Abstract
Background
The symptoms that contribute to the clinical diagnosis of depression likely emerge from, or are related to, underlying cognitive deficits. To understand this relationship further, we examined the relationship between self-reported somatic and cognitive-affective Beck’s Depression Inventory-II (BDI-II) symptoms and aspects of cognitive control reflected in error event-related potential (ERP) responses.
Methods
Task and assessment data were analyzed within 51 individuals. The group contained a broad distribution of depressive symptoms, as assessed by BDI-II scores. ERP’s were collected following error responses within a go/no-go task. Individual error ERP amplitudes were estimated by conducting group independent component analysis (ICA) on the electroencephalographic (EEG) time series and analyzing the individual reconstructed source epochs. Source error amplitudes were correlated with the subset of BDI-II scores representing somatic and cognitive-affective symptoms.
Results
We demonstrate a negative relationship between somatic depression symptoms (i.e. fatigue or loss of energy) (after regressing out cognitive-affective scores, age and IQ) and the central-parietal ERP response that peaks at 359 ms. The peak amplitudes within this ERP response were not significantly related to cognitive-affective symptom severity (after regressing out the somatic symptom scores, age, and IQ).
Limitations
These findings were obtained within a population of female adults from a maximum-security correctional facility. Thus, additional research is required to verify that they generalize to the broad population.
Conclusions
These results suggest that individuals with greater somatic depression symptoms demonstrate a reduced awareness of behavioral errors, and help clarify the relationship between clinical measures of self-reported depression symptoms and cognitive control.
Keywords: depression, somatic symptoms, cognitive-affective symptoms, error ERP’s, go/no-go, cognitive control
1. Introduction
There is considerable heterogeneity among the symptoms expressed by individuals diagnosed with major depression disorder (MDD). This heterogeneity appears to emerge from the wide range of cognitive deficits associated with depression and their potential interrelationship with negative mood or negative affect (Joormann et al., 2007; Joormann and Siemer, 2004; Mathews and MacLeod, 2005). In order to understand these factors, additional research should be directed to understand the interrelationship of cognitive and affective factors with the symptoms that contribute to the clinical diagnosis of depression, and with the symptoms that individual’s report. These investigations may help further characterize the constellation of symptoms that contribute to or emerge with depression, leading to more accurate diagnosis and treatment.
A broad range of cognitive deficits have been reported within individuals diagnosed with depression, including impairments in cognitive control (Channon and Green, 1999; Joormann and Gotlib, 2010). The electrophysiological characteristics of cognitive control have been well studied by examining cortical responses to rare non-target stimuli that appear among frequent targets (i.e. in go/no-go tasks). The event-related potential (ERP) to incorrect responses to rare non-targets partially reflects the cascade of neural events that include the processes which may lead to errors, the awareness of errors, and post-error adjustments in processing (Falkenstein, 2004; Yeung et al., 2004). These aspects of error processing are reflected in part within the complex of ERP peaks that follow erroneous responses to rare stimuli, including a negative peak that appears approximately 50 ms – 150 ms following the error response (termed the error related negativity (ERN) or Ne), and the complex of positive peaks that appear between 200 and 500 ms after the response (termed the Pe) (Falkenstein et al., 1991; Falkenstein, 2004; Gehring et al., 1993; Nieuwenhuis et al., 2001; Overbeek et al., 2005).
The relationship between error ERP responses and depression appears complex. This complexity may result in part due to differences in the severity of depressive symptoms within a population, and the broad range of depression-like psychopathologies present (Lux and Kendler, 2010). For example, a reduced Pe response was observed within a group of elderly medicated patients with major depression (Alexopoulos et al., 2007), and within a group of severely depressed patients (Olvet et al., 2010; Schrijvers et al., 2008), while others didn’t report on potential differences in the Pe response within depressed patients (Ruchsow et al., 2006, 2004) or failed to find statistically significant differences (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2008). Researchers may obtain a better understanding of these inconsistencies in error ERP modulations by focusing on their relationship with depressive symptoms. For example, individuals with greater anxiety (General Distress Anxious Symptom subscale) demonstrate reduced Pe amplitudes (Olvet et al., 2010), and Ne amplitudes appear to be associated with psychomotor retardation (Schrijvers et al., 2008). These findings help clarify specific elements of depression that may contribute to inconsistencies among studies, and highlight the potential benefits of focusing on the relationship between subsets of depressive symptoms and error ERP’s.
Depressive symptoms can generally be categorized along the cognitive-affective or somatic dimensions (Storch et al., 2004). The cognitive-affective dimension of symptoms includes negative mood or negative affect, while the somatic dimension includes symptoms such as fatigue or loss of energy (Kapfhammer, 2006). These two categories of symptoms can be empirically evaluated using the Beck Depression Inventory-II (BDI-II) (Beck et al., 1996), a widely used protocol for examining self-reported depression symptoms within healthy and clinical populations. Factor analysis of the 21 item symptom information collected with BDI-II validates the use of BDI-II for providing summary measures of somatic and cognitive-affective symptoms, and further supports the existence of these two dimensions (Steer et al., 1999; Storch et al., 2004; Whisman et al., 2000).
The relationship between somatic and cognitive-affective BDI-II symptoms has been unexplored with respect to the aspects of cognitive control reflected in error ERP responses. Our goal was to examine the relationship between individual somatic and cognitive-affective BDI-II scores with individual error ERP amplitudes obtained within a traditional go/no-go task. These findings provide further clarification on the relationship between clinical measures of self-reported depression symptoms and cognitive function.
