and consolidation of extinction memories (Laurent and Westbrook, 2008; Milad and Quirk, 2012; Orsini and Maren, 2012; Quirk and Mueller, 2008)
LTP at excitatory synapses on either LA or ITC interneurons may be a mechanism by which extinction increases inhibition in the amygdala to suppress fear. Indeed, NMDA receptor activation is required for both LTP at excitatory synapses on interneurons as well as extinction learning (Falls et al., 1992; H. Lee and Kim, 1998; Zimmerman and Maren, 2010). Of course, much remains to be learned about the behavioral contingencies that lead to the induction of LTP at excitatory synapses on LA interneurons and whether, for example, this form of synaptic plasticity is induced by extinction training. Computational models incorporating LTP on inhibitory interneurons have been proposed to account for several extinction phenomena (Franklin B Krasne, 2011; Li et al., 2009; Vlachos et al., 2011), and subtle changes in the balance of excitation and inhibition in the amygdala are likely to regulate fear after extinction.
Inhibitory LTP at GABAergic synapses on LA principal neurons
Another mechanism for recruiting inhibition is to potentiate inhibitory synaptic transmission, a form of synaptic plasticity termed “inhibitory” LTP (LTPi; Figure 1). In the amygdala, it has recently been shown that high-frequency stimulation of LA neurons evokes a heterosynaptic LTPi of monosynaptic inhibitory postsynaptic currents (IPSCs) in BLA principal neurons (Lange et al., 2011). Potentiation of inhibitory GABAergic synapses on BLA principal neurons was dependent on both AMPA and NMDA receptors, thereby revealing the heterosynaptic nature of this form of plasticity. Interestingly, the expression of LTPi was mediated by increases in presynaptic GABA release whereas inhibitors of neuronal nitric oxide (NO) synthase, which is expressed by many BLA principal neurons, prevented the induction of LTPi. This suggests that NO generated by principal neurons may serve as a retrograde messenger to regulate synaptic plasticity at inhibitory synapses contacting BLA principal neurons.
What role might LTPi play in extinction learning? It has been reported that NO signaling in the amygdala is involved in the acquisition of auditory fear conditioning (Schafe et al., 2005), although systemic inhibition of NO synthase with 7-nitroindazole (7-NI) does not impair contextual fear conditioning (Maren, 1998). Interestingly, in the latter study, 7-NI administration prior to a context extinction test resulted in greater levels of freezing suggestive of an extinction impairment. However, whether this outcome results from impairments in LTPi is not clear. Indeed, intra-BLA administration of AMPA receptor antagonists does not prevent fear extinction (Falls et al., 1992; Zimmerman and Maren, 2010). Of course, even in the presence of AMPA receptor antagonists, there is apparently sufficient postsynaptic depolarization to induce NMDA receptor-dependent LTP and LTPi under some conditions (Kim et al., 2007; Lange et al., 2011; Muller et al., 1988), and this is apparently sufficient for extinction learning to proceed in the presence of AMPA receptor antagonism in the BLA. Clearly, further work formally exploring the contribution of NO signaling and LTPi to extinction is needed.
The possibility that increases in inhibitory synaptic transmission in the BLA contribute to extinction is bolstered by a recent ex vivo cellular imaging study (Kim et al., 2007; Trouche et al., 2013). These investigators used a transgenic mouse expressing a conditional and enduring Fos-GFP tag to label neurons active during contextual fear conditioning and then asked whether those same neurons were activated by extinction by examining co-localization of Fos with extinction-induced Zif268 (Zif), another activity-dependent transcription factor. They found that in the basal amygdala a number of GFP-positive neurons activated by fear conditioning were less likely to be active after extinction conditioning. Importantly, these GFP+/Zif- neurons exhibited greater levels of perisomatic GAD67 and parvalbumin immunolabeling, suggestive of an increase in the number or size of inhibitory synaptic terminals on these cells. Although it is not clear whether structural changes at inhibitory synaptic terminals accompany LTPi, these data nonetheless suggest that plasticity at inhibitory synaptic terminals (i.e., ‘Inhibitory’ LTP in Figure 1) may accompany extinction. Of course, a critical question is how inhibition in the amygdala is itself dampened to allow for the return of fear, for example, during renewal or spontaneous recovery (Hong et al., 2009; Maren, 2013; 2011).
