Abstract
The majority of ovarian cancer patients acquire resistance to standard platinum chemotherapy and novel therapies to reduce tumor burden and ascites accumulation are needed. Pregnancy-associated plasma protein-A (PAPP-A) plays a key role in promoting insulin-like growth factor (IGF) pathway activity, which directly correlates to ovarian cancer cell transformation, growth and invasiveness. Herein, we evaluate PAPP-A expression in tumors and ascites of women with ovarian cancer, and determine the anti-tumor efficacy of a neutralizing monoclonal PAPP-A antibody (mAb-PA) in ovarian cancer using primary patient ovarian tumorgrafts (“Ovatars”).
PAPP-A mRNA expression in patient ovarian tumors correlated with poor outcome and was validated as a prognostic surrogate in Ovatar tumors. Following confirmation of mAb-PA bioavailability and target efficacy in vivo, the anti-tumor efficacy of mAb-PA in multiple Ovatar tumor models was examined and the response was found to depend on PAPP-A expression. Strikingly, the addition of mAb-PA to standard platinum chemotherapy effectively sensitized platinum-resistant Ovatar tumors. PAPP-A protein in ascites was also assessed in a large cohort of patients and very high levels were evident across the entire sample set. Therefore, we evaluated targeted PAPP-A inhibition as a novel approach to managing ovarian ascites, and found that mAb-PA inhibited the development, attenuated the progression and induced the regression of Ovatar ascites.
Together, these data indicate PAPP-A as a potential palliative and adjunct therapeutic target for women with ovarian cancer.
Keywords: Ovarian cancer, pregnancy-associated plasma protein-A, insulin-like growth factor, ascites, predictive biomarker, antibody therapy
INTRODUCTION
Epithelial ovarian cancer (OvCa) is the leading cause of death among gynecologic malignancies (1). While generally curable if detected at an early stage, the symptoms of OvCa are vague and patients often present with advanced disease. The current standard for OvCa includes primary surgical intervention to remove macroscopic disease burden (both solid tumor and any accompanying ascites) proceeded by a platinum-based chemotherapy regimen to abrogate any remaining microscopic deposits. Unfortunately, the majority of patients will relapse with platinum-resistant disease and develop intractable ascites, thereby contributing to the historically poor median overall survival rate. Thus, there is a critical need to identify novel targets and ways to predict response of the individual patient to new therapeutic agents in order to improve outcomes for women with this disease.
The role of insulin-like growth factors (IGFs) in the development and progression of a broad range of epithelial cancers, including OvCa, is well documented (2-4), and investigational compounds targeting the IGF axis have been developed for cancer therapeutics. In particular, monoclonal antibodies directed against the IGF-I receptor (IGF-IR), which transduces IGF-I and IGF-II signaling in cells, have been evaluated. Although conceptually sound, the clinical experience to date using these antibodies in unselected patients with various cancers has been largely disappointing. This can be explained by the broad-based activity of said antibodies (IGF-IR is ubiquitous and serves essential functions in normal tissues), lack of effect on IGF-II mitogenic signaling through insulin receptor isoform A (InsR-A), and secondary hormonal and metabolic derangements (3-7). Nevertheless, there remains the possible benefit to patient subgroups and, therefore, a need of predictive biomarkers to identify those patients who would be most likely to respond to specific IGF-targeted therapies. More importantly, alternative means of inhibiting IGF signaling to avoid the above side effects is desirable. We propose targeting pregnancy-associated plasma protein-A (PAPP-A).
PAPP-A is a novel metalloprotease of the metzincin superfamily (8). PAPP-A enhances IGF action through specific cleavage of inhibitory IGF binding proteins, primarily IGFBP-4, resulting in increased IGF bioavailability (reviewed in 9). The secreted protease is tethered to the surface of the secreting and neighboring cells thereby acting in an autocrine/paracrine manner to increase local IGF available for IGF-IR activation. It also has the potential to increase local IGF-II available for activation of InsR-A, an insulin receptor that is prevalent in tumor tissue and mediates a mitogenic signal (10). Conversely, therapeutic inhibition of PAPP-A proteolytic activity would effectively suppress both IGF-IR and InsR-A signaling, while insulin signaling through the classic InsR-B would not be affected by this approach. Thus, there are several advantages of targeting PAPP-A: it is a novel enzyme lending itself to specificity; it is present extracellularly and therefore accessible; its expression is both condition- and cell-specific and therefore selective; and its inhibition would suppress both IGF-I and IGF-II (but not insulin) signaling, thereby enhancing efficacy but limiting metabolic toxicity.
There are compelling data supporting PAPP-A inhibition of IGF signaling in OvCa as a viable therapeutic approach. Several ovarian cancer cell lines and primary cultures express IGF-IR and have been shown to be responsive to IGFs (11,12). In addition, IGF-II demonstrates mitogenic signaling through the InsR-A in human ovarian cancer cells (13). Wang et al. (14) found that an IGF-IR antibody inhibited OvCa tumor growth in a xenograft model, and Gest et al. (15) suggest a role for IGF signaling in ovarian cancer aggressiveness. Moreover, we have recently shown that a relatively non-tumorigenic OvCa cell line becomes highly tumorigenic upon overexpression of PAPP-A (16). Conversely, decreased PAPP-A expression was associated with lowered invasive potential and tumor growth rate of an OvCa cell line in vivo (17). Unfortunately, screening for PAPP-A expression in primary OvCa has been limited (18,19).
