Abstract
Anti-angiogenic therapies target the tumor vasculature, impairing its development and growth. It was hypothesized over 40 years ago by the late Judah Folkman and Julie Denekamp that depriving a tumor of oxygen and nutrients, by targeting the tumor vasculature, could have therapeutic benefits. Identification of growth factors and signaling pathways important in angiogenesis subsequently led to the development of a series of anti-angiogenic agents that over the past decade have become part of the standard of care in several disease settings. Unfortunately not all patients respond to the currently available anti-angiogenic therapies while others become resistant to these agents following prolonged exposure. Identification of new pathways that may drive angiogenesis led to the development of second-generation anti-angiogenic agents such as those targeting the Ang-2/Tie2 axis. Recently, it has become clear that combination of first and second generation agents targeting the blood vessel network can lead to outcomes superior to those using either agent alone. The present review focuses on the current status of VEGF and Ang-2 targeted agents and the potential utility of using them in combination to impair tumor angiogenesis.
Keywords: Ang-2 targeting, Angiogenesis, Anti-angiogenic therapy, VEGF targeting, Combination therapy
Introduction
The formation of new blood vessels from pre-existing ones, angiogenesis, is a physiological event that occurs during embryonic vascular development as well as in adult vasculature as part of wound healing, reproduction and the menstrual cycle [1]. Angio-genesis also is an important process in pathological conditions including cancer, diabetic retinopathy, atherosclerosis and rheumatoid arthritis [2]. In cancer, it is a fundamental process involved in tumor development, progression and dissemination [3–5]. As a consequence, key growth factors in angiogenesis have been exploited for anti-cancer therapies [3,6,7].
Tumor vasculature is distinct from normal vasculature. Normal vessels are quiescent with minimal endothelial cell proliferation, tight endothelial–endothelial cell and endothelial–peri-endothelial cell (e.g. smooth muscle cells and pericytes) contacts to regulate vessel permeability [8]. Tumor vessels, on the other hand, have highly proliferative endothelial cells with loose to minimal endothelial– endothelial and endothelial–peri-endothelial cell contacts yielding a leaky vascular phenotype [9,10]. Yet despite being highly active, the leaky tumor vasculature is unable to provide a tumor with its oxygen and nutritional needs leading to aberrant tumor microenvironments that negatively impact the ability to control the cancer with conventional anticancer therapies [10,11]. Given a tumor's dependence on angiogenesis, agents targeting the ability of tumors to initiate new vessel formation rapidly advanced into the clinical realm of cancer management.
Today there are ten agents with direct anti-angiogenic activity (primarily antibody and small molecule inhibitors) that have been approved by the FDA for the treatment of solid tumors (Table 1). Although outcomes have been generally encouraging, patients often fail to respond or become resistant to the currently available therapies [12,13]. Consequently, efforts to develop optimal approaches to realize the true potential of vascular-targeted therapies as cancer treatment are actively being pursued. Although a number of angiogenesis targeting approaches are under active preclinical and clinical development (discussed later), the focus of this review is on the status of inhibitors that target the VEGF and Ang-2 axes. Due to the unique mode of action and apparent interplay between VEGF and Ang-2 pathways there has been tremendous recent effort to combine these two modalities to achieve enhanced treatment outcomes. The rationale for such combination and advances in therapies will be discussed in this review.
Table 1.
VEGF and Ang-2 axis targeted agents in the clinic and preclinical settings. (Due to the scope of this review, this table only shows the VEGF/VEGFR axis targeted agents that have been FDA approved.).
| Agent | Target | Clinical Status | Tumor Setting |
|---|---|---|---|
| VEGF Targeted | |||
| Bevacizumab (Avastin®) | VEGF | FDA Approved | mCRC, RCC, NSCLC, Glioblastoma |
| Sorafenib (Nexavar®) | VEGFR, PDGFR, Raf, RET, cKIT, Flt3 | FDA Approved | RCC, HCC |
| Sunitinib (Sutent®) | VEGFR, PDGFR, Flt3, RET, CSF-1 | FDA Approved | RCC, GIST, Pancreatic cancer |
| Pazopanib (Votrient®) | VEGFR, PDGFR, cKIT | FDA Approved | RCC, Sarcoma |
| Vandetanib (Caprelsa®) | VEGFR, EGFR, RET | FDA Approved | Medullary thyroid cancer |
| Cabozantinib (Cometriq®) | cMET, VEGFR-2 | FDA Approved | Medullary thyroid cancer |
| Axitinib (Inlyta®) | VEGFR, PDGFR, cKIT | FDA Approved | RCC |
| Ziv-Aflibercept (Zaltrap®) | VEGF | FDA Approved | mCRC |
| Ramucirumab (Cyramza®) | VEGFR-2 | FDA Approved | Stomach cancer |
| Angiopoietin Targeted | |||
| Trebananib (AMG386) | Ang-1/Ang-2 | Phase III | Ovarian cancer |
| Phase I | AML, CNS | ||
| AMG780 | Ang-2 | Phase I | Solid tumors |
| MEDI3617 | Ang-2 | Phase I | Solid tumors |
| Nesvacumab (REGN910) | Ang-2 | Phase I | Solid tumors |
| CVX-060 | Ang-2 | Phase I | Solid tumors |
| DX-2240 | Tie1 | Preclinical | |
| scFv-Ang-2 | Ang-2 | Preclinical | |
| LC-06 | Ang-2 | Preclinical | |
| VEGF and angiopoietin targeted | |||
| Regorafenib (Stivarga®) | VEGFR-2, PDGFR, Tie2, KIT, RET, Raf | FDA Approved | mCRC, GIST |
| CEP-11981 | Tie2, VEGFR | Phase I | Solid tumors |
| ARRY-614 | Tie2, VEGFR-2, p38, Abl | Phase I | Myelodysplastic syndromes |
| CVX-241 | Ang-2, VEGF | Phase I | Solid tumors |
| RO5520985/RG7221 | Ang-2-VEGF CrossMab | Phase I | Solid tumors |
| ACTB-1003 | Tie2, VEGFR-2, FGFR-1, RSK, S6K A | Preclinical | |
| DAAP | Ang-2, VEGF | Preclinical |
mCRC: metastatic colorectal cancer, RCC: renal cell carcinoma, NSCLC: non-small cell lung cancer, HCC: hepatocellular carcinoma, GIST: gastrointestinal stromal tumor, AML: acute myeloid leukemia, CNS: central nervous system tumor.
Ang-2/Tie2 signaling disrupts normal vasculature
The angiopoietin/Tie system was identified nearly two decades ago [14–18]. It is an endothelial cell specific system that is responsible for the maintenance and disruption of normal vasculature [19]. It is composed of three ligands in humans, Ang-1, -2, and -4; Ang-1 and Ang-2 are the best characterized of the three and will be discussed here. The angiopoietin/Tie axis is essential for vasculogenesis, normal vascular development, as well as angiogenesis; however vasculogenesis will not be discussed in depth in this review (for reviews see [19–21]).
Tyrosine kinase with Ig and EGF homology domains-2 (Tie2) is a transmembrane tyrosine kinase receptor and is expressed by both vascular and lymphatic endothelial cells [14,16]. Tie1 remains an orphan receptor with no known direct ligand binding. Ang-1 and Ang-2 are both secreted proteins with similar structures and 60% amino acid sequence homology. They have however, opposing functions upon binding the Tie2 receptor [18]. Ang-1 paracrine signaling to Tie2, from peri-endothelial cells, maintains a mature-quiescent vessel with tight endothelial–endothelial and endothelial–periendothelial cell interactions, shown in Fig. 1A [23,24]. In the adult, Ang-1 is found in various tissues and secretion occurs continuously at low levels; signaling mechanisms by Ang-1 mediated Tie2 phosphorylation involves Rho and mDia leading to inhibition of VEGF/VEGFR-2 mediated vascular permeability through src [25]. The endothelium-specific vascular endothelial–phosphotyrosine phosphatase (VE-PTP) has also been shown to mediate cross talk between the Ang-1/Tie2 and VEGF/VEGFR pathways [26]. Ang-1 phosphorylation of Tie2 stimulates pro-survival pathways, such as PI3K-AKT and MAPK/ERK, as well as represses pro-inflammatory pathways through NF-κβ [27–29] indicating that Ang-1 can promote a stable, quiescent vasculature through various signaling modalities.
