Abstract
Cell proliferation and cell death are integral elements in maintaining homeostatic balance in metazoans. Disease pathologies ensue when these processes are disturbed. A plethora of evidence indicates that malfunction of cell death can lead to inflammation, autoimmunity or immuno-deficiency. Programmed necrosis or necroptosis is a form of non-apoptotic cell death driven by the receptor interacting protein kinase 3 (RIPK3) and its substrate mixed lineage kinase domain-like (MLKL). RIPK3 partners with its upstream adaptors RIPK1, TRIF or DAI to signal for necroptosis in response to death receptor or toll-like receptor stimulation, pathogen infection, or sterile cell injury. Necroptosis promotes inflammation through leakage of cellular contents from damaged plasma membrane. Intriguingly, many of the signal adaptors of necroptosis have dual functions in innate immune signaling. This unique signature illustrates the cooperative nature of necroptosis and innate inflammatory signaling pathways in managing cell and organismal stresses from pathogen infection and sterile tissue injury.
Keywords: Necroptosis, RIP1, RIP3, MLKL, TNF, inflammation, TRIF, DAI, MCMV, Vaccinia virus
Introduction
Cell death is an important biological process that sculpts the development of multicellular organisms. In the immune system, cell death has critical roles in immune cell development and pathogen defense. Apoptosis is an orderly form of cell death marked by chromatin condensation, DNA fragmentation and membrane blebbing into “apoptotic bodies”. Apoptosis can be triggered by receptors in the TNF superfamily (extrinsic pathway) or through direct activation of mitochondrial effectors (intrinsic pathway). Caspases are cysteine proteases that drive apoptosis. Effector caspases cleave and inactivate the “flippase” adenosine triphosphatase type 11C (1). This results in exposure of phosphatidyl serine (PS) on the cell surface, which flags the dying cell for uptake and clearance by professional phagocytes such as macrophages (2). The rapid clearance of apoptotic cells ensures minimal risk of detrimental inflammation. This explains why apoptosis is the preferred and dominant pathway by which multi-cellular organisms eliminate unwanted cells during development. In contrast, necrosis is marked by rapid loss of plasma membrane integrity. Plasma membrane leakage in necrosis is widely thought to occur prior to or concomitant with exposure of PS and other “eat-me” signals. This early rupture of plasma membrane releases endogenous danger signals or “danger-associated molecular patterns” (DAMPs), which are potent stimulants of inflammation (3). As such, necrosis is often detected in infections and inflammatory diseases. This association has led to the popular view that necrosis represents pathological cell death while apoptosis is more central for development.
Pathologists have historically relied on morphology to distinguish between apoptosis and necrosis. Apoptosis is marked by cell shrinking, appearance of membrane blebs called apoptotic bodies, and condensation of chromatin. In contrast, necrosis is associated with cell and organelle swelling, and limited to no chromatin condensation. Biologists have long considered necrosis as the consequence of trauma or accidental injury. This view has now been revised with recent advances showing the existence of dedicated molecular pathways controlling necrotic cell death. It is noteworthy that the classical markers that define apoptosis can sometimes be detected in necrosis. For example, Annexin V staining is a commonly used method to detect exposure of PS on the outer leaflet of the plasma membrane in early apoptotic cells. In some necrotic cells, PS exposure can be detected without significant plasma membrane leakage (4). PS exposure is supposed to mark apoptotic cells for clearance by phagocytes. However, scavenger receptors that recognize necrotic cells have also been described (5, 6). Moreover, TUNEL staining, which detects DNA strand breaks in apoptosis, is also detected in necrotic cells (7). These observations suggest that the morphological definition of apoptosis and necrosis is insufficient to distinguish between these two cell death modules. Instead, we favor a molecular definition based on genetic pathways. Terms such as “programmed necrosis”, “regulated necrosis” and “necroptosis” have now been used to describe necrosis induced by the receptor interacting protein kinases (RIPK) (8). In addition, certain forms of regulated necrosis can occur without the RIP kinases (9, 10). Here, we will focus our discussion on RIP kinase-driven necrosis. The term “necroptosis” will be used throughout to distinguish RIPK-dependent necrosis from other forms of regulated necrosis. As we shall see below, pathways that control necroptosis and inflammation often utilize overlapping signaling adaptors. The sharing of common signal adaptors establishes an intimate link between inflammation and necroptosis that goes beyond their association in disease pathologies. Hence, necroptosis and inflammation can be mutually reinforcing processes that govern not only inflammatory diseases, but also immune and organismal homeostasis.
The molecular machinery of programmed necrosis
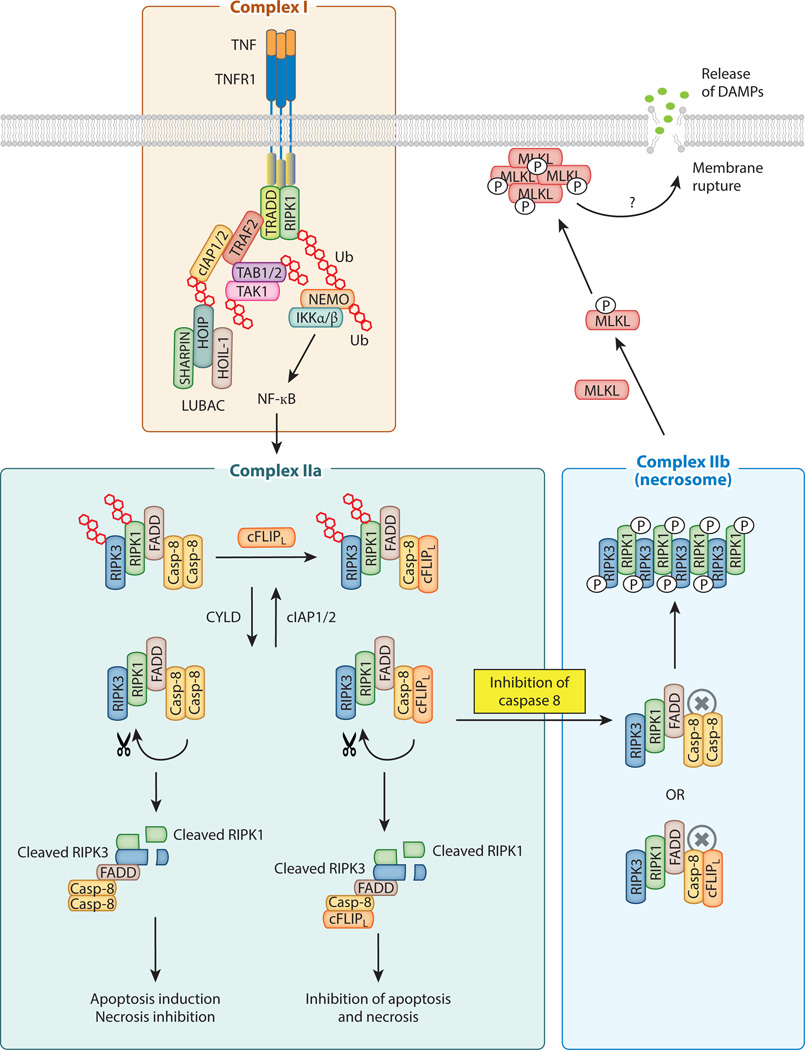
Necroptosis can be activated by death receptors in the TNF superfamily, toll-like receptor 3 (TLR3) and TLR4, and the interferon receptors (11). The signaling pathway for necroptosis is best characterized for TNF receptor 1 (TNFR1). TNFR1 is the prototypic member of a sub-family within the TNF receptor superfamily that contains an essential protein interaction domain called the “death domain” (DD). DD-containing death receptors include Fas/CD95/APO-1, TRAIL receptor 1 and 2, death receptor 3 (DR3), DR6 and ectodysplasin A receptor (EDAR). However, cell death is not the only signaling outcome for the death receptors. In fact, NF-κB activation is often the dominant response emanating from these receptors. TNFR1 is a prime example of such a receptor. Ligation of the pre-assembled TNFR1 trimer (12) with TNF causes conformation change to promote formation of a short-lived membrane-signaling complex termed “Complex I” by Micheau and Tschopp (13). This membrane complex is comprised of the adaptors TRADD, TRAF2, RIPK1, cIAP1, cIAP2 and the linear ubiquitin chain assembly complex (LUBAC), which is made up of the subunits HOIL-1, HOIP and SHARPIN (14). The E3 ligases cIAP1, cIAP2 and HOIL-1 within LUBAC critically control ubiquitination of many of the adaptors within Complex I. Ubiquitin linkages of different types have been found with various adaptors in Complex I. This ubiquitin network within Complex I is essential for recruitment and activation of the inhibitor of κB kinase (IKK) complex. The activated IKK phosphorylates IκBα, leading to its degradation by the proteasome and nuclear translocation of NF-κB dimers.
NF-κB is a key transcription factor for many pro-inflammatory and survival genes. Proper NF-κB response is crucial for cell survival and to counteract the cytotoxic effects of TNF. The pro-survival effect of NF-κB is mediated in part by its transcriptional targets cFLIPL, cIAP1 and cIAP2 (15, 16). As we will see below, cFLIPL and the cIAPs critically regulate cellular sensitivity to apoptosis and necroptosis, Hence, Complex I is a critical checkpoint for cell death versus cell survival signaling (Fig. 1). In addition, Complex I adaptors appear to have NF-κB-independent survival functions. For example, mice lacking both cIAP1 and cIAP2 die at an earlier stage in embryonic development than RelA/p65-deficient mice (17). The adaptor TRAF2 stabilizes cIAP1 expression by preventing its autoubiquitination and protesomal degradation (18). Consistent with this association, Traf2−/− mice also exhibit embryonic lethality (19). In contrast, cpdm mice that lack the LUBAC subunit SHARPIN show defective NF-κB activation but are nonetheless viable (20–22). These results demonstrate that the cyto-protective effects of TRAF2, cIAP1, cIAP2 and XIAP are mediated through NF-kB-dependent and independent functions.
Figure 1. Schematic diagram of TNF-induced signaling complexes.
The membrane associated Complex I is chiefly responsible for NF-κB activation. The ubiquitin chains are represented by the red hexagons. Induction of cFLIPL expression by NF-κB inhibits apoptosis and necroptosis. Active caspase 8 in Complex IIa promotes apoptosis and inhibits necroptosis by cleavage of RIPK1, RIPK3 and CYLD. When caspase 8 is inactive, RIPK1 and RIPK3 initiate Complex IIb assembly, amyloid conversion, and recruitment of MLKL. Both Complex IIa and Complex IIb are also regulated by protein ubiquitination. CYLD acts as the de-ubiquitinase that promotes Complex II activity by removing ubiquitin chains on RIPK1 and RIPK3.
Unlike conventional death receptors such as Fas or TRAIL receptors, FADD and caspase 8 are not recruited to the TNFR1-associated Complex I (13, 23). Instead, rapid receptor internalization is important for docking of the adaptor FADD and the initiator caspase, caspase 8, to the complex. Although there is evidence that FADD and caspase 8 can be recruited to the TNFR1 complex (24), standard biochemical pull-down supports a model in which TNFR1 dissociation occurs prior to docking of FADD and caspase 8 (13). The cytosolic complex that contains FADD and caspase 8 is often referred to as Complex IIa (Fig. 1). Normally, apoptosis is prevented by dimerization between caspase 8 and the long form of cFLIP (cFLIPL), an enzyme-inactive homolog of caspase 8. Caspase 8/cFLIPL heterodimer inhibits full activation of caspase 8 and apoptosis, but retains cleavage of essential necrosis regulators such as RIPK1, RIPK3 and CYLD (25–29). Hence, NF-κB dependent induction of cFLIPL inhibits apoptosis as well as necroptosis.
Active caspase 8 in Complex IIa not only initiates the caspase cascade and the apoptotic program, but also cleaves and inactivates essential necroptosis mediators such as RIPK1, RIPK3 and CYLD. Hence, inhibition of caspase 8 or its upstream adaptor FADD primes cells for necroptosis by preserving the integrity of RIPK1 and RIPK3. Stabilization of RIPK1 and recruitment of RIPK3 convert Complex IIa to Complex IIb or the “necrosome” (Fig. 1). The cytosolic complexes that contain the RIP kinases have also been referred to as the “ripoptosome” (30, 31), although this term does not make the distinction between apoptosis versus necroptosis. RIPK1 and RIPK3 interact via the “RIP homotypic interaction motif (RHIM)” to form an amyloid-like complex that is essential for recruitment and activation of the downstream RIPK3 substrate mixed lineage kinase domain-like (MLKL) (32–34). RIPK3 phosphorylates MLKL at Thr357 and Ser358 to stimulate its oligomerization and translocation to intracellular and plasma membranes (33, 35–38) (Fig. 1). The precise mechanism by which MLKL induces membrane rupture is controversial, with some reports implicating disruption of calcium or sodium ion channels (35, 36) and others showing direct binding to membrane phospholipids and disruption of membrane integrity (37, 38). In contrast to MLKL, another reported substrate of RIPK3, the mitochondrial phosphatase phosphorylate mutase family member 5 (Pgam5) (39), may not be crucial as shRNA-mediated knock-down of Pgam5 did not consistently confer protection against TNF-induced necroptosis (40, 41). In agreement with the notion that Pgam5 is not a core component of the necroptosis machinery, widespread depletion of mitochondria did not impair necroptosis (42). The differential requirement for mitochondrial signaling further distinguishes necroptosis from apoptosis.