A substantial barrier to the study of OvCa is the paucity of translationally and clinically relevant models. The development of primary patient ovarian tumorgrafts (“Ovatars”), with availability of source patient biospecimens (germline DNA, serum, frozen and formalin-fixed paraffin-embedded tissue) and prospective clinical annotations, helps to overcome these hurdles. We have shown that intraperitoneal-derived Ovatars recapitulate patient tumor in terms of histologic, genomic, transcriptomic and therapeutic heterogeneity (20). Thus, Ovatars represent a practical medium to study the effects of novel targets in OvCa. Rather than selecting for clonal population of patient-derived cells able to grow in vitro, the generation of individualized orthotopic models allows for development and interaction of the tumor cells with the stroma in an environment similar to the source patient (20-22). As a result, experiments in Ovatars are more likely to produce clinically-relevant outcome parameters. To this end, we examined the potential role of PAPP-A as a prognostic surrogate of clinical outcome and predictive index of anti-PAPP-A targeted therapy in patient OvCa tumors and their respective Ovatars. Herein, we describe the efficacy of a novel PAPP-A neutralizing antibody to limit tumor growth, prevent ascites accumulation and reverse platinum resistance in Ovatars.
MATERIALS AND METHODS
Neutralizing PAPP-A monoclonal antibody (mAb-PA)
We have developed a high-affinity IgG monoclonal antibody against a substrate-binding exosite of PAPP-A required for proteolysis of IGFBP-4 (23). The development and characterization of this antibody and its effectiveness in inhibiting IGFBP-4 proteolysis and xenograft tumor growth has been published recently (24).
Ovatar model
The generation and expansion of viable ovarian tumor tissue obtained from consenting patients at the time of surgery has been described previously (20). Briefly, fresh patient tumor tissue was injected intraperitoneally (IP) into severe combined immunodeficient (SCID) mice (Harlan, Madison, WI). Upon engraftment, solid tumor (surgically resected and minced) or ascites was reimplanted into 20 to 80 mice, depending on the experiment, to generate biological Ovatar replicates for in vivo experiments. The use of all human subject material was approved by the Institutional Review Board of Mayo Clinic. All animal studies were approved by the Institutional Animal Care and Use Committee of Mayo Clinic.
Treatments were initiated upon confirmation of tumors measuring ≥ 0.2 cm2 cross-sectional area or the presence of ascites as measured by trans-abdominal ultrasound (SonoSite S-series, SonoSite Inc., Bothell, WA). Unless otherwise indicated, mice were treated weekly with mAb-PA (30 mg/kg) or IgG2a isotype control (Bio × Cell, West Lebanon, NH) via IP delivery. For the platinum studies, Ovatars were randomized to receive IP saline or carboplatin plus paclitaxel (CP; NOVAPLUS) at 50 mg/kg and 15 mg/kg, respectively, as described (20). Disease burden was assessed in tumor bearing animals up to three times per week. After four weeks (or if clinical endpoints of tumor size, ascites burden, or morbidity were reached), mice were euthanized and blood and tumor tissue harvested. Final tumor weights were recorded and tumor sections snap frozen in liquid nitrogen. Where appropriate, ascites was collected, centrifuged and acelluar and cellular components independently stored at -80°C. Personnel involved with acquisition of ultrasound measurements and subsequent tumor and/or ascites analyses were blinded to the treatments.
Microarray
For analysis of public microarray data sets, normalized gene expression data were obtained from The Cancer Genome Atlas (TCGA) Research Network and Gene Expression Omnibus (GEO) database for the following independent studies: GSE13876, GSE14764, GSE49997 and GSE9891. Patient tumors within each cohort were ranked according to PAPPA expression and split evenly into two cohorts, defining the top 50% as “PAPPA High” and the bottom 50% as “PAPPA Low”. Ovatar tumors (n = 118) were analyzed by Affymetrix HG U133 plus 2.0 arrays (Santa Clara, TX) at the Mayo Medical Genome Facility according to manufacturer’s protocol. Gene expression arrays were preprocessed and normalized by frozen multi-chip analysis (25). Patients were ranked according to their matched Ovatar PAPPA expression, split evenly into two groups (“PAPP-A High” vs. “PAPP-A Low”) and assessed for outcome.
Immunohistochemistry
Formalin-fixed paraffin-embedded Ovatar tumor tissue samples were processed and immunostained for PAPP-A using a recombinant anti-human PAPP-A monoclonal antibody as previously described (26)
Human PAPP-A ELISA
Snap frozen Ovatar tumor tissues were pulverized in liquid nitrogen using the Cellcrusher (Cork, Ireland). PAPP-A protein levels in pulverized tumor tissue [lysed in M-PER extraction buffer (Thermo Scientific, Waltham, MA)] or acellular ascites were quantified using a highly sensitive PAPP-A ELISA (picoPAPP-A) generously provided by Ansh Labs (Webster, TX). Of special note, this assay does not recognize mouse PAPP-A.
IGFBP-4 proteolysis
Aliquots of tumor lysates or cell-free ascites were incubated overnight at 37°C with IGFBP-4 and IGF-II [IGFBP-4 must bind IGF to be susceptible to cleavage by PAPP-A (27)]. IGFBP-4 proteolysis was assessed by Western blot analysis using primary antibodies towards the C- and N-termini of IGFBP-4 (Abcam, Cambridge, MA) and fluorescently-labeled secondary antibodies (LI-COR, Lincoln, NE). Images were captured using the LI-COR Odyssey scanner and intensities quantitated using Image J software (28). IGFBP-4 proteolysis was also assessed using ELISA kits for total and intact IGFBP-4 (kindly provided by Ansh Labs). Total IGFBP-4 minus intact IGFBP-4 provides a quantitatively accurate measure of proteolyzed IGFBP-4.
IgG2 Immunofluorescence
Five μm cryosections of Ovatar tumor were fixed in methanol and dried to glass slides. Sections were rehydrated in phosphate-buffered saline, blocked in protein-free buffer (Dako, Carpinteria, CA) and penetrated mAb-PA detected using a FITC-labeled anti-mouse IgG2.
IGF-I and IGFBP assessment
Total mouse IGF-I and total and active human IGF-I were measured using ELISA kits from Ansh Labs. The serum IGFBP profile was assessed by Western ligand blot analysis using radiolabeled IGF-I, as described previously (29).
Statistical analyses
Statistical significance between two groups was tested using ANOVA with Bonferroni post hoc test for multiple comparison analysis using GraphPad Prism 6.0. Univariate analyses for progression-free and overall survival were performed using the Kaplan–Meier method and corresponding log-rank test for intergroup differences. All analyses were conducted using JMP 9.0 (SAS Institute).