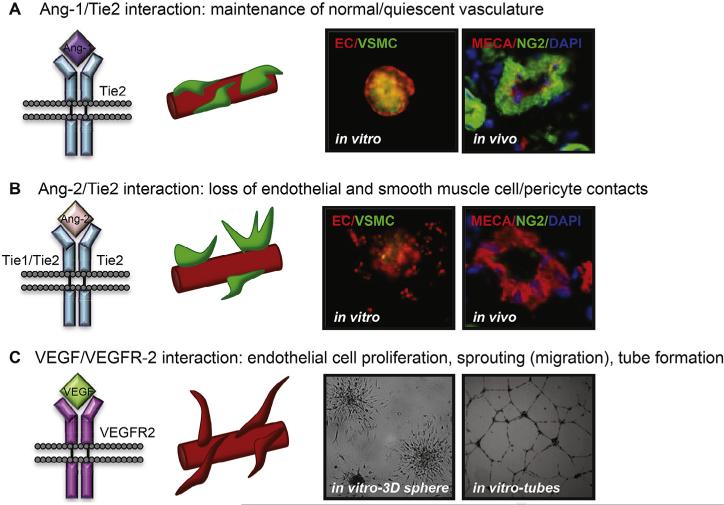
Fig. 1.
The Angiopoietin/Tie2 and VEGF/VEGFR signaling axis in angiogenesis. (A) Ang-1/Tie2 interaction maintains normal-quiescent vasculature with tight endothelial– endothelial and endothelial–smooth muscle cell/pericyte contacts as demonstrated by an in vitro, unstimulated, co-culture endothelial and smooth muscle cell sphere and in vivo normal vasculature with smooth muscle cell coverage. (B) Ang-2/Tie2 interaction with Tie2 homodimers or heterodimers results in loss of endothelial–endothelial and endothelial–smooth muscle cell contacts (i.e. destabilization of the vasculature) as demonstrated by an in vitro, PMA (stimulant of Ang-2 secretion) stimulated, co-culture sphere and in vivo vasculature from tumor tissue with loss of smooth muscle cell coverage. (C) VEGF/VEGFR-2 interaction stimulates endothelial cell survival, proliferation and sprouting of new vessels. Endothelial cell sprouting is illustrated by the collagen embedded 3D HUVEC spheres (image taken at 24 hrs) and tube formation by matrigel plated HUVEC (image taken at 12 hrs). EC: endothelial cell (HUVEC); VSMC: vascular smooth muscle cell (HUASMC); MECA: endothelial cell marker; NG2: peri-endothelial cell marker (ex. smooth muscle cell, pericyte); DAPI: nuclear marker (modified from Microvasc. Res. 2012: 83 (3): 290–297. Molnar N. and Siemann D.W., fig. 3D [22]; and Biel N.M. and Siemann D.W., unpublished results).
Ang-2 is stored in Weibel–Palade bodies in endothelial cells and secreted upon microenvironmental changes [30–33]. Contrary to Ang-1/Tie2 signaling Ang-2 autocrine signaling via the Tie2 receptor results in loss of cell–cell contacts and vascular destabilization (Fig. 1B) [18,34]. Consequently Ang-2 is usually referred to as the Tie2 antagonist [18,35–37]. However, Ang-2 may also act as a Tie2 agonist in a context dependent manner [38,39]. Furthermore the ratios of Ang-1 to Ang-2 as well as Tie1 to Tie2 in the microenvironment can affect signaling activities through Tie2, clearly making this system highly complex [40]. In general Ang-1 appears to prefer Tie2 homodimer interactions as the presence of Tie1 can hinder Ang-1 mediated signaling, while the absence of Tie1 can enhance Ang-1 mediated signaling through Tie2 [40]. Tie 1 and Tie2 receptors have been shown to form heterodimers, and even though no ligands are known to bind Tie1, the receptor can interact with Ang2 through the heterodimers [39,41]. However, the presence or absence of Tie1 does not alter Ang-2's interaction with Tie2 nor the antagonistic characteristics of Ang-2 toward Ang-1 [40].
Ang-2 binding to Tie2 homodimers can lead to agonistic interactions similar to Ang-1/Tie2 interactions, mainly as shown in the lymphatic vasculature where Tie1 is sparsely expressed [38]. Tie1 is commonly expressed in blood endothelial cells and has been shown to be upregulated in the tumor microenvironment [42]. Elevated Tie1 expression can increase Tie1/Tie2 heterodimer formation resulting in increased Ang-2 binding to the receptors which antagonizes Ang-1 interactions and results in vessel destabilization [39,41]. However, current evidence suggests that Ang-2's antagonistic role cannot be solely attributed to Tie1 involvement, therefore making this axis highly unique and complex. Ang-1's inability but Ang-2's ability to interact with both Tie2 homodimers and Tie1/ Tie2 heterodimers is a consequence of a difference of three amino acids (proline, glutamine, arginine) in the protein sequence outside the receptor binding site of Ang-2 as compared to Ang-1 (threo-nine, alanine, glycine) [43].
VEGF/VEGFR signaling stimulates new vessel growth
The VEGF/VEGFR system was identified three decades ago [44,45]. The Vascular Endothelial Growth Factor (VEGF) family has several members including VEGF-A, -B, -C, -D, -E and placental growth factor (PlGF) that bind to various transmembrane tyrosine kinase VEGF receptors VEGFR-1, -2, and -3. The following discussion will focus on the major angiogenic factor, VEGF-A, and its receptors VEGFR-1 and VEGFR-2. VEGF-A has several isoforms resulting from alternative splicing; the most abundant isoform being VEGF-A165 consisting of 165 amino acids and will be referred to as VEGF for the rest of this review. VEGF binds both VEGFR-1 and VEGFR-2 with higher affinity for VEGFR-1 but lower kinase activity than binding to VEGFR-2 [46,47].
VEGF has numerous effects on the endothelial cells of the vasculature; it acts as a pro-survival factor and stimulator of endothelial cell proliferation, invasion and migration ultimately leading to new vessel growth (Fig. 1C) [46]. VEGF signaling enhances endothelial cell survival by activation of the PI3K-Akt pathway and upregulation of anti-apoptotic proteins such as Bcl-2 [48,49]. VEGF is also known as vascular permeability factor for its ability to loosen endothelial cell–cell contacts through mediating the loss of VE-cadherin via activation of src signaling [50,51]. Effects on endothelial cell proliferation is mainly through activation of Erk1/2 signaling and endothelial cell migration through activation of focal adhesion kinase (FAK) as well as induction of several extracellular matrix degrading proteins including metalloproteinases, urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (TTPA) [46,52]. It should be noted that many other growth factors and their receptors, including fibroblast growth factor (FGF/FGFR), epidermal growth factor (EGF/EGFR), insulin-like growth factor (IGF/ IGFR), and platelet derived growth factor (PDGF/PDGFR), similarly exert stimulating effects on endothelial cells; however their review is outside the scope of the present article (for excellent comprehensive reviews of those factors see [53–57].
Along with its survival, migratory and proliferative effects, VEGF also interacts with the Notch-Delta like 4 (Dll4) pathway affecting tip and stalk cell selection as new vessels sprout [58]. In general, tip cells are migratory and follow pro-angiogenic signaling cues originating from the tumor while stalk cells are proliferative and produce the newly growing vessel [58].
Pro-angiogenic factors, VEGF and Ang-2, in the tumor microenvironment
Solid tumors require active angiogenesis for their development and growth. Tumor cells have the ability to secrete key proangiogenic factors to directly stimulate this process. Although the present discussion is focused on two primary factors, VEGF and Ang-2, found in the tumor microenvironment, it is clear that many other factors can affect tumor angiogenesis and are also being explored as therapeutic targets (for review see [59–65]. VEGF is secreted by virtually all solid tumor cells although some tumor types may secrete heightened levels compared to others. Elevated levels of VEGF have been correlated to disease progression and prognosis in various cancers including colorectal, lung, hepatocellular, breast, pancreatic and gastrointestinal cancers [66–71].
