Abstract
Background
Radical pleurectomy (RP) for mesothelioma is often considered either technically infeasible or an operation limited to patients who would not tolerate a pneumonectomy. The purpose of this study was to review our experience using RP and intraoperative photodynamic therapy (PDT) for mesothelioma.
Methods
38 patients (42–81 years) underwent RP-PDT. 35/38 (92%) patients also received systemic therapy. Standard statistical techniques were employed for analysis.
Results
37/38 (97%) patients had Stage III/IV (AJCC) cancer and 7/38 (18%) patients had nonepithelial subtypes. Macroscopic complete resection was achieved in 37/38 (97%) patients. There was one postoperative mortality (stroke). At a median follow-up of 34.4 months, the median survival was 31.7 months for all 38 patients, 41.2 months for the 31/38 (82%) epithelial patients and 6.8 months for the 7/38 (18%) nonepithelial patients. The median progression free survivals were 9.6, 15.1 and 4.8 months, respectively. The median and progression free survivals for the 20/31 (64%) epithelial patients with N2 disease were 31.7 and 15.1 months, respectively.
Conclusions
It was possible to achieve a macroscopic complete resection utilizing lung-sparing surgery in 97% of these stage III/IV patients. The survival we observed with this approach was unusually long for the epithelial subtype patients but, interestingly, the progression free survival was not. The reason for this prolonged survival in spite of recurrence is not clear, but is potentially related to preservation of the lung and/or some PDT-induced effect. We conclude that the results of this lung-sparing approach are safe, encouraging and warrant further investigation.
Keywords: mesothelioma, pleura, lasers, immunology, thoracotomy
INTRODUCTION
Malignant pleural mesothelioma remains an incurable cancer for which all treatments, including surgery, are palliative. Although the role of surgery for mesothelioma remains investigational, the evidence is compelling that surgery-based multimodal treatments are the ones most likely to have the greatest impact on the course of the disease [1].
The goal of surgery in treating mesothelioma is to remove all gross disease, achieving a macroscopic complete resection (MCR). Other modalities are used to treat the residual microscopic disease that is always present. There are two surgical approaches, extrapleural pneumonectomy (EPP) and lung-sparing surgery. Lung-sparing surgery results in more debrided surface area and, hence, almost certainly results in more residual microscopic disease.
Whereas EPP is defined by a standardized technique and nomenclature, lung-sparing surgery enjoys neither. Reported goals of lung-sparing surgery range from a palliative debulking to an attempted MCR [2]. Some surgeons reserve lung-sparing surgery for patients who would not tolerate pneumonectomy. Some decide preoperatively while some decide intraoperatively based, primarily, on the degree of invasion into the pulmonary fissures. There is even variability in the nomenclature used to describe lung-sparing surgery: pleurectomy, palliative pleurectomy, radical pleurectomy, or pleurectomy/decortication.
In this study all patients underwent lung-sparing surgery. This was a preoperative decision for every patient, even with evidence of extensive involvement of the fissures, bulky tumors and patients who could tolerate pneumonectomy. The goal of every operation was to achieve MCR. Each operation included sparing of the lung and as much of the surrounding normal structures as possible. Thus, every effort was made to preserve the phrenic nerve and as much of the diaphragm and pericardium as possible, without leaving behind any visible or palpable cancer. When necessary, due to extensive full thickness invasion, prosthetic reconstruction of the diaphragm, chest wall and/or pericardium was performed. The term we use to define this procedure is “radical pleurectomy” (RP).
As part of a multimodal approach, our group has employed photodynamic therapy (PDT), a light-based cancer treatment, as an intraoperative adjuvant. In PDT, a patient receives a nontoxic photosensitizing agent that is activated with visible laser light, triggering a variety of tumoricidal cascades. The currently known mechanisms of PDT include direct cell kill, destruction of tumor neovasculature and provocation of a tumor-directed immune response [3]. Because the activating energy for PDT is visible light, and because visible light penetrates several millimeters into tissue, PDT will treat for a short depth below the surface.
Inspired by the intuitive appeal of lung-sparing surgery, with respect to safety and quality of life, we conducted a small pilot study comparing two similar cohorts of mesothelioma patients [4]. Half underwent EPP-PDT and half underwent RP-PDT. Our hypothesis was that PDT would be effective at controlling the increased residual microscopic disease in the RP group. As with the current study, the decision to perform RP in the pilot study was a preoperative decision for every patient, regardless of tumor bulk or cardiopulmonary reserve. It was a small retrospective study with only 14 patients in each arm and, arguably, only valid for establishing trends rather than definitive conclusions. That said, we observed a significant difference in the survival for the two groups. The median survival for the EPP group was 8.4 months, with the RP group not reaching median survival at a follow-up of 2.1 years. There was a higher percentage of nonepithelial patients in the EPP group but, overall, both groups were quite similar with 86% AJCC stage III/IV disease. Stage adjusted, the survival for EPP group was similar, and the RP group longer, than what is typically reported [2]. Interestingly, local control was far superior in the EPP group, the group with the shorter survival.