Since excessive necrosis in FADD- or caspase 8-deficient mice was rescued by inactivation of RIPK1 or RIPK3 (27, 43, 44), FADD and caspase 8 are paradoxically pro-survival factors during development. This “yin-yang” relationship between the RIP kinases and FADD/caspase 8 is also played out in the skin keratinocytes, intestinal epithelium and T cells (45–48). The genetic evidence also provides a mechanistic explanation for the biochemical interaction between FADD, caspase 8 and the RIP kinases. Intriguingly, although caspase 8-deficient Jurkat T cells are sensitized to necroptosis induced by TNF, FasL and TRAIL, FADD-deficient Jurkat are only sensitized to TNF-induced necroptosis (49). The molecular basis for the resistance of FADD-deficient Jurkat cells to FasL- and TRAIL-induced necrosis is unknown. One possibility is that since FADD is the apical adaptor recruited to Fas and TRAIL receptor, downstream signaling will be completely blunted in its absence. While this is certainly the case for FADD-mediated apoptosis, it is insufficient to explain RIPK3 signaling in T cells. Fadd−/− T cells undergo RIPK1- and RIPK3-dependent necroptosis in response to T cell receptor (TCR) stimulation (44, 47, 48). Since Fadd−/−Ripk3−/− mice developed lymphoproliferation resembling that caused by the Fas mutant lpr mice (50), one can argue that Fas triggers necroptosis of Fadd−/− T cells through RIPK3. Alternatively, necroptosis of Fadd−/− T cells could be the consequence of direct TCR signaling. As we shall see below, RIPK3 is also capable of signaling for necroptosis in the absence of RIPK1 under certain conditions. These perplexing results highlight that fact that the traditional model of Complex I to Complex II transition may not be adequate to account for signaling in necroptosis.
Ubiquitination: A critical checkpoint for necroptosis
RIPK1 and other Complex I adaptors are key substrates of the E3 ubiquitin ligases cIAP1 and cIAP2. As discussed already, the ubiquitin network within Complex I functions to recruit the IKK complex and to promote survival through NF-κB dependent and independent mechanisms (51–55). Bivalent IAP antagonists or Smac mimetics (SM) are often used to deplete cIAP1, cIAP2 and XIAP. SMs are small peptide mimetic of second mitochondrial-derived activator of caspases (Smac) that trigger auto-ubiquitination and degradation of the IAPs. Since RIPK1 ubiquitination does not occur in the absence of the IAPs, SM tips the balance of TNF signaling towards cell death. Moreover, because the NF-κB inducing kinase (NIK) is constitutively targeted for ubiquitination and degradation by the IAPs (56–59), SM can additionally stabilize NIK, leading to non-canonical NF-κB activation and autocrine TNF production. Thus, SM primes cells to cell death through two mutually reinforcing mechanisms: elimination of a cyto-protective ubiquitin network and induction of TNF. Given that many tumors over-express cIAPs and are resistant to traditional chemotherapies, SM can provide a powerful one-two punch to trigger cancer cell death through either apoptosis or necroptosis (60, 61). Physiologically, IAP depletion occurs in response to stimulation of TNFR2, the TNF receptor whose expression is highly inducible (62). Although TNFR2 does not contain a cytoplasmic death domain, it recruits TRAF2 and the cIAPs and triggers their proteasomal degradation. Hence, similar to the action of SMs, TNFR2 also skews TNF signaling towards cell death (63–66). Hence, the IAPs and ubiquitination have important roles in fending off the cytotoxic effects of TNF.
The importance of cIAPs and the ubiquitin machinery in regulating RIP kinase activities and necroptosis is illustrated by the partial rescue of embryonic lethality of ciap1−/−xiap−/− or ciap1−/−ciap2−/− embryos by loss of Ripk3 or a single Ripk1 allele (17). Systemic auto-inflammatory disease of mice with myeloid-specific deletion of cIAP1, cIAP2 and X-linked IAP (XIAP) was also corrected by inactivation of RIPK1 or RIPK3 (67). These results provide strong evidence that the IAPs are crucial guardians that keep RIPK1 and RIPK3 in check to prevent deleterious cell injury and inflammation. Further evidence that the ubiquitin network within Complex I serves critical functions in limiting cell death and inflammation comes from mice lacking the LUBAC components SHARPIN or HOIL-1. Cells lacking SHARPIN or HOIL-1 are sensitized to apoptosis as well as necroptosis (20, 68), and mice lacking these components develop systemic autoinflammatory diseases (20–22, 69). Interestingly, the severe skin and multi-organ inflammation in SHARPIN-deficient cpdm mice was corrected by crosses to “knock-in” mice expressing kinase inactive RIPK1 (Ripk1-K45A) (70). Fibroblasts and macrophages from RIPK1-KI mice exhibit normal MAP kinase and NF-κB responses, but are resistant to TNF-induced necroptosis (70, 71). Hence, excessive cell death appears to be the major driver for RIPK1-dependent inflammation in cpdm mice. However, since the kinase activity of RIPK1 is required for apoptosis under certain conditions (72), it remains to be determined if RIPK1-induced inflammation in cpdm mice is driven by apoptosis or necroptosis.
In addition to mouse models, human patients with mutations in the E3 ligase subunit of LUBAC HOIL-1 exhibit chronic inflammation, increased cytokine expression in response to IL-1β, cardiomyopathy, and susceptibility to pyogenic bacteria due to impaired NF-κB activation (73, 74). Consistent with the key role for the LUBAC complex for recruitment of the IKK complex, mutations in the IKK regulatory subunit NEMO/IKKγ cause Incontinentia Pigmenti (IP), a disease marked by skin lesions and multi-organ inflammation. Because Nemo is an X-linked gene, male mice lacking NEMO are embryonic lethal (75–77), and male patients of IP are rarely found. In the few rare cases of male patients harboring mild mutations in NEMO, patients develop a variant form of the disease called Hypohidrotic ectodermal dysplasia (HED), which is marked by abnormalities in the teeth, hair and eccrine sweat glands (78). The chronic inflammatory phenotypes caused by mutations in the cIAPs, LUBAC and NEMO seem to contradict with the fact that cells lacking these adaptors are impaired in cytokine-induced NF-κB responses. Since cells lacking these components in the ubiquitin network are also highly sensitive to death signals, heightened cell death is likely the driver of the chronic inflammation. It will be interesting to determine if the kinase function of RIPK1 is similarly responsible for driving the lethal inflammatory disease of Nemo−/− mice as in cpdm mice (70).
The NF-κB transcriptional target A20 and the tumor suppressor cylindromatosis (CYLD) are believed to facilitate Complex I transition to Complex II by promoting de-ubiquitination of RIPK1. Although both A20 and CYLD are recruited to Complex I, siRNA knock-down of CYLD, but not A20, protects cells against TNF-induced necroptosis (68, 79, 80). Surprisingly, RIPK1 ubiquitination in Complex I was not altered in Cyld−/− cells. Rather, RIPK1 and RIPK3 ubiquitination within the necrosome was greatly elevated in Cyld−/− cells (80). Hence, rather than regulating RIPK1 ubiquitination in Complex I, CYLD acts within the necrosome to de-ubiquitinate RIPK1 and RIPK3. Moreover, these results suggest that in addition to regulating NF-κB activation within Complex I, the E3 ligases cIAP1 and cIAP2 may also control necrosome activation through ubiqutination of RIPK1 and RIPK3.
The Janus nature of RIPK1
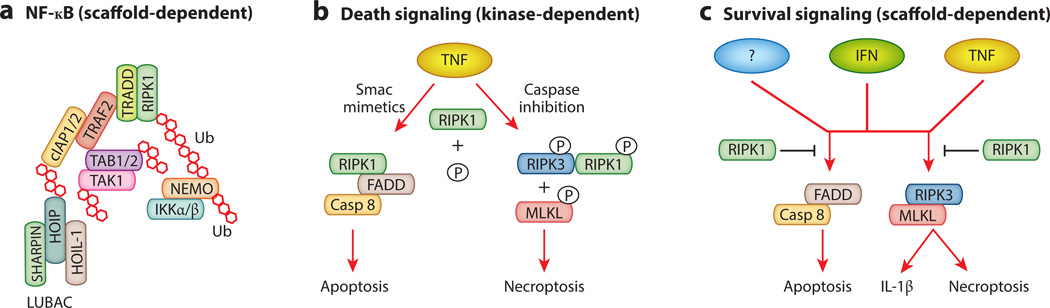
Besides acting as the upstream activator of TNF-induced, RIPK3-mediated necroptosis, RIPK1 is also required for Smac mimetic-primed, TNF-induced apoptosis (72). Paradoxically, RIPK1 also functions as an inhibitor of RIPK3- and caspase 8-mediated cell injury and inflammation. Ripk1−/− mice suffer from perinatal lethality that was originally believed to be due to defective NF-κB-mediated induction of survival genes (81). However, Ripk1−/− mice are born alive while RelA−/− mice die in utero at e15.5. In addition, a recent report argues that RIPK1 does not have a significant role in NF-κB activation (82). These results suggest that defective NF-κB activity may not fully account for the perinatal lethality of Ripk1−/− mice. Mice that lack multiple cellular IAPs, such as ciap1−/− Xiap−/− mice, suffered from embryonic lethality at E10.5. The lethality at E10.5 is eerily similar mice lacking Fadd, caspase 8 or cFlip. Strikingly, hemizygous Ripk1 deficiency significantly prolonged survival of ciap1−/− Xiap−/− mice till weaning age (17). Thus, an alternative model has been proposed that RIPK1 ubiquitination sterically hinders recruitment of downstream cell death effectors (54, 55).
Recently, the molecular basis that underlies the perinatal lethality of Ripk1−/− mice was examined in further details. Ripk1−/− mice exhibit extensive cleaved caspase 3 in multiple tissues and systemic increase in inflammatory cytokines. The increase in caspase 3 activation and apoptosis appears to be partly due to failure to upregulate cFLIPL expression (83). Cleaved caspase 3 and apoptosis was significantly reduced in Ripk1−/−Casp8−/− mice. However, the Ripk1−/−Casp8−/− mice still succumbed to perinatal lethality (84), indicating that apoptosis is not the only driver for the lethal phenotype. Deletion of Ripk3 also had minimal effect on survival of Ripk1−/− mice (83). However, Ripk1−/−Ripk3−/−Casp8−/− mice survived till adulthood and developed an lpr-like autoimmune disease that is also observed in Ripk3−/−Casp8−/− and Ripk3−/−Fadd−/− mice (83–85). Tissue-specific deletion of Ripk1 and bone marrow reconstitution experiments show that RIPK1 is essential for survival of hematopoietic stem cells, skin keratinocytes and intestinal epithelial cells (84, 86, 87). Inactivation of TNFR1 and IFN receptor significantly increased survival of Ripk1−/− mice (83), suggesting that RIPK1 inhibits TNFR1- and IFN-induced cell death in multiple cell types (Fig. 2). Surprisingly, “knock-in” mice expressing kinase inactive RIPK1 are viable and do not exhibit the abnormalities found in Ripk1−/− mice (70, 71). Hence, while its kinase activity promotes cell death through apoptosis and necroptosis, RIPK1 has a separate scaffolding function that curbs the death signals emanating from multiple innate immune and death receptors (Fig. 2).
Figure 2. RIPK1 mediates cell survival and cell death through distinct mechanisms.
(A) RIPK1 facilitates assembly of the ubiquitin scaffold that stimulates NF-κB activation. This function does not require the kinase activity of RIPK1. (B) The kinase activity of RIPK1 promotes apoptosis and necroptosis. (C) The scaffolding function of RIPK1 promotes survival and suppresses inflammation by inhibiting FADD-Caspase 8 and RIPK3-MLKL activation. This RIPK1 function is required to neutralize deleterious signals from interferon receptor, TNF receptor and other yet to be identified receptors. The kinase activity of RIPK1 is dispensable for this survival function.
RHIM-mediated amyloid conversion in necroptosis
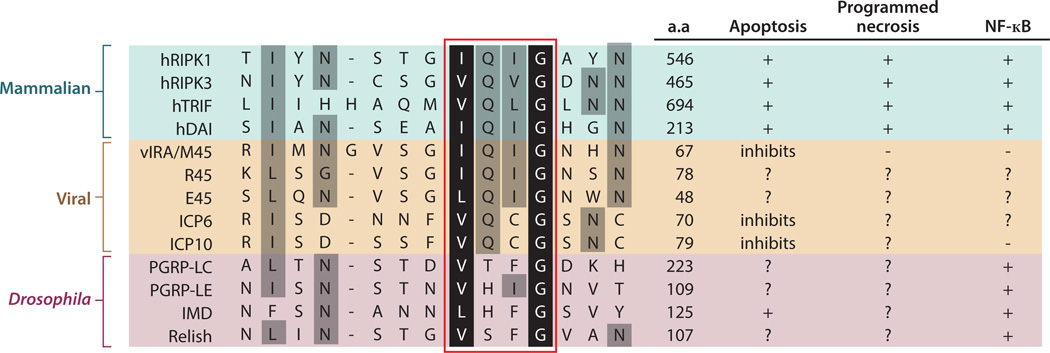
In addition to avoiding caspase 8-mediated cleavage and de-ubiquitination by CYLD, induction of necroptosis also requires “RIP homotypic interaction motif” (RHIM)-mediated interaction between RIPK1 and RIPK3 (88, 89). The RHIM is defined by a highly conserved tetra-peptide core flanked by hydrophobic residues that predominantly form β-sheet (Fig. 3). RHIM-like adaptors are found in viruses and in Drosophila, arguing for a critical role for RHIM-mediated interaction immunity throughout evolution (see below). Strikingly, RHIM-containing adaptors exhibit strong propensity to adopt an amyloid-like conformation either alone or in complex with another RHIM-containing adaptor. This unique structural scaffold is important for signaling. In the case of RIPK1 and RIPK3, disruption of this amyloid scaffold severely impairs autophosphorylation and activation of RIPK1 and RIPK3, and downstream execution of necroptosis (32).