Out with the Old and In With the New
Extinction might involve synaptic changes that either eliminate (depotentiation) or spare (inhibitory plasticity) synaptic changes associated with fear conditioning. However, there is also evidence that metaplasticity at synapses modified by conditioning and extinction allows for a greater dynamic range of synaptic change that could account for both rapid fear recovery (e.g., renewal) after extinction, as well as temporal windows within which fear memory is susceptible to erasure. Regulation of both the subunit composition and phosphorylation state of glutamate receptors at BLA synapses may support these forms of metaplasticity.
Re-potentiation as a renewal mechanism
There are a number of conditions that promote the return of fear after extinction. One mechanism to support return of fear is to gate the expression of conditioning- and extinction-related plasticity in networks that maintain representations of both experiences (Maren, 2011; Maren et al., 2013; Maren and Quirk, 2004; Orsini and Maren, 2012). However, another possibility is that the return of fear is mediated by a re-potentiation of synapses that were initially potentiated by conditioning and subsequently depotentiated after extinction. By this view, synapses undergoing both LTP and depotentiation after conditioning and extinction, respectively, might maintain a metaplastic tag that allows them to re-potentiate under conditions that promote fear return.
Consistent with this possibility, Choi and colleagues (2013) have recently described a form of metaplasticity at BLA synapses that contributes to the renewal of conditioned fear to an extinguished CS (i.e., a return of fear to the CS when it is encountered outside the extinction context) in mice. After extinction, LA synapses were found to exhibit a form of low-threshold potentiation that was occluded by behavioral procedures that yielded renewal (i.e., presenting the extinguished CS outside the extinction context)((S. Lee et al., 2013). This suggests that renewal re-potentiates LA synapses that were depotentiated after extinction training thereby promoting the return of fear to the extinguished CS. Interestingly, the renewal of fear after extinction was associated with increased GluA1 phosphorylation in LA, and renewal was prevented by intra-LA infusions of a peptide inhibitor that impaired GluA1 phosphorylation. Collectively, these results suggest that although extinction has the capacity to depotentiate LA synapses, it may leave them particularly susceptible to re-potentiation by enabling low-threshold potentiation. This increased capacity for LTP after extinction might serve as a synaptic mechanism to promote the rapid return of conditional responding during renewal and other recovery phenomena.
Retrieval-extinction and long-term depression in the BLA
Recently, an intriguing paper reported that delivering extinction trials soon after reactivation of a fear memory impaired renewal, reinstatement, and spontaneous recovery of fear (Monfils et al., 2009). Unlike a standard extinction protocol, it was suggested that this “retrieval-extinction” procedure erased fear memories, because fear responses did not recover after extinction. While there is debate about the behavioral contingencies that promote memory erasure by retrieval-extinction procedures (Auber et al., 2013; Chan et al., 2010; Maren, 2014; 2011), it does appear that retrieval-extinction procedures alter conditioning-related plasticity in the BLA under some conditions.
In a recent series of ex vivo slice electrophysiology experiments, Clem and Huganir (2010) have shown that retrieval-extinction procedures in mice limit both renewal and spontaneous recovery of extinguished fear and are accompanied by depotentiation in the BLA (Clem and Huganir, 2010). First, they demonstrated that fear conditioning is associated with the insertion of GluA2-lacking calcium-permeable AMPA receptors, a change that peaked one day after conditioning. Interestingly, insertion of this form of AMPA receptor into LA synapses increased their capacity for long-term depression after paired-pulse low frequency stimulation of thalamic afferents. Thus, removal of calcium-permeable AMPA receptors at potentiated synapses in LA might serve as a mechanism for fear suppression. Indeed, the authors found that a retrieval-extinction procedure (but not extinction alone, curiously) resulted in synaptic and molecular (e.g., GluA1 phosphorylation) changes suggestive of LTD induction. Moreover, transgenic mice that lacked the capacity to traffic calcium-permeable AMPA receptors showed both spontaneous recovery and renewal of fear after the retrieval-extinction procedure. In other words, LTD and consequent synaptic removal of calcium-permeable AMPA receptors appear to be a mechanism for generating a long-term reversal if not erasure of the synaptic potentiation in LA neurons associated with fear conditioning.