Our hypothesis was that, despite poorer local control, there appeared to be a benefit to RP-PDT, at least with that specific photosensitizer. Expanding the RP cohort with another 24 consecutive patients, to confirm or refute this hypothesis, was the purpose of this study.
PATIENTS AND METHODS
This study represents a retrospective review performed with approval of the University of Pennsylvania Institutional Review Board under a protocol entitled, “Treatment Parameters and Outcomes in Pleural Photodynamic Therapy (PDT).”
Patient characteristics and workup
From 2005 to 2010, 38 patients underwent radical pleurectomy and PDT for mesothelioma (median age 65, range 42–81, 28/10 male/female). The first 14 patients in this series are from a prior pilot study comparing lung-sparing to lungsacrificing surgery [4]. All 24 of the subsequent patients were consecutive. A multidisciplinary team evaluated all patients prior to enrollment.
Twenty-eight patients were treatment naïve and went directly to RP-PDT, with the intention of following with four cycles of pemetrexed-based chemotherapy. Ten patients were referred after chemotherapy, primarily for progression, and underwent RP-PDT with individualized adjuvant treatment recommendations. Patient characteristics are summarized in Table 1.
Table 1.
Patient demographics and survival outcome
| Survival | Progression Free Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| N | 2yr rate ± SE |
Median (months) |
Log rank P value |
2yr rate ± SE |
Median (months) |
Log rank P value |
||
| All patients | 38 | 52%±9% | 31.7 | 31%±8% | 9.6 | |||
| Gender | ||||||||
| Male | 28 | 41%±10% | 17.8 | 0.07 | 18%±8% | 8.8 | 0.02 | |
| Female | 10 | 79%±13% | 54.7 | 53%±18% | 28.4 | |||
| Age | ||||||||
| < 65 | 18 | 53%±13% | 54.7 | 0.99 | 27%±12% | 9.0 | 0.31 | |
| ≥ 65 | 20 | 54%±11% | 31.7 | 34%±11% | 10.3 | |||
| Cell type | ||||||||
| Epithelial | 31 | 64%±9% | 41.2 | <0.001 | 38%±9% | 15.1 | <0.001 | |
| Non-epithelial | 7 | 0% | 6.8 | 0% | 4.8 | |||
| N stage | ||||||||
| N0 | 9 | 67%±16% | 57.1 | 0.10 | 56%±17% | 26.9 | 0.21 | |
| N+(4 N1; 24 N2) | 28 | 49%±10% | 22.7 | 24%±9% | 9.6 | |||
| Unknown Nx | 1 | |||||||
| AJCC stage | ||||||||
| 1 | 1 | -- | -- | -- | -- | |||
| 3 | 28 | 50%±10% | 22.7 | 0.83 | 30%±9% | 9.2 | 0.95 | |
| 4 | 9 | 52%±18% | 36.6 | 3 vs. 4 | 26%±16% | 10.3 | 3 vs. 4 | |
| Chemotherapy | ||||||||
| None | 3 | 67%±27% | 42.5 | ** | 67%±27% | Undefined | ** | |
| Pre-op | 4 | 0% | 2.5 | 0% | 5.0 | |||
| Post-op | 25 | 33%±10% | 9.6 | 58%±10% | 31.7 | |||
| Pre & post-op | 6 | 17%±15% | 10.3 | 44%±22% | 22.7 | |||
| N2 disease only | ||||||||
| Cell type | ||||||||
| Epithelial | 20 | 55%±12% | 31.7 | 0.001 | 27%±11% | 15.1 | <0.001 | |
| Non-epithelial | 4 | 0% | 6.8 | 0% | 4.7 | |||
No comparison, patients were not randomized to treatment
The radiographic staging workup included: PET scan, CT scan of the chest/abdomen and brain imaging. Thirty-six patients underwent invasive staging with a bronchoscopy and laparoscopy +/− contralateral thoracoscopy. All patients without a fused abdomen were taken to the operating room for an outpatient laparoscopy with peritoneal lavage to exclude radiographically occult peritoneal disease. Contralateral thoracoscopy was used only when there was concern of contralateral cancer. Mediastinoscopy was not performed, as mediastinal metastastes was not an exclusion criterion. Enrollment was broad, including multiple patients with obvious nodal metastases, chest wall/rib invasion and/or massive tumor bulk. In addition, two patients with cancer detected on the invasive staging workup (one contralateral pleural/one abdominal) were enrolled after chemotherapy and repeat workup revealed no detectable extrahemithoracic disease.
Surgery
The operative technique was consistent by virtue of the same surgeon (JSF) performing all operations. The operative plan was established preoperatively and was to achieve MCR while preserving the lung, phrenic nerve and as much of the diaphragm and pericardium as possible. A detailed and illustrated description of this technique can be found elsewhere [5], but the essential elements are as follows:
Approach
As all patients were light sensitive, light precautions were taken throughout the operation. Patients were positioned in lateral decubitus position and the chest was typically entered through a serratus-sparing thoracotomy through either the sixth interspace or the bed of the resected seventh rib. Rib removal was limited to when interspace contracture precluded access to the extrapleural plane.