2. Methods
2.1 Participants
Task and assessment data were collected on 63 female adults from a maximum-security correctional facility in New Mexico. Five individuals were excluded due to the absence of error responses, two individuals were excluded due to experimental error, and five individuals were excluded for excessive EEG artifacts, reducing the number of participants to 51. Individuals ranged in age from 21 to 57 (M = 35.41 yrs, SD = 7.84) with an average IQ of 95.08 (SD = 11.00). Of the 51 participants, 12 declined to state their ethnic category, 5 self-identified as American Indian or Alaskan Native, 4 as Black or African American, 1 as Native Hawaiian or Pacific Islander, and 29 as white.
Participants were informed of their right to terminate participation at any point and were advised that their participation was not associated with institutional benefits or their facility or parole status. Participants received remuneration at the hourly labor wage of the facility. The work was approved by the University of New Mexico Health Science Center Human Research Review Committee and the Office of the Human Research Protections (OHRP). All subjects provided written informed consent prior to data collection.
2.2 Drug dependence and personality disorder profile
Structured Clinical Interviews for DSM-IV Axis I Disorders – Patient Version (SCID I–P) (First et al., 1995) were conducted to provide an overview of the distribution of substance dependence and personality disorders within the group. Sixteen individuals were threshold for major depression, five were threshold for panic disorder, and at least two individuals were threshold for 7 of the 25 personality disorders. None of the individuals were threshold or sub-threshold for schizophrenia, schizophreniform, or schizoaffective disorder (SCID I–P). There was notable alcohol, stimulant, cocaine, and opioid dependence within the group (N = 22 24, 23, and 17, respectively). Table 1 demonstrates the full distribution of substance dependence and personality disorders within the group.
Table 1.
The number of individuals present with absent, sub-threshold, and threshold personality disorders, or with drug absence, abuse or dependence (Structured Clinical Interviews for DSM-IV Axis I Disorders – Patient Version (SCID I–P) (First et al., 1995)).
| SCID I Category | # absent | # sub-threshold | # threshold |
|---|---|---|---|
| Bipolar PD | 50 | 0 | 1 |
| Bipolar II | 51 | 0 | 0 |
| Other Bipolar | 50 | 0 | 1 |
| Major Depression | 31 | 4 | 16 |
| Dysthymia | 48 | 1 | 2 |
| Depressive Disorder NOS | 51 | 0 | 0 |
| Mood Disorder | 51 | 0 | 0 |
| Substance Induced Mood Disorder | 51 | 0 | 0 |
| Schizophrenia | 51 | 0 | 0 |
| Schizophreniform | 51 | 0 | 0 |
| Schizoaffective Disorder | 51 | 0 | 0 |
| Delusional Disorder | 51 | 0 | 0 |
| Brief Psychotic Disorder | 51 | 0 | 0 |
| Psychotic Disorder due to Med. | 51 | 0 | 0 |
| Substance Induced Psychotic Disorder | 49 | 0 | 2 |
| Psychotic Disorder | 51 | 0 | 0 |
| Anxiety Disorder NOS | 49 | 0 | 2 |
| Panic Disorder | 45 | 1 | 5 |
| Agoraphobia | 50 | 1 | 0 |
| Social Phobia | 50 | 1 | 0 |
| Specific Phobia | 49 | 2 | 0 |
| OCD | 48 | 2 | 1 |
| PTSD | 48 | 2 | 1 |
| Generalized Anxiety Disorder | 51 | 0 | 0 |
| Substance Induced Anxiety Disorder | 51 | 0 | 0 |
| SCID I Category | # absent | # abuse | # dependence |
| Alcohol | 22 | 7 | 22 |
| Sedative | 43 | 4 | 4 |
| Cannabis | 23 | 18 | 10 |
| Stimulant | 24 | 3 | 24 |
| Opioids | 34 | 0 | 17 |
| Cocaine | 22 | 6 | 23 |
| Hallucinogens / PCP | 44 | 5 | 2 |
| Poly-Drug | 48 | 1 | 2 |
NOS: not otherwise specified, PD: personality disorder, Med: medication, OCD: obsessive compulsive disorder, PTSD: post-traumatic stress disorder, PCP: Phencyclidine
2.3 BDI Assessment and Somatic and cognitive-affective symptoms
Depressive symptoms were assessed using the BDI-II (Beck et al., 1996), a 21-item measure that assesses the severity of depressive symptoms. BDI-II scores are highly correlated with clinical measures of depression severity, including the Revised Hamilton Psychiatric Rating Scale for Depression (Hamilton, 1960), and the number of SCID-I threshold symptoms (Beck et al., 1996; Sprinkle et al., 2002). Within the BDI-II, each symptom is rated by the individual between 0 and 3, with greater scores indicating greater symptom severity. The average total BDI-II score within the sample was 17.47 (SD = 9.68), with a Chronbach's alpha of 0.88. Twenty-one individuals had total BDI-II scores within approximate threshold for “minimum depression” (BDI-II between 0 and 13), 7 within “mild depression” (BDI-II between 14 and 19), 16 within ”moderate depression” (BDI-II within 20 and 28), and 7 within “severe depression” (BDI-II between 29 and 63).
The 21 depressive symptoms are often grouped into the two interrelated symptom categories “somatic” and “cognitive-affective” (Steer et al., 1999; Storch et al., 2004; Whisman et al., 2000). These different symptoms were summarized in the following study by summing the subset of the 21 symptom scores that reflect somatic symptoms (sadness, loss of pleasure, crying, agitation, loss of interest, indecisiveness, loss of energy, changes in sleep pattern, irritability, changes in appetite, concentration difficulties, tiredness or fatigue, and loss of interest in sex) and the subset of the symptom scores which reflect cognitive-affective symptoms (pessimism, past failure, guilty feelings, punishment feelings, self-dislike, self-criticalness, suicidal thoughts, and worthlessness). The average total somatic symptom score was 10.69 (SD = 6.63), and the average total cognitive-affective symptom score was 6.78 (SD = 3.94). Age and IQ were regressed out of the total BDI score and the separate symptom scores prior to statistical analysis. The somatic symptom score was additionally regressed out of the cognitive-affective symptom score (and vice versa) prior to analysis.