In general, heightened secretion of VEGF by tumor cells is a response to hypoxic conditions inherent to all developing solid tumors. As the tumor grows, cells existing further than the oxygen diffusion distance (70–100 μm) from blood vessels become hypoxic [72]. Hypoxia Inducible Factor-1α (HIF-1α) is stabilized under low oxygen conditions and dimerizes with HIF-1β; the HIF complex then trans-locates to the nucleus and binds to the VEGF promoter and increases VEGF transcription [73,74]. Elevated VEGF levels, in turn, stimulate endothelial cells of nearby vessels to initiate new vessel growth to the tumor [47,75].
Ang-2 serum levels in patients with solid tumors are typically elevated compared to measurements in healthy individuals [76,77] and increase with disease progression [78–82]. Additionally, Ang-2 levels in the tumor microenvironment have been correlated with increased recruitment of Tie2 expressing macrophages (TEMs) that correlate with pro-angiogenic activity [83]. Unlike VEGF, the specific mechanisms for Ang-2 elevation in the microenvironment are unclear. Our preclinical studies indicate that renal cell carcinoma cells (Caki-1, Caki-2, 786-0, A498) do not secrete Ang-2 directly under aerobic nor hypoxic conditions but that they do secrete factors, currently unidentified, that can stimulate the release of Ang-2 from the endothelial cells (Biel NM and Siemann DW, unpublished 2013); suggesting that the presence of Ang-2 and VEGF in the tumor microenvironment occurs through distinct but perhaps complementary actions. Still, the presence of both VEGF and Ang-2 in the tumor microenvironment and their respective importance in angiogenesis has made them attractive targets as anti-angiogenic cancer therapy.
First generation anti-angiogenic agents: targeting the VEGF signaling pathway
Agents targeting the VEGF pathway exert their effects by inhibition of endothelial cell proliferation, migration and tube formation (Fig. 2). The first FDA approved anti-angiogenic agent was Bevacizumab (Avastin®; Genetech/Roche) a humanized monoclonal antibody against VEGF-A. Initially approved in 2004 for metastatic colorectal cancer and is now approved for use in a number of oncologic settings. Sorafenib (Nexavar®, Bayer/Onyx), a multi-tyrosine kinase small molecule inhibitor, was approved in 2005 for the treatment of renal cell carcinoma. The following year the small molecule inhibitor Sunitinib (Sutent®, Pfizer) was approved for renal cell carcinoma.
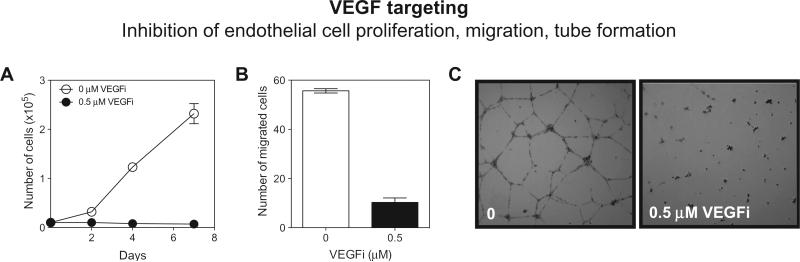
Fig. 2.
VEGF axis targeting in angiogenesis. Interference of the VEGF/VEGFR-2 signaling axis leads to inhibition of human umbilical vein endothelial cell (HUVEC) proliferation. (A) Transwell migration at 24 hrs (B) and tube formation on matrigel at 24 hrs ± VEGFi (Cediranib/AZD2171, AstraZeneca) (C) (Biel N.M. and Siemann D.W., unpublished results).
Small molecule tyrosine kinase inhibitors Pazopanib (Votrient®, GlaxoSmithKline), Vandetanib (Caprelsa®, AstraZeneca), Cabozantinib (Cometriq®, Exelixis) and Axitinib (Inlyta®, Pfizer) were approved for renal cell carcinoma and thyroid cancers. The VEGF-Trap Ziv-Aflibercept (Zaltrap®, Regeneron) has been approved for metastatic colorectal cancer and the most recent FDA approval was the human monoclonal VEGFR-2 antibody Ramucirumab (Cyramza®, Eli Lilly) for stomach cancer (Table 1). Many other agents are currently in clinical trials in a wide range of oncologic settings. To date, Bevacizumab has proven to be most successful having been approved for use in the treatment of glioblastoma, metastatic colorectal, non-small cell lung cancer, renal cell carcinoma and until recently metastatic breast cancer [84–86].
Although these agents show minimal direct antitumor effects when administered alone, they can enhance the impairment of tumor growth when combined with conventional anticancer therapies [87–89]. Such combination treatments also resulted in somewhat greater chemotherapy and radiation therapy induced tumor cell kill. It's been proposed that anti-angiogenic treatments yield a window of vascular normalization that leads to improved chemotherapeutic drug delivery to the tumor core as well as a reduction in tumor hypoxia resulting in better cell kill by radiation therapy [87,90,91]. However, tumor oxygenation status following anti-angiogenic agent therapy can vary greatly with changes ranging from decreased oxygenation to no change in oxygenation to increased oxygenation having been observed post treatment [88].
Second generation anti-angiogenic agents: targeting the Ang-2 signaling pathway
Unlike the VEGF axis targeted agents, which predominantly affect endothelial cell proliferation, migration and tube formation, the inhibition of the Ang-2/Tie2 axis is more complex and multifactorial. For example, Ang-2 inhibition impairs endothelial and smooth muscle cell contact loss leading to reduced tumor vasculature and increased vessel normalization. Ang-1 inhibition alone has little effect on the tumor vasculature. However, combined Ang-2 and Ang-1 signal inhibition leads to reduced tumor vasculature without vessel normalization. These findings suggest that normalization of the vasculature is Ang-1 dependent and Ang-2 blockade allows for enhanced Ang-1/Tie2 interactions. While the reduction of tumor vessels is Ang-1 independent, the maintenance of tumor vessel reduction in the presence of both Ang-1 and Ang-2 blockade most likely accounts for the fact that Ang-2 can interact with integrins, especially on sprouting tip cells devoid of Tie2 [92–94]. Furthermore, there is evidence that Ang-2 blockade reduces the recruitment of tumor-associated macrophages (TAMs) that express Tie2, and aid angiogenesis [83,95]. This in turn may significantly reduce angio-genesis [96].
Consequently, Ang-2 targeted agents may promote both the maintenance of a normal vascular phenotype (Fig. 3) and reduction of vessel sprouts. The recognized importance of Ang-1 and Ang-2 in vascular integrity has resulted in great interest in the possibility of targeting the Ang-2/Tie2 axis to inhibit tumor angiogenesis; both for the development of novel antiangiogenic agents and as a means to circumvent the lack of response or adaptive resistance that has been reported with VEGF directed agents [12,13,97]. A variety of approaches have been undertaken (Table 1).
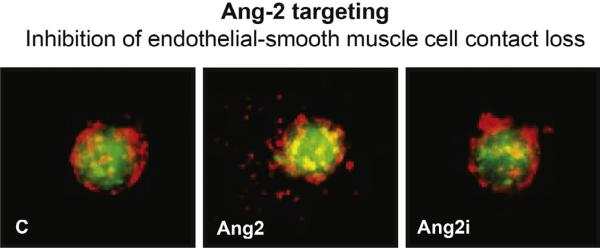
Fig. 3.
Ang-2 axis targeting in angiogenesis. Interference with Ang-2/Tie2 interaction inhibits the Ang-2 dependent loss of endothelial and smooth muscle cell contacts. Red: human umbilical vein endothelial cells (HUVEC); Green: human umbilical artery smooth muscle cells (HUASMC); Ang2i: MEDI3617 (MedImmune) (modified from Microvasc. Res. 2012: 83 (3): 290–297. Molnar N. and Siemann D.W., fig. 2B [22]). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The Ang-2 specific antibody LC06 (Roche) primarily serves as the Ang-2 specific arm of the Ang-2-VEGF CrossMab, to be discussed later. Zhang et al. demonstrated anti-tumor effects of an Ang-2 specific single-chain antibody (scFv-Ang-2) [98]. DX-2240 (Sanofi-Aventis) is a human monoclonal antibody targeting the Tie1 receptor based on the interaction of Ang-2 with Tie1/Tie2 heterodimers in vessel destabilization. Recently, D'Amico et al. have shown direct evidence for the benefit of targeting the Tie1 receptor using a Tie1 deficient endothelial cell specific mouse model [99].