RESULTS
PAPP-A expression correlates with poor outcome in OvCa tumors
To date, the prognostic value of PAPP-A expression in OvCa tumors has not been reported. As an initial screen, five publicly available datasets were interrogated (TCGA, GSE13876, GSE14764, GSE49997 and GSE9891) for PAPPA gene expression split evenly into two cohorts, defining the top 50% as “PAPPA High” and the bottom 50% as “PAPPA Low”, and correlated to patient outcome. Univariate hazard ratios (HR) with corresponding 95% confidence intervals (CI) for progression-free survival (PFS) and overall survival (OS) were calculated and depicted in a forest plot (Fig. 1A). These data support high PAPPA as a direct correlate of poor outcome in terms of PFS and OS in OvCa tumors. Moreover, univariate analysis of the combined cohorts yielded highly significant differences in PFS (HR = 1.581; 95% CI 1.316-1.901; P < 0.001) and OS (HR = 1.558; 95% CI 1.348-1.800; P < 0.0001; Fig. 1B).
Figure 1.
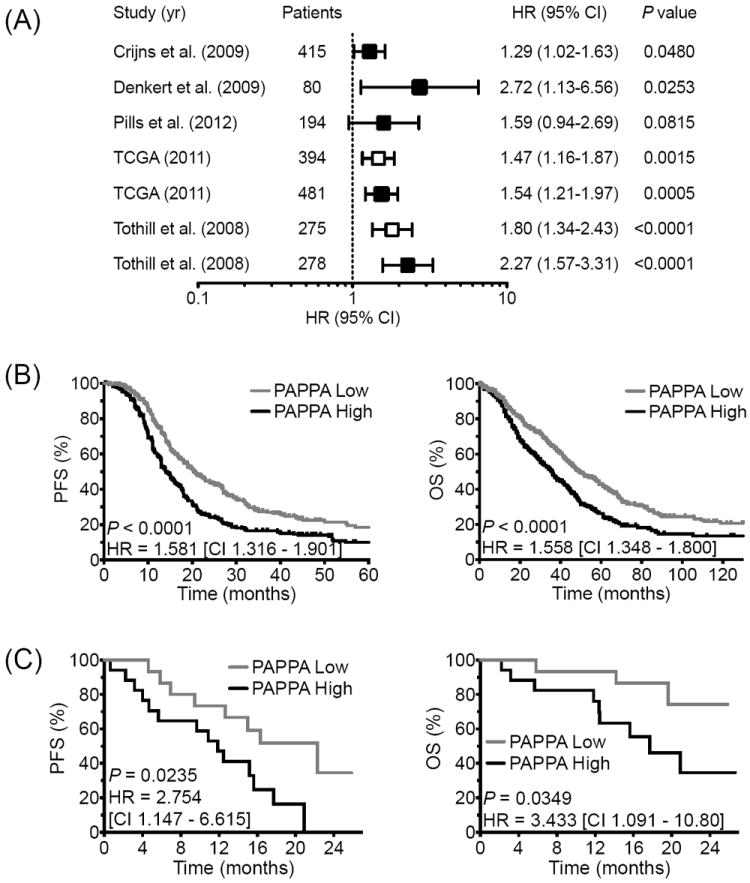
PAPPA expression correlates with poor outcome in ovarian cancer patients and represents a prognostic surrogate in Ovatar tumors.
(A) Forest plot of PFS (open) and OS (filled) by PAPPA expression in multiple ovarian carcinoma tumor cohorts, showing HRs (PAPPA Low/High) and 95% CIs where a HR >1 implies a higher risk of recurrence and mortality in the PAPPA High group. Kaplan Meier analyses of the combined cohorts depicting PFS and OS in the PAPPA Low (gray line) vs. PAPPA High (black line) for patient (B) and Ovatar (C) tumor expression.
We recently demonstrated that Ovatar response to standard carboplatin/paxlitaxel chemotherapy recapitulates donor patient response (20). To further validate Ovatars as molecular surrogates, we sought to determine if Ovatar PAPPA expression correlates to donor patient outcome. Patients were evenly stratified into two groups according to their Ovatar PAPPA expression as described above (PAPPA High vs. Low) and univariate analysis revealed significant differences in PFS (HR = 2.754; 95% CI 1.147-6.615; P = 0.0235) and OS (3.433; 95% CI 1.091-10.80; P = 0.0349; Fig. 1C).
Targeted PAPP-A inhibition confers anti-tumor efficacy in Ovatars
The following three Ovatars were selected from a cohort of 118 models, based on relative PAPPA expression, to test the effects of an inhibitory monoclonal antibody specific to PAPP-A (mAb-PA): PH006 (moderate expression, PH042 (low expression) and PH070 (high expression). Elevated PAPP-A expression was observed in 15-20% of ovarian tumors and was reflected in the Ovatars (Fig 2A). In addition, PAPP-A protein levels were determined by ELISA (Table 1) and immunohistochemistry (Fig. 2B) and found to correlate with PAPPA gene expression.
Figure 2.