2.4 Task
Participants performed a classic go/no-go task (Kiehl et al., 2000). A series of targets (i.e. go stimuli) (a white “X”) and non-targets (i.e. no-go stimuli) (a white “K”) were presented on the computer screen with Presentation software (Neurobehavioral Systems, http://www.neurobs.com). Individuals were instructed to respond to targets as “quickly and accurately as possible” with their right index finger on a keyboard, and responses were recorded if they occurred within 1 second after a stimulus. The stimuli were approximately 3 × 5 visual degrees, and were presented for 240ms against a black background. Targets appeared with higher frequency (84%, 412 trials) than non-targets (16%, 78 trials) to establish a strong stimulus-response mapping on “Go” trials. The stimuli were presented randomly, except two “K’s” or “No/Go” trials were never presented sequentially. The interstimulus interval was 1 second (48% of stimuli), 2 seconds (37% of stimuli), or 3 seconds (15% of stimuli). Prior to recording, each participant performed a block of 10 practice trials to ensure that the instructions were clearly understood.
2.5 EEG acquisition and preprocessing
EEG data was collecting using a 64-channel BioSemi Active Two system (http://www.biosemi.com). Signals were low-pass filtered using a fifth-order sinc filter with a half-power cutoff of 204.8 Hz and sampled at 512 Hz. EEG activity was recorded using sintered Ag-AgCl active electrodes placed in accordance with the 10–20 International System, with a nose reference. All offsets were kept below 10 kΩ.
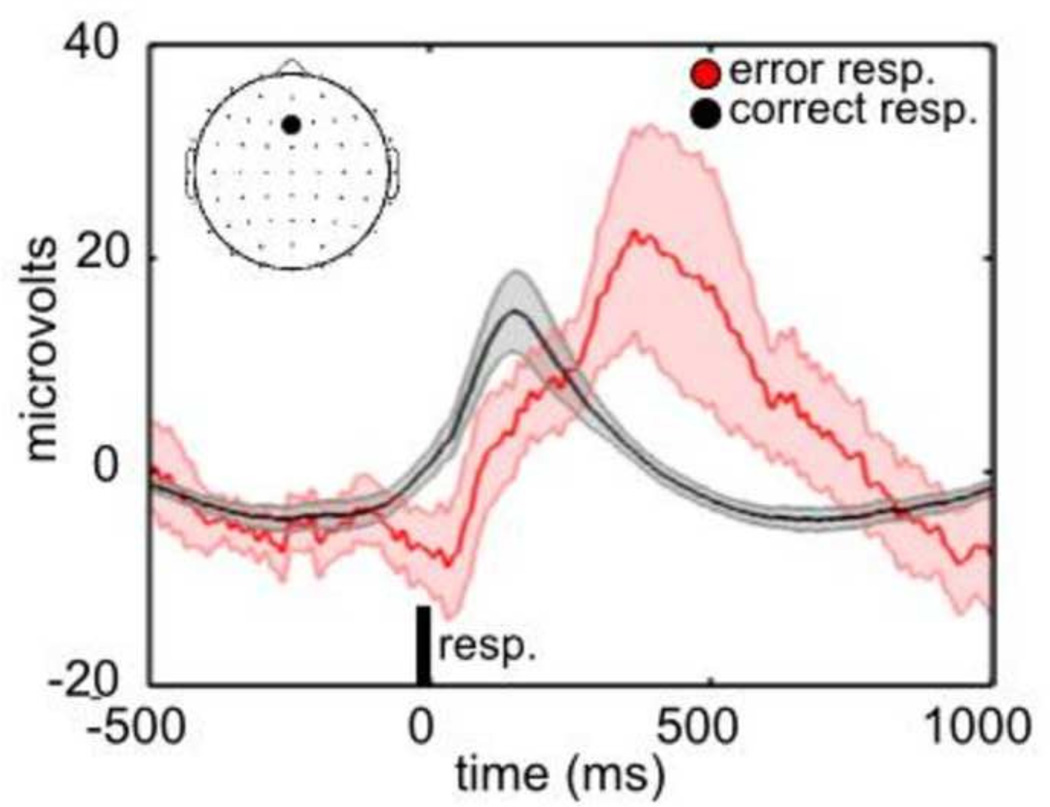
EEG preprocessing was conducted in Matlab (http://www.mathworks.com) using custom functions, built-in functions, and the EEGLAB toolbox (http://sccn.ucsd.edu/eeglab). The EEG data was linearly detrended, forward and backward filtered with a Butterworth filter (bandpass: 0.01 to 50 Hz), and referenced to channel CZ for bad channel identification. Bad channels were identified based on the data distribution and variance of channels, as implemented in EEGLAB’s pop_rejchan function (Delorme and Makeig, 2004) and the FASTER toolbox (Nolan et al., 2010), and spherically interpolated. An average of 2.71 channels were interpolated (Min: 0; Max: 5; SD: 0.97). The EEG data was average referenced and blink artifacts were attenuating by conducting a temporal ICA decomposition on the individual recordings (extended Infomax algorithm in EEGLAB (Bell and Sejnowski, 1995; Lee et al., 1999), detecting artifactual sources with the ADJUST toolbox (Mognon et al., 2011), and reconstructing to the original data space. An average of 1.04 sources were eliminated (Min: 0; Max: 3; SD: 0.60). Artifactual epochs were identified using the automatic artifact epoch detection algorithm in EEGLAB (function: pop_autoreg.m), and the single trial peak amplitudes within these epochs were excluded in the subsequent analysis of the group temporal ICA individual reconstructed sources (described below). Figure 1 indicates the average ERP for correct and incorrect behavioral responses.