There are three fully human monoclonal antibodies against Ang-2 that are being evaluated in phase I clinical trials, MEDI3617 (MedImmune/AstraZeneca), Nesvacumab (REGN910; Regeneron/ Sanofi) and AMG780 (Amgen) [100–102]. CVX-060 (Pfizer) is a monospecific Ang-2 CovX-Body that was evaluated in phase I clinical trials [103]. Trebananib (AMG386), the Ang-1/Ang-2 peptibody, is the currently most clinically advanced agent having entered phase III trials in ovarian cancer as well as being evaluated in several phase I/II trials in a variety of oncologic settings, particularly in combination with conventional chemotherapeutic agents.
Challenges of current anti-angiogenic therapies
Despite the strong scientific evidence supporting the use of blood vessel targeted agents, resistance to these agents can develop [12,13,97,104]. Furthermore lack of improvement in overall patient survival has curtailed their use; as in the case of the removal of Bevacizumab for the treatment of metastatic breast cancer [13,84,86,97]. Tumors can develop acquired resistance to anti-angiogenic therapies by upregulation of pathways in angiogenesis that are not inhibited by the therapy [12]. Even though VEGF is a major driver of angiogenesis there are other growth factors such as basic FGF, interleukin-8 (IL-8), PlGF, PDGF, and IGF that can compensate in the absence of VEGF pathway activation [12,59]. The lack of tumor responses and potential to l for compensatory mechanisms that could lead to treatment resistance clearly emphasize the continued need to develop more effective anti-angiogenic therapeutic strategies in order to fulfill the promise of vascular-targeted therapeutic strategies.
Currently several multikinase inhibitors targeting FGFRs, VEGFRs and RDGFRs are undergoing active preclinical and clinical development [61]. Notch-Delta-like 4 (Dll4) targeted agents are also being evaluated and studies suggest that Dll4 inhibition could be beneficial in VEGF resistant tumors [62,105,106]. The future of PlGF inhibitors is somewhat less clear as recent studies have suggested that such agents may offer little benefit alone or in combination [64,107]. Selective PDGFR inhibitors also are in development as anti-angiogenic agents. However, it is worth noting that many of the small molecule tyrosine kinase inhibitors approved by the FDA also target PDGFR (Table 1) [108–110]. Inhibitors of IGF are being actively pursued as well but the complexity of this signaling axis has somewhat limited the ability to achieve maximal therapeutic potential [65,111].
The most common side effects associated with anti-angiogenic therapy are hypertension and occasional bleeding along with impaired physiological angiogenesis such as wound healing, reproduction and fetal development [112–114]. These side effects limit the population of patients that can be treated and affect the dosing and scheduling of agents that are administered. However, the removal of these agents from the patients’ therapeutic regimen quickly restores physiological angiogenesis, making it feasible to remove or resume treatment before or after major surgeries.
Combination of Ang-2 and VEGF axis inhibitors
The increased understanding of the angiopoietin system coupled with the development of selective Ang-2/Tie2 axis targeted agents has led to the possibility of combining such agents with anti-VEGF therapies. Since effective tumor angiogenesis requires (a) destabilization of normal vasculature (mediated by the Ang-2/ Tie2 axis) followed by (b) endothelial cell activation by proangiogenic factors (such as VEGF), the combination of therapies targeting both steps ought to provide enhanced impairment of the angiogenesis process. Indeed, several preclinical studies have shown the benefit of combining Ang-2 and VEGF targeted agents; in general, targeting both pathways shows superior anti-angiogenic and antitumor effects compared to treatments with either Ang-2 or VEGF targeted agents alone (Fig. 4) [101,102,115,116].
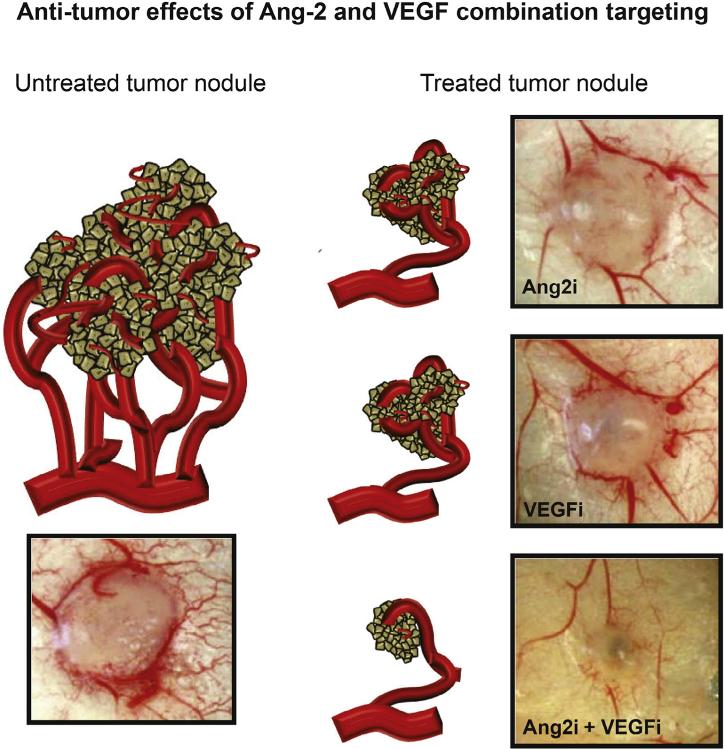
Fig. 4.
Combination of Ang-2 and VEGF targeting in vivo. Both Ang-2 and VEGF targeting agents inhibit tumor angiogenesis and reduce tumor growth but the combination of these therapies results in greater anti-angiogenic and anti-tumor effects. Ang2i: MEDI3617; VEGFi: Sunitinib (modified from J. Cancer Ther. 2013: 4: 1–6. Molnar N. and Siemann D.W., fig. 2A [115]).
There are several ongoing clinical trials evaluating the combination of Ang-2 and VEGF targeted agents. Trebananib is being evaluated in a number of phase II trials in combination with the leading anti-VEGF agents, Bevacizumab, Pazopanib, Sorafenib, and Sunitinib in various solid tumor settings. The Ang-2 specific monoclonal antibodies, MEDI3617 and Nesvacumab (REGN910), are being evaluated in phase I trials with Bevacizumab and Aflibercept respectively (Table 2). There was a recently completed phase I/II clinical trial with the monospecific Ang-2 CovX-Body (CVX-060; Pfizer) in combination with Sunitinib. A phase II trial in combination with Axitinib was terminated due to adverse toxicities; new strategies for dosing and scheduling are being evaluated.
Table 2.
Clinical trials with Ang-2 and VEGF-axis combination therapies (active and/or recruiting).
| Angiopoietin-targeted agent | VEGF-targeted Agent | Tumor Setting | Clinical Trial |
|---|---|---|---|
| Phase I | |||
| MEDI3617 | Bevacizumab | Solid tumors | NCT01248949 |
| Nesvacumab (REGN910) | Ziv-Aflibercept | Solid tumors | NCT01688960 |
| Phase II | |||
| Trebananib | Bevacizumab | mCRC | NCT01249521 |
| Trebananib | Bevacizumab | RCC | NCT01664182 |
| Pazopanib | |||
| Sorafenib | |||
| Sunitinib | |||
| Trebananib | Bevacizumab | Glioblastoma | NCT01609790 |
| Trebananib | Bevacizumab | Metastatic breast cancer | NCT00511459 |
| Trebananib | Sorafenib | HCC | NCT00872014 |
| Trebananib | Sunitinib | RCC | NCT00853372 |
| Trebananib | Sorafenib | RCC | NCT00467025 |
mCRC: metastatic colorectal cancer, RCC: renal cell carcinoma, HCC: hepatocellular carcinoma.