Chest wall/posterior-superior mediastinal mobilization
The first step of the operation was to mobilize the cancer off the bony hemithorax, followed by the posterior and superior mediastinum, identical to the initial steps of an EPP. In T4 cases where the cancer extended through the ribs, but without rib destruction, a “bird cage” procedure was performed by resecting the cancer from the interspaces and to grossly normal chest wall soft tissue, leaving the skeletonized ribs intact. When rib destruction was present, a chest wall resection was performed and reconstructed with a 2mm Gore-Tex patch after PDT.
Anterior mediastinum
The anterior mediastinum was approached by sweeping off all pericardial fat in an antero-posterior direction and then attempting to separate the cancer from the pericardium such that there was no visible or palpable disease. If this was not possible, an attempt was made to resect the cancer with the fibrous pericardium, leaving the underlying serous pericardium. If this was not possible, then the areas of incompletely debulked pericardium were left intact until after the PDT, to avoid directly illuminating the heart. After PDT these areas were resected and reconstructed with a Gore-Tex pericardial patch. The dissection was continued to the anterior hilum, attempting to preserve the phrenic nerve by skeletonizing it from the encasing tumor.
Diaphragm
The diaphragm dissection was started in the costophrenic recess, attempting to bluntly separate the pleura from the underlying bare musculature. Often this would require sharp dissection and was best accomplished with broad-tipped scissors, allowing the scissors to “find” the plane between the hard cancer and the soft underlying normal tissue. Limited areas of full thickness invasion were resected and the diaphragm was primarily reconstructed. If too much diaphragm was invaded full thickness for primary reconstruction, then gross tumor was left attached until after the PDT to avoid directly illuminating the abdominal viscera. Subsequently the diaphragm was resected, as with an EPP, preserving the peritoneum and reconstructing with a 2mm Gore-Tex patch.
Lung
At this point in the dissection the entire tumor was tethered solely to the lung, which was placed on 10–30 cm water positive pressure through a separate oxygen supply. The tumor was sharply incised, extending through the visceral pleura. The plane between the undersurface of the visceral pleura and bare lung parenchyma was then developed. The vast majority of the time, this was best accomplished with the lung still under positive pressure and using a broad Cobb dissector. On several occasions this required dissection with electrocautery. This dissection was continued on all surfaces of the lung and tracking toward, but not into, the fissure. The fissure was only entered when both sides had been dissected off the lung and the only remaining area of adherence of the tumor to the lung was within the fissure itself. The lung was then deflated and the fissure was developed evenly from both sides. When the fissure was incomplete it could be liberated using the Cobb dissector. When it was anatomically complete the tumor would be tracked all the way down to the pulmonary artery, which required skeletonization (Figure 1). Depending upon the bulk and firmness of the cancer, the specimen was either removed piecemeal or as a continuous specimen (Figure 2). At this point MCR would be confirmed, often inspecting the hemithorax with the same thoracoscope used liberally for supplemental visualization during the dissection. Reconstructions, as mentioned above, were conducted after completion of PDT.
Figure 1.
(A) corresponds to the tumor extending into the fissure, visualized in the CT image in (B). (C) shows the appearance of the right pulmonary fissure after removing the tumor – note the skeletonized branches of the right pulmonary artery.
Figure 2.
(A) shows a tumor that was excised to achieve a macroscopic complete resection that was removed piecemeal, while the tumor on the right (B) came out as a unified specimen. The rulers in both images are 15 centimeters. Multiple patients had similar tumors, which exceeded one liter in volume.
Lymphadenectomy
A thoracic lymphadenectomy was then performed, dissecting all standard nodal stations as well as the posterior intercostal nodes, accessed by opening the intercostal space near the level of the rib heads and considered N1 in our analyses.
Intraoperative PDT
Each patient received intravenous porfimer sodium 2 mg/kg 24 hours preoperatively. 630 nm laser light was delivered to a measured dose of 60 J/cm2, as registered on seven strategically placed isotropic light detectors using a custom-built dosimetry system. The chest was filled with 0.01% dilute intralipid solution to facilitate light dispersion. Light delivery typically took approximately one hour to complete.
Follow-up and statistics
Standard follow-up was an office visit and chest CT every three months. Patient demographics and treatment variables were described by frequencies and percentages. Survivals were calculated from the time of surgery, not from time of enrollment, diagnosis or initiation of any other treatment. Survival was defined as the time from surgery to death due to any cause or last patient contact alive. Progression free survival (PFS) was defined as the time from surgery to first documented recurrence, death due to any cause or last patient contact alive. Survival and PFS were estimated by the method of Kaplan and Meier. Group differences in survival and PFS were tested by the log rank test. Statistical significance was set at a 0.05 level. All statistical analyses were performed in SPSS version 19.0 (SPSS Inc, Chicago, IL).
RESULTS
Patient demographics and survival statistics are summarized in Table 1. MCR was achieved in 37/38 (97%) of the cases. Prosthetic reconstructions included: five pericardium, one diaphragm and one chest wall. The one MCR failure was a patient with aortic and esophageal invasion who presented with a diagnosis of epithelial subtype but subsequently diagnosed biphasic, predominantly desmoplastic, on the surgical specimen.