Figure 1.
ERP’s to correct and error responses. The average ERP (N = 51 subjects) is plotted separately following correct (in black) and incorrect (in red) behavioral responses. The ERP’s were averaged after epoching with respect to the onset of the behavioral response (0 ms). The responses are plotted for electrode Fz, as indicated in the upper left hand corner. The error bars represent the 95% confidence interval of the mean.
2.6 Group temporal ICA of EEG
Group temporal ICA was used to decompose the multiplexed ERP response into distinct sources which potentially reflect the distinct ERP peaks (Bridwell et al., 2014; Eichele et al., 2011). The temporal ICA model assumes that scalp voltage fluctuations within electrodes reflect a linear mixture of independent temporal sources. Principle components analysis (PCA) was conducted on the aggregate group [[channel X subjects] X time points] matrix and group ICA was conducted on the [[pca components × 51 subjects] × 422760 time points] matrix, as implemented in the EEGIFT toolbox (http:/mialab.mrn.org/software/eegift/) (for a detailed description of the Group ICA implementation, please see: Calhoun et al., 2001; Eichele et al., 2011). Infomax ICA was conducted on the aggregate matrix after an initial PCA reduction to 10, 15 or 20 components, generating 10, 15, or 20 group components. The components and results are consistent across the three model orders, suggesting that the findings are relatively robust to the ICA model order. For simplicity, we only report the results with 10 components. Separate source and mixing matrices were generated for each individual by back-reconstructing the group components (Calhoun et al., 2001).
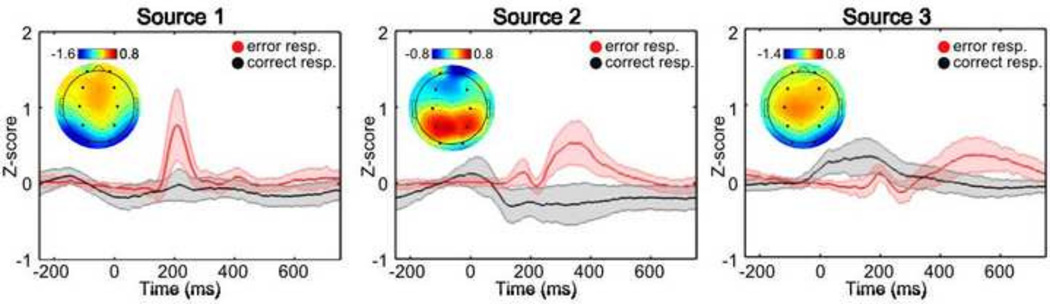
The individual time courses were converted to z-scores and averaged separately for error responses and correct responses. The individual error ERP’s were then averaged for each of the 10 sources and 3 sources were selected for further analysis since they visually demonstrate ERP differences to error and correct responses (Fig. 2). Source topographies were determined by averaging the individual mixing matrices across subjects (Fig. 2). The full width half maximum (fwhm) of the peak error response was determined for the prominent peak within the 3 selected sources (see Table 2). The average amplitude of individual subject responses was then calculated within the fixed window for each peak.
Figure 2.
Source ERP’s to correct and error responses. The ERP’s are plotted separately for each source following correct (in black) and incorrect (in red) behavioral responses. The source number is indicated above each plot. The topographic plots indicate the average spatial loadings (N = 51 subjects) for each group temporal source. The large black circles within each topographic plot denote electrodes Fp1, Fp2, F3, F4, C3, C4, P3, P4, O1, and O2 (from top left to bottom right).
Table 2.
Peak time, window (full width half maximum, fwhm), and peak location of the overall average ERP response for the 3 selected sources. O = occipital, T = temporal, P = parietal, C = central, F = frontal.
| Source | Peak time (ms) | Peak window (fwhm) (ms) |
Spatial peak Min (z-score) |
Spatial peak Max |
|---|---|---|---|---|
| 1 | 207 | 180 – 256 | O / T (−1.78) | F / C (0.93) |
| 2 | 359 | 287 – 525 | F (−0.72) | O / C (0.91) |
| 3 | 678 | 510 – 725 | O (−1.38) | C (0.81) |
2.7 Statistical Analysis
Significance tests were conducted examining the correlation between individual error ERP amplitudes, the total BDI score (after regressing out age and IQ), and the two separate symptom scores (after regressing out the other symptom score, age and IQ). A total of 3 error amplitudes were calculated for each individual (1 peak ERP response within each of the 3 selected components), generating a total of 3 × 3 = 9 Pearson’s r statistical tests. These statistical tests are reported as “significant” if they pass Holm-Bonferroni correction for the 9 planned comparisons (alpha = 0.05) (Holm, 1979).
3. Results
3.1 Behavior
The average individual correct reaction time was 441 ms (SD: 59 ms) and the average individual incorrect reaction time was 383 ms (SD: 49 ms). Individuals correctly responded to 96.07 % of the target stimuli, and incorrectly responded to 20.61 % of the non-target stimuli. Thus, there were an average of 16.08 error trials (min: 1; max: 47; SD: 9.80) and 395.82 correct responses (min: 234; max: 412; SD: 29.27) across subjects. 2.05 % of the ERP error epochs were identified as artifactual, reducing the average number of errors in the subsequent ERP analysis to 13.45 trials per subject (min: 1, max: 40; SD: 9.38).
3.2 Average ERP following target and non-target responses
The average ERP is indicated in Fig. 1 for correct behavioral responses to targets (in black) and the incorrect (i.e. error) behavioral responses to non-targets (in red). The ERP to error trials diverges negatively from the ERP to correct trials within the 0 – ~200 ms interval after the behavioral response, and diverges positively at later intervals, with a peak difference at ~ 400 ms. The time course of these ERP differences are consistent with the ERP peaks that have been labeled “ERN/Ne” and “Pe” in previous studies (Falkenstein, 2004; Gehring et al., 1993; Overbeek et al., 2005; Ullsperger et al., 2010).