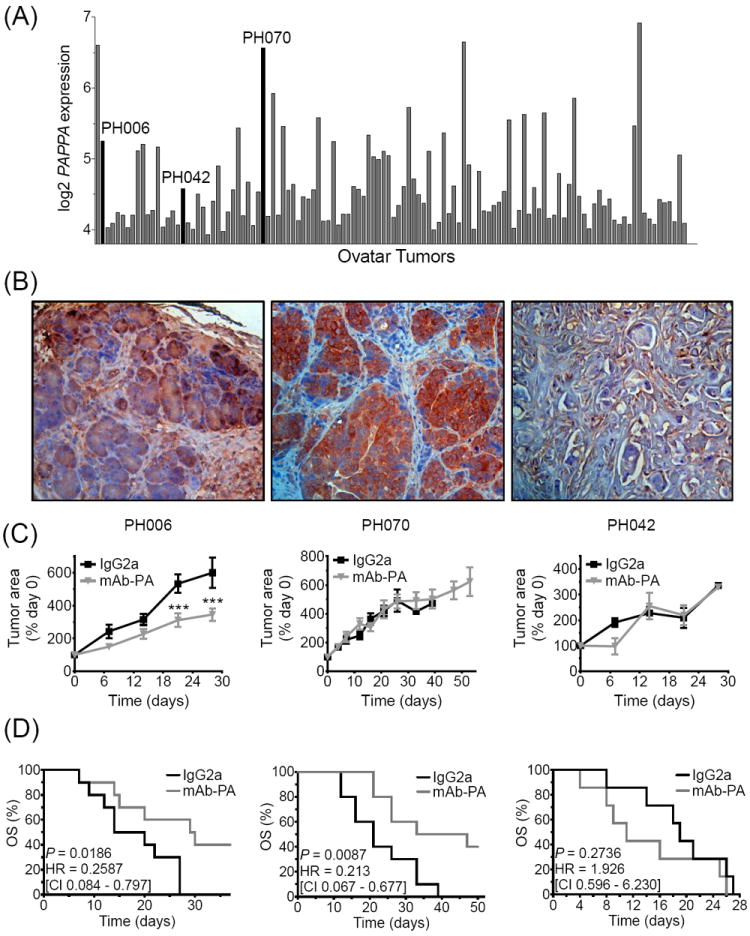
Anti-tumor efficacy of mAb-PA in Ovatars is dependent on PAPP-A levels.
(A) PAPPA expression in a cohort of Ovatar tumors (n=118). Three Ovatar tumors (PH006, PH070, PH042) were selected for in vivo testing (denoted by black bars). (B) PAPP-A immunostaining of tumors from Ovatar PH006, PH070, PH042 (C) Tumor growth (normalized to pretreatment Day 0) and (D) corresponding Kaplan Meier analysis depicting OS in the mAb-PA treated (gray line) vs. IgG2a treated (black line) PH006 (n=7/group), PH070 (n=10/group), and PH042 (n=10/group) Ovatar models. Error bars represent SEM, asterisks denote statistical significance; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Table 1.
PAPP-A Expression in Ovatar tumors and ascites
relative mRNA expression (Fig. 2A)
ng/50 μg tumor
ng/ml ascites
no ascites available
nd: not detectable
PAPP-A protein levels in Ovatar tumors and ascites as measured by ultra-sensitive PAPP-A ELISA
For the first experiment, Ovatar PH006 mice were treated with mAb-PA at varying doses (10 mg/kg vs. 30 mg/kg, once vs. twice weekly) for a total of four weeks. At necropsy, all mice presented with multilobular tumors adherent to the pelvic floor. Final tumor weight was significantly reduced in mice receiving 30 mg/kg mAb-PA compared to IgG2a isotype control; there was no significant difference between the once vs. twice weekly 30 mg/kg dose. In subsequent studies, significant inhibition of tumor growth (Fig. 2C) and increased overall survival (P = 0.0119; HR = 0.26; Fig. 2D) was demonstrated in Ovatar PH006 mice that received mAb-PA once weekly at 30 mg/kg compared to mice given IgG2a.
Treatment of Ovatar PH070 mice with mAb-PA once weekly at 30 mg/kg mAb-PA did not inhibit tumor growth (Fig. 2C), but significantly increased overall survival (P = 0.009; HR = 0.21; Fig. 2D). Investigation into the patient history of PH070 revealed that this Ovatar model recapitulated the clinical disease in terms of bowel adhesions and ascites development. PH070 Ovatars treated weekly with 30 mg/kg mAb-PA demonstrated decreased adhesions and ascites development (data not shown). Assessment of Ovatar tumors indicated penetrance of the mAb-PA (Fig. 3A) and effective inhibition of PAPP-A proteolytic activity towards IGFBP-4 (Fig. 3B).
Figure 3.

Bioavailability and target efficacy of mAb-PA in Ovatar tumors.
(A) Immunofluorescent staining for the nucleus (blue) and IgG2a (green) in mAb-PA treated (Right) versus untreated (Left) PH070 Ovatar tumors, indicating penetrance of the antibody into the tumor. (B) Western blot analysis of intact (upper yellow) and proteolyzed (lower green/red) IGFBP-4 in IgG2a and mAb-PA treated Ovatar tumors, indicating effective inhibition of PAPP-A activity in the tumor by mAb-PA.
As a confirmatory measure of PAPP-A as a biomarker of therapeutic efficacy, we also evaluated the response of Ovatar PH042, which had relatively low PAPP-A mRNA expression and little or no PAPP-A protein (Table 1, Fig. 2B). mAb-PA did not significantly impact tumor progression (Fig. 2C) or outcome (Fig. 2D) in this Ovatar.
No apparent secondary effects of mAb-PA were observed
In all Ovatar models analyzed there were no significant differences in circulating levels of IGF-I (128 ± 7 ng/ml for mAb-PA vs.120 ± 10 ng/ml for IgG2a) or IGFBPs, the latter assessed by Western ligand blotting (data not shown).
mAb-PA treatment reduces ascites
To investigate the effect of mAb-PA on ascites accumulation and progression, it was determined that ultrasound was an accurate and precise measure of actual tumor weight and ascites volume (Supplemental Fig. 1). PH070 Ovatars were analyzed following tumor cell implantation. Ascites-free survival (AFS) was significantly increased (P < 0.0001; HR = 0.2351) and ascites volume significantly decreased (P = 0.0317) in response to mAb-PA treatment (Fig. 4A). To assess the effect of mAb-PA on ascites progression, a fraction of ascites containing active tumor cells from Ovatar PH070 was injected IP into mice, and mAb-PA or IgG2a treatments initiated four days later. Overall survival was significantly increased (P = 0.0458; HR = 0.310) and time for tumor-to-ascites appearance was significantly delayed (P < 0.0001) with mAb-PA treatment (Fig. 4B). Moreover, mAb-PA treatment was found to induce ascites regression (P = 0.0157; Fig. 4C).