There was one postoperative mortality from a stroke on postoperative day four. The most common complication attributable to PDT was transient respiratory insufficiency requiring reintubation in 6/38 patients. This was thought secondary to the systemic inflammatory response that accompanies PDT and manifested as a transient ARDS-type picture in both lungs, starting on postoperative days 1–3, usually lasting about a week and treated with diuresis. Other complications included: deep venous thrombosis 8/38, pulmonary embolism 1/38, atrial fibrillation 9/38 and chyle leak 2/38.
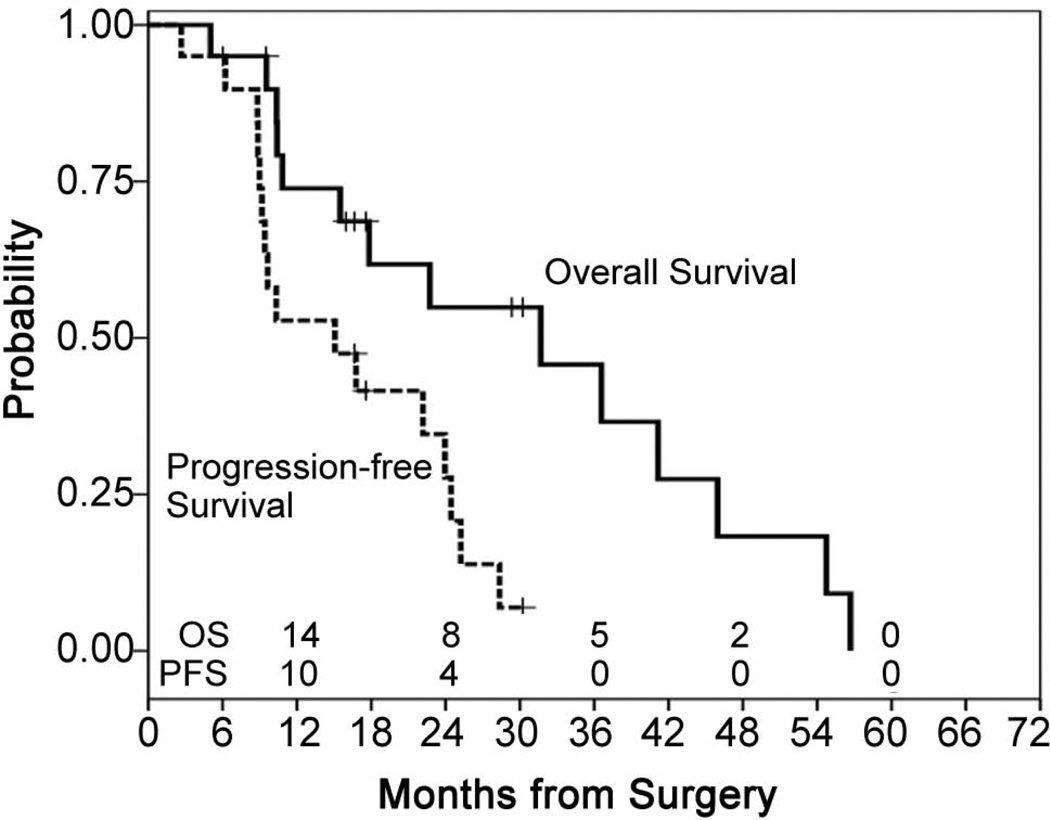
Survival and PFS for all patients
At a median follow-up of 34.4 months from the time of surgery for all 38 patients and 17.6 months (minimum of 9.5 months) for 14 living patients, the survival was 31.7 months (95% CI 9.0 – 54.3 months). Six patients are currently alive beyond two years. The median PFS for all patients was 9.6 months (95% CI 6.8 – 12.4 months) (Figure 3).
Figure 3.
Progression-free and survival for all 38 patients.