3.3 Group temporal ICA of EEG and individual error amplitudes
Group ICA was used to decompose the multiplexed ERP response into distinct sources which potentially reflect the distinct ERP peaks. The sources were averaged across epochs for error and correct responses and 3 out of 10 sources were selected for further analysis since they demonstrate an average peak following the error response. The selected average source ERP’s and the topography (i.e. the average mixing matrix across subjects) (N = 51) are indicated in Fig. 2. ICASSO analysis indicated that the selected sources were stable across multiple ICA iterations, with stability indices (Iq ) > 0.95 (Himberg et al., 2004).
3.4 Error ERP amplitudes and depression symptoms
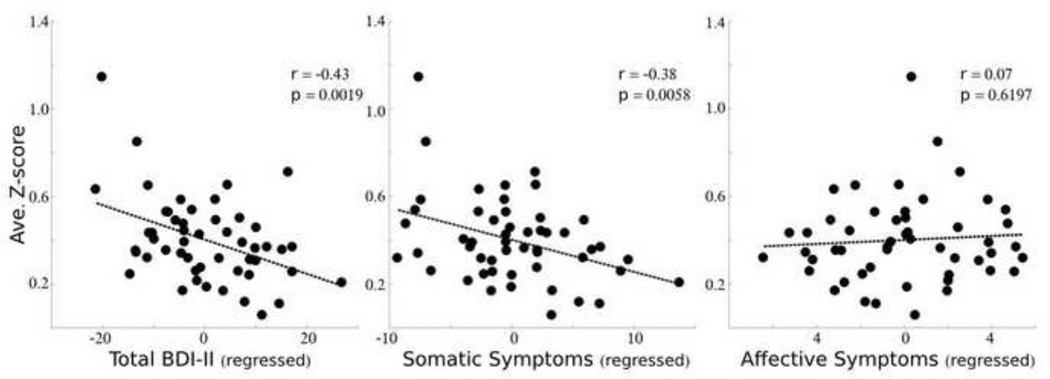
There was a significant negative relationship between source 2 error amplitudes and total BDI-II scores (r(49) = −0.43; p = 0.0019). Thus, individuals with greater depression symptoms demonstrate a reduced ERP response ~287–525 ms following an error. The relationship between source 1 and total BDI scores was significant without correction for multiple comparisons (r(49) = −0.31; p = 0.0278) and there was insufficient evidence of a relationship between source 3 amplitudes and BDI total scores (r(49) = −0.01; p = 0.9570).
Somatic and cognitive-affective symptoms may uniquely contribute to the relationship between source 2 (~287–525 ms) error responses and total BDI scores. Their potentially distinct contributions were demonstrated by a significant correlation between source 2 amplitudes and the total somatic symptom scores (r(49) = −0.38; p = 0.0058), and insufficient evidence of a relationship between source 2 amplitudes and total cognitive-affective symptom scores (r(49) = 0.07; p = 0.6197). Thus, individuals with greater somatic symptoms demonstrate a reduced ERP response ~287–525 ms following an error.
4. Discussion
In the present study, we examine the relationship between different depression symptoms and the cortical responses that follow behavioral errors. We demonstrate that the average error ERP amplitude between 287 – 525 ms is significantly correlated with somatic depression symptom severity but not with the cognitive-affective dimension of depression symptoms. Reductions within this error ERP component were associated with increases in the severity of somatic depression symptoms. These results highlight the utility of considering the self-reported depression symptom profile in further studies that examine differences in cognitive and affective processing in depression. In addition, they help characterize the relationship between symptoms of depression measured clinically and cortical measures of cognitive control.
The temporal and topographic characteristics of this source are consistent with the Pe ERP responses that have been described previously in go/no-go or flanker tasks (Falkenstein, 2004; Falkenstein et al., 1991; Gehring et al., 1993; Nieuwenhuis et al., 2001). Thus, it is important to understand the aspects of error processing that may be associated with the Pe response. The influence of experimental manipulations and individual differences on Pe amplitudes is relatively understudied, but the emerging evidence indicates that Pe responses are related to individuals awareness of errors (Endrass et al., 2007; Leuthold and Sommer, 1999; Nieuwenhuis et al., 2001; O’Connell et al., 2007; Overbeek et al., 2005; Shalgi et al., 2009). For example, (Nieuwenhuis et al., 2001) demonstrate greater Pe amplitudes when individuals are aware of error antisaccades compared to when they fail to report their awareness of antisaccade errors. In the context of the present study, this suggests that individuals with greater somatic depression symptoms demonstrate a reduced awareness of behavioral errors. Thus, somatic symptoms appear to represent a general non-responsiveness to the environment, and this non-responsiveness may extend, or be related to, a compromised perceptual experience of errors. With regards to the cognitive-affective depression symptoms, the absence of a relationship with Pe is consistent with the theory that the Pe response is invariant to the emotional aspects of the error response (Overbeek et al., 2005).