Figure 3. The RHIM is a conserved signaling motif in innate immune and death signaling adaptors.
Sequence alignment of mammalian (human), viral and Drosophila RHIM-containing adaptors. R45 and E45 are the M45 homologues in the Maastricht and English isolates of rat CMV, respectively. ICP6 and ICP10 are M45 homologues of herpes simplex virus 1 (HSV-1) and HSV-2 respectively. Although all the viral RHIM adaptors encode a ribonucleotide reductase domain, not all of them are active enzymes (146). The Drosophila “RHIM-like” adaptors and receptors are included for comparison, although there is no evidence currently to indicate that they function like the mammalian RHIMs. Other than the PGRPs, the Drosophila IMD and Relish also contain RHIM-like motifs (A. Kleino and N. Silverman, personal communication). The red box indicates the tetra-peptide core of the RHIM. The black and grey shades represent highly conserved and moderately conserved residues as defined by functional side chains. a.a. = amino acid position of the last residue shown in the sequence alignment.
Although RHIM-mediated interaction is essential for RIPK1 and RIPK3 dependent necroptosis downstream of TNFR1, it is noteworthy that not all RHIM-mediated interactions lead to cell death. For example, TRIF and RIPK1 interact via their respective RHIM to mediate NF-κB activation downstream of TLR3 or TLR4 (90, 91). By contrast, RIPK3 inhibits this response, apparently through disruption of RHIM-RHIM interaction between or RIPK1 and TRIF. Similarly, the murine cytomegalovirus (MCMV) necrosis inhibitor M45/vIRA inhibits premature necroptosis in infected cells by binding to RIPK3 and preventing it from interacting with its partner DAI (92, 93) (see below). Since all these RHIM-containing adaptors have the propensity to form amyloid fibrils in vitro (32), it will be vital to determine whether amyloid conversion also occurs in situations that do not result in cell death.
Amyloid complexes are widely perceived to be the etiological agents for age-related dementia such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). The neurological pathologies in these diseases are often marked by necrosis and inflammation. Recent evidence indicates that signaling through the NLRP3 inflammasome drives the disease pathology in AD (94, 95). Interestingly, the NLRP3 inflammasome is activated by agonists that are aggregate in nature including silica, uric acid crystals and Aβ peptides. This raises the tantalizing possibility that the RIPK1-RIPK3 amyloid fibrils may stimulate neuronal injury and inflammation through activating the NLRP3 inflammasome. Alternatively, released RIPK1-RIPK3 amyloid fibrils from dying neurons may seed the amyloid conversion of pathologenic amyloid proteins such as Aβ, α-synuclein or tau. RIPK1 has been implicated in several models of ischemia-reperfusion induced brain injury (96, 97). It will be interesting to determine if there is any interaction between the RIPK1-RIPK3 complex with any of the neurotoxic amyloid-like peptides and to determine whether the RIP kinases contribute to age-related neuro-degeneration.
Non-canonical necrosomes
As we have alluded to in the previous section, the RHIM is also found in the TLR3/4 adaptor TIR domain-containing adaptor inducing interferon-beta (TRIF) and the DNA activator of interferon (DAI). A common link for the mammalian RHIM-containing adaptors is that they share functions in innate immune and/or cell death signaling (Fig. 3).
In the presence of caspase inhibition, TLR3 and TLR4 stimulation causes necroptosis mediated by TRIF and RIPK3 (98, 99). Similar to RIPK1 and RIPK3, TRIF is a cleavage substrate of caspase 8. TRIF cleavage by caspase 8 inhibits its ability to stimulate NF-κB-dependent cytokine gene expression (100). However, it is not known whether caspase 8 cleavage of TRIF also inhibits necroptosis. TRIF-dependent necroptosis requires binding to RIPK3 via the RHIM. In contrast to TRIF and RIPK3, the role of RIPK1 in TLR3- and TLR4-induced necroptosis is enigmatic. The RIPK1 inhibitor Nec-1 inhibited TLR3- and TLR4-induced necroptosis in primary bone marrow derived macrophages, the macrophage cell line J774, and to a lesser extent the endothelial cell line SVEC4-10 (98, 99). However, Ripk1−/− fibroblasts or siRNA knock-down of RIPK1 in 3T3 fibroblasts and SVEC4-10 did not rescue TLR3-induced necroptosis. Since Nec-1 was able to enhance survival of TLR3- and TLR4-induced necroptosis in J774 macrophages with silenced expression of RIPK1 (99), the protection conferred by Nec-1 might be due to off-target effects (101, 102).
Unlike RIPK1, TRIF does not possess kinase activity. This implies that the mechanism by which TRIF activates RIPK3 is different from that used by RIPK1. We therefore propose the term “non-canonical” necrosome to distinguish pro-necrotic RIPK3 complexes that do not contain RIPK1. In addition to TRIF, RIPK3 can also partner with DAI to induce necroptosis during MCMV infection (see below). Canonical necrosome activation requires RIPK1-dependent phosphorylation of RIPK3 at specific sites including Ser199, Ser357 and Ser358 (33, 103). Will these modifications also be required for non-canonical necrosome activation? If they are, what are the kinases that mediate these events in the absence of RIPK1? These are some of the questions that will need to be addressed in the future.
Necroptosis is controlled by phosphorylation
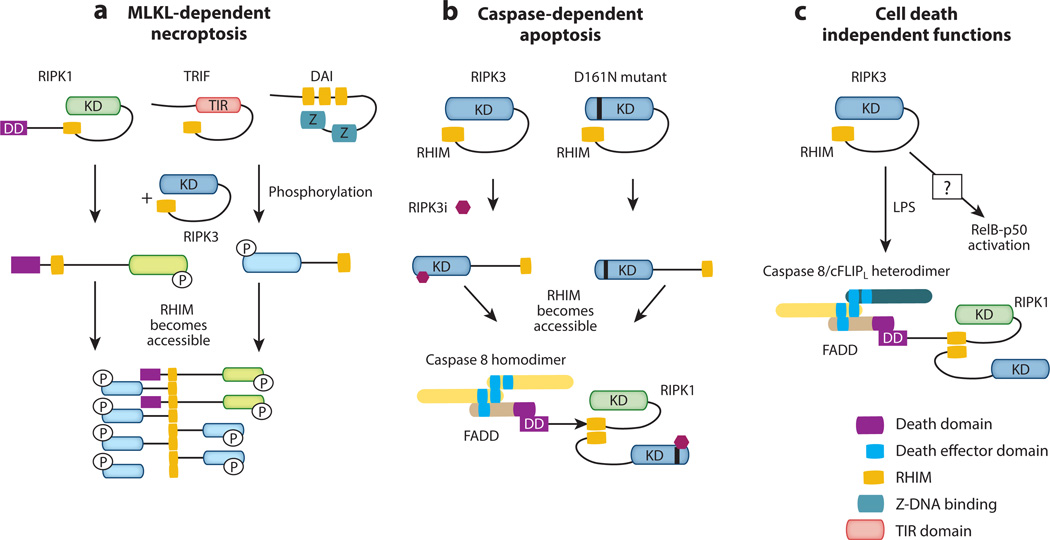
Both RIPK1 and RIPK3 are heavily phosphorylated in the necrosome. Mass spectrometry analyses have identified multiple phosphorylation sites on RIPK1 and RIPK3 (33, 104), with the majority of these phosphorylation sites localized within the amino-terminal kinase domains. Interestingly, expression of truncated RIPK1 or RIPK3 lacking the kinase domain, but not full-length proteins, results in spontaneous formation of amyloid fibrils. Since alanine substitutions of individual serine or threonine residues on RIPK1 had little effects on RIPK1 kinase activity and TNF-induced necroptosis (103), these results are most consistent with a model in which the kinase domain inhibits RIP kinase activation by masking the RHIM. In this model, phosphorylation of RIPK1 in the kinase domain will alter the conformation of the kinase, perhaps through charge repulsion, to allow RHIM-mediated interaction with downstream signal adaptors (Fig. 4A).
Figure 4. RIPK3 signals for cell death and inflammation through diverse mechanisms.
(A) RIPK3 mediates necroptosis by binding to RIPK1 or other RHIM-containing adaptors. This causes amyloid conversion of RIPK3, which serves as a platform for docking and recruitment of the RIPK3 substrate MLKL. (B) Binding of RIPK3 kinase inhibitor or introduction of the D161N mutation causes a conformational change that promotes a different form of RHIM-mediated interaction between RIPK1 and RIPK3 that leads to FADD and caspase 8 binding and apoptosis. (C) Although the mechanisms are yet to be defined, RIPK3 can also induce pro-IL-1β processing through caspase 1 and caspase 8. In over-expression studies, RIPK3 has also been shown to either enhance or inhibit NF-κB signaling.
Although it is widely accepted that RIPK1 is the upstream kinase that activates RIPK3, there is evidence that RIPK3 can signal for necroptosis independent of RIPK1. For example, inducible dimerization of RIPK3 drives RHIM- and MLKL-dependent necroptosis independent of RIPK1 (105–107). These results argue that the major function of RIPK1 is to initiate the nucleation event for RIPK3 oligomerization. Indeed, the phosphomimetic mouse RIPK3 mutant S204D (S199D in human RIPK3) restored TNF-induced necroptosis in Ripk3−/− fibroblasts that is no longer sensitive to inhibition by the RIPK1 kinase inhibitor necrostatin-1 or siRNA knock-down of RIPK1 (103). Moreover, over-expression of RIPK3 also leads to TNF-induced necroptosis that is independent of RIPK1 (108). Since RIPK3 expression is highly inducible by different activation signals (8, 89), the result from RIPK3 over-expression suggests that TNF-induced necroptosis can indeed proceed under certain physiological conditions. Since the current model predicates that RIPK1 is essential for recruitment and activation of RIPK3, these results do raise questions about the veracity of the traditional Complex I to Complex II transition model downstream of TNFR1 signaling.
In addition to Ser204, phosphorylation of RIPK3 at Ser227 was reported to mediate MLKL binding (33). Although alanine substitution at this residue abrogated RIPK3 function (33), a phosphomimetic mutant was unable to restore TNF-induced necroptosis (103). Therefore, the negative charge that results from phosphorylation of RIPK3 at Ser227 is not crucial for MLKL recruitment. Rather, Ser227 phosphorylation may convert RIPK3 into a “permissive” conformation to interact with MLKL. This type of conformation-sensitive interaction involving RIPK3 is also found in the kinase inactive RIPK3 mutant D161N. Mice and cells that express RIPK3-D161N undergo apoptosis due to assembly of an alternative caspase 8 activating complex that contains RIPK1, RIPK3-D161N, FADD and caspase 8 (71). However, not all kinase inactive mutants of RIPK3 drive assembly of this apoptosis-inducing complex. For example, expression of RIPK3-K51A and RIPK3-D143N are not toxic to cells. Surprisingly, high doses of RIPK3-specific kinase inhibitors can drive assembly of this apoptosis complex in cells that express wild type RIPK3 or the kinase inactive RIPK3 mutants K51A or D143N (manuscript under review) (Fig. 4B). Because an intact RHIM is also required to drive assembly of this caspase 8 activating complex, RHIM-mediated interaction alone is not sufficient to determine the cell death mode. Additional factors such as differences in conformation or recruitment of distinct adaptors are likely important in determining the cell death module being activated.
RIP kinases, NF-κB activation and IL-1β
The receptors that induce necroptosis are also potent inducers of the pro-inflammatory transcription factor NF-κB. NF-κB induces expression of pro-survival genes such as cFLIP and cIAPs and hence is generally considered to be a mutually exclusive signaling outcome from apoptosis or necroptosis. However, this is not always the case. For example, activated T cells upregulate expression of TNFR2 and are highly sensitive to cell death signals (109, 110). The sensitization to cell death can be recapitulated in Jurkat T cell leukemia by expression of TNFR2. Under these conditions, enhanced TNF-induced apoptosis or necroptosis was accompanied by strong NF-κB activation (63). In addition, Smac mimetics, which sensitize cells to death cytokines, also cause non-canonical NF-κB activation (56, 57, 111). Hence, NF-κB and necroptosis can synergize with each other to maximize the inflammatory response to stress signals.
To further highlight the cross-talk between necroptosis and inflammation signaling, both RIPK1 and RIPK3 can promote NF-κB activation. As we have already discussed, RIPK1 facilitates NF-κB downstream of TNFR1 and other innate immune receptors such as TLR3 and TLR4 (112, 113). Because of its homology to RIPK1, early studies on RIPK3 also focused on its ability to modulate NF-κB signaling. Over-expression of RIPK3 either stimulates or inhibits NF-κB activation in a context-dependent manner (91, 114–117). However, embryonic fibroblasts and macrophages from Ripk3−/− mice were normal for TNF- and TLR4-induced IκBα phosphorylation and degradation, and cytokine expression was unaffected (89, 118). Although these results suggests that RIPK3 is not a core component of the NF-κB pathway, we found that RIPK3 can indeed modulate NF-κB signaling, especially that of RelB and p50, in certain dendritic cell subsets (manuscript under review) (Fig. 4C). Taken together, these results indicate that RIPK1 and RIPK3 can promote inflammation in vivo through necrosis-dependent and independent mechanisms.