Figure 4.

Targeting PAPP-A in Ovatar models with ascites development.
(A) PAPP-A blockade inhibits ovarian ascites development. Left - Kaplan Meier analysis depicting ascites-free survival (AFS) in mAb-PA treated (gray line) vs. IgG2a treated (black line) PH070 Ovatars (n=40/group). Right - Final ascites tumor burden depicted as volume in mL at the time of necropsy. (B) PAPP-A blockade attenuates ovarian ascites progression. Left - Kaplan Meier analysis depicting OS in PH070 Ovatars (n=11/group) wherein treatment (mAb-PA vs IgG2a) was initiated at the time of confirmed (ultrasound) subclinical ascites burden (Day 0). Right - Time (days) from solid tumor detection to the development of ascites in mAb-PA vs IgG2a treated mice. (C) PAPP-A blockade induces ovarian ascites regression. Ascites burden as measured by ultrasound (area in cm2) before treatment (Pre Tx) and after treatment (Post Tx) with mAb-PA vs IgG2a (n=5/group).
PAPP-A levels in OvCa patient ascites
The ascites collected from PH070 Ovatars had measureable levels of human PAPP-A, indicating production by the patient tumor. Although ascites from patient PH070 was not available, PAPP-A levels were evaluated in a large cohort of additional patient ascites. All patient ascites (N = 33) had high levels of PAPP-A, with mean ± SEM of 45 ± 3 ng/ml (normal serum levels < 1 ng/ml). Greater than 90% of the endogenous IGFBP-4 in the ascites was proteolyzed, and levels of bioactive IGF-I were 2-50% that of total IGF-I (mean ± SEM, 18 ± 3%). Proteolytic activity in patient ascites could be inhibited with mAb-PA (Fig. 5), demonstrating that PAPP-A is responsible for IGFBP-4 cleavage in this fluid.
Figure 5.
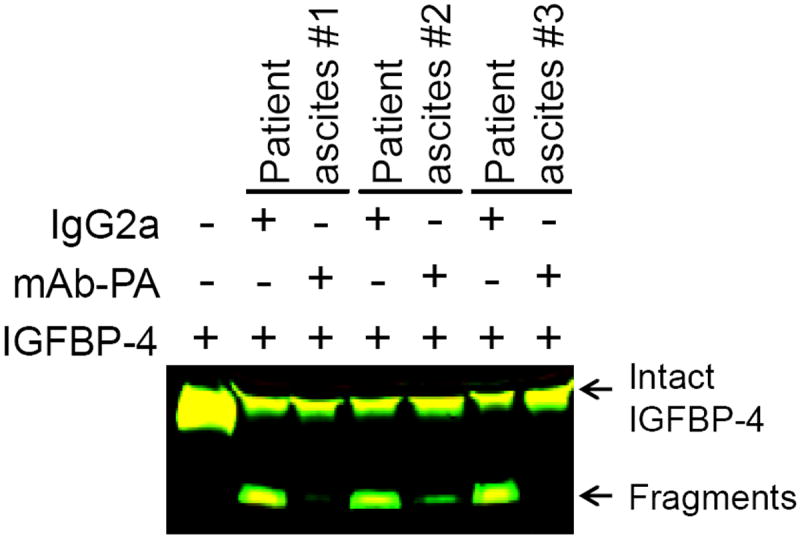
mAb-PA inhibits IGFBP-4 proteolysis in patient ascites.
Aliquots of ascites from three different patients were incubated with IGFBP-4 and IgG2a or mAb-PA. Western blot analysis indicates effective inhibition of IGFBP-4 proteolysis by mAb-PA.
Combination mAb-PA and adjunct carboplatin/paclitaxel therapy
Patient PH070 had chemo-resistant OvCa (20). To determine if targeted inhibition of PAPP-A could affect platinum resistance, standard platinum chemotherapy (CP) combined with mAb-PA therapy was tested (Fig. 6A). The combination CP plus mAb-PA vs. CP plus IgG2a was initiated upon ultrasound confirmation of engrafted PH070 Ovatar tumors. Chemotherapy was ceased after four weeks and Ovatars continued to receive weekly treatments of mAb-PA or IgG2a for up to 100 days post initial treatment. As expected, CP treatment alone did not regress tumors below baseline during chemotherapy and the addition of IgG2a did not alter tumor growth trajectory. However, mAb-PA treatment enhanced sensitivity to platinum-based chemotherapy, as further supported by the highly significant reduction in final tumor weight (Fig. 6B).
Figure 6.

Adjuvant mAb-PA therapy sensitizes platinum-resistant Ovatars.
(A) Tumor growth in response to platinum chemotherapy combined with mAb-PA [CP + mAb-PA, gray line] vs. IgG2a [CP + IgG2a, black line] (n = 10/group). (B) Final tumor burden (solid tumor) at the time of necropsy.
DISCUSSION
In this study, we demonstrate the efficacy of a novel neutralizing antibody against PAPP-A (mAb-PA) in primary patient ovarian tumorgrafts (Ovatars) to significantly: 1) inhibit intraperitoneal OvCa tumor growth, 2) delay ascites development, 3) inhibit ascites accumulation, and 4) induce ascites regression. Ovatars were selected for predictive response to treatment based on elevated PAPP-A expression in matched primary patient tumors. In contrast to the adverse effects reported in response to IGF-IR directed antibodies (3-7), secondary endocrine disruption resulting from mAb-PA monotherapy was not evident. Furthermore, the addition of mAb-PA to standard frontline carboplatin/paclitaxel chemotherapy markedly improved tumor regression to effectively sensitize chemo-resistant Ovatars.