Given the complementary effects of anti-Ang-2 and anti-VEGF therapies, there has also been interest in developing agents that simultaneously target both axes. Regorafenib (Stivarga®, Bayer AG) is a small molecule inhibitor that was approved for metastatic colorectal cancer in 2012 and for gastrointestinal cancer in 2013. Although it is reported to have both VEGFR-1, -2, -3 and Tie2 activity, the former is more potent with little impact on Tie2 phosphorylation [117]. Currently, there are 57 reported clinical trials with this agent in patients in all phases and with various tumor types used alone or in combination with chemotherapy. The Ang-2/ VEGF-A binding peptide antibody fusion protein (CVX-241, Pfizer) was evaluated in phase I clinical trials (NCT01004822). This trial was terminated in 2011 due to shorter half life of VEGF-A binding than expected. In addition, several small molecule agents with dual action against both VEGFR and Tie2 receptor tyrosine kinase activity have been developed. CEP-11981 (Cephalon, Inc) has completed a phase I clinical trial (NCT00875264) in 2012 with no other trials active. ARRY-614 (Array BioPharma, Inc) is currently being evaluated in phase I clinical trials in myelodysplastic syndromes (NCT00916227, NCT01496495). The agent ACTB-1003 (ACT Biotech/Bayer AG) has also been reported to have Tie2 activity; however this agent is advertised as an FGFR/PI3K inhibitor.
Another strategy to combine Ang-2 and VEGF targeting into one agent comes from the chimeric decoy receptor, DAAP, and the Ang-2/VEGF-A bispecific antibody (Ang-2-VEGF-A CrossMab). DAAP was evaluated in preclinical studies and showed greater antitumor effects than treatment with either VEGF-Trap or Tie2-Fc alone [118]. However no follow-up studies have been reported since the initial publication. The Ang-2-VEGF-A CrossMab (RO5520985/RG7221, Roche) is a cross between VEGF-A based on Bevacizumab and the Ang-2 specific human IgG1, LC06. It showed antitumor, anti-angiogenic and anti-metastatic effects in xenograft models and has recently completed phase I clinical trial evaluation (NCT01688206). Phase II evaluations in colorectal cancer are planned [119,120].
Future considerations for Ang-2 and VEGF anti-angiogenic therapy
Currently, the Angiopoietin/Tie2 pathway inhibitors are being evaluated in combination with chemotherapeutic agents and/or VEGF targeted anti-angiogenic agents [100]. While such combinations are logical and meritorious, there are additional possibilities for the application of Angiopoietin/Tie-2 axis targeting agents that have yet to be explored.
Radiation therapy
Radiation therapy is a standard form of therapy for many malignancies [121]. Hypoxic tumor microenvironments which are common in most solid tumors can negatively impact the curative potential of this therapy because tumor cells lacking oxygen are radiation resistant [122]. Several studies have shown that combining radiation therapy with anti-angiogenic agents, primarily anti-VEGF agents [123,124], results in superior tumor responses but the timing of the agents is crucial for favorable outcomes [125,126]. To date no clinical studies combining radiation with anti-Ang-2 therapies have been reported. Given the positive anti-angiogenic effects reported for Ang-2/Tie2 axis targeting agents, their therapeutic potential when applied with radiation treatment ought to be considered. Additionally, with the apparent beneficial effects of combining anti-VEGF and anti-Ang-2 therapeutics, the future evaluation of such a combination in conjunction with radiation therapy would appear logical.
Vascular disrupting agents
An alternative strategy to enhance the anti-vascular effects in tumors would be the combination of anti-angiogenic agents with vascular disrupting agents (VDAs) [127,128] given the distinctively different modes of action of these classes of agents. VDAs damage existing tumor vasculature rather than inhibit the formation of new blood vessels as do anti-angiogenics. VDAs selectively target the expanding tumor vascular network by exerting their effects against proliferating endothelial cells, a phenomenon that is absent in normal vasculature [129,130]. VDAs have been shown to be beneficial in preclinical settings both in combination with anti-VEGF agents as well as conventional anti-cancer therapies [131,132]. In the clinic, the combination of Bevacizumab plus Combretastatin has recently been reported to yield promising results in ovarian cancer [133]. Taking into consideration the complementary anti-angiogenic actions of Ang-2 and VEGF targeted agents alongside the complementary anti-vascular actions of VDAs and anti-angiogenics, a combination of such agents also is feasible. The study of Welford and colleagues [134] which showed superior outcomes when combining the VDA Combretastatin with the CXCR4 inhibitor AMD3100 which depletes Tie2 expressing macrophages in the tumor microenvironment lends support to a VDA plus anti-Ang-2 targeting strategy.
Concluding remark
Despite significant advances during the past 40 years, the true potential of treatment strategies targeting the tumor vascular network may not as yet have been realized. Vascular involvement in tumor development and growth is a complex interaction of various pathways. The ability to identify the appropriate pro-angiogenic axes in individual patients will lead to better selection of targeting agents to yield maximal efficacy. Furthermore, better understanding of the intricacies of the tumor vasculature and the development of novel tumor vessel directed targeting agents when applied alone or in combination or in conjunction with conventional anticancer therapies such as radiation or chemotherapy may ultimately achieve the goal of improving treatment outcomes.
Highlights.
Anti-angiogenic therapies such as Ang-2 and VEGF targeted agents have been heavily pursued in oncologic settings.
Angiopoietin/Tie2 system plays a role in vascular destabilization.
VEGF/VEGFR system plays a role in endothelial cell survival, proliferation, and migration.
The combination of Ang-2 and VEGF targeted agents have recently shown to be complimentary and superior to either pathway targeted agent alone.
Recently, development of dual Ang-2 and VEGF targeted agents have been developed.
Acknowledgments
Funding
This work was supported in part by grants from the NCI (US Public Health Service Grants R01 CA089655 and R01 CA084408) and NIH T32 training grant (5T32 CA009126-33).
Footnotes
Conflict of interest
The authors declare no conflict of interests.
References/Uncited reference
- 1.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Cell Physiol. 2002;282:C947–C970. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 3.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 4.Denekamp J. Endothelial cell proliferation as a novel approach to targeting tumour therapy. Br. J. Cancer. 1982;45:136–139. doi: 10.1038/bjc.1982.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denekamp J. Vascular endothelium as the vulnerable element in tumours. Acta Radiol. 1984;23:217–225. doi: 10.3109/02841868409136015. [DOI] [PubMed] [Google Scholar]
- 6.Young RJ, Reed MW. Anti-angiogenic therapy: concept to clinic. Microcirculation. 2012;19:115–125. doi: 10.1111/j.1549-8719.2011.00147.x. [DOI] [PubMed] [Google Scholar]
- 7.Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. In: Klagsburn M, D'Amore P, editors. Perspectives in Medicine. Cold Spring Harbor Laboratory Press; New York: 2012. pp. 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDonald DM. Angiogenesis and vascular remodeling in inflammation and cancer: biology and architecture of the vasculature. In: Folkman J, Figg WD, editors. Angiogenesis: an Integrative Approach from Science to Medicine. Springer; New York: 2008. [Google Scholar]
- 9.Vaupel P. Abnormal microvasculature and defective microcirculatory function in solid tumors. In: Siemann DW, editor. Vascular Targeted Therapies in Oncology. West Sussex, UK; Wiley Ltd: 2006. [Google Scholar]
- 10.Minchinton AI, Kyle AH. Drug penetration and therapeutic resistance. In: Siemann DW, editor. Tumor Microenvironment. Wiley-Blackwell Ltd; West Sussex, UK: 2011. [Google Scholar]