Besides necroptosis and NF-κB activation, RIPK3 has also been implicated to facilitate pro-IL-1β processing in macrophages and dendritic cells. IL-1β is an innate inflammatory cytokine that requires NF-κB-dependent de novo synthesis as well as cleavage and maturation by caspase 1. Caspase 1 cleavage of pro-IL-1β happens as a result of activation of a macro-molecular complex termed the inflammasome, which consists of a sensor such as AIM2 or NLRP3, the adaptor ASC-1 and caspase 1. This basic signaling scheme is eerily similar to that used by death receptors, suggesting that the apoptosis and inflammasome signaling pathways share common evolutionary ancestry. Although the necroptosis signaling pathway does not utilize similar signaling scheme, the necrosome and the inflammasome both require the assembly of higher order filamentous complex for activation. For the AIM2 and NLRP3 inflammasomes, cryoelectron microscopy revealed that activation of the inflammasome sensor causes a nucleation reaction driven by the pyrin domain of the adaptor ASC, leading to multimerization of caspase 1 and formation of an elongated, filamentous complex (119). This prion-like property again highlights the potential link between cell death, inflammation and neuro-degeneration. This multimerization model of caspase 1 activation contrasts that of the widely accepted model of proximity-induced dimerization apoptosis-inducing caspases (120). However, it is noteworthy that more recent work reveals that oligomerization is also important for caspase 8 activation by TNFR-like death receptors (121, 122). Interestingly, although the filamentous inflammasome complex is not amyloid in nature, it apparently can “seed” further polymerization reaction in neighboring cells as it is released from cells undergoing pyroptosis (123, 124). Higher order oligomerization appears to be an emerging theme in innate and cell death signaling, since other intracellular pattern recognition receptors/sensors including RIG-I and MAVS are also activated by similar polymerization mechanisms (125–127).
In addition to caspase 1-associated inflammasome, pro-IL-1β can also be processed by caspase 8 in certain situations (128–133). For example, the chemotherapeutic agent doxorubicin exclusively induces caspase 8-mediated pro-IL-1β processing in bone marrow derived DCs (BMDCs) (130). Moreover, in LPS-primed macrophages that lack cIAP1, cIAP2 and XIAP, pro-IL-1β processing was mediated through caspase 1 and caspase 8 in a RIPK3-dependent manner (134). The mechanism by which RIPK3 promotes IL-1β processing is unclear at present. As we have discussed earlier, RIPK3 inhibitors and the kinase inactive RIPK3 mutant D161N can drive formation of an alternative caspase 8 activating complex. Could a similar complex be involved in caspase 8-mediated pro-IL-1β processing (Fig. 4C)? In the case of the D161N mutant, this complex promotes apoptosis. However, if caspase 8 is paired with its inhibitor cFLIPL, this complex may no longer promote apoptosis but instead facilitate pro-IL-1β processing. This model is consistent with published report that caspase 8/cFLIPL heterodimer exhibits altered substrate specificity compared with caspase 8 homodimer (28). Because these effects are manifested when the cIAPs are depleted, the IAPs are crucial gatekeepers of RIPK3 activity in cell death and inflammation.
In addition to caspase 8, RIPK3 can also promote caspase 1-mediated pro-IL-1β processing. Fadd−/− and caspase 8−/− macrophages and dendritic cells produced greatly elevated levels of IL-1β that was reversed by deletion of Ripk3 (135, 136). However, there is disagreement on whether the enhanced IL-1β production was due to increased necrosis-associated release of DAMPs or direct effects of RIPK3 on caspase 1 activation. Regardless of the mechanism, it is clear that RIPK3 can promote caspase 1- and caspase 8-mediated pro-IL-1β processing via distinct mechanisms.
The necrosis-independent effects of RIPK1 and RIPK3 on NF-κB and pro-IL-1β processing illustrates an important principle: that the RIP kinases facilitate inflammation through multiple means. It also reinforces the notion that death-signaling adaptors often have important functions beyond cell death. The multi-faceted nature of death-inducing adaptors is not a novel concept. FADD, for example, has been implicated to regulate cell cycle entry (137–139), and caspases have important functions in cell differentiation, wound repair and pruning of neuronal dendrites (140–142). The diverse functions of RIPK3 remind us that inhibition of necroptosis is not the only possible explanation why Ripk3−/− mice often show protection in many inflammatory disease models.
Necroptosis in viral infections
Because of the release of DAMPs that can stimulate pattern recognition receptors such as TLRs, necroptosis is widely recognized to be beneficial in innate immune responses against pathogens. However, studies also show that necrosis-dependent inflammation can lead to detrimental pathology in sterile injury-induced diseases (Table 1). Given the fact that caspase inhibition is a priming signal for necroptosis, perhaps it is not surprising that viruses that encode caspase inhibitors are susceptible to host cell necroptosis (143). Poxviruses are master evaders of the host cell death machinery. In the case of vaccinia virus, the viral serpin Spi2/B13R is a potent inhibitor of caspase 1 and caspase 8. As in the case of most pathogens, vaccinia virus infection causes an early wave of TNF expression, which triggers RIPK1/RIPK3-dependent necroptosis in different infected tissues (64, 89). In vitro experiments confirmed that while wild type cells infected with vaccinia virus were sensitized to TNF-induced cytotoxicity, infected Ripk1−/− and Ripk3−/− cells were highly resistant to TNF-induced necroptosis (64, 89). Ripk3−/− mice had reduced necrosis and inflammation in infected tissues, and ultimately succumbed to the infection due to failure to control viral replication. In agreement with these results, mice expressing a kinase inactive RIPK1 (D138N) were also partially impaired in clearance of vaccinia virus (144). These results established necroptosis as an important anti-viral response against certain viral pathogens. This innate immune defense mechanism may be important to tamp down viral replication before robust virus-specific T cell responses are mobilized (Fig. 5A).
Table 1.
Necroptosis-related diseases.
| Cause of cell injury | Disease | Model | Reference |
|---|---|---|---|
| Virus infection | vaccinia virus | Ripk3−/− mice and Ripk1-D138N mice | 64,89,144 |
| MCMV | Ripk3−/− mice and DAI−/− mice | 92,93 | |
| Bacterial infection | Mycobacterium tuberculosis | zebrafish | 155 |
| Salmonella enterica serovar Typhimurium | Ripk3−/− mice and macrophages; necrostatin-1 | 154 | |
| Sterile injury-induced inflammation | psoriasis | keratinocyte-specific deletion of Fadd or casp8; cpdm mice | 46,70,184 |
| Inflammatory bowel disease | intestinal epithelium-specific deletion of Fadd or casp8 | 7,45 | |
| myocardial infarction | necrostatin-1 | 167 | |
| hypoxia-ischemia induced brain injury | necrostatin-1 | 96 | |
| ischemia-reperfusion kidney injury | necrostatin-1 and Ripk3−/− mice | 158,185 | |
| Retinal degeneration | retinal detachment; interphotoreceptor retinoid-binding protein-deficient mice; poly(I:C)-induced retina injury; rd10 mice; pde6c mutant zebrafish | 172–175 | |
| Pancreatitis | Cerulein-induced pancreatitis in Ripk3−/− mice | 150,165,186 | |
| atherosclerosis | Ripk3 deletion in Apolipoprotein E or low-density lipoprotein cholesterol receptor deficient mice | 171 | |
| Gaucher's disease | conduritol B epoxide inhibition of gluocerebrosidase (Gba) in Ripk3−/− mice | 166 |
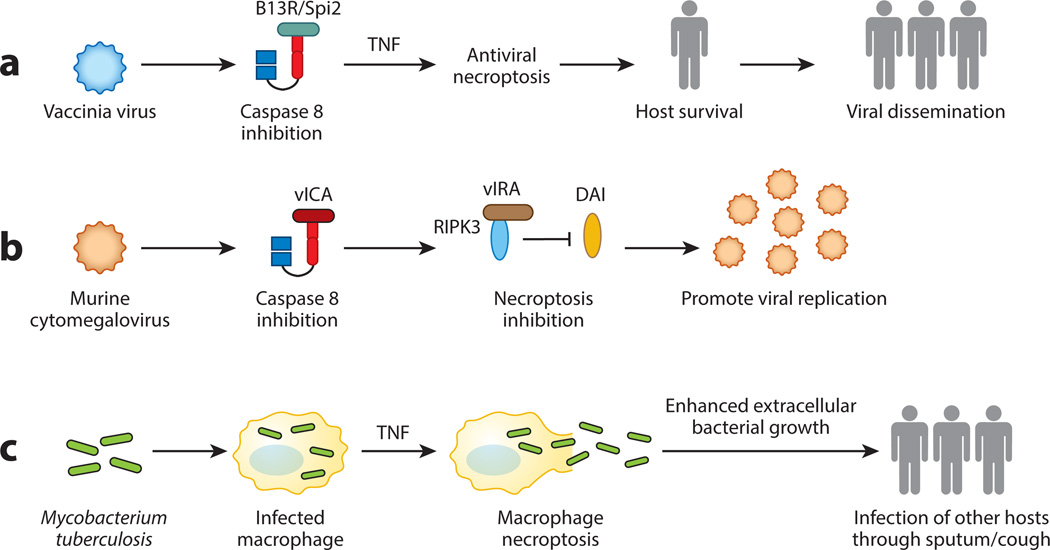
Figure 5. Necroptosis in host-pathogen interaction.
(A) Vaccinia virus inhibits caspase 8 via the viral inhibitor B13R/Spi2. This primes the cells towards necroptosis. Although necroptosis and the ensuing inflammation have anti-viral effects, it may in fact promote viral dissemination to another host by avoiding premature death of the infected host. (B) MCMV inhibits caspase 8 and necroptosis via the vICA and vIRA. Genetic experiments show that vIRA is essential to prevent premature death of the infected cells. Hence, vIRA-mediated necroptosis inhibition is important for the virus to complete its replication cycle and to generate more viral progenies. (C) Mycobacterium tuberculosis uses RIPK3-dependent necroptosis to release the bacteria into growth permissive environment, which in turn enhances spread of the pathogen to uninfected hosts via the sputum.
The results from vaccinia virus are surprising because they seem to indicate that by blocking caspase activation, viruses set themselves up for destruction by the host. Because ablation of necroptosis led to rapid death of the host, blocking necroptosis may actually deprive the virus the opportunity to disseminate and infect another host. From this perspective, one can argue that necroptosis is beneficial not only to the host, but also the invading virus. Moreover, it is noteworthy that when compared to other naturally occurring poxviruses, vaccinia virus contains large deleted gene segments (145). Thus, a tantalizing possibility is that the gene that inhibits necroptosis is lost in vaccinia virus due to these gene deletions. In this scenario, triggering of necroptosis may be an exception rather than a rule for vaccinia virus. To this end, it will be important to determine if other poxviruses also causes infected cells to undergo necroptosis.
Viral inhibition of apoptosis is widely perceived to give the virus an edge in its struggle with the host. Because of its anti-viral effects, it stands to reason that some viruses may also have developed strategies to counteract necroptosis. Herpeviruses are highly adept at countering the host cell death machinery. Murine cytomegalovirus (MCMV) encodes several viral cell death inhibitors, one of which is the viral caspase inhibitor (vICA). Because inhibition of caspase 8 is a priming signal for necroptosis, one would expect that cells infected with MCMV would become susceptible to necroptosis. However, MCMV-infected cells are spared from necroptosis because the virus also encodes the viral inhibitor of RIPK activation (vIRA), the product of the M45 gene. M45/vIRA is a RHIM-containing viral cell death inhibitor that binds to RIPK3 to prevent virus-induced necroptosis (93, 143). Recombinant MCMV expressing a tetra-alanine substitution RHIM mutant of vIRA fails to inhibit RIPK3 and succumbs to rapid necrosis. Because of this premature cell death, the mutant virus fails to establish productive infection in cells and in mice. Productive infection was re-established with the mutant virus in Ripk3−/− cells and Ripk3−/− mice (92). In contrast to RIPK3, TNF signaling and RIPK1 are both dispensable for mutant MCMV-induced necroptosis. Instead, RIPK3 interacts with another RHIM-containing adaptor DAI to form a non-canonical necrosome that drives virus-induced necrosis (Fig. 5B). M45 encodes a ribonucleotide reductase (RNR) with no enzymatic activity. Interestingly, many RNRs from other herpesviruses also encode a RHIM (Fig. 3) (146). This suggests that viral inhibitors that target the RIP kinases via the RHIM are a common viral immune evasion strategy for herpesviruses. The results from vaccinia virus and MCMV highlight the importance of necroptosis in acute viral infections. Yet, questions still remain whether necroptosis can influence the quality and magnitude of adaptive immune responses, generation of immunological memory, and viral latency.
Bacterial and parasitic infections
TNF is a major driver of bacterial sepsis, a life-threatening condition marked by systemic cytokine storm and multi-organ failure. In agreement with the idea that RIP kinase-dependent necroptosis promotes damaging inflammation, Ripk3−/− mice are resistant to TNF-induced systemic inflammatory syndrome (SIRS) (147, 148). In contrast to RIPK3, the role of RIPK1 in TNF-induced SIRS is more controversial. While several reports show that mice expressing kinase inactive RIPK1 or wild type mice treated with RIPK1 kinase inhibitors are protected from TNF-induced SIRS (70, 148) (149), another report found that necrostatin-1 exacerbates the disease (147). Furthermore, the response of Ripk3−/− and Mlkl−/− mice against cecal ligation and puncture (CLP)-induced sepsis is also variable (148, 150). Since Ripk3−/− mice and Ripk3−/− macrophages exhibit normal response to LPS (unpublished observation) (118), RIPK1 and RIPK3 likely have minor roles in acute bacterial sepsis.