Patterns of relapse
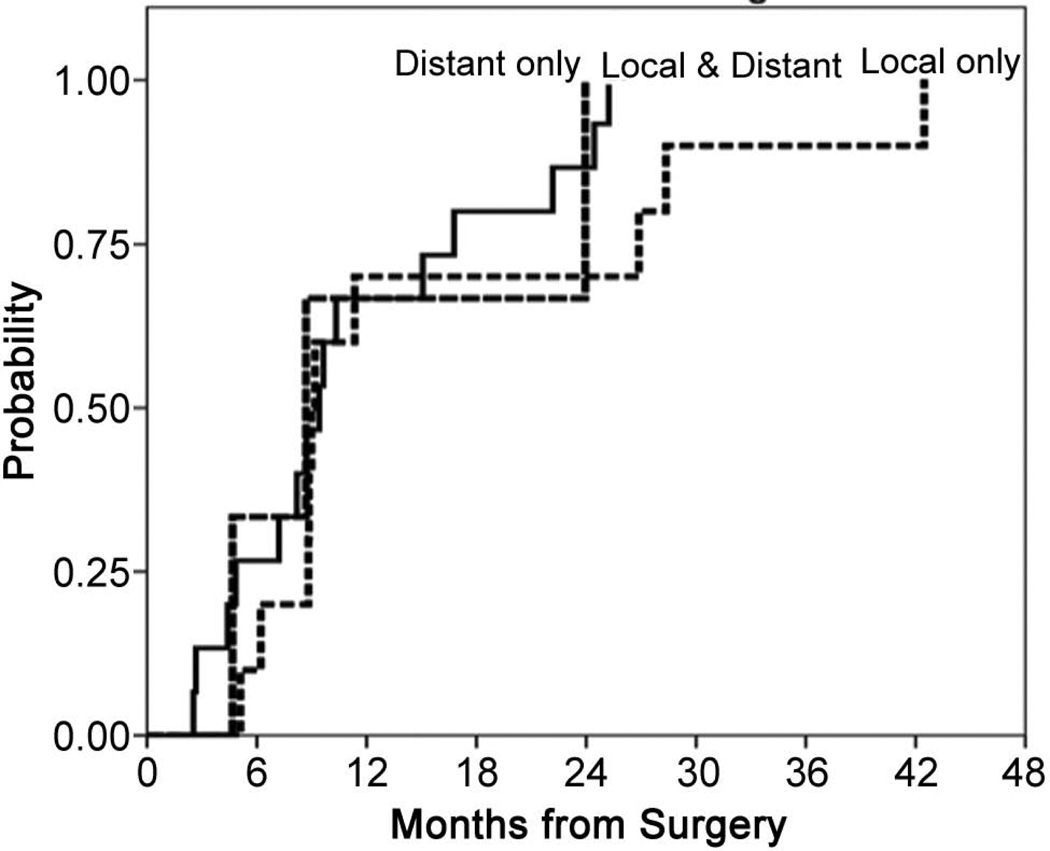
At the time of this analysis, 14 patients are alive, 24 are dead (2 having died without any evidence of cancer). 30 patients progressed, or died without evidence of progression, and 8 are alive without any evidence of disease. Of the 28 patients who progressed, the pattern of recurrence was: 10 local only, 3 distant only and 15 local and distant at a median time to first progression of 9.0, 8.7 and 9.4 months, respectively (Figure 4).
Figure 4.
Time from surgery to first progression for patients who progressed locally (n=10), distantly (n=3) or with simultaneous local and distant progression (n=15).
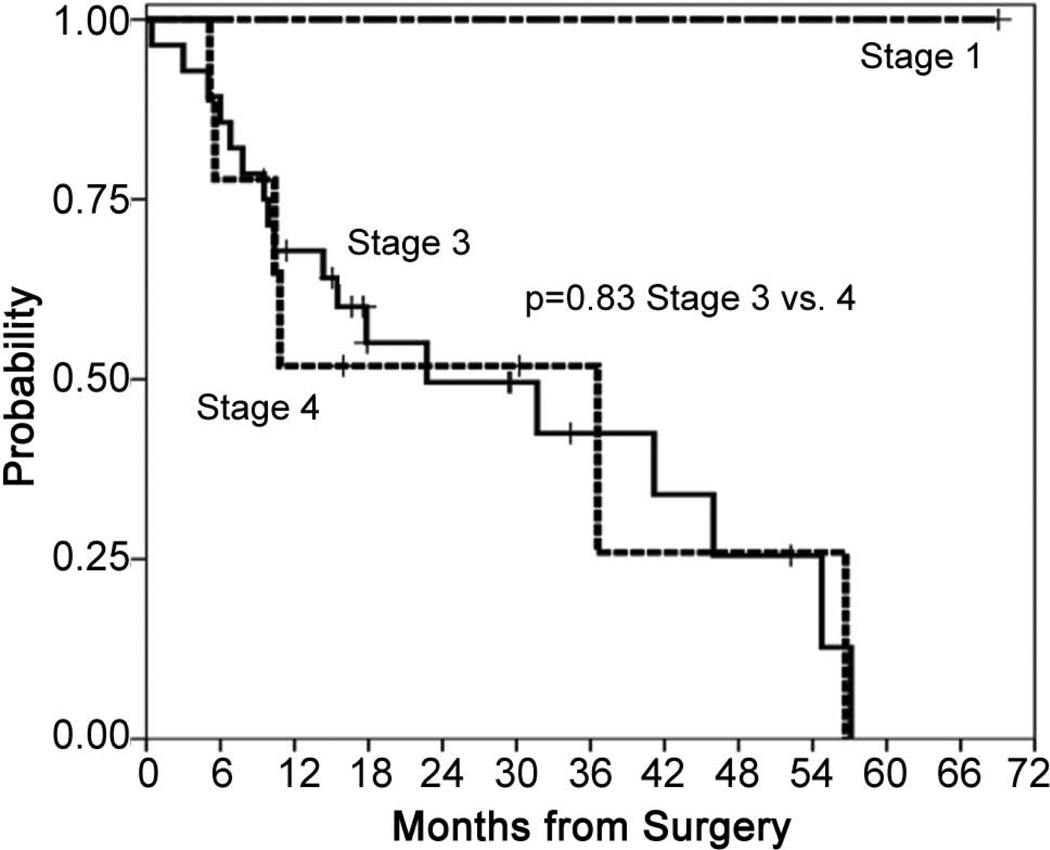