Importance of the Ovatar model
Orthotopic implantation of patient OvCa tissue in SCID mice results in the formation a primary tumor that is commonly localized to the mouse pelvis and/or ovaries. As these primary tumors develop and progress, cancer cell growth, proliferation and metastatic dissemination within the peritoneal cavity is comparable to that of the patient. We have previously demonstrated that Ovatar tumors maintain the histopathologic and molecular diversity (genomic, transcriptomic, proteomic) of the donor patient tumor (20). Moreover, Ovatars recapitulate the patient experience in terms of metastasis and ascites-related complications. Indeed, the Ovatar models (PH006, PH042, PH070) employed herein exhibited markedly different characteristics across models while conserving the patient disease phenotype. For example, Patient PH006 presented with a solid Stage 3 tumor with no ascites or signs of metastases at the time of primary debulking surgery while Patient PH070 presented with adhesions, bowel involvement and ascites. Thus, these orthotopic Ovatars more accurately resemble the patient experience in terms of clinical complications as compared to subcutaneous xenografts, and present a more relevant and directly translatable medium towards analyzing ovarian cancer disease progression and metastasis. More importantly, therapeutic response in Ovatars (standard and targeted therapeutics) should more likely reflect how the patient would respond, and, therefore, is a step closer towards translation into clinical benefit.
Necessity of identifying anti-PAPP-A therapy biomarkers
Arguably the greatest impedance to the advancement of IGF-IR directed antibodies is a lack of predictive biomarkers, as the clinical value of IGF-IR remains controversial (2,30). In this study, we utilized patient tumor PAPP-A gene expression to select candidate Ovatar models for testing mAb-PA therapy. DNA microarray data identified a subset of patient tumors (15-20%) reporting high PAPP-A expression. Elevated PAPP-A protein expression in both solid tumors and in ascites was confirmed using a highly sensitive PAPP-A ELISA. Strikingly, one hundred percent of patients’ ascites tested reported high PAPP-A, and, with the exception of a single sample, levels were increased ≥100-fold compared to the average serum level of non-pregnant women. Bioactive IGF-I accounts for less than 1% of total IGF-I and high levels of free IGF-I are associated with disease progression (12). Virtually all of the endogenous IGFBP-4 in patient ascites samples was found to be proteolyzed and, as a result, bioactive IGF-I levels were 2-50% of total IGF-I. Furthermore, we verified that PAPP-A present in the ascites remains proteolytically active. This was important as the expression of a naturally occurring, irreversible PAPP-A inhibitor (proMBP) has been shown to be increased in ovarian tumors and transformed ovarian epithelial cells (19).
Advantages of targeting PAPP-A via mAb-PA
The mAb-PA presented herein is uniquely specific to PAPP-A as it targets a substrate-binding exosite required for IGFBP-4 proteolysis (23). Importantly, the epitope of mAb-PA is not present in other enzymes, including homologous PAPP-A2 [primarily an IGFBP-5 protease (31)]. The specificity of this anti-PAPP-A therapy would, therefore, serve to improve the safety and tolerability via reducing off-target and potentially harmful side effects, and target inhibition to the site(s) of local (supraphyiologic) PAPP-A expression, in this case the tumor. This is in contrast to strategies targeting IGF-IR, IGF ligands, and IGFBPs that are ubiquitous and therefore nonspecific to site or condition. Another advantage of mAb-PA is its potential to reduce both IGF-I and IGF-II binding to IGF-IR, IGF-IR:InsR hybrids and InsR-A, while sparing any effect on insulin-stimulated InsR-B signaling. Therefore, there should be preferential reduction in proliferative/metastatic effects versus metabolic dysregulation.
Compensatory increases in circulating growth hormone (GH) and IGF-I have been observed with IGF-IR antibodies (4). There did not appear to be any secondary consequences of PAPP-A inhibition in the Ovatars resulting from mAb-PA. There were no apparent effects on either serum levels of IGF-I or profiling of IGFBPs, thus indirectly supporting that there were no effects on growth hormone (GH), which regulates serum IGF-I and IGFBP-3 (32). GH can also induce insulin resistance (33). We did not perform specific insulin sensitivity tests in Ovatars treated with mAb-PA, but previous studies in PAPP-A knockout mice did not indicate insulin resistance (34).
Ascites attenuation in response to mAb-PA treatment
Perhaps the most exciting finding in this study was the effect of mAb-PA therapy on inhibiting ascites development and accumulation as well as promoting regression of established ascites. Ascites produces significant morbidity in OvCa, and palliative treatment options to reduce ascites burden are limited (35). Paracentesis via percutaneous drainage is frequently employed for short-term symptom relief. Unfortunately, this invasive procedure carries many risks and frequently requires hospitalization. Thus, our findings suggest the potential use of mAb-PA monotherapy for palliative benefit, with minimal risk, toxicity and discomfort.
In conjunction with previously cited work (16,17), our Ovatar data implicate PAPP-A attenuation (expression/activity) as a potential strategy to limit and/or suppress OvCa metastasis. Indeed, PAPP-A has recently been identified as a metastasis-related target gene (36,37). It is of interest that ascites, which promotes tumor growth and metastases, has high levels of PAPP-A. Furthermore, ascites has been found to be rich in proinflammatory cytokines (38), which have been shown to upregulate PAPP-A expression in several cell types (39,40). Further studies will be necessary to establish a role for PAPP-A in OvCa metastases.
mAb-PA in platinum-resistant OvCa
The application of potentially harmful and cytotoxic targeted therapeutics in combination with standard chemotherapy in the frontline and/or maintenance setting has been rationalized by the promise of extending historically poor progression-free disease intervals and improving overall survival. For a subset of primary OvCa tumors with elevated PAPP-A expression, anti-PAPP-A therapy presents as a viable adjuvant option in terms of favorable pharmacokinetic (e.g. easily administered, extensive tumor uptake, long half-life, little to no toxicity) and pharmacodynamic (e.g. low Kd, low IC50) attributes. Increased IGF signaling can potentially limit the efficacy of cytotoxic agents, and IGF-IR inhibition to overcome platinum-resistance in OvCa is by no means a novel concept (41,42). PAPP-A is proposed as a better therapeutic target with greater tumor specificity and lower risk of side effects than other IGF system targets.