Although the role of the RIP kinases in LPS-induced responses is ambiguous, they are nonetheless crucial in controlling certain bacterial pathogens. Yersinia pestis, the etiological agent of the “Black Death” pandemic, causes rapid RIPK1- and caspase 8-dependent macrophage apoptosis. As in TNF and SM induced apoptosis, Y. pestis induced macrophage apoptosis requires intact RIPK1 kinase activity. In addition, RIPK1 is required for inflammatory cytokine production in response to Y. pestis infection (151, 152). However, RIPK3 appears to have minimal role in Y. pestis infection. Salmonella enterica, a flagellated Gram-negative bacterium, is also a potent inducer of macrophage cell death. While it is widely accepted that macrophage cell death induced by Salmonella is caused by inflammasome activation and caspase 1-mediated pyroptosis (153), a recent report argues that RIPK3-dependent necroptosis is also involved (154). These discrepant conclusions could be reconciled by the fact that RIPK3 can also modulate inflammasome and caspase 1 activation (134, 136).
Host control of Mycobacterium tuberculosis (Mtb) critically requires TNF. One of the major protective functions of TNF is to promote granuloma formation, which is thought to be crucial in containment of the bacteria. Using zebrafish as model, Roca and Ramankrishnan show that RIPK1 and RIPK3 are both required to trigger TNF-induced ROS production and necroptosis in response to tuberculosis infection (155). Although necroptosis of infected macrophages initially inhibits bacterial growth, it later enhances bacteria growth as they are released into the growth permissive extracellular environment. Hence, unlike the situation with vaccinia virus and MCMV, one can view Mtb as a pathogen that hijacks the host necroptosis machinery to promote its growth and dissemination (Fig. 5C). Consistent with this thesis, necrosis is often associated with severe Mtb infection (156).
Mechanistically, TNF-induced necroptosis in Mtb-infected macrophages requires mitochondrial cyclophilin D (CypD) and acid sphingomyelinase-induced ceramide production. Ceramide has long been implicated in death receptor induced apoptosis. However, its role in mammalian cell necroptosis has yet to be thoroughly tested. CypD is an inner mitochondrial protein and an important component of the mitochondria permeability transition pore. CypD is required for certain forms of necrosis, such as that induced by calcium and ROS (157). CypD and RIPK3 act in synergy to mediate acute kidney injury in an ischemia-reperfusion model (158). However, CypD deficiency did not rescue excessive necroptosis of Caspase 8−/− T cells (47). Moreover, widespread elimination of mitochondria through induced mitophagy did not alter cellular response to TNF-induced necroptosis (42). Hence, rather than being a core component of the necroptosis machinery, the CypD pathway appears to be uniquely involved in Mtb-induced necroptosis. It will be interesting to determine whether similar mechanisms involving RIPK1, RIPK3, CypD and ceramide are involved in immune defense against Mtb infections in mammals.
Parasitic diseases such as Malaria and Leishmaniasis target red blood cells, leading to anemia, hemolysis and bleeding in some cases. These symptoms are caused by red blood cell lysis, which releases cell-free hemoglobin into the circulation. Oxidation of hemoglobin releases heme to trigger the Fenton reaction and generation of highly reactive oxygen radicals. The oxidative stress drives lipid and protein peroxidation, DNA damage and other insults to the cell (159). Free heme greatly sensitizes hepatocytes to TNF-induced apoptosis in Plasmodium infection (160). In addition to hepatocytes, marcophages are also highly susceptible to heme-induced cytotoxicity. Through a poorly defined mechanism, heme directly activates TLR4, leading to autocrine TNF and ROS production, which synergize with each other to induce RIPK1- and RIPK3-dependent necroptosis (161). Through inducing macrophage necroptosis, RIPK1 and RIPK3 may restrict the niche within which parasites can replicate. In vivo infections will be required to validate the biological role of necroptosis in parasitic infections.
Necroptosis in sterile inflammation
Besides pathogen infections, necrosis is also a hallmark of acute and chronic sterile inflammation. In agreement with its induced expression in response to acute and chronic exposure to alcohol, Ripk3−/− mice are protected from alcoholic liver disease (162). Moreover, Ripk3−/− mice were protected from acetaminophen-induced liver injury (163), and elevated phospho-MLKL signals were detected in drug-induced liver diseases (37). Repeated doses of cerulein led to a biphasic cell death reaction in the acinar cells that is partially dependent on TNF, RIPK3 and MLKL (164). As such, Ripk3−/− and Mlkl−/− mice are partially protected from cerulein-induced acute pancreatitis (150, 165). RIPK3 deficiency also improves the neurological manifestation of Gaucher’s disease, a lysosomal storage disease caused by mutations in glucocerebrosidase (166).
Necroptosis appears to be an important mechanism of cell injury in ischemia-reperfusion (IR)-induced tissue injury. As we have already discussed, RIPK3-dependent necroptosis is partially responsible for IR-induced kidney injury (158). The RIPK1 kinase inhibitor necrostatin-1 (Nec-1) is effective in alleviating hypoxia-ischemia induced oxidative brain injury and inflammation in neonatal mice (96). Nec-1 also reduced mouse and rat models of IR-induced myocardial cell death and infarct formation (167). However, in a model of permanent left anterior descending coronary artery ligation, the resulting inflammation and tissue remodeling were impaired in Ripk3−/− mice (168). Since Nec-1 has been shown to exhibit off-target effects (101, 169), the results obtained with Nec-1 need to be interpreted with caution since. As mice expressing kinase inactive RIPK1 have recently been generated (70, 71), they will be useful in further dissecting the kinase-dependent necroptotic signaling versus scaffold-dependent non-necroptotic signaling in these disease models.
In addition to drug- and trauma-induced tissue injury and inflammation, RIPK3-dependent necroptosis also contributes to chronic inflammatory diseases such as atherosclerosis. Macrophage necrosis is widely viewed as a key factor in atherosclerotic plaque formation (170). Mice deficient in apolipoprotein E or low-density lipoprotein receptor (LDL-R) that are fed with high-fat diet developed atherosclerosis marked by macrophage necrosis in the atherosclerotic plaques. Strikingly, RIPK3 deletion ameliorates macrophage necrosis in the plaques and atherosclerosis in ApoE−/− and Ldl-r−/− mice (171). Given that Ripk3−/− macrophages are resistant to oxidized LDL-induced necroptosis, these results strongly suggest that RIPK3-dependent macrophage necroptosis is a direct driver of atherosclerotic plaque formation. Finally, RIPK3-dependent necroptosis has also been shown to be causative of mouse models of retinal injury (172–175). These examples point to the emerging role of the RIP kinases in diverse inflammatory diseases. However, one needs to consider both necroptosis-dependent and independent effects of the RIP kinases when interpretating these results.
Evolutionary perspectives
Studies in Caenorhabditis elegans and Drosophila melanogaster have contributed greatly to our knowledge of apoptosis signaling mechanisms. The conservation of apoptosis machinery through evolution illustrates its importance in the maintenance of organismal homeostasis. Is the mammalian necroptosis pathway also conserved in C. elegans and Drosophila? Interestingly, RHIM-like adaptors are found in Drosophila (Fig. 3). In response to Gram-negative bacteria, the innate immune receptors peptidoglycan recognition protein (PGRP) PGRP-LC and PGRP-LE stimulate anti-microbial peptide expression through the Immune deficiency (IMD), a RIPK1-like adaptor, and Relish, a Drosophila NF-κB (176). The tetra-peptide core sequences of Drosophila RHIM-containing adaptors differ from those in the mammalian RHIMs (Fig. 3). Mutations of the RHIM-like motif in PGRP-LC and PGRP-LE compromised anti-microbial peptide expression in response to peptidoglycan stimulation (177), indicating that these variant RHIMs are functional. The structural similarity between mammalian and Drosophila RHIM adaptors argues that they may have evolved from a common primordial pathway. Although the PGRP-IMD-Relish pathway is generally not known to promote cell death, over-expression of IMD has been shown to result in cell death. Interestingly, IMD-induced cell death was only partially rescued by the caspase inhibitor p35 (178), suggesting the possibility non-apoptotic cell death may be involved.
In addition to the IMD pathway, transgenic over-expression of the Drosophila TNF ortholog Eiger in the developing eye primordium led to Jnk-dependent necrosis-like cell death (179). Interestingly, suppression of genes involved in glycolysis and mitochondrial respiration inhibits Eiger-induced cell death (179). The apoptosis protease activating factor 1 (Apaf1) interacts with caspase 9 and cytochrome c to form the apoptosome, a macromolecular structure essential for mitochondria-mediated apoptosis. Surprisingly, Apaf1 hypomorph mutant also exhibits progressive wing cell necrosis, which triggers a systemic inflammatory response, wasting, and expression of anti-microbial peptides (180). It is interesting that in both Eiger and Apaf1 mediated necrosis, the cell death phenotype is associated with changes in energy metabolism. This is in contrast to mammalian necroptosis, which does not require Jnk or the mitochondria (42, 101). Other pathways of non-apoptotic cell death have recently been described in Drosophila Nurse cells (181) and the developing neuroblasts (182). It will be interesting to determine if similar principles that govern mammalian necroptosis are conserved in these situations.
Closing thoughts
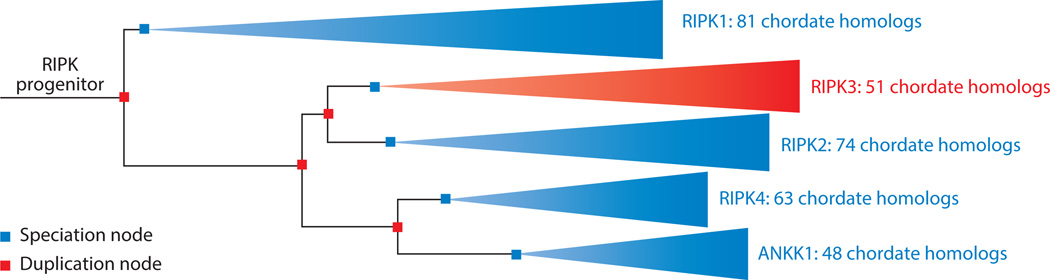
In considering the molecular machineries that control necroptosis and its roles in different diseases, perhaps it will be helpful to step back and ponder why evolution has preserved this unique cell death module. Phylogenetic analysis indicates that modern day RIP kinases evolved through a series of gene duplication events. The relatively short branch between RIPK1 and the RIPK progenitor suggests that RIPK1 is probably the most ancient RIPK (Fig. 6). This evolutionary model is appealing as RIPK1 is the only RIP kinase that is crucial for embryonic survival and beyond. Although the Drosophila IMD was once thought to be a RIPK1 ortholog (178), it shares homology only in the death domain and lacks the essential kinase domain. The absence of RIP-like kinases in lower organisms such as Drosophila or C elegans argues that they are relatively novel products of evolution. The earliest example of RIPK1-like kinases is found in bony fish (Fig. 6). How are we supposed to make sense of this? One possible explanation is that necroptosis is the product of co-evolution with certain viruses that target vertebrates. The strongest support for this argument comes from the examples of vaccinia virus and MCMV. According to this model, sterile injury-induced necroptosis is the price we pay in this evolutionary struggle with viruses. This is appealing since necroptosis tends to associate with detrimental pathologies in sterile inflammation. Study in more viruses will be crucial to validate this hypothesis.
Figure 6. The RIPK gene family evolved through a series of gene duplication events.
The reconstructed phylogeny was generated by the Ensembl genome browser (183). Internal nodes correspond to key speciation (purple) and gene duplication (red) events. Branch lengths correspond to rates of evolutionary change. ANKK1 (ankyrin repeat and protein kinase domain-containing protein 1) does not function as a RIP kinase but is closely related to RIPK4 and other RIPKs.
Summary points.
The receptors that stimulate programmed necrosis or necroptosis are also apoptosis inducers.
Necroptosis is regulated by RIPK3 and MLKL.
Protein phosphorylation, ubiquitination and FADD/caspase-8 mediated proteolytic processing are the three major post-translational mechanisms that control the induction of necroptosis.
RIPK3 promotes inflammation through necroptosis, NF-κB activation and caspase 1/8-mediated pro-IL-β maturation.
RIP kinase-dependent necroptosis contributes to pathogen-induced and sterile inflammation.
Necroptosis can promote or suppress anti-viral immune responses in a pathogen-specific manner.
The existence of viral inhibitors of necroptosis argues for an important role for necroptosis in host-pathogen interaction.
Acknowledgement
We thank N. Silverman, D. Caffrey and R. Siegel for discussion and critical reading of the manuscript, and many other colleagues for discussion and ideas. We apologize to our colleagues whose work we cannot cite due to space limitation. This work is supported by NIH grant AI083497 (F.K-M.C).