- 11.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat. Rev. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 12.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ebos JM, Lee CR, Kerbel RS. Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin. Cancer Res. 2009;15:5020–5025. doi: 10.1158/1078-0432.CCR-09-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Partanen J, Armstrong E, Makela TP, Korhonen J, Sandberg M, Renkonen R, et al. A novel endothelial cell surface receptor tyrosine kinase with extracellular epidermal growth factor homology domains. Mol. Cell. Biol. 1992;12:1698–1707. doi: 10.1128/mcb.12.4.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 16.Schnurch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957–968. doi: 10.1242/dev.119.3.957. [DOI] [PubMed] [Google Scholar]
- 17.Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1904–1909. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 19.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 20.Thomas M, Augustin HG. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125–137. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- 21.Loughna S, Sato TN. Angiopoietin and Tie signaling pathways in vascular development. Matrix Biol. 2001;20:319–325. doi: 10.1016/s0945-053x(01)00149-4. [DOI] [PubMed] [Google Scholar]
- 22.Molnar N, Siemann DW. Inhibition of endothelial/smooth muscle cell contact loss by the investigational angiopoietin-2 antibody MEDI3617. Microvasc. Res. 2012;83:290–297. doi: 10.1016/j.mvr.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, et al. Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
- 24.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, et al. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat. Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 25.Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev. Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi M, Majumdar A, Li X, Adler J, Sun Z, Vertuani S, et al. VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat. Commun. 2013;4:1672. doi: 10.1038/ncomms2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujikawa K, de Aos Scherpenseel I, Jain S, Presman E, Varticovski L. The role of PI3-Kinase in Angiopoietin-1-mediated migration and attachment-dependent survival of endothelial cells. Exp. Cell Res. 1999;253:663–672. doi: 10.1006/excr.1999.4693. [DOI] [PubMed] [Google Scholar]
- 28.Kim I, Kim H, So J, Kim J, Kwak H, Koh G. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Circ. Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 29.Hughes D, Marron M, Brindle N. The antiinflammatory endothelial tyrosine kinase Tie2 interacts with a novel nuclear factor-kappaB inhibitor ABIN-2. Circ. Res. 2003;92:630–636. doi: 10.1161/01.RES.0000063422.38690.DC. [DOI] [PubMed] [Google Scholar]
- 30.Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, et al. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 31.Huang YQ, Li JJ, Hu L, Lee M, Karpatkin S. Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood. 2002;99:1646–1650. doi: 10.1182/blood.v99.5.1646. [DOI] [PubMed] [Google Scholar]
- 32.Dixit M, Bess E, Fisslthaler B, Hartel FV, Noll T, Busse R, et al. Shear stress-induced activation of the AMP-activated protein kinase regulates FoxO1a and angiopoietin-2 in endothelial cells. Cardiovasc. Res. 2008;77:160–168. doi: 10.1093/cvr/cvm017. [DOI] [PubMed] [Google Scholar]
- 33.Jang C, Koh YJ, Lim NK, Kang HJ, Kim DH, Park SK, et al. Angiopoietin-2 exocytosis is stimulated by sphingosine-1-phosphate in human blood and lymphatic endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2009;29:401–407. doi: 10.1161/ATVBAHA.108.172676. [DOI] [PubMed] [Google Scholar]
- 34.Scharpfenecker M, Fiedler U, Reiss Y, Augustin HG. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J. Cell Sci. 2005;118:771–780. doi: 10.1242/jcs.01653. [DOI] [PubMed] [Google Scholar]
- 35.Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev. Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 36.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 37.Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 38.Song SH, Kim KL, Lee KA, Suh W. Tie1 regulates the Tie2 agonistic role of angiopoietin-2 in human lymphatic endothelial cells. Biochem. Biophys. Res. Commun. 2012;419:281–286. doi: 10.1016/j.bbrc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Seegar TC, Eller B, Tzvetkova-Robev D, Kolev MV, Henderson SC, Nikolov DB, et al. Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol. Cell. 2010;37:643–655. doi: 10.1016/j.molcel.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen T, Singh H, Tahir T, Brindle N. Effects of Angiopoietin-1 and -2 on the receptor tyrosine kinase Tie2 are differentially regulated at the endothelial cell surface. Cell. Signal. 2010;22:527–532. doi: 10.1016/j.cellsig.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim KL, Shin IS, Kim JM, Choi JH, Byun J, Jeon ES, et al. Interaction between Tie receptors modulates angiogenic activity of angiopoietin2 in endothelial progenitor cells. Cardiovasc. Res. 2006;72:394–402. doi: 10.1016/j.cardiores.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Brown LF, Dezube BJ, Tognazzi K, Dvorak HF, Yancopoulos GD. Expression of Tie1, Tie2, and angiopoietins 1, 2, and 4 in Kaposi's sarcoma and cutaneous angiosarcoma. Am. J. Pathol. 2000;156:2179–2183. doi: 10.1016/S0002-9440(10)65088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu X, Seegar TC, Dalton AC, Tzvetkova-Robev D, Goldgur Y, Rajashankar KR, et al. Structural basis for angiopoietin-1-mediated signaling initiation. Proc. Natl. Acad. Sci. U.S.A. 2013;110:7205–7210. doi: 10.1073/pnas.1216890110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219:983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 45.Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- 46.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 47.Shibuya M. Vascular permeability/vascular endothelial growth factor. In: Folkman J, Figg WD, editors. Angiogenesis: an Integrative Approach from Science to Medicine. Springer Science + Business Media, LLC; New York: 2008. [Google Scholar]
- 48.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J. Biol. Chem. 1998;273:13313–13316. doi: 10.1074/jbc.273.21.13313. [DOI] [PubMed] [Google Scholar]
- 49.Gerber HP, McMurtrey A, Kowalski J, Yan M, Keyt BA, Dixit V, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3’-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J. Biol. Chem. 1998;273:30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 50.Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. doi: 10.1016/s0092-8674(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 51.Dejana E, Spagnuolo R, Bazzoni G. Interendothelial junctions and their role in the control of angiogenesis, vascular permeability and leukocyte transmigration. Thromb. Haemost. 2001;86:308–315. [PubMed] [Google Scholar]
- 52.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 53.Figg W, Folkman J. Angiogenesis: an Integrative Approach from Science to Medicine. Springer Science + Business Media, LLC; New York: 2008. p. 591. [Google Scholar]
- 54.Klagsbrun M, D'Amore P. Angiogenesis: Biology and Pathology. Cold Spring Harbor Laboratory Press; New York: 2012. p. 522. [Google Scholar]
- 55.Ellis L. Epidermal growth factor receptor in tumor angiogenesis. Hematol. Oncol. Clin. North Am. 2004;18:1007–1021. doi: 10.1016/j.hoc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 56.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello M, Aldinucci D, et al. The role of EGFR signaling in tumor microenvironment. J. Cell. Physiol. 2008;214:559–567. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 57.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 58.Blanco R, Gerhardt H. VEGF and notch in tip and stalk cell selection. In: Klagsburn M, Amore PAD, editors. Angiogenesis: Biology and Pathology. Cold Spring Harbor Laboratory Press; New York: 2012. pp. 41–59. [Google Scholar]