A key question, however, is why this NMDA- and mGluR-dependent LTD only accompanies a retrieval-extinction procedure (Lin et al., 2003b). To the extent that standard extinction procedures (i.e., repeated CS presentations) reproduce some features of the repetitive electrical stimulation trains used to induce LTD, one would expect extinction (without a prior reminder) to induce LTD as well. Understanding why a retrieval event opens a temporal window to allow depotentiation or LTD to reverse memory is obviously central to appreciating the synaptic and molecular mechanisms underlying extinction and erasure. Moreover, it will be important to understand whether these changes are truly irreversible, or whether there remain metaplasticity mechanisms that can rapidly return depotentiated synapses to a potentiated state under some conditions (S. Lee et al., 2013).
Conclusions
There are a variety of synaptic mechanisms in the amygdala that might support the extinction of fear. If one accepts that extinction preserves the content of conditioning, at least in part, then a synaptic mechanism that encodes new information alongside the conditioning memory (e.g., LTP at excitatory inputs to interneurons or inhibitory LTP) might be favored over one that erases (e.g., LTD or depotentiation) conditioning-induced plasticity. However, because extinction likely involves both the erasure of the conditioning memory as well as new learning, it is conceivable that all three of the mechanisms illustrated in Figure 1 might contribute to extinction learning. Moreover, metaplasticity at BLA synapses may complement these mechanisms to allow for dynamic regulation of synaptic gain to promote the rapid return (or erasure) of fear after extinction. Of course, the relative contribution of each mechanism to extinction remains to be determined.
In conclusion, the synaptic analysis of extinction provides important new insight into how neural networks encode different memories about the same external stimulus. How these different synaptic engrams are read out to produce adaptive behavior remains an intriguing question, but likely involves hippocampal-prefrontal networks that contextualize the meaning of the CS when it is encountered. Consistent with this idea, a recent report reveals changes in the synaptic efficacy of medial prefrontal inputs to BLA after extinction training (Cho et al., 2013). Ultimately, the synaptic mechanism underlying extinction in the amygdala must involve plasticity at synapses in the amygdala that inform the animals not only what to fear (thalamo-cortical inputs), but also when and where that fear should be expressed (hippocampal-prefrontal inputs).
Acknowledgments
This work was supported by a grant from the National Institutes of Health (RO1 MH065961). I would like to thank Dr. Naomi Nagaya for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amano T, Unal CT, Paré D. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci. 2010;13:489–494. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auber A, Tedesco V, Jones CE, Monfils MH, Chiamulera C. Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions? Psychopharmacology (Berl) 2013:1–17. doi: 10.1007/s00213-013-3004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE. Heterosynaptic long-term potentiation of inhibitory interneurons in the lateral amygdala. J Neurosci. 2004;24:9507–9512. doi: 10.1523/JNEUROSCI.3567-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EP, LeDoux JE, Nader K. Fear conditioning and LTP in the lateral amygdala are sensitive to the same stimulus contingencies. Nat Neurosci. 2001;4:687–688. doi: 10.1038/89465. [DOI] [PubMed] [Google Scholar]
- Blair HT, Schafe GE, Bauer EP, Rodrigues SM, LeDoux JE. Synaptic plasticity in the lateral amygdala: a cellular hypothesis of fear conditioning. Learn Mem. 2001;8:229–242. doi: 10.1101/lm.30901. [DOI] [PubMed] [Google Scholar]
- Bouton M, Bolles R. Contextual control of the extinction of conditioned fear. Learn Motiv. 1979;10:445–466. [Google Scholar]
- Bouton ME. Context and Behavioral Processes in Extinction. Learn Mem. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Chan WYM, Leung HT, Westbrook RF, Mcnally GP. Effects of recent exposure to a conditioned stimulus on extinction of Pavlovian fear conditioning. Learn Mem. 2010;17:512–521. doi: 10.1101/lm.1912510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman P, Bellavance L. Induction of long-term potentiation in the basolateral amygdala does not depend on NMDA receptor activation. Synapse. 1992;11:310–318. doi: 10.1002/syn.890110406. [DOI] [PubMed] [Google Scholar]
- Chapman P, Kairiss E, Keenan C, Brown T. Long-term synaptic potentiation in the amygdala. Synapse. 1990;6:271–278. doi: 10.1002/syn.890060306. [DOI] [PubMed] [Google Scholar]