Literature cited
- 1.Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344:1164–1168. doi: 10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- 2.Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harb Perspect Biol. 2013;5:a008748. doi: 10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawai H, Domae N. Discrimination between primary necrosis and apoptosis by necrostatin-1 in Annexin V-positive/propidium iodide-negative cells. Biochem Biophys Res Commun. 2011;411:569–573. doi: 10.1016/j.bbrc.2011.06.186. [DOI] [PubMed] [Google Scholar]
- 5.Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K, Saito T. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol. 2008;9:1179–1188. doi: 10.1038/ni.1651. [DOI] [PubMed] [Google Scholar]
- 6.Sancho D, Joffre OP, Keller AM, Rogers NC, Martinez D, Hernanz-Falcon P, Rosewell I, Reis e Sousa C. Identification of a dendritic cell receptor that couples sensing of necrosis to immunity. Nature. 2009;458:899–903. doi: 10.1038/nature07750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunther C, Martini E, Wittkopf N, Amann K, Weigmann B, Neumann H, Waldner MJ, Hedrick SM, Tenzer S, Neurath MF, Becker C. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moriwaki K, Chan FK. RIP3: a molecular switch for necrosis and inflammation. Genes Dev. 2013;27:1640–1649. doi: 10.1101/gad.223321.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sosna J, Voigt S, Mathieu S, Lange A, Thon L, Davarnia P, Herdegen T, Linkermann A, Rittger A, Chan FK, Kabelitz D, Schutze S, Adam D. TNF-induced necroptosis and PARP-1-mediated necrosis represent distinct routes to programmed necrotic cell death. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1381-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fu D, Jordan JJ, Samson LD. Human ALKBH7 is required for alkylation and oxidation-induced programmed necrosis. Genes Dev. 2013;27:1089–1100. doi: 10.1101/gad.215533.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thapa RJ, Nogusa S, Chen P, Maki JL, Lerro A, Andrake M, Rall GF, Degterev A, Balachandran S. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proc Natl Acad Sci U S A. 2013;110:E3109–E3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chan FK, Chun HJ, Zheng L, Siegel RM, Bui KL, Lenardo MJ. A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science. 2000;288:2351–2354. doi: 10.1126/science.288.5475.2351. [DOI] [PubMed] [Google Scholar]
- 13.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 14.Rieser E, Cordier SM, Walczak H. Linear ubiquitination: a newly discovered regulator of cell signalling. Trends Biochem Sci. 2013;38:94–102. doi: 10.1016/j.tibs.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Papa S, Zazzeroni F, Bubici C, Jayawardena S, Alvarez K, Matsuda S, Nguyen DU, Pham CG, Nelsbach AH, Melis T, De Smaele E, Tang WJ, D'Adamio L, Franzoso G. Gadd45 beta mediates the NF-kappa B suppression of JNK signalling by targeting MKK7/JNKK2. Nat Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- 16.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moulin M, Anderton H, Voss AK, Thomas T, Wong WW, Bankovacki A, Feltham R, Chau D, Cook WD, Silke J, Vaux DL. IAPs limit activation of RIP kinases by TNF receptor 1 during development. Embo Journal. 2012;31:1679–1691. doi: 10.1038/emboj.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csomos RA, Brady GF, Duckett CS. Enhanced cytoprotective effects of the inhibitor of apoptosis protein cellular IAP1 through stabilization with TRAF2. J Biol Chem. 2009;284:20531–20539. doi: 10.1074/jbc.M109.029983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, de la Pompa JL, Ferrick D, Hum B, Iscove N, Ohashi P, Rothe M, Goeddel DV, Mak TW. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda F, Deribe YL, Skanland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, van Wijk SJ, Goswami P, Nagy V, Terzic J, Tokunaga F, Androulidaki A, Nakagawa T, Pasparakis M, Iwai K, Sundberg JP, Schaefer L, Rittinger K, Macek B, Dikic I. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tokunaga F, Nakagawa T, Nakahara M, Saeki Y, Taniguchi M, Sakata S, Tanaka K, Nakano H, Iwai K. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 22.Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WW, Nachbur U, Gangoda L, Warnken U, Purcell AW, Silke J, Walczak H. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 23.Harper N, Hughes M, MacFarlane M, Cohen GM. Fas-associated death domain protein and caspase-8 are not recruited to the tumor necrosis factor receptor 1 signaling complex during tumor necrosis factor-induced apoptosis. J Biol Chem. 2003;278:25534–25541. doi: 10.1074/jbc.M303399200. [DOI] [PubMed] [Google Scholar]
- 24.Schneider-Brachert W, Tchikov V, Neumeyer J, Jakob M, Winoto-Morbach S, Held-Feindt J, Heinrich M, Merkel O, Ehrenschwender M, Adam D, Mentlein R, Kabelitz D, Schutze S. Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity. 2004;21:415–428. doi: 10.1016/j.immuni.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 25.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, Castanares M, Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 27.Oberst A, Dillon CP, Weinlich R, McCormick LL, Fitzgerald P, Pop C, Hakem R, Salvesen GS, Green DR. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pop C, Oberst A, Drag M, Van Raam BJ, Riedl SJ, Green DR, Salvesen GS. FLIP(L) induces caspase 8 activity in the absence of interdomain caspase 8 cleavage and alters substrate specificity. Biochem J. 2011;433:447–457. doi: 10.1042/BJ20101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, Green DR, Ting AT. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–1442. doi: 10.1038/ncb2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenev T, Bianchi K, Darding M, Broemer M, Langlais C, Wallberg F, Zachariou A, Lopez J, MacFarlane M, Cain K, Meier P. The Ripoptosome, a signaling platform that assembles in response to genotoxic stress and loss of IAPs. Molecular Cell. 2011;43:432–448. doi: 10.1016/j.molcel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Feoktistova M, Geserick P, Kellert B, Dimitrova DP, Langlais C, Hupe M, Cain K, MacFarlane M, Hacker G, Leverkus M. cIAPs block Ripoptosome formation, a RIP1/caspase-8 containing intracellular cell death complex differentially regulated by cFLIP isoforms. Molecular Cell. 2011;43:449–463. doi: 10.1016/j.molcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK, Wu H. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 2012;150:339–350. doi: 10.1016/j.cell.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 34.Zhao J, Jitkaew S, Cai Z, Choksi S, Li Q, Luo J, Liu ZG. Mixed lineage kinase domain-like is a key receptor interacting protein 3 downstream component of TNF-induced necrosis. Proc Natl Acad Sci U S A. 2012;109:5322–5327. doi: 10.1073/pnas.1200012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai Z, Jitkaew S, Zhao J, Chiang HC, Choksi S, Liu J, Ward Y, Wu LG, Liu ZG. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Li W, Ren J, Huang D, He WT, Song Y, Yang C, Li W, Zheng X, Chen P, Han J. Translocation of mixed lineage kinase domain-like protein to plasma membrane leads to necrotic cell death. Cell Res. 2014;24:105–121. doi: 10.1038/cr.2013.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Dondelinger Y, Declercq W, Montessuit S, Roelandt R, Goncalves A, Bruggeman I, Hulpiau P, Weber K, Sehon CA, Marquis RW, Bertin J, Gough PJ, Savvides S, Martinou JC, Bertrand MJ, Vandenabeele P. MLKL Compromises Plasma Membrane Integrity by Binding to Phosphatidylinositol Phosphates. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 39.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 40.Murphy JM, Czabotar PE, Hildebrand JM, Lucet IS, Zhang JG, Alvarez-Diaz S, Lewis R, Lalaoui N, Metcalf D, Webb AI, Young SN, Varghese LN, Tannahill GM, Hatchell EC, Majewski IJ, Okamoto T, Dobson RC, Hilton DJ, Babon JJ, Nicola NA, Strasser A, Silke J, Alexander WS. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 41.Remijsen Q, Goossens V, Grootjans S, Van den Haute C, Vanlangenakker N, Dondelinger Y, Roelandt R, Bruggeman I, Goncalves A, Bertrand MJ, Baekelandt V, Takahashi N, Berghe TV, Vandenabeele P. Depletion of RIPK3 or MLKL blocks TNF-driven necroptosis and switches towards a delayed RIPK1 kinase-dependent apoptosis. Cell Death Dis. 2014;5:e1004. doi: 10.1038/cddis.2013.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tait SW, Oberst A, Quarato G, Milasta S, Haller M, Wang R, Karvela M, Ichim G, Yatim N, Albert ML, Kidd G, Wakefield R, Frase S, Krautwald S, Linkermann A, Green DR. Widespread mitochondrial depletion via mitophagy does not compromise necroptosis. Cell Rep. 2013;5:878–885. doi: 10.1016/j.celrep.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaiser WJ, Upton JW, Long AB, Livingston-Rosanoff D, Daley-Bauer LP, Hakem R, Caspary T, Mocarski ES. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Zhou X, McQuade T, Li J, Chan FK, Zhang J. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernandez-Majada V, Ermolaeva M, Kirsch P, Sterner-Kock A, van Loo G, Pasparakis M. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 46.Bonnet MC, Preukschat D, Welz PS, van Loo G, Ermolaeva MA, Bloch W, Haase I, Pasparakis M. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Ch'en IL, Tsau JS, Molkentin JD, Komatsu M, Hedrick SM. Mechanisms of necroptosis in T cells. J Exp Med. 2011 doi: 10.1084/jem.20110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu JV, Weist BM, van Raam BJ, Marro BS, Nguyen LV, Srinivas P, Bell BD, Luhrs KA, Lane TE, Salvesen GS, Walsh CM. Complementary roles of Fas-associated death domain (FADD) and receptor interacting protein kinase-3 (RIPK3) in T-cell homeostasis and antiviral immunity. Proc Natl Acad Sci U S A. 2011;108:15312–15317. doi: 10.1073/pnas.1102779108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 50.Dillon CP, Oberst A, Weinlich R, Janke LJ, Kang TB, Ben-Moshe T, Mak TW, Wallach D, Green DR. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity. 2000;12:301–311. doi: 10.1016/s1074-7613(00)80183-1. [DOI] [PubMed] [Google Scholar]
- 52.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 53.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 54.O'Donnell MA, Hase H, Legarda D, Ting AT. NEMO inhibits programmed necrosis in an NFkappaB-independent manner by restraining RIP1. Plos One. 2012;7:e41238. doi: 10.1371/journal.pone.0041238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.O'Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, Zobel K, Dynek JN, Elliott LO, Wallweber HJ, Flygare JA, Fairbrother WJ, Deshayes K, Dixit VM, Vucic D. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 57.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 58.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, Gillard JW, Jaquith JB, Morris SJ, Barker PA. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Dueber EC, Schoeffler AJ, Lingel A, Elliott JM, Fedorova AV, Giannetti AM, Zobel K, Maurer B, Varfolomeev E, Wu P, Wallweber HJ, Hymowitz SG, Deshayes K, Vucic D, Fairbrother WJ. Antagonists induce a conformational change in cIAP1 that promotes autoubiquitination. Science. 2011;334:376–380. doi: 10.1126/science.1207862. [DOI] [PubMed] [Google Scholar]
- 60.Petersen SL, Peyton M, Minna JD, Wang X. Overcoming cancer cell resistance to Smac mimetic induced apoptosis by modulating cIAP-2 expression. Proc Natl Acad Sci U S A. 2010;107:11936–11941. doi: 10.1073/pnas.1005667107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chromik J, Safferthal C, Serve H, Fulda S. Smac mimetic primes apoptosis-resistant acute myeloid leukaemia cells for cytarabine-induced cell death by triggering necroptosis. Cancer Lett. 2014;344:101–109. doi: 10.1016/j.canlet.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 62.Kim EY, Teh HS. Critical role of TNF receptor type-2 (p75) as a costimulator for IL-2 induction and T cell survival: a functional link to CD28. J Immunol. 2004;173:4500–4509. doi: 10.4049/jimmunol.173.7.4500. [DOI] [PubMed] [Google Scholar]
- 63.Chan FK, Lenardo MJ. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 64.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, Orenstein J, Moss B, Lenardo MJ. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 65.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 66.Fotin-Mleczek M, Henkler F, Samel D, Reichwein M, Hausser A, Parmryd I, Scheurich P, Schmid JA, Wajant H. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757–2770. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- 67.Wong WW, Vince JE, Lalaoui N, Lawlor KE, Chau D, Bankovacki A, Anderton H, Metcalf D, O'Reilly L, Jost PJ, Murphy JM, Alexander WS, Strasser A, Vaux DL, Silke J. cIAPs and XIAP regulate myelopoiesis through cytokine production in an RIPK1- and RIPK3-dependent manner. Blood. 2014;123:2562–2572. doi: 10.1182/blood-2013-06-510743. [DOI] [PubMed] [Google Scholar]
- 68.Vanlangenakker N, Vanden Berghe T, Bogaert P, Laukens B, Zobel K, Deshayes K, Vucic D, Fulda S, Vandenabeele P, Bertrand MJ. cIAP1 and TAK1 protect cells from TNF-induced necrosis by preventing RIP1/RIP3-dependent reactive oxygen species production. Cell Death Differ. 2011;18:656–665. doi: 10.1038/cdd.2010.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tokunaga F, Sakata S, Saeki Y, Satomi Y, Kirisako T, Kamei K, Nakagawa T, Kato M, Murata S, Yamaoka S, Yamamoto M, Akira S, Takao T, Tanaka K, Iwai K. Involvement of linear polyubiquitylation of NEMO in NF-kappaB activation. Nat Cell Biol. 2009;11:123–132. doi: 10.1038/ncb1821. [DOI] [PubMed] [Google Scholar]