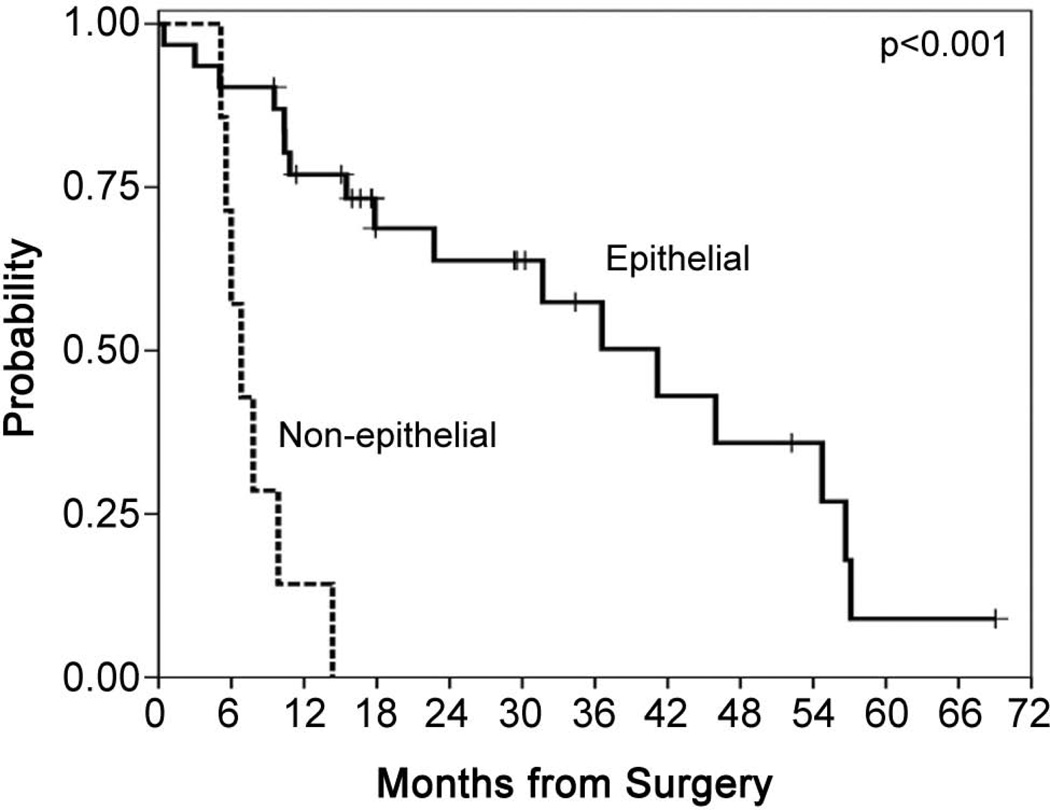
Stage and subtype
There was no difference in outcome by stage, though this analysis is a comparison between the 28 stage III patients, 9 stage IV patients and only 1 stage I patient (Figure 5). Subtype was a significant factor for both survival and PFS, though the results should be viewed with caution as only 7/38 patients had nonepithelial subtypes. The median PFS for the nonepithelial/epithelial patients was 4.8 months (95% CI 4.4 – 5.3 months)/15.1 months (95% CI 0.8 – 29.3 months). The median OS for the nonepithelial/epithelial patients was 6.8 months (95% CI 4.7 – 8.9 months)/41.2 months (95% CI 25.9 – 56.4 months)(Figure 6).
Figure 5.
Survival by stage
Figure 6.