- Cho JH, Deisseroth K, Bolshakov VY. Synaptic encoding of fear extinction in mPFC-amygdala circuits. Neuron. 2013;80:1491–1507. doi: 10.1016/j.neuron.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clugnet M, LeDoux J. Synaptic plasticity in fear conditioning circuits: induction of LTP in the lateral nucleus of the amygdala by stimulation of the medial geniculate body. J Neurosci. 1990;10:2818–2824. doi: 10.1523/JNEUROSCI.10-08-02818.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen P. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Delamater AR. Experimental extinction in Pavlovian conditioning: Behavioural and neuroscience perspectives. Quarterly Journal of Experimental Psychology Section B. 2004;57:97–132. doi: 10.1080/02724990344000097. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Paré D. Amygdala microcircuits controlling learned fear. Neuron. 2014;82:966–980. doi: 10.1016/j.neuron.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Lüthi A. Amygdala inhibitory circuits and the control of fear memory. Neuron. 2009;62:757–771. doi: 10.1016/j.neuron.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Falls W, Miserendino M, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow M, Poulos A. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Krasne Franklin B, F MS, Z M. Design of a Neurally Plausible Model of Fear Learning. Front Behav Neurosci. 2011;5 doi: 10.3389/fnbeh.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosens KA, Maren S. Long-term potentiation as a substrate for memory: Evidence from studies of amygdaloid plasticity and Pavlovian fear conditioning. Hippocampus. 2002;12:592–599. doi: 10.1002/hipo.10099. [DOI] [PubMed] [Google Scholar]
- Goosens KA, Maren S. NMDA receptors are essential for the acquisition, but not expression, of conditional fear and associative spike firing in the lateral amygdala. Eur J Neurosci. 2004;20:537–548. doi: 10.1111/j.1460-9568.2004.03513.x. [DOI] [PubMed] [Google Scholar]
- Harris J, Jones M, Bailey G, Westbrook R. Contextual control over conditioned responding in an extinction paradigm. J Exp Psychol Anim Behav Process. 2000;26:174–185. doi: 10.1037//0097-7403.26.2.174. [DOI] [PubMed] [Google Scholar]
- Hong I, Song B, Lee S, Kim J, Kim J, Choi S. Extinction of cued fear memory involves a distinct form of depotentiation at cortical input synapses onto the lateral amygdala. Eur J Neurosci. 2009;30:2089–2099. doi: 10.1111/j.1460-9568.2009.07004.x. [DOI] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler K. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. American Journal of Psychiatry. 2010;167:648. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Park K, Hong I, Song B, Son G, Park H, Kim WR, Park E, Choe HK, Kim H, Lee C, Sun W, Kim K, Shin KS, Choi S. Amygdala depotentiation and fear extinction. Proc Natl Acad Sci USA. 2007;104:20955–20960. doi: 10.1073/pnas.0710548105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konorski J. Integrative activity of the brain: An interdisciplinary approach. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Lange MD, Doengi M, Lesting J, Pape HC, Jungling K. Heterosynaptic long-term potentiation at interneuron-principal neuron synapses in the amygdala requires nitric oxide signalling. J Physiol (Lond) 2011;590:131–143. doi: 10.1113/jphysiol.2011.221317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook R. Distinct contributions of the basolateral amygdala and the medial prefrontal cortex to learning and relearning extinction of context conditioned fear. Learn Mem. 2008;15:657–666. doi: 10.1101/Lm.1080108. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim J. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Song B, Kim J, Park K, Hong I, An B, Song S, Lee J, Park S, Kim J. GluA1 phosphorylation at serine 831 in the lateral amygdala is required for fear renewal. Nat Neurosci. 2013 doi: 10.1038/nn.3491. [DOI] [PubMed] [Google Scholar]
- Li G, Nair SS, Quirk GJ. A biologically realistic network model of acquisition and extinction of conditioned fear associations in lateral amygdala neurons. J Neurophysiol. 2009;101:1629–1646. doi: 10.1152/jn.90765.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yeh S, Leu T, Chang W, Wang S, Gean P. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003a;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Lee CC, Gean PW. Involvement of a calcineurin cascade in amygdala depotentiation and quenching of fear memory. Mol Pharmacol. 2003b;63:44–52. doi: 10.1124/mol.63.1.44. [DOI] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Maren S. Effects of 7-nitroindazole, a neuronal nitric oxide synthase (nNOS) inhibitor, on locomotor activity and contextual fear conditioning in rats. Brain Res. 1998;804:155–158. doi: 10.1016/s0006-8993(98)00668-4. [DOI] [PubMed] [Google Scholar]
- Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22:561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–786. doi: 10.1016/j.neuron.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Maren S. Seeking a spotless mind: extinction, deconsolidation, and erasure of fear memory. Neuron. 2011;70:830–845. doi: 10.1016/j.neuron.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Putting the Brakes on Fear. Neuron. 2013 doi: 10.1016/j.neuron.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Nature and causes of the immediate extinction deficit: A brief review. Neurobiol Learn Mem. 2014;113:19–24. doi: 10.1016/j.nlm.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Synaptic plasticity in the basolateral amygdale induced by hippocampal formation stimulation in vivo. J Neurosci. 1995;15:7548–7564. doi: 10.1523/JNEUROSCI.15-11-07548.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McConnell BL, Miller RR. Associative Accounts of Recovery-from-Extinction Effects. Learn Motiv. 2014;46:1–15. doi: 10.1016/j.lmot.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEchron MD, McCabe PM, Green EJ, Llabre MM, Schneiderman N. Simultaneous single unit recording in the medial nucleus of the medial geniculate nucleus and amygdaloid central nucleus throughout habituation, acquisition, and extinction of the rabbit's classically conditioned heart rate. Brain Res. 1995;682:157–166. doi: 10.1016/0006-8993(95)00331-j. [DOI] [PubMed] [Google Scholar]
- McKernan M, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Milad M, Quirk G. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserendino M, Sananes C, Melia K, Davis M. Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature. 1990;345:716–718. doi: 10.1038/345716a0. [DOI] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D, Joly M, Lynch G. Contributions of quisqualate and NMDA receptors to the induction and expression of LTP. Science. 1988;242:1694–1697. doi: 10.1126/science.2904701. [DOI] [PubMed] [Google Scholar]
- Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014 doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Pare D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiological Reviews. 2010;90:419–463. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Dover Publications; New York: 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/Sj.Npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk G, Repa C, LeDoux J. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Racine R, Milgram N, Hafner S. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983;260:217–231. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. Appleton Century Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- Rogan M, Stäubli U, LeDoux J. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Royer S, Martina M, Paré D. An inhibitory interface gates impulse traffic between the input and output stations of the amygdala. J Neurosci. 1999;19:10575–10583. doi: 10.1523/JNEUROSCI.19-23-10575.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Paré D. Bidirectional synaptic plasticity in intercalated amygdale neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Rumpel S. Postsynaptic Receptor Trafficking Underlying a Form of Associative Learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- Sah P, Westbrook RF, Lüthi A. Fear Conditioning and Long-term Potentiation in the Amygdala. Ann N Y Acad Sci. 2008;1129:88–95. doi: 10.1196/annals.1417.020. [DOI] [PubMed] [Google Scholar]
- Schafe G, Bauer E, Rosis S, Farb C, Rodrigues S, LeDoux J. Memory consolidation of Pavlovian fear conditioning requires nitric oxide signaling in the lateral amygdala. European Journal of Neuroscience. 2005;22:201–211. doi: 10.1111/j.1460-9568.2005.04209.x. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Doyère V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–227. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Trouche S, Sasaki JM, Tu T, Reijmers LG. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron. 2013;80:1054–1065. doi: 10.1016/j.neuron.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkov E, Carlezon WA, Benes FM, Kandel ER, Bolshakov VY. Fear conditioning occludes LTP-induced presynaptic enhancement of synaptic transmission in the cortical pathway to the lateral amygdala. Neuron. 2002;34:289–300. doi: 10.1016/s0896-6273(02)00645-1. [DOI] [PubMed] [Google Scholar]
- Vlachos I, Herry C, Lüthi A, Aertsen A, Kumar A. Context-Dependent Encoding of Fear and Extinction Memories in a Large-Scale Network Model of the Basal Amygdala. PLoS Comp Biol. 2011;7:e1001104. doi: 10.1371/journal.pcbi.1001104.g008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JM, Maren S. NMDA receptor antagonism in the basolateral but not central amygdala blocks the extinction of Pavlovian fear conditioning in rats. Eur J Neurosci. 2010;31:1664–1670. doi: 10.1111/j.1460-9568.2010.07223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]