- 70.Berger S, Kasparcova V, Hoffman S, Swift B, Dare L, Schaeffer M, Capriotti C, Cook M, Finger J, Hughes-Earle A, Harris P, Kaiser WJ, Mocarski ES, Bertin J, Gough P. Cutting Edge: RIP1 Kinase Activity is Dispensable for Normal Development but is a Key Regulator of Inflammation in SHARPIN-Deficient Mice. Journal of Immunology. 2014 doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Newton K, Dugger D, Wickliffe KE, Kapoor N, de-Almagro C, Vucic D, Komuves L, Ferrando RE, French DM, Webster J, Roose-Girma M, Warming S, Dixit V. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014 doi: 10.1126/science.1249361. this issue. [DOI] [PubMed] [Google Scholar]
- 72.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 73.Boisson B, Laplantine E, Prando C, Giliani S, Israelsson E, Xu Z, Abhyankar A, Israel L, Trevejo-Nunez G, Bogunovic D, Cepika AM, MacDuff D, Chrabieh M, Hubeau M, Bajolle F, Debre M, Mazzolari E, Vairo D, Agou F, Virgin HW, Bossuyt X, Rambaud C, Facchetti F, Bonnet D, Quartier P, Fournet JC, Pascual V, Chaussabel D, Notarangelo LD, Puel A, Israel A, Casanova JL, Picard C. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat Immunol. 2012;13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nilsson J, Schoser B, Laforet P, Kalev O, Lindberg C, Romero NB, Davila Lopez M, Akman HO, Wahbi K, Iglseder S, Eggers C, Engel AG, Dimauro S, Oldfors A. Polyglucosan body myopathy caused by defective ubiquitin ligase RBCK1. Ann Neurol. 2013;74:914–919. doi: 10.1002/ana.23963. [DOI] [PubMed] [Google Scholar]
- 75.Makris C, Godfrey VL, Krahn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, Feng L, Johnson RS, Karin M. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell. 2000;5:969–979. doi: 10.1016/s1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- 76.Rudolph D, Yeh WC, Wakeham A, Rudolph B, Nallainathan D, Potter J, Elia AJ, Mak TW. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt-Supprian M, Bloch W, Courtois G, Addicks K, Israel A, Rajewsky K, Pasparakis M. NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol Cell. 2000;5:981–992. doi: 10.1016/s1097-2765(00)80263-4. [DOI] [PubMed] [Google Scholar]
- 78.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, Bodemer C, Kenwrick S, Dupuis-Girod S, Blanche S, Wood P, Rabia SH, Headon DJ, Overbeek PA, Le Deist F, Holland SM, Belani K, Kumararatne DS, Fischer A, Shapiro R, Conley ME, Reimund E, Kalhoff H, Abinun M, Munnich A, Israel A, Courtois G, Casanova JL. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nat Genet. 2001;27:277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 79.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFalpha-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS One. 2013;8:e76841. doi: 10.1371/journal.pone.0076841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 82.Wong WW, Gentle IE, Nachbur U, Anderton H, Vaux DL, Silke J. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ. 2010;17:482–487. doi: 10.1038/cdd.2009.178. [DOI] [PubMed] [Google Scholar]
- 83.Dillon CP, Weinlich R, Rodriguez DA, Cripps JG, Quarato G, Gurung P, Verbist KC, Brewer TL, Llambi F, Gong YN, Janke LJ, Kelliher MA, Kanneganti TD, Green DR. RIPK1 Blocks Early Postnatal Lethality Mediated by Caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rickard JA, O'Donnell JA, Evans JM, Lalaoui N, Poh AR, Rogers T, Vince JE, Lawlor KE, Ninnis RL, Anderton H, Hall C, Spall SK, Phesse TJ, Abud HE, Cengia LH, Corbin J, Mifsud S, Di Rago L, Metcalf D, Ernst M, Dewson G, Roberts AW, Alexander WS, Murphy JM, Ekert PG, Masters SL, Vaux DL, Croker BA, Gerlic M, Silke J. RIPK1 Regulates RIPK3-MLKL-Driven Systemic Inflammation and Emergency Hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 85.Kaiser WJ, Daley-Bauer LP, Thapa RJ, Mandal P, Berger SB, Huang C, Sundararajan A, Guo H, Roback L, Speck SH, Bertin J, Gough PJ, Balachandran S, Mocarski ES. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takahashi N, Vereecke L, Bertrand MJ, Duprez L, Berger SB, Divert T, Goncalves A, Sze M, Gilbert B, Kourula S, Goossens L, Lefebvre S, Gunther C, Becker C, Bertin J, Gough PJ, Declercq W, van Loo G, Vandenabeele P. RIPK1 is a guardian of intestinal homeostasis protecting the epithelium against apoptosis. Nature. 2014 doi: 10.1038/nature13706. in press. [DOI] [PubMed] [Google Scholar]
- 87.Dannappel M, Vlantis K, Kumari S, Polykratis A, Kim C, Wachsmuth L, Eftychi C, Lin J, Corona T, Hermance N, Zelic M, Kirsch P, Basic M, Bleich A, Kelliher M, Pasparakis M. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014 doi: 10.1038/nature13608. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cho Y, Challa S, Chan FK. A RNA interference screen identifies RIP3 as an essential inducer of TNF-induced programmed necrosis. Adv Exp Med Biol. 2011;691:589–593. doi: 10.1007/978-1-4419-6612-4_62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 91.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-kappa B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 92.Upton JW, Kaiser WJ, Mocarski ES. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Upton JW, Kaiser WJ, Mocarski ES. DAI/ZBP1/DLM-1 complexes with RIP3 to mediate virus-induced programmed necrosis that is targeted by murine cytomegalovirus vIRA. Cell Host Microbe. 2012;11:290–297. doi: 10.1016/j.chom.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, Fitzgerald KA, Latz E, Moore KJ, Golenbock DT. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, Gelpi E, Halle A, Korte M, Latz E, Golenbock DT. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Northington FJ, Chavez-Valdez R, Graham EM, Razdan S, Gauda EB, Martin LJ. Necrostatin decreases oxidative damage, inflammation, and injury after neonatal HI. J Cereb Blood Flow Metab. 2011;31:178–189. doi: 10.1038/jcbfm.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chavez-Valdez R, Martin LJ, Flock DL, Northington FJ. Necrostatin-1 Attenuates Mitochondrial Dysfunction in Neurons and Astrocytes Following Neonatal Hypoxia-Ischemia. Neuroscience. 2012;219:192–203. doi: 10.1016/j.neuroscience.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He S, Liang Y, Shao F, Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3-mediated pathway. Proc Natl Acad Sci U S A. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaiser WJ, Sridharan H, Huang C, Mandal P, Upton JW, Gough PJ, Sehon CA, Marquis RW, Bertin J, Mocarski ES. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. J Biol Chem. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rebsamen M, Meylan E, Curran J, Tschopp J. The antiviral adaptor proteins Cardif and Trif are processed and inactivated by caspases. Cell Death Differ. 2008;15:1804–1811. doi: 10.1038/cdd.2008.119. [DOI] [PubMed] [Google Scholar]
- 101.Cho Y, McQuade T, Zhang HB, Zhang JK, Chan FKM. RIP1-Dependent and Independent Effects of Necrostatin-1 in Necrosis and T Cell Activation. Plos One. 2011:6. doi: 10.1371/journal.pone.0023209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takahashi N, Duprez L, Grootjans S, Cauwels A, Nerinckx W, DuHadaway JB, Goossens V, Roelandt R, Van Hauwermeiren F, Libert C, Declercq W, Callewaert N, Prendergast GC, Degterev A, Yuan J, Vandenabeele P. Necrostatin-1 analogues: critical issues on the specificity, activity and in vivo use in experimental disease models. Cell Death Dis. 2012;3:e437. doi: 10.1038/cddis.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McQuade T, Cho Y, Chan FK. Positive and negative phosphorylation regulates RIP1- and RIP3-induced programmed necrosis. Biochem J. 2013;456:409–415. doi: 10.1042/BJ20130860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y, Chen X, Shao J, Han J. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death and Differentiation. 2014 doi: 10.1038/cdd.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Orozco S, Yatim N, Werner MR, Tran H, Gunja SY, Tait SW, Albert ML, Green DR, Oberst A. RIPK1 both positively and negatively regulates RIPK3 oligomerization and necroptosis. Cell Death and Differentiation. 2014 doi: 10.1038/cdd.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cook WD, Moujalled DM, Ralph TJ, Lock P, Young SN, Murphy JM, Vaux DL. RIPK1- and RIPK3-induced cell death mode is determined by target availability. Cell Death and Differentiation. 2014 doi: 10.1038/cdd.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moujalled DM, Cook WD, Okamoto T, Murphy J, Lawlor KE, Vince JE, Vaux DL. TNF can activate RIPK3 and cause programmed necrosis in the absence of RIPK1. Cell Death Dis. 2013;4:e465. doi: 10.1038/cddis.2012.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 110.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 111.Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, Harran P, Wang X. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vivarelli MS, McDonald D, Miller M, Cusson N, Kelliher M, Geha RS. RIP links TLR4 to Akt and is essential for cell survival in response to LPS stimulation. J Exp Med. 2004;200:399–404. doi: 10.1084/jem.20040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cusson-Hermance N, Khurana S, Lee TH, Fitzgerald KA, Kelliher MA. Rip1 mediates the Trif-dependent toll-like receptor 3- and 4-induced NF-{kappa}B activation but does not contribute to interferon regulatory factor 3 activation. J Biol Chem. 2005;280:36560–36566. doi: 10.1074/jbc.M506831200. [DOI] [PubMed] [Google Scholar]
- 114.Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC. The RIP-like kinase, RIP3, induces apoptosis and NF-kappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 2000;473:285–291. doi: 10.1016/s0014-5793(00)01473-3. [DOI] [PubMed] [Google Scholar]
- 115.Yu PW, Huang BC, Shen M, Quast J, Chan E, Xu X, Nolan GP, Payan DG, Luo Y. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol. 1999;9:539–542. doi: 10.1016/s0960-9822(99)80239-5. [DOI] [PubMed] [Google Scholar]
- 116.Pazdernik NJ, Donner DB, Goebl MG, Harrington MA. Mouse receptor interacting protein 3 does not contain a caspase-recruiting or a death domain but induces apoptosis and activates NF-kappaB. Mol Cell Biol. 1999;19:6500–6508. doi: 10.1128/mcb.19.10.6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- 118.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NF-kappa Bs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schleich K, Warnken U, Fricker N, Ozturk S, Richter P, Kammerer K, Schnolzer M, Krammer PH, Lavrik IN. Stoichiometry of the CD95 death-inducing signaling complex: experimental and modeling evidence for a death effector domain chain model. Mol Cell. 2012;47:306–319. doi: 10.1016/j.molcel.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 122.Dickens LS, Boyd RS, Jukes-Jones R, Hughes MA, Robinson GL, Fairall L, Schwabe JW, Cain K, Macfarlane M. A death effector domain chain DISC model reveals a crucial role for caspase-8 chain assembly in mediating apoptotic cell death. Mol Cell. 2012;47:291–305. doi: 10.1016/j.molcel.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, Mangan MS, Zimmer S, Monks BG, Fricke M, Schmidt RE, Espevik T, Jones B, Jarnicki AG, Hansbro PM, Busto P, Marshak-Rothstein A, Hornemann S, Aguzzi A, Kastenmuller W, Latz E. The adaptor ASC has extracellular and 'prionoid' activities that propagate inflammation. Nat Immunol. 2014 doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, Bujan S, Couillin I, Brough D, Arostegui JI, Pelegrin P. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014 doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 125.Cai X, Chen J, Xu H, Liu S, Jiang QX, Halfmann R, Chen ZJ. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peisley A, Wu B, Yao H, Walz T, Hur S. RIG-I forms signaling-competent filaments in an ATP-dependent, ubiquitin-independent manner. Mol Cell. 2013;51:573–583. doi: 10.1016/j.molcel.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 127.Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, Hur S. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152:276–289. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 128.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. Journal of Experimental Medicine. 2008;205:1967–1973. doi: 10.1084/jem.20071632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gringhuis SI, Kaptein TM, Wevers BA, Theelen B, van der Vlist M, Boekhout T, Geijtenbeek TB. Dectin-1 is an extracellular pathogen sensor for the induction and processing of IL-1beta via a noncanonical caspase-8 inflammasome. Nature Immunology. 2012;13:246–254. doi: 10.1038/ni.2222. [DOI] [PubMed] [Google Scholar]