There are a range of tasks that elicit positive ERP’s that appear ~ 200 – 500 ms following a stimulus or behavioral response. This response is commonly termed the P3 and it is often observed after averaging EEG epochs that follow rare irrelevant stimuli (i.e. within oddball tasks). The Pe has similar temporal and spatial characteristics as the P3 (Leuthold and Sommer, 1999), and the different terminology is used to distinguish that the response is observed by averaging EEG epochs that follow the erroneous behavioral response. The P3 and Pe should not be considered distinct due to their distinct titles however, as they appear to overlap to some degree in their neural significance and psychological attributes (Davies et al., 2001; Ridderinkhof et al., 2009; Shalgi et al., 2009). Given this potential similarity, it is important to consider the relationship between depression symptoms and P3 amplitudes (Bruder et al., 2012). The predominant findings appear to be a robust negative relationship between P3 amplitudes within individuals with melancholic depression features (Gangadhar et al., 1993; Urretavizcaya et al., 2003), and within individuals with psychotic depression (Karaaslan et al., 2003; Kaustio et al., 2002). The negative relationship between melancholic depression and P3 amplitudes is consistent with the present findings given the considerable overlap between melancholic features (i.e. including loss of interest, lack of reactivity to pleasurable stimuli, and psychomotor retardation (“American Psychiatric Association,” 1994)) and somatic symptoms.
In addition to ERP’s, different depression symptoms are related to differences in resting blood flow/metabolism (Videbech, 2000) and resting EEG (Pizzagalli et al., 2002). For example, (Graff-Guerrero et al., 2004) demonstrate a positive correlation between right dorsolateral prefrontal cortex (DLPFC) blood flow and Hamilton Ratings for depressed mood, somatic anxiety, and paranoid symptoms, and a negative relationship with work and interest. Negative affect (as assessed with PANAS-neg or the assessment of negative symptoms (SANS)) appears positively correlated with right amygdala activity (Abercrombie et al., 1998) and negatively correlated with activity within frontal brain regions (Galynker et al., 1998). Collectively, these results suggest that different symptom profiles are related to differences in resting activity within frontal and limbic regions.
4.1 Limitations and further considerations
As far as we are aware, this is the first study to examine the relationship between error ERP's and somatic and cognitive-affective BDI-II symptom scores. However, a few studies have reported an non-significant relationship between total BDI-II symptoms and Pe amplitudes within a modified flanker task (Schrijvers et al., 2008; Schrijvers et. al., 2009; Chiu and Deldin 2007) or a go/no-go task (Kaiser et al., 2003). These studies had a smaller sample size (min = 15; max = 26) than the present study (N = 51). The studies also focused on a sample of individuals with clinical major depressive disorder (MDD), and thus may have a comparably restricted range of BDI-II scores. Thus, it is possible that the broad range of BDI scores within the current study (i.e. with a minimum BDI score of 0, and a maximum of 42) and the larger sample size may have improved the ability to detect a relationship between BDI-II symptom scores and error ERP's.
The blind source separation (BSS) approach implemented in the present study facilitates the ability to estimate ERP amplitudes, especially in cases where only a limited number of epochs are present (i.e. when analyzing error trials or single trial amplitudes) (Beauducel, et al., 2000; Bridwell et al., 2014). The multiplexed ERP response was decomposed in the present study with group temporal ICA and analysis was conducted on the individual reconstructed sources (as implemented in EEGIFT (Eichele et al., 2011)). Temporal group ICA integrates the temporal information across all of the individuals when deriving group source time courses. Aggregating information across subjects is advantageous for emphasizing commonalities across subjects, but it is important to note that low frequency ERP's (such as the positive potential appearing 250–500 ms post-response) may be preserved across subjects to a greater extent than the higher frequency ERP's (such as the negative potential between 50 – 150 ms). In order to understand this further, additional studies may examine the degree in which individual variation is preserved within the individual sources that are reconstructed with this approach.
The data in the present study was collected from a sample of female adults from a maximum-security correctional facility. On average, the participants demonstrated mild depression symptoms with a mean BDI-II score of 17.47. Depression was the predominant mood disorder within the sample, and there was limited presence of other disorders (see Table 1). However, there is considerable drug dependence within the sample, consistent with comorbidities between personality disorders and drug dependence (see Table 1). In an exploratory analysis, we were unable to find a statistical relationship between dependence within the 8 SCID-I substance categories (see Table 1) and somatic or cognitive-affective symptom severity (one-way ANOVA; max F(2,48) = 1.21; min p = 0.3055, among the 16 tests). Thus, the severity of somatic and cognitive-affective depression symptoms appears unrelated to drug dependence within this sample, reducing the influence of this potential confound on the observed results. Nevertheless, these findings highlight the potential utility of focusing on specific symptoms instead of specific diagnosis. For example individuals diagnosed with schizophrenia often present with depressive symptoms (Buckley et al., 2009), and it would be interesting to determine whether the relationship between symptoms and neural measures of cognitive function is retained within different clinical populations. These findings would help disentangle the overlapping cognitive deficits present within different patient populations, potentially contributing to improved assessment and diagnosis.
5. Conclusion
The relationship between self-report somatic and cognitive-affective depressive symptoms were examined with respect to error ERP amplitudes in a go/no-go task. Individual error ERP amplitudes were estimated by conducting group ICA on the EEG time series and analyzing the individual reconstructed epochs. We demonstrate a negative relationship between somatic depression symptoms and the central-parietal ERP response that peaks at 359 ms. The peak amplitudes within this ERP response were not significantly related to cognitive-affective symptom severity. These findings further clarify the relationship between clinical measures of self-reported depression symptoms and cognitive control.
Figure 3.
Source amplitude and BDI symptoms. The average z-scored amplitude (around the peak full width half maximum (fwhm)) of source 2 is plotted against the individual total BDI score (left), the total somatic symptom score (middle) or the total cognitive-affective symptom score (right). The influence of age and IQ were regressed out of the total BDI-II score, and the cognitive-affective symptom score was additionally regressed out of the somatic symptom score (and vice versa).
Temporal group ICA was applied to error ERP’s.
Increases in somatic depression symptoms were associated with error ERP’s.
Affective depression symptoms were not significantly related to error ERP’s.
Increases in somatic symptoms appear related to reduced error awareness.
Acknowledgments
The authors would like to thank the participants.