Conclusion
Identification of women with ovarian cancer who are most likely to respond is critical to the success of PAPP-A inhibition in clinical trials. In this study, we illustrate the potential power of patient selection through appropriate and easily obtainable biomarkers of PAPP-A in terms of expression and bioactivity, the efficacy of a novel neutralizing monoclonal antibody against PAPP-A, and proof-of-concept in a relevant patient Ovatar model supporting a multitude of therapeutics applications for PAPP-A as a promising new target in OvCa.
Supplementary Material
Acknowledgments
Financial support: This work was supported by National Institutes of Health Endocrine Training Grant T32 DK07352 (MA Becker), Mayo Clinic Ovarian SPORE CA136393 (MA Becker, P Haluska, CA Conover), and unrestricted research funding from Ansh Laboratories (CA Conover).
References
- 1.Romero I, Bast RC., Jr Minireview: Human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology. 2012;153:1593–1602. doi: 10.1210/en.2011-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev. 2007;28:20–47. doi: 10.1210/er.2006-0001. [DOI] [PubMed] [Google Scholar]
- 3.Weroha SJ, Haluska P. IGF system in Cancer. Endocrinol Metab Clin North Am. 2012;41:335–350. doi: 10.1016/j.ecl.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev. 2012;12:159–69. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 5.King H, Aleksic A, Haluska P, Macaulay VM. Can we unlock the potential of IGF-1R inhibition in cancer therapy? Cancer Treat Rev. 2014 doi: 10.1016/j.ctrv.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater? J Natl Cancer Inst. 2012;104:975–81. doi: 10.1093/jnci/djs258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao J, Chang YS, Jallal B, Viner J. Targeting the insulin-like growth factor axis for the development of novel therapeutics in oncology. Cancer Res. 2012;72:3–12. doi: 10.1158/0008-5472.CAN-11-0550. [DOI] [PubMed] [Google Scholar]
- 8.Boldt HB, Overgaard MT, Laursen LS, Weyer K, Sottrup-Jensen L, Oxvig C. Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin. Biochem J. 2001;358:359–67. doi: 10.1042/0264-6021:3580359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conover CA. Key questions and answers about pregnancy-associated plasma protein-A. Trends Endocrinol Metab. 2012;23:242–9. doi: 10.1016/j.tem.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 11.Kalli KR, Conover CA. The insulin-like growth factor/insulin system in epithelial ovarian cancer. Front Biosci. 2003;8:d714–22. doi: 10.2741/1034. [DOI] [PubMed] [Google Scholar]
- 12.Brokaw J, Katsaros D, Wiley A, Lu L, Su D, Sochirca O, et al. IGF-I in epithelial ovarian cancer and its role in disease progression. Growth Factors. 2007;346:54. doi: 10.1080/08977190701838402. [DOI] [PubMed] [Google Scholar]
- 13.Kalli KR, Falowo OI, Bale LK, Zschunke MA, Roche PC, Conover CA. Functional insulin receptors on human epithelial ovarian carcinoma cells: implications for IGF-II mitogenic signaling. Endocrinology. 2002;143:3259–67. doi: 10.1210/en.2001-211408. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Hailey J, Williams D, Wang Y, Lipari P, Malkowski M, et al. Inhibition of insulin-like growth factor-I receptor (IGF-IR) signaling and tumor cell growth by a fully human neutralizing anti-IGF-IR antibody. Mol Cancer Ther. 2005;4:1214–21. doi: 10.1158/1535-7163.MCT-05-0048. [DOI] [PubMed] [Google Scholar]
- 15.Gest C, Mirshahi P, Li H, Pritchard L-L, Joimel U, Blot E, et al. Ovarian cancer: Stat3, RhoA and IGF-IR as therapeutic targets. Cancer Lett. 2012;317:207–17. doi: 10.1016/j.canlet.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Boldt HB, Conover CA. Overexpression of pregnancy-associated plasma protein-A in ovarian cancer cells promotes tumor growth in vivo. Endocrinology. 2011;152:1470–8. doi: 10.1210/en.2010-1095. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Kobayashi H, Suzuki M, Hirashima Y, Kanayama N, Terao T. Genetic downregulation of pregnancy-associated plasma protein-A (PAPP-A) by bikunin reduces IGF-I-dependent Akt and ERK1/2 activation and subsequently reduces ovarian cancer cell growth, invasion and metastasis. Int J Cancer. 2004;109:336–47. doi: 10.1002/ijc.11700. [DOI] [PubMed] [Google Scholar]
- 18.Alexiadis M, Mamers P, Chu S, Fuller PJ. Insulin-like growth factor, insulin-like growth factor-binding protein-4, and pregnancy-associated plasma protein-A gene expression in human granulosa cell tumors. Int J Gynecol Cancer. 2006;16:1973–9. doi: 10.1111/j.1525-1438.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 19.Kalli KR, Chen B-K, Bale LK, Gernand E, Overgaard MT, Oxvig C, et al. Pregnancy-associated plasma protein-A (PAPP-A) expression and insulin-like growth factor binding protein-4 protease activity in normal and malignant ovarian surface epithelial cells. Int J Cancer. 2004;110:633–640. doi: 10.1002/ijc.20185. [DOI] [PubMed] [Google Scholar]
- 20.Weroha SJ, Becker MA, Enderica-Gonzalez S, Harrington S, Oberg AL, Maurer MJ, Perkins SE, Hilli MA, Butler KA, McKinstry S, Fink S, Jenkins R, Hou X, Kalli K, Goodman K, Sarkaria J, Karlan B, Kumar A, Kaufmann SH, Hartmann LC, Haluska P. Tumorgrafts as in vivo surrogates for women with ovarian cancer. Clin Cancer Res. 2014;20:1288–1297. doi: 10.1158/1078-0432.CCR-13-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siolas D, Hannon GJ. Patient-derived tumor xenografts: transforming clinical samples into mouse models. Cancer Res. 2013;73:5315–5319. doi: 10.1158/0008-5472.CAN-13-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehner C, Radisky DC. Triggering the landslide: the tumor-promotional effects of myofibroblasts. Exp Cell Res. 2013;319:1657–1662. doi: 10.1016/j.yexcr.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mikkelsen JH, Gyrup C, Kristensen P, Overgaard MT, Poulsen CB, Laursen LS, et al. Inhibition of the proteolytic activity of pregnancy-associated plasma protein-A by targeting substrate exosite binding. J Biol Chem. 2008;283:16772–80. doi: 10.1074/jbc.M802429200. [DOI] [PubMed] [Google Scholar]