- 130.Antonopoulos C, El Sanadi C, Kaiser WJ, Mocarski ES, Dubyak GR. Proapoptotic chemotherapeutic drugs induce noncanonical processing and release of IL-1beta via caspase-8 in dendritic cells. J Immunol. 2013;191:4789–4803. doi: 10.4049/jimmunol.1300645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S, Fitzgerald P, Oberst A, Dillon CP, Green DR, Cerundolo V, Sher A. Cutting edge: Endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. Journal of Immunology. 2014;192:2029–2033. doi: 10.4049/jimmunol.1302549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, Bryant CE. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1beta production. Journal of Immunology. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gurung P, Anand PK, Malireddi RK, Vande Walle L, Van Opdenbosch N, Dillon CP, Weinlich R, Green DR, Lamkanfi M, Kanneganti TD. FADD and caspase-8 mediate priming and activation of the canonical and noncanonical Nlrp3 inflammasomes. Journal of Immunology. 2014;192:1835–1846. doi: 10.4049/jimmunol.1302839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vince JE, Wong WW, Gentle I, Lawlor KE, Allam R, O'Reilly L, Mason K, Gross O, Ma S, Guarda G, Anderton H, Castillo R, Hacker G, Silke J, Tschopp J. Inhibitor of apoptosis proteins limit RIP3 kinase-dependent interleukin-1 activation. Immunity. 2012;36:215–227. doi: 10.1016/j.immuni.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 135.Young DA, Hegen M, Ma HL, Whitters MJ, Albert LM, Lowe L, Senices M, Wu PW, Sibley B, Leathurby Y, Brown TP, Nickerson-Nutter C, Keith JC, Jr., Collins M. Blockade of the interleukin-21/interleukin-21 receptor pathway ameliorates disease in animal models of rheumatoid arthritis. Arthritis Rheum. 2007;56:1152–1163. doi: 10.1002/art.22452. [DOI] [PubMed] [Google Scholar]
- 136.Kang TB, Yang SH, Toth B, Kovalenko A, Wallach D. Caspase-8 blocks kinase RIPK3-mediated activation of the NLRP3 inflammasome. Immunity. 2013;38:27–40. doi: 10.1016/j.immuni.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 137.Alappat EC, Feig C, Boyerinas B, Volkland J, Samuels M, Murmann AE, Thorburn A, Kidd VJ, Slaughter CA, Osborn SL, Winoto A, Tang WJ, Peter ME. Phosphorylation of FADD at serine 194 by CKIalpha regulates its nonapoptotic activities. Mol Cell. 2005;19:321–332. doi: 10.1016/j.molcel.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 138.Hua ZC, Sohn SJ, Kang C, Cado D, Winoto A. A function of Fas-associated death domain protein in cell cycle progression localized to a single amino acid at its C-terminal region. Immunity. 2003;18:513–521. doi: 10.1016/s1074-7613(03)00083-9. [DOI] [PubMed] [Google Scholar]
- 139.Osborn SL, Sohn SJ, Winoto A. Constitutive phosphorylation mutation in Fas-associated death domain (FADD) results in early cell cycle defects. J Biol Chem. 2007;282:22786–22792. doi: 10.1074/jbc.M703163200. [DOI] [PubMed] [Google Scholar]
- 140.Zermati Y, Garrido C, Amsellem S, Fishelson S, Bouscary D, Valensi F, Varet B, Solary E, Hermine O. Caspase activation is required for terminal erythroid differentiation. J Exp Med. 2001;193:247–254. doi: 10.1084/jem.193.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee P, Lee DJ, Chan C, Chen SW, Ch'en I, Jamora C. Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature. 2009;458:519–523. doi: 10.1038/nature07687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hyman BT, Yuan J. Apoptotic and non-apoptotic roles of caspases in neuronal physiology and pathophysiology. Nat Rev Neurosci. 2012;13:395–406. doi: 10.1038/nrn3228. [DOI] [PubMed] [Google Scholar]
- 143.Upton JW, Chan FK. Staying alive: cell death in antiviral immunity. Molecular Cell. 2014;54:273–280. doi: 10.1016/j.molcel.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Polykratis A, Hermance N, Zelic M, Roderick J, Kim C, Van TM, Lee TH, Chan FK, Pasparakis M, Kelliher MA. Cutting Edge: RIPK1 Kinase Inactive Mice Are Viable and Protected from TNF-Induced Necroptosis In Vivo. J Immunol. 2014 doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kotwal GJ, Moss B. Analysis of a large cluster of nonessential genes deleted from a vaccinia virus terminal transposition mutant. Virology. 1988;167:524–537. [PubMed] [Google Scholar]
- 146.Lembo D, Brune W. Tinkering with a viral ribonucleotide reductase. Trends Biochem Sci. 2009;34:25–32. doi: 10.1016/j.tibs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 147.Linkermann A, Brasen JH, De Zen F, Weinlich R, Schwendener RA, Green DR, Kunzendorf U, Krautwald S. Dichotomy between RIP1- and RIP3-mediated necroptosis in tumor necrosis factor-alpha-induced shock. Mol Med. 2012;18:577–586. doi: 10.2119/molmed.2011.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Duprez L, Takahashi N, Van Hauwermeiren F, Vandendriessche B, Goossens V, Vanden Berghe T, Declercq W, Libert C, Cauwels A, Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 149.Harris PA, Bandyopadhyay D, Berger SB, Campobasso N, Capriotti CA, Cox JA, Dare L, Finger JN, Hoffman SJ, Kahler KM, Lehr R, Lich JD, Nagilla R, Nolte RT, Ouellette MT, Pao CS, Schaeffer MC, Smallwood A, Sun HH, Swift BA, Totoritis RD, Ward P, Marquis RW, Bertin J, Gough PJ. Discovery of Small Molecule RIP1 Kinase Inhibitors for the Treatment of Pathologies Associated with Necroptosis. ACS Med Chem Lett. 2013;4:1238–1243. doi: 10.1021/ml400382p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu J, Huang Z, Ren J, Zhang Z, He P, Li Y, Ma J, Chen W, Zhang Y, Zhou X, Yang Z, Wu SQ, Chen L, Han J. Mlkl knockout mice demonstrate the indispensable role of Mlkl in necroptosis. Cell Res. 2013;23:994–1006. doi: 10.1038/cr.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, Zwack EE, Hu B, Fitzgerald L, Mauldin EA, Copenhaver AM, Shin S, Wei L, Parker M, Zhang J, Oberst A, Green DR, Brodsky IE. Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc Natl Acad Sci U S A. 2014;111:7385–7390. doi: 10.1073/pnas.1403252111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, Kaiser WJ, Mocarski ES, Pouliot K, Chan FK, Kelliher MA, Harris PA, Bertin J, Gough PJ, Shayakhmetov DM, Goguen JD, Fitzgerald KA, Silverman N, Lien E. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Broz P, Ruby T, Belhocine K, Bouley DM, Kayagaki N, Dixit VM, Monack DM. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature. 2012;490:288–291. doi: 10.1038/nature11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Robinson N, McComb S, Mulligan R, Dudani R, Krishnan L, Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat Immunol. 2012;13:954–962. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roca FJ, Ramakrishnan L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell. 2013;153:521–534. doi: 10.1016/j.cell.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Divangahi M, Behar SM, Remold H. Dying to live: how the death modality of the infected macrophage affects immunity to tuberculosis. Adv Exp Med Biol. 2013;783:103–120. doi: 10.1007/978-1-4614-6111-1_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 158.Linkermann A, Brasen JH, Darding M, Jin MK, Sanz AB, Heller JO, De Zen F, Weinlich R, Ortiz A, Walczak H, Weinberg JM, Green DR, Kunzendorf U, Krautwald S. Two independent pathways of regulated necrosis mediate ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2013;110:12024–12029. doi: 10.1073/pnas.1305538110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chiabrando D, Vinchi F, Fiorito V, Mercurio S, Tolosano E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharmacol. 2014;5:61. doi: 10.3389/fphar.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seixas E, Gozzelino R, Chora A, Ferreira A, Silva G, Larsen R, Rebelo S, Penido C, Smith NR, Coutinho A, Soares MP. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc Natl Acad Sci U S A. 2009;106:15837–15842. doi: 10.1073/pnas.0903419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fortes GB, Alves LS, de Oliveira R, Dutra FF, Rodrigues D, Fernandez PL, Souto-Padron T, De Rosa MJ, Kelliher M, Golenbock D, Chan FK, Bozza MT. Heme induces programmed necrosis on macrophages through autocrine TNF and ROS production. Blood. 2012;119:2368–2375. doi: 10.1182/blood-2011-08-375303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Roychowdhury S, McMullen MR, Pisano SG, Liu X, Nagy LE. Absence of receptor interacting protein kinase 3 prevents ethanol-induced liver injury. Hepatology. 2013;57:1773–1783. doi: 10.1002/hep.26200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ramachandran A, McGill MR, Xie Y, Ni HM, Ding WX, Jaeschke H. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–2108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Malleo G, Mazzon E, Genovese T, Di Paola R, Muia C, Centorrino T, Siriwardena AK, Cuzzocrea S. Etanercept attenuates the development of cerulein-induced acute pancreatitis in mice: a comparison with TNF-alpha genetic deletion. Shock. 2007;27:542–551. doi: 10.1097/01.shk.0000246900.50445.1d. [DOI] [PubMed] [Google Scholar]
- 165.He S, Wang L, Miao L, Du F, Zhao L, Wang X. Receptor Interacting Protein Kinase-3 Determines Cellular Necrotic Response to TNF-α. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 166.Vitner EB, Salomon R, Farfel-Becker T, Meshcheriakova A, Ali M, Klein AD, Platt FM, Cox TM, Futerman AH. RIPK3 as a potential therapeutic target for Gaucher's disease. Nat Med. 2014;20:204–208. doi: 10.1038/nm.3449. [DOI] [PubMed] [Google Scholar]
- 167.Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 168.Luedde M, Lutz M, Carter N, Sosna J, Jacoby C, Vucur M, Gautheron J, Roderburg C, Borg N, Reisinger F, Hippe HJ, Linkermann A, Wolf MJ, Rose-John S, Lullmann-Rauch R, Adam D, Flogel U, Heikenwalder M, Luedde T, Frey N. RIP3, a kinase promoting necroptotic cell death, mediates adverse remodelling after myocardial infarction. Cardiovasc Res. 2014 doi: 10.1093/cvr/cvu146. [DOI] [PubMed] [Google Scholar]
- 169.Vandenabeele P, Grootjans S, Callewaert N, Takahashi N. Necrostatin-1 blocks both RIPK1 and IDO: consequences for the study of cell death in experimental disease models. Cell Death Differ. 2013;20:185–187. doi: 10.1038/cdd.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36–46. doi: 10.1038/nri2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lin J, Li H, Yang M, Ren J, Huang Z, Han F, Huang J, Ma J, Zhang D, Zhang Z, Wu J, Huang D, Qiao M, Jin G, Wu Q, Huang Y, Du J, Han J. A role of RIP3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 172.Trichonas G, Murakami Y, Thanos A, Morizane Y, Kayama M, Debouck CM, Hisatomi T, Miller JW, Vavvas DG. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1009179107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Murakami Y, Matsumoto H, Roh M, Suzuki J, Hisatomi T, Ikeda Y, Miller JW, Vavvas DG. Receptor interacting protein kinase mediates necrotic cone but not rod cell death in a mouse model of inherited degeneration. Proc Natl Acad Sci U S A. 2012;109:14598–14603. doi: 10.1073/pnas.1206937109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Murakami Y, Matsumoto H, Roh M, Giani A, Kataoka K, Morizane Y, Kayama M, Thanos A, Nakatake S, Notomi S, Hisatomi T, Ikeda Y, Ishibashi T, Connor KM, Miller JW, Vavvas DG. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2014;21:270–277. doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Viringipurampeer IA, Shan X, Gregory-Evans K, Zhang JP, Mohammadi Z, Gregory-Evans CY. Rip3 knockdown rescues photoreceptor cell death in blind pde6c zebrafish. Cell Death Differ. 2014;21:665–675. doi: 10.1038/cdd.2013.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kleino A, Silverman N. The Drosophila IMD pathway in the activation of the humoral immune response. Dev Comp Immunol. 2014;42:25–35. doi: 10.1016/j.dci.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH, Kurata S, Silverman N. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. doi: 10.1038/ni1356. [DOI] [PubMed] [Google Scholar]
- 178.Georgel P, Naitza S, Kappler C, Ferrandon D, Zachary D, Swimmer C, Kopczynski C, Duyk G, Reichhart JM, Hoffmann JA. Drosophila immune deficiency (IMD) is a death domain protein that activates antibacterial defense and can promote apoptosis. Dev Cell. 2001;1:503–514. doi: 10.1016/s1534-5807(01)00059-4. [DOI] [PubMed] [Google Scholar]
- 179.Kanda H, Igaki T, Okano H, Miura M. Conserved metabolic energy production pathways govern Eiger/TNF-induced nonapoptotic cell death. Proc Natl Acad Sci U S A. 2011;108:18977–18982. doi: 10.1073/pnas.1103242108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Obata F, Kuranaga E, Tomioka K, Ming M, Takeishi A, Chen CH, Soga T, Miura M. Necrosis-Driven Systemic Immune Response Alters SAM Metabolism through the FOXO-GNMT Axis. Cell Rep. 2014 doi: 10.1016/j.celrep.2014.03.046. [DOI] [PubMed] [Google Scholar]
- 181.Jenkins VK, Timmons AK, McCall K. Diversity of cell death pathways: insight from the fly ovary. Trends Cell Biol. 2013;23:567–574. doi: 10.1016/j.tcb.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kuang C, Golden KL, Simon CR, Damrath J, Buttitta L, Gamble CE, Lee CY. A novel Fizzy/Cdc20-dependent mechanism suppresses necrosis in neural stem cells. Development. 2014;141:1453–1464. doi: 10.1242/dev.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vilella AJ, Severin J, Ureta-Vidal A, Heng L, Durbin R, Birney E. EnsemblCompara GeneTrees: Complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 2009;19:327–335. doi: 10.1101/gr.073585.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. Journal of Experimental Medicine. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Linkermann A, Brasen JH, Himmerkus N, Liu S, Huber TB, Kunzendorf U, Krautwald S. Rip1 (receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012;81:751–761. doi: 10.1038/ki.2011.450. [DOI] [PubMed] [Google Scholar]
- 186.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an Energy Metabolism Regulator that Switches TNF-Induced Cell Death from Apoptosis to Necrosis. Science. 2009 doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]