Role of the Funding Source
The data was collected under NIMH 1R01MH085010-01A1 (PI: Kiehl), and analysis was supported by NIH grant NIBIB 1R01EB006841 (Dr. Calhoun).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no interest to declare.
Contributors
KK contributed to experimental design. DB conducted analysis and wrote the manuscript. VS, MM, and KK, and VC edited the manuscript and assisted with interpretation of the results. All authors have contributed to and approved the final manuscript.
References
- Abercrombie HC, Schaefer SM, Larson CL, Oaks TR, Lindgren KA, Holden JE, Perlman SB, Turski PA, Krahn DD, Benca RM, Davidson RJ. Metabolic rate in the right amygdala predicts negative affect in depressed patients. NeuroReport. 1998;9:3301–3307. doi: 10.1097/00001756-199810050-00028. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Kalayam B, Katz R, Kanellopoulos D, Etwaroo GR, Klimstra S, Foxe JJ. Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport. 2007;18:217–221. doi: 10.1097/WNR.0b013e328013ceda. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington DC: APA; 1994. [Google Scholar]
- Beauducel A, Debener S, Brocke B, Kayser J. On the reliability of augmenting/reducing: peak amplitudes and principle component analysis of auditory evoked potentials. J. Psychophysiol. 2000;14:226–240. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio TX: Psychol. Corp; 1996. [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bridwell DA, Kiehl KA, Pearlson GD, Calhoun VD. Patients with schizophrenia demonstrate reduced cortical sensitivity to auditory oddball regularities. Schizophr. Res. 2014;2014 doi: 10.1016/j.schres.2014.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder GE, Kayser J, Tenke CE. Event-related brain potentials in depression: Clinical, cognitive and neurophysiologic implications. Oxf. Handb. Event-Relat. Potential Compon. 2012;2012:563–592. [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric Comorbidities and Schizophrenia. Schizophr. Bull. 2009;35:383–402. doi: 10.1093/schbul/sbn135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum. Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channon S, Green PS. Executive function in depression: the role of performance strategies in aiding depressed and non-depressed participants. J. Neurol. Neurosurg. Psychiatry. 1999;66:162–171. doi: 10.1136/jnnp.66.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P, Deldin P. Neural evidence for enhanced error detection in major depressive disorder. Am. J. Psychiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Davies PL, Segalowitz SJ, Dywan J, Pailing PE. Error-negativity and positivity as they relate to other ERP indices of attentional control and stimulus processing. Biol. Psychol. 2001;56:191–206. doi: 10.1016/s0301-0511(01)00080-1. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Eichele T, Rachakonda S, Brakedal B, Eikeland R, Calhoun VD. EEGIFT: group independent component analysis for event-related EEG data. Comput. Intell. Neurosci. 2011:1–9. doi: 10.1155/2011/129365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: aware and unaware errors in an antisaccade task: ERP correlates of conscious error recognition. Eur. J. Neurosci. 2007;26:1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- Falkenstein M. Current Opinions on Performance Monitoring. Leipzig: Max-Plank-Institut fur Kognitions- und Neurowissenschaften; 2004. ERP correlates of erroneous performance, in: Errors, Conflicts, and the Brain; pp. 5–14. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr. Clin. Neurophysiol. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders - Patient edition (SCID-I/P) N. Y. Biom. Res. Dep. NY State Psychiatr. Inst. 1995 [Google Scholar]
- Galynker II, Cai J, Ongseng F, Finestone H, Dutta E, Serseni D. Hypofrontality and negative symptoms in major depressive disorder. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1998;39:608–612. [PubMed] [Google Scholar]
- Gangadhar BN, Ancy J, Janakiranaiah N, Umpathy C. P300 amplitude in non-bipolar melancholic depression. J. Affect. Disord. 1993;28:57–60. doi: 10.1016/0165-0327(93)90077-w. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol. Sci. 1993;4:385–390. [Google Scholar]
- Graff-Guerrero A, González-Olvera J, Mendoza-Espinosa Y, Vaugier V, García-Reyna JC. Correlation between cerebral blood flow and items of the Hamilton Rating Scale for Depression in antidepressant-naive patients. J. Affect. Disord. 2004;80:55–63. doi: 10.1016/S0165-0327(03)00049-1. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J, Hyvärinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage. 2004;22:1214–1222. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch. Gen. Psychiatry. 2008;65:179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Joormann J, Gotlib IH. Emotion regulation in depression: Relation to cognitive inhibition. Cogn. Emot. 2010;24:281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Siemer M. Memory Accessibility, Mood Regulation, and Dysphoria: Difficulties in Repairing Sad Mood With Happy Memories? J. Abnorm. Psychol. 2004;113:179–188. doi: 10.1037/0021-843X.113.2.179. [DOI] [PubMed] [Google Scholar]
- Joormann J, Siemer M, Gotlib IH. Mood regulation in depression: Differential effects of distraction and recall of happy memories on sad mood. J. Abnorm. Psychol. 2007;116:484–490. doi: 10.1037/0021-843X.116.3.484. [DOI] [PubMed] [Google Scholar]
- Kaiser S, Unger J, Kiefer M, Markela J, Mundt C, Weisbrod M. Executive control deficit in depression: event-related potentials in a Go/Nogo task. Psychiatry Res. Neuroimaging. 2003;122:169–184. doi: 10.1016/s0925-4927(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Kapfhammer H-P. Somatic symptoms in depression. Dialogues Clin. Neurosci. 2006;8:227. doi: 10.31887/DCNS.2006.8.2/hpkapfhammer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaaslan F, Gonul AS, Oguz A, Erdinc E, Esel E. P300 changes in major depressive disorders with and without psychotic features. J. Affect. Disord. 2003;73:283–287. doi: 10.1016/s0165-0327(01)00477-3. [DOI] [PubMed] [Google Scholar]