- 24.Mikkelsen JH, Resch ZT, Kalra B, Savjani G, Kumar A, Conover CA, Oxvig C. Indirect targeting of IGF receptor signaling in vivo by substrate-selective inhibition of PAPP-A. Oncotarget. 2014;5:1014–1025. doi: 10.18632/oncotarget.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCall MN, Bolstad BM, Irizarry RA. Frozen robust multiarray analysis (fRMA) Biostatistics. 2010;11:242–253. doi: 10.1093/biostatistics/kxp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mansfield AS, Visscher DW, hart SN, Wang C, Goetz MP, Oxvig C, Conover CA. Pregnancy-associated plasma protein-A expression in human breast cancer. GH & IGF Res. 2014;24:264–267. doi: 10.1016/j.ghir.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin X, Byun D, Lau K-H, Baylink DJ, Mohan S. Evidence that the interaction between insulin-like growth factor (IGF)-II and IGF binding protein (IGFBP)-4 is essential for the action of the IGF-II-dependent IGFBP-4 protease. Arch Biochem Biophys. 2000;379:209–16. doi: 10.1006/abbi.2000.1872. [DOI] [PubMed] [Google Scholar]
- 28.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to Image J: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khosla S, Hassoun AAK, Baker BK, Liu F, Zein NN, Whyte MP, Reasner CA, Nippoldt TB, Tiegs RD, Hintz RL, Conover CA. Insulin-like growth factor system abnormalities in hepatitis-associated osteosclerosis. J Clin Invest. 1998;101:2165–2173. doi: 10.1172/JCI1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werner H, Maor S. The insulin-like growth factor-I receptor gene: a downstream target for oncogene and tumor suppressor action. Trends Endocrinol Metab. 2006;17:236–42. doi: 10.1016/j.tem.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. PAPP-A2, a novel insulin-like growth factor binding protein-5 proteinase. J Biol Chem. 2001;276:21849–53. doi: 10.1074/jbc.M102191200. [DOI] [PubMed] [Google Scholar]
- 32.Clemmons DR. Value of insulin-like growth factor system markers in the assessment of growth hormone status. Endocrinology and Metabolism Clinics. 2007;36:109–129. doi: 10.1016/j.ecl.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 33.Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI, Turyen D. Influence of the crosstalk between growth hormone and insulin signaling on the modulation of insulin sensitivity. GH & IGF Res. 2005;15:324–336. doi: 10.1016/j.ghir.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Conover CA, Mason MA, Levine JA, Novak CM. Metabolic consequences of pregnancy-associated plasma protein-A deficiency in mice: exploring possible relationship to the longevity phenotype. J Endocrinol. 2008;198:599–605. doi: 10.1677/JOE-08-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kipps E, Tan DSP, Kaye SB. Meeting the challenge of ascites in ovarian cancer: new avenues for therapy and research. Nat Rev. 2013;13:273–82. doi: 10.1038/nrc3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang J, Tabata S, Kakiuchi S, Van T, Goto H, Hanibuchi M, et al. Identification of pregnancy-associated plasma protein A as a migration-promoting gene in malignant pleural mesothelioma cells: a potential therapeutic target. Oncotarget. 2013;4:1172–1184. doi: 10.18632/oncotarget.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salim H, Arvanitis A, de Petris L, Kanter L, Haag P, Zovko A, et al. miRNA-214 is related to invasiveness of human non-small cell lung cancer and directly regulates alpha protein kinase 2 expression. Genes Chromosomes Cancer. 2013;52:895–911. doi: 10.1002/gcc.22085. [DOI] [PubMed] [Google Scholar]
- 38.Robinson Smith TM, Isaacsohn I, Mercer CA, Zhou M, Van Rooijen N, Husseinzadeh N, McFarland-Mancini MM, Drew AF. Macrophages mediate inflammation-enhanced metastasis of ovarian tumors in mice. Cancer Res. 2007;67:5708–5716. doi: 10.1158/0008-5472.CAN-06-4375. [DOI] [PubMed] [Google Scholar]
- 39.Resch ZT, Chen B-K, Bale LK, Oxvig C, Overgaard MT, Conover CA. Pregnancy-associated plasma protein A gene expression as a target of inflammatory cytokines. Endocrinology. 2004;145:1124–1129. doi: 10.1210/en.2003-1313. [DOI] [PubMed] [Google Scholar]
- 40.Boldt HB, Conover CA. Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res. 2007;17:10–18. doi: 10.1016/j.ghir.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 41.Friedbichler K, Hofmann MH, Kroez M, Ostermann E, Lamche HR, Koessl C, Borges E, Pollack MN, Adolf G, Adam PJ. Pharmacodynamic and antineoplastic activity of BI 836845, a fully human IGF ligand-neutralizing antibody, and mechanistic rationale for combination with rapamycin. Mol Cancer Ther. 2014;13:399–409. doi: 10.1158/1535-7163.MCT-13-0598. [DOI] [PubMed] [Google Scholar]
- 42.Eckstein N, Servan K, Hildebrandt B, Politz A, von Jonquieres G, Wolf-Kummeth S, et al. Hyperactivation of the insulin-like growth factor receptor I signaling pathway is an essential event for cisplatin resistance of ovarian cancer cells. Cancer Res. 2009;69:2996–3003. doi: 10.1158/0008-5472.CAN-08-3153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