- 59.Ferrara N. Pathways mediating VEGF-independent tumor angiogenesis. Cytokine Growth Factor Rev. 2010;21:21–26. doi: 10.1016/j.cytogfr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Lieu C, Heymach J, Overman M, Tran H, Kopetz S. Beyond VEGF: inhibition of the fibroblast growth factor pathway and antiangiogenesis. Clin. Cancer Res. 2011;17:6130–6139. doi: 10.1158/1078-0432.CCR-11-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daniele G, Corral J, Molife LR, de Bono JS. FGF receptor inhibitors: role in cancer therapy. Curr. Oncol. Rep. 2012;14:111–119. doi: 10.1007/s11912-012-0225-0. [DOI] [PubMed] [Google Scholar]
- 62.Yan M, Plowman GD. Delta-like 4/Notch signaling and its therapeutic implications. Clin. Cancer Res. 2007;13:7243–7246. doi: 10.1158/1078-0432.CCR-07-1393. [DOI] [PubMed] [Google Scholar]
- 63.Aster JC, Blacklow SC. Targeting the Notch pathway: twists and turns on the road to rational therapeutics. J. Clin. Oncol. 2012;30:2418–2420. doi: 10.1200/JCO.2012.42.0992. [DOI] [PubMed] [Google Scholar]
- 64.Bais C, Wu X, Yao J, Yang S, Crawford Y, McCutcheon K, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141:166–177. doi: 10.1016/j.cell.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 65.Bid HK, Zhan J, Phelps DA, Kurmasheva RT, Houghton PJ. Potent inhibition of angiogenesis by the IGF-1 receptor-targeting antibody SCH717454 is reversed by IGF-2. Mol. Cancer Ther. 2012;11:649–659. doi: 10.1158/1535-7163.MCT-11-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y, Yao X, Ge J, Hu F, Zhao Y. Can vascular endothelial growth factor and microvessel density be used as prognostic biomarkers for colorectal cancer? A systemic review and meta-analysis. ScientificWorldJournal. 2014;2014:102736. doi: 10.1155/2014/102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhan P, Wang J, Lv XJ, Wang Q, Qiu LX, Lin XQ, et al. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systemic review with meta-analysis. J. Thorac. Oncol. 2009;4:1094–1103. doi: 10.1097/JTO.0b013e3181a97e31. [DOI] [PubMed] [Google Scholar]
- 68.Smith RA, Tang J, Tudur-Smith C, Neoptolemos JP, Ghaneh P. Meta-analysis of immunohistochemical prognostic markers in resected pancreatic cancer. Br. J. Cancer. 2011;104:1140–1151. doi: 10.1038/bjc.2011.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systemic review and meta-analysis. Br. J. Cancer. 2009;100:1385–1392. doi: 10.1038/sj.bjc.6605017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systemic review of the literature and meta-analysis. Cancer Res. 2004;64:2941–2955. doi: 10.1158/0008-5472.can-03-1957. [DOI] [PubMed] [Google Scholar]
- 71.Chen J, Li T, Wu Y, He L, Zhang LC, Shi T, et al. Prognostic significance of vascular endothelial growth factor expression in gastric carcinoma: a meta-analysis. J. Cancer Res. Clin. Oncol. 2011;137:1799–1812. doi: 10.1007/s00432-011-1057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 73.Charlesworth PJS, Harris AL. Hypoxic regulation of angiogenesis by HIF-1. In: Folkman J, Figg WD, editors. Angiogenesis: an Integrative Approach from Science to Medicine. Springer Science + Business Media, LLC; New York: 2008. [Google Scholar]
- 74.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–685. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leung DW. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 76.Bach F, Uddin FJ, Burke D. Angiopoietins in malignancy. Eur. J. Surg. Oncol. 2007;33:7–15. doi: 10.1016/j.ejso.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 77.Molnar N, Siemann DW. Angiopoietin-2 axis inhibitors: current status and future considerations for cancer therapy. Curr. Angiogenes. 2013;2:2–12. [Google Scholar]
- 78.Lind AJ, Wikstrom P, Granfors T, Egevad L, Stattin P, Bergh A. Angiopoietin 2 expression is related to histological grade, vascular density, metastases, and outcome in prostate cancer. Prostate. 2005;62:394–399. doi: 10.1002/pros.20163. [DOI] [PubMed] [Google Scholar]
- 79.Helfrich I, Edler L, Sucker A, Thomas M, Christian S, Schadendorf D, et al. Angiopoietin-2 levels are associated with disease progression in metastatic malignant melanoma. Clin. Cancer Res. 2009;15:1384–1392. doi: 10.1158/1078-0432.CCR-08-1615. [DOI] [PubMed] [Google Scholar]
- 80.Detjen KM, Rieke S, Deters A, Schulz P, Rexin A, Vollmer S, et al. Angiopoietin-2 promotes disease progression of neuroendocrine tumors. Clin. Cancer Res. 2010;16:420–429. doi: 10.1158/1078-0432.CCR-09-1924. [DOI] [PubMed] [Google Scholar]
- 81.Sie M, Wagemakers M, Molema G, Mooij JJ, de Bont ES, den Dunnen WF. The angiopoietin 1/angiopoietin 2 balance as a prognostic marker in primary glioblastoma multiforme. J. Neurosurg. 2009;110:147–155. doi: 10.3171/2008.6.17612. [DOI] [PubMed] [Google Scholar]
- 82.Park JH, Park KJ, Kim YS, Sheen SS, Lee KS, Lee HN, et al. Serum angiopoietin-2 as a clinical marker for lung cancer. Chest. 2007;132:200–206. doi: 10.1378/chest.06-2915. [DOI] [PubMed] [Google Scholar]
- 83.De Palma M, Naldini L. Angiopoietin-2 TIEs up macrophages in tumor angiogenesis. Clin. Cancer Res. 2011;17:5226–5232. doi: 10.1158/1078-0432.CCR-10-0171. [DOI] [PubMed] [Google Scholar]
- 84.Montero AJ, Escobar M, Lopes G, Gluck S, Vogel C. Bevacizumab in the treatment of metastatic breast cancer: friend or foe? Curr. Oncol. Rep. 2012;14:1–11. doi: 10.1007/s11912-011-0202-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 86.Pollack A. F.D.A. Revokes Approval of Avastin for Use as Breast Cancer Drug. The New York Times; New York: 2011. p. B1. [Google Scholar]
- 87.Siemann DW. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011;37:63–74. doi: 10.1016/j.ctrv.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Horsman MR, Siemann DW. Pathophysiological effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 2006;66:11520–11539. doi: 10.1158/0008-5472.CAN-06-2848. [DOI] [PubMed] [Google Scholar]
- 89.Kerbel RS. Antiangiogenic therapy: a universal chemosensitization strategy for cancer? Science. 2006;312:1171–1175. doi: 10.1126/science.1125950. [DOI] [PubMed] [Google Scholar]
- 90.Bottsford-Miller JN, Coleman RL, Sood AK. Resistance and escape from antiangiogenesis therapy: clinical implications and future strategies. J. Clin. Oncol. 2012;30:4026–4034. doi: 10.1200/JCO.2012.41.9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology. 2005;19:7–16. [PubMed] [Google Scholar]
- 92.Coxon A, Bready J, Min H, Kaufman S, Leal J, Yu D, et al. Context-dependent role of angiopoietin-1 inhibition in the suppression of angiogenesis and tumor growth: implications for AMG 386, an angiopoietin-1/2-neutralizing peptibody. Mol. Cancer Ther. 2010;9:2641–2651. doi: 10.1158/1535-7163.MCT-10-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Falcón BL, Hashizume H, Koumoutsakos P, Chou J, Bready JV, Coxon A, et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am. J. Pathol. 2009;175:2159–2170. doi: 10.2353/ajpath.2009.090391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Felcht M, Luck R, Schering A, Seidel P, Srivastava K, Hu J, et al. Angiopoietin-2 differentially regulates angiogenesis through TIE2 and integrin signaling. J. Clin. Invest. 2012;122:1991–2005. doi: 10.1172/JCI58832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 96.Mazzieri R, Pucci F, Moi D, Zonari E, Ranghetti A, Berti A, et al. Targeting the ANG2/TIE2 axis inhibits tumor growth and metastasis by impairing angiogenesis and disabling rebounds of proangiogenic myeloid cells. Cancer Cell. 2011;19:512–526. doi: 10.1016/j.ccr.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 97.Loges S, Schmidt T, Carmeliet P. Mechanisms of resistance to anti-angiogenic therapy and development of third-generation anti-angiogenic drug candidates. Genes Cancer. 2010;1:12–25. doi: 10.1177/1947601909356574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang ZL, Zhang JF, Yuan YF, He YM, Liu QY, Mao XW, et al. Supression of angiogenesis and tumor growth in vitro and in vivo using an anti-angiopoietin-2 single-chain antibody. Exp. Ther. Med. 2014;7:543–552. doi: 10.3892/etm.2014.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.D'Amico G, Korhonen EA, Anisimov A, Zarkada G, Holopainen T, Hagerling R, et al. Tie1 deletion inhibits tumor growth and improves angiopoietin antagonist therapy. J. Clin. Invest. 2014;124:824–834. doi: 10.1172/JCI68897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.U.S. National Institutes of Health. [PubMed]