Survival by subtype
Epithelial subtype subanalyses
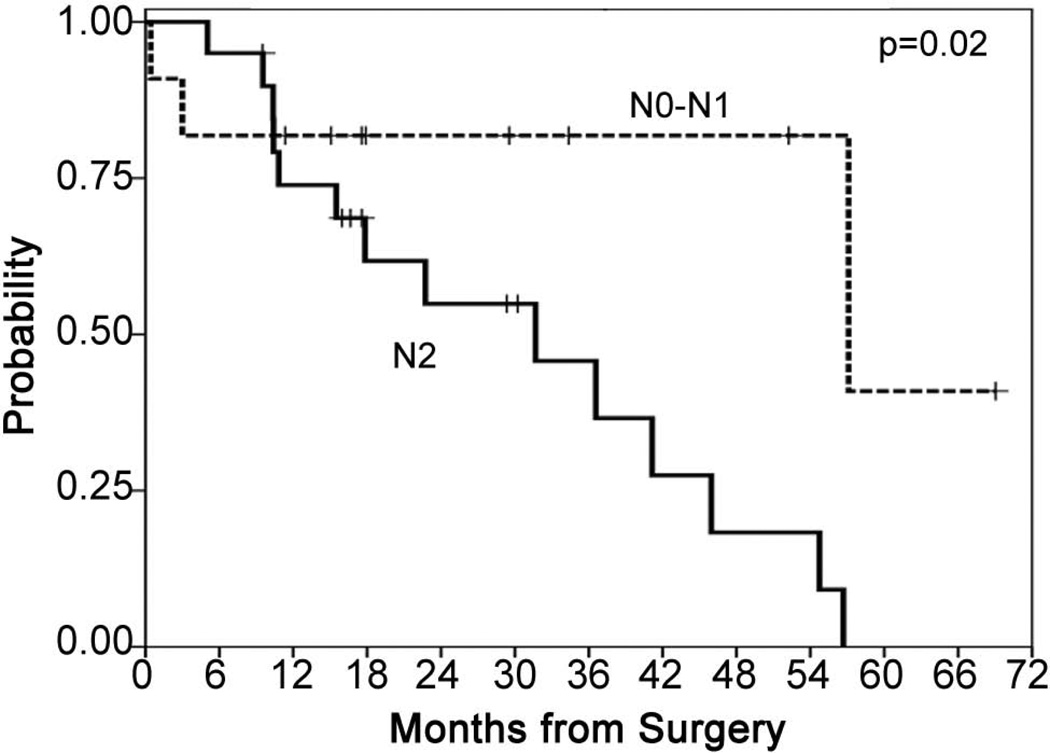
The median survival for 20/31 epithelial patients with N2 disease was 31.7 months (95% CI 12.5 – 50.9 months) and the PFS was 15.1 months (95% CI 5.3 – 24.8 months) (Figure 7). Of the 11/31 epithelial patients with N0 or N1 disease, of which 8 are currently alive, the median survival was 57.1 months (95% CI 0 – 133.6 months). The survival for the 20 epithelial N2 patients was statistically significantly different (p = 0.02) from the 11 epithelial patients with N0–1 disease (Figure 8). Of the 21/31 epithelial patients with recurrence, 9/21 demonstrated isolated local recurrence with median survival from disease progression of 22.5 months (95% CI 4.4 – 40.6 months) and 12/21 recurred with distant +/− local disease and demonstrated a survival from disease progression of 14.4 months (95% CI 0 – 40.3 months). There was no statistically significant difference between survivals by pattern of recurrence and there was no statistically significant difference in pattern of recurrence with respect to tumor characteristics, patient age or gender (Table 2).
Figure 7.
Progression-free and survival in epithelial patients with N2 disease
Figure 8.
Survival in epithelial patients with N0 or N1 disease (n=13) or N2 disease (n=24)
Table 2.
Statistics for the 21/31 epithelial patients with local or distant recurrent diseas
| Local only progression n = 9 |
Any distant progression n = 12 |
Fisher’s exact P value |
||||
|---|---|---|---|---|---|---|
| # | % | # | % | |||
| Gender | F | 3 | 33 | 2 | 25 | 1.0 |
| M | 6 | 67 | 9 | 75 | ||
| Age | < 65 | 6 | 67 | 3 | 25 | 0.09 |
| ≥ 65 | 3 | 33 | 9 | 75 | ||
| T stage | T2 | 3 | 33 | 3 | 25 | 0.35* |
| T3 | 5 | 56 | 5 | 42 | ||
| T4 | 1 | 11 | 4 | 33 | ||
| N stage | N0 – N1 | 3 | 33 | 2 | 17 | 0.61 |
| N2 | 6 | 67 | 10 | 83 | ||
| AJCC stage | Stage 3 | 8 | 89 | 8 | 67 | 0.34 |
| Stage 4 | 1 | 11 | 4 | 33 | ||
Trend test
COMMENT
The results of this study conflict with some commonly held beliefs and observations regarding surgical approach, prognostic factors and survival for surgery-based mesothelioma treatments. These observations are intriguing in that they are based on the outcomes for a particularly advanced stage cohort for a surgery series, 97% stage III/IV disease and 63% N2 metastases.
A surprise finding was that it was possible to achieve a MCR in 97% of these patients, of which the entire 24 patient expansion cohort was consecutive. The decision to perform lung-sparing surgery was made preoperatively in every case and even if the patient could tolerate pneumonectomy. Many of the patients had disease typically thought to preclude MCR with RP, including bulky extension into the fissure (Figure 1). It is our belief that this was accomplished by employing the described systematic approach to RP and that any mesothelioma in which it is thought that MCR could be achieved with EPP could be achieved with RP. The exception would be a case of overt invasion of the major bronchovascular structures. This, however, was not encountered in any of the 52 patients comprising this and our previous series and, in our experience, is an unusual isolated finding in any patient thought to be a surgical candidate. We believe there may be patients who would benefit more from EPP than RP as part of a surgery-based treatment, but we conclude from this series that RP is almost always going to be an option to achieve MCR.