- Kaustio O, Partanen J, Valkonen-Korhonen M, Viinamäki H, Lehtonen J. Affective and psychotic symptoms relate to different types of P300 alteration in depressive disorder. J. Affect. Disord. 2002;71:43–50. doi: 10.1016/s0165-0327(01)00410-4. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Lee TW, Girolami M, Sejnowski TJ. Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput. 1999;11:417–441. doi: 10.1162/089976699300016719. [DOI] [PubMed] [Google Scholar]
- Leuthold H, Sommer W. ERP correlates of error processing in spatial S–R compatibility tasks. Clin. Neurophysiol. 1999;110:342–357. doi: 10.1016/s1388-2457(98)00058-3. [DOI] [PubMed] [Google Scholar]
- Lux V, Kendler KS. Deconstructing major depression: a validation study of the DSM-IV symptomatic criteria. Psychol. Med. 2010;40:1679–1690. doi: 10.1017/S0033291709992157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive Vulnerability to Emotional Disorders. Annu. Rev. Clin. Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L, Buiatti M. ADJUST: An automatic EEG artifact detector based on the joint use of spatial and temporal features: Automatic spatio-temporal EEG artifact detection. Psychophysiology. 2011;48:229–240. doi: 10.1111/j.1469-8986.2010.01061.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response erorrs: evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- Nolan H, Whelan R, Reilly RB. FASTER: Fully Automated Statistical Thresholding for EEG artifact Rejection. J. Neurosci. Methods. 2010;192:152–162. doi: 10.1016/j.jneumeth.2010.07.015. [DOI] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, Robertson IH, Foxe JJ. The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study: Error awareness. Eur. J. Neurosci. 2007;25:2571–2579. doi: 10.1111/j.1460-9568.2007.05477.x. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Klein DN, Hajcak G. Depression symptom severity and error-related brain activity. Psychiatry Res. 2010;179:30–37. doi: 10.1016/j.psychres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable Components of Error Processing. J. Psychophysiol. 2005;19:319–329. [Google Scholar]
- Pizzagalli DA, Nitschke JB, Oaks TR, Hendrick AM, Horras KA, Larson CL, Abercrombie HC, Schaefer SM, Koger JV, Benca RM, Pascual-Marqui RD, Davidson RJ. Brain electrical tomography in depression: the importance of symptom severity, anxiety, and melancholic features. Biol. Psychiatry. 2002;52:73–85. doi: 10.1016/s0006-3223(02)01313-6. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ramautar JR, Wijnen JG. To Pe or not to Pe: A P3-like component reflecting the processing of response errors. Psychophysiology. 2009;46:531–538. doi: 10.1111/j.1469-8986.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Beschoner P, Grön G, Spitzer M, Kiefer M. Error processing in major depressive disorder: Evidence from event-related potentials. J. Psychiatr. Res. 2006;40:37–46. doi: 10.1016/j.jpsychires.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Herrnberger B, Wiesend C, Gron G, Spitzer M, Kiefer M. The effect of erroneous responses on response monitoring in patients with major depressive disorder: A study with event-related potentials. Psychophysiology. 2004;41:833–840. doi: 10.1111/j.1469-8986.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, de Bruijn ERA, Maas Y, De Grave C, Sabbe BGC, Hulstijn W. Action monitoring in major depressive disorder with psychomotor retardation. Cortex. 2008;44:569–579. doi: 10.1016/j.cortex.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Shalgi S, Barkan I, Deouell LY. On the positive side of error processing: error-awareness positivity revisited. Eur. J. Neurosci. 2009;29:1522–1532. doi: 10.1111/j.1460-9568.2009.06690.x. [DOI] [PubMed] [Google Scholar]
- Sprinkle SD, Lurie D, Insko SL, Atkinson G, Jones GL, Logan AR, Bissada NN. Criterion validity, severity cut scores, and test-retest reliability of the Beck Depression Inventory-II in a university counseling center sample. J. Couns. Psychol. 2002;49:381–385. [Google Scholar]
- Steer RA, Ball R, Ranieri WF, Beck A. Dimensions of the Beck Depression Inventory-II in clinically depressed outpatients. J. Clin. Psychol. 1999;55:117–128. doi: 10.1002/(sici)1097-4679(199901)55:1<117::aid-jclp12>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Storch EA, Roberti JW, Roth DA. Factor structure, concurrent validity, and internal consistency of the beck depression inventory? second edition in a sample of college students. Depress. Anxiety. 2004;19:187–189. doi: 10.1002/da.20002. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, Harsay HA, Wessel JR, Ridderinkhof KR. Conscious perception of errors and its relation to the anterior insula. Brain Struct. Funct. 2010;214:629–643. doi: 10.1007/s00429-010-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urretavizcaya M, Moreno I, Benlloch L, Cardoner N, Serrallonga J, Menchón J, Vallejo J. Auditory event-related potentials in 50 melancholic patients: increased N100, N200 and P300 latencies and diminished P300 amplitude. J. Affect. Disord. 2003;74:293–297. doi: 10.1016/s0165-0327(02)00016-2. [DOI] [PubMed] [Google Scholar]
- Videbech P. PET measurements of brain glucose metabolism and blood flow in major depressive disorder: a critical review. Acta Psychiatr. Scand. 2000;101:11–20. doi: 10.1034/j.1600-0447.2000.101001011.x. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Perez JE, Ramel W. Factor structure of the Beck Depression Inventory- Second Edition (BDI-II) in a student sample. J. Clin. Psychol. 2000;56:545–551. doi: 10.1002/(sici)1097-4679(200004)56:4<545::aid-jclp7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Yeung N, Botvinick MM, Cohen JD. The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol. Rev. 2004;111:931–959. doi: 10.1037/0033-295x.111.4.939. [DOI] [PubMed] [Google Scholar]