- 101.Daly C, Eichten A, Castanaro C, Pasnikowski E, Adler A, Lalani AS, et al. Angiopoietin-2 functions as a Tie2 agonist in tumor models, where it limits the effects of VEGF inhibition. Cancer Res. 2013;73:108–118. doi: 10.1158/0008-5472.CAN-12-2064. [DOI] [PubMed] [Google Scholar]
- 102.Leow CC, Coffman K, Inigo I, Breen S, Czapiga M, Soukharev S, et al. MEDI3617, a human anti-angiopoietin 2 monoclonal antibody, inhibits angiogenesis and tumor growth in human tumor xenograft models. Int. J. Oncol. 2012;40:1321–1330. doi: 10.3892/ijo.2012.1366. [DOI] [PubMed] [Google Scholar]
- 103.Doppalapudi VR, Huang J, Liu D, Jin P, Liu B, Li L, et al. Chemical generation of bispecific antibodies. Proc. Natl. Acad. Sci. U.S.A. 2010;107:22611–22616. doi: 10.1073/pnas.1016478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: reassessing the target. Cancer Res. 2012;72:1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 106.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 107.Niels U, Chinot O, McBain C, Sorensen M, Larsen V, Barrie M, et al. Phase I study of anti-PlGF monoclonal antibody (mAb) RO5323441 (RO) and anti-VEGF mab bevacizumab (BV) in patients with recurrent glioblastoma (GBM). American Society of Clinical Oncology Annual Meeting; Chicago, IL. 2013. [Google Scholar]
- 108.Ostman A, Heldin CH. PDGF receptors as targets in tumor treatment. Adv. Cancer Res. 2007;97:247–274. doi: 10.1016/S0065-230X(06)97011-0. [DOI] [PubMed] [Google Scholar]
- 109.Kampa-Schittenhelm KM, Heinrich MC, Akmut F, Döhner H, Döhner K, Schittenhelm MM. Quizartinib (AC220) is a potent second generation class III tyrosine kinase inhibitor that displays a distinct inhibition profile against mutant-FLT3, -PDGFRA and -KIT isoforms. Mol. Cancer. 2013;12:19. doi: 10.1186/1476-4598-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xi Y, Chen M, Liu X, Lu Z, Ding Y, Li D. CP-673451, a platelet-derived growth-factor receptor inhibitor, suppresses lung cancer cell proliferation and migration. Onco. Targets Ther. 2014;7:1215–1221. doi: 10.2147/OTT.S62946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Soh CL, McNeil K, Owczarek CM, Hardy MP, Fabri LJ, Pearse M, et al. Exogenous administration of protease-resistant, non-matrix-binding IGFBP-2 inhibits tumour growth in a murine model of breast cancer. Br. J. Cancer. 2014;110:2855–2864. doi: 10.1038/bjc.2014.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Elice F, Rodeghiero F. Side effects of anti-angiogenic drugs. Thromb. Res. 2012;129(Suppl. 1):S50–S53. doi: 10.1016/S0049-3848(12)70016-6. [DOI] [PubMed] [Google Scholar]
- 113.Hayman SR, Leung N, Grande JP, Garovic VD. VEGF inhibition, hypertension, and renal toxicity. Curr. Oncol. Rep. 2012;14:285–294. doi: 10.1007/s11912-012-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pralhad T, Madhusudan S, Rajendrakumar K. Concept, mechanisms and therapeutics of angiogenesis in cancer and other diseases. J. Pharm. Pharmacol. 2003;55:1045–1053. doi: 10.1211/0022357021819. [DOI] [PubMed] [Google Scholar]
- 115.Molnar N, Siemann DW. Combined Ang-2 and VEGF targeting therapies in renal cell carcinoma. J. Cancer Ther. 2013;4:1–6. [Google Scholar]
- 116.Brown JL, Cao ZA, Pinzon-Ortiz M, Kendrew J, Reimer C, Wen S, et al. A human monoclonal anti-ANG2 antibody leads to broad antitumor activity in combination with VEGF inhibitors and chemotherapy agents in preclinical models. Mol. Cancer Ther. 2010;9:145–156. doi: 10.1158/1535-7163.MCT-09-0554. [DOI] [PubMed] [Google Scholar]
- 117.Wilhelm SM, Dumas J, Adnane L, Lynch M, Carter CA, Schutz G, et al. Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer. 2011;129:245–255. doi: 10.1002/ijc.25864. [DOI] [PubMed] [Google Scholar]
- 118.Koh YJ, Kim HZ, Hwang SI, Lee JE, Oh N, Jung K, et al. Double antiangiogenic protein, DAAP, targeting VEGF-A and angiopoietins in tumor angiogenesis, metastasis, and vascular leakage. Cancer Cell. 2010;18:171–184. doi: 10.1016/j.ccr.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 119.Kienast Y, Klein C, Scheuer W, Raemsch R, Lorenzon E, Bernicke D, et al. Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin. Cancer Res. 2013;19:6730–6740. doi: 10.1158/1078-0432.CCR-13-0081. [DOI] [PubMed] [Google Scholar]
- 120.Hidalgo M, Le Tourneau C, Massard C, Boni V, Calvo E, Albanell J, et al. Results from the first-in-human (FIH) phase I study of RO5520985 (RG7221), a novel bispecific human anti-Ang-2/anti-VEGF-A antibody, administered as an intravenous infusion to patients with advanced solid tumors. American Society of Clinical Oncology Annual Meeting; Chicago, IL. 2014. [Google Scholar]
- 121.Bristow RG, Hill RP. Molecular and Cellular Basis of Radiotherapy. In: Hill R, Tannock IF, Bristow RG, Harrington L, editors. The Basic Science of Oncology. McGraw-Hill Medical Publishing Division; 2005. [Google Scholar]
- 122.Giaccia AJ, Hall EJ. Cell survival curves. In: Giaccia AJ, Hall EJ, editors. Radiobiology for the Radiologist. Lippincott Williams and Wilkins; Philadelphia PA: 2006. [Google Scholar]
- 123.Vogel J, Camphausen K. Angiogenesis inhibitors and radiation in multimodality cancer therapy: preclinical and clinical studies. Curr. Angiogenes. 2012;1:157–167. [Google Scholar]
- 124.Schmidt B, Lee HJ, Ryeom S, Yoon SS. Combining bevacizumab with radiation or chemoradiation for solid tumors: a review of the scientific rationale, and clinical trials. Curr. Angiogenes. 2012;1:169–179. doi: 10.2174/2211552811201030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dings RP, Loren M, Heun H, McNiel E, Griffioen AW, Mayo KH, et al. Scheduling of radiation with angiogenesis inhibitors anginex and Avastin improves therapeutic outcome via vessel normalization. Clin. Cancer Res. 2007;13:3395–3402. doi: 10.1158/1078-0432.CCR-06-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Geng L, Donnelly E, McMahon G, Lin PC, Sierra-Rivera E, Oshinka H, et al. Inhibition of vascular endothelial growth factor receptor signaling leads to reversal of tumor resistance to radiotherapy. Cancer Res. 2001;61:2413–2419. [PubMed] [Google Scholar]
- 127.Siemann DW, Shi W. Efficacy of combined antiangiogenic and vascular disrupting agents in treatment of solid tumors. Int. J. Radiat. Oncol. Biol. Phys. 2004;60:1233–1240. doi: 10.1016/j.ijrobp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 128.Shaked Y, Nathan P, Daenen LGM, Kerbel RS. Combining antiangiogenic drugs with vascular disrupting agents rationale and mechanisms of action. In: Meyer T, editor. Vascular Disruptive Agents for the Treatment of Cancer. Springer Science + Business Media LLC; New York: 2010. [Google Scholar]
- 129.Siemann DW, Bibby MC, Dark GG, Dicker AP, Eskens FA, Horsman MR, et al. Differentiation and definition of vascular-targeted therapies. Clin. Cancer Res. 2005;11:416–420. [PubMed] [Google Scholar]
- 130.Siemann DW, Chaplin DJ, Horsman MR. Vascular-targeting therapies for treatment of malignant disease. Cancer. 2004;100:2491–2499. doi: 10.1002/cncr.20299. [DOI] [PubMed] [Google Scholar]
- 131.Siemann DW, Shi W. Dual targeting of tumor vasculature: combining avastin and vascular disrupting agents (Ca4P or Oxi4503) Anticancer Res. 2008;28:2027–2032. [PMC free article] [PubMed] [Google Scholar]
- 132.Chen F, Feng Y, Zheng K, De Keyzer F, Li J, Feng Y, et al. Enhanced antitumor efficacy of a vascular disrupting agent combined with an antiangiogenic in a rat liver tumor model evaluated by multiparametric MRI. PLoS ONE. 2012;7:e41140. doi: 10.1371/journal.pone.0041140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nathan P, Zweifel M, Padhani AR, Koh DM, Ng M, Collins DJ, et al. Phase I trial of combretastatin A4 phosphate (CA4P) in combination with bevacizumab in patients with advanced cancer. Clin. Cancer Res. 2012;18:3428–3439. doi: 10.1158/1078-0432.CCR-11-3376. [DOI] [PubMed] [Google Scholar]
- 134.Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, et al. TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J. Clin. Invest. 2011;121:1969–1973. doi: 10.1172/JCI44562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]