The limitations of this study are that it was small, retrospective and likely subject to selection bias, typical of numerous mesothelioma surgery studies. The study strengths include the particularly advanced stage population, our practice of starting with surgery and not limiting enrollment to patients who successfully complete chemotherapy and the calculation of survival from the time of surgery, not diagnosis or other treatments. Viewed within this framework, the median survival for the patients in this study were unusually long, particularly the epithelial patients: 31.7 months for all patients including nonepithelial, 41.2 months for all epithelial patients, 31.7 months for the N2 epithelial patients and 57.1 months for the N0–1 epithelial patients. Although based on only 7 patients, median survival for the nonepithelial patients was only 6.8 months. Based upon these results we feel this treatment should be limited to patients with epithelial subtype, at least with this photosensitizer. Given the favorable survival we would not exclude patients with N2 disease. Because of the significant difference we found in survival between N0–1 versus N2 disease, however, we plan to change our invasive staging routine to include the mediastinum.
Also noteworthy was the interval between PFS and survival. Again focusing on the epithelial patients, the PFS was 15.1 months, approximately a third of the survival of 41.2 months. Our previous experience with EPP, and that commonly reported, typically reveals only several months between relapse and death. Overall, the trends we saw indicate that this treatment combination was not particularly effective at local control, but somehow made the recurrences less imminently lethal. We speculate that this trend, if accurate, was potentially related to saving the operative lung and/or to PDT.
It is possible, perhaps likely, that having two lungs played a significant role. Essentially all patients with mesothelioma recur. If they are in a more robust state of health, as one would expect with two lungs compared to one, then it stands to reason they will have more treatment options when they recur. Another possibility is that patients with two lungs simply have more reserve, and therefore more time to expend that reserve, before they succumb to their recurrent cancer. Another series of 35 patients undergoing RP for mesothelioma, of which approximately 50% were stage III/IV, reported a survival of 30.0 months and a PFS of 15.8 months [6]. As those patients did not receive PDT, but did display a significant interval between PFS and survival, it supports the hypothesis that sparing the lung plays a role in this phenomenon.
With respect to the possibility that the recurrence was rendered more indolent, there is evidence to believe that PDT could play a role in such a phenomenon. Clearly our initial hypothesis, that PDT could be combined with lung-sparing surgery to achieve excellent local control, was proven wrong. Local control in this series was poor, but that did not appear to adversely affect survival compared to other surgery-based treatments of which many report superior local control. The immunostimulatory effects of PDT are well known [3]. It is our hypothesis that there may be an autologous tumor vaccine-type response provoked by the PDT-treated residual microscopic disease. As we did not see the same benefit in our pilot study in the patients who underwent pneumonectomy and PDT, it is our secondary hypothesis that the lung may somehow play a role in an immune response [4].
In conclusion, we feel that radical pleurectomy is an operation that can be used to achieve MCR and, combined with intraoperative PDT, can be done with a very low mortality and acceptable morbidity. This operation can be planned preoperatively, even in patients generally not thought to be candidates for lung-sparing surgery, and does not have to be reserved for patients who would not tolerate pneumonectomy. PDT did not appear to have a positive impact on local control, but may have played a role in extending survival for a duration well beyond what would commonly be expected for recurrence after surgery in this cohort of patients. This particular combination of treatments yielded an unexpectedly long survival for advanced stage epithelial patients, the majority with N2 disease. It appeared to have little impact against, and is not recommended for, patients with non-epithelial histology. We believe this lung-sparing approach to mesothelioma is promising and warrants further investigation.
Acknowledgements
This work was supported, in part, by a grant from the NIH/NCI (P01 CA-87971). We gratefully acknowledge the independent review of our results and patient data by Dr. Lee Krug prior to revising this manuscript.
Abbreviations and Acronyms
- MCR
macroscopic complete resection
- MPM
malignant pleural mesothelioma
- EPP
extrapleural pneumonectomy
- PDT
photodynamic therapy
- PFS
progression free survival
- RP
radical pleurectomy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: STS 47th Annual Meeting – January 2011
References
- 1.Sugarbaker DJ, Wolf AS. Surgery for malignant pleural mesothelioma. Expert Rev Respir Med. 2010;4(3):363–372. doi: 10.1586/ers.10.35. [DOI] [PubMed] [Google Scholar]
- 2.Flores RM, Pass H, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg. 2008;135(3):620–626. doi: 10.1016/j.jtcvs.2007.10.054. [DOI] [PubMed] [Google Scholar]
- 3.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nature Rev Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedberg JS, Mick R, Culligan M, et al. Photodynamic Therapy and the Evolution of a Lung Sparing Surgical Treatment for Mesothelioma. Ann Thorac Surg. 2011;91:1738–1746. doi: 10.1016/j.athoracsur.2011.02.062. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg JS. Photodynamic therapy as an innovative treatment for malignant pleural mesothelioma. Sem Thorac & Cardiovasc Surg. 2009;21(2):177–187. doi: 10.1053/j.semtcvs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Bolukbas S, Manegold C, Eberlein M, Bergmann T, Fisseler-Eckhoff A, Schirren J. Survival after trimodality therapy for malignant pleural mesothelioma: Radical Pleurectomy, chemotherapy with Cisplatin/Pemetrexed and radiotherapy. Lung Cancer. 2011;71:75–81. doi: 10.1016/j.lungcan.2009.08.019. [DOI] [PubMed] [Google Scholar]