Abstract
PI3K/AKT/mTOR pathway which is aberrantly stimulated in many cancer cells, has emerged as a target for therapy. However, mTORC1/S6K also mediates negative feedback loops that attenuate upstream signaling. Suppression of these feedback loops opposes the growth-suppressive effects of mTOR inhibitors and leads to drug resistance. Here, we demonstrate that treatment of PANC-1 or MiaPaCa-2 pancreatic ductal adenocarcinoma (PDAC) cells with the dual PI3K/mTOR kinase inhibitor (PI3K/TOR-KI) NPV-BEZ235 blocked mTORC1/S6K activation (scored by S6 phosphorylation at Ser240/244), mTORC1/4E-BP1 (assayed by 4E-BP1 phosphorylation at Thr37/46) and mTORC2-mediated AKT phosphorylation at Ser473, in a concentration-dependent manner. Strikingly, NPV-BEZ235 markedly enhanced the MEK/ERK pathway in a dose-dependent manner. Maximal ERK over-activation coincided with complete inhibition of phosphorylation of AKT and 4E-BP1. ERK over-activation was induced by other PI3K/TOR-KIs, including PKI-587 and GDC-0980. The MEK inhibitors U126 or PD0325901 prevented ERK over-activation induced by PI3K/TOR-KIs. The combination of NPV-BEZ235 and PD0325901 caused a more pronounced inhibition of cell growth than that produced by each inhibitor individually. Mechanistic studies assessing PI3K activity in single PDAC cells indicate that PI3K/TOR-KIs act through a PI3K-independent pathway. Doses of PI3K/TOR-KIs that enhanced MEK/ERK activation coincided with those that inhibited mTORC2-mediated AKT phosphorylation on Ser473, suggesting a role of mTORC2. Knockdown of Rictor via transfection of siRNA markedly attenuated the enhancing effect of NVP-BEZ235 on ERK phosphorylation. We propose that dual PI3K/mTOR inhibitors suppress a novel negative feedback loop mediated by mTORC2 thereby leading to enhanced MEK/ERK pathway activity in pancreatic cancer cells.
Keywords: NVP-BEZ235, PKI-587, GDC-0980, PD0325901, AKT, Rictor, RAF
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the most lethal human diseases. The estimated incidence of PDAC in the US has increased to 44,000 new cases in 2012 and is now the fourth leading cause of cancer mortality in both men and women (1). Novel targets and strategies for therapeutic intervention in PDAC are urgently needed and will most likely arise from a more detailed understanding of the signaling mechanisms that promote survival, proliferation and invasiveness and of the complex feedback mechanisms that mediate drug resistance in these cells.
The phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR pathway, a key module in the regulation of metabolism, migration, survival, autophagy and growth (2), plays a pivotal role in the pancreas, mediating acinar-to-ductal metaplasia, and PDAC formation (3,4) and is active in premalignant pancreatic lesions and pancreatic cancer tissues (4–6). The mammalian target of rapamycin (mTOR) functions as a catalytic subunit in two distinct multi-protein complexes, mTORC1 and mTORC2 (7). mTORC1, a complex including Raptor, phosphorylates and controls at least two regulators of protein synthesis, the 40S ribosomal protein subunit S6 kinase (S6K) and the translational repressor 4E-binding protein 1, referred as 4E-BP1. mTORC2, characterized by Rictor, phosphorylates several AGC protein kinases, including AKT at Ser473. The PI3K/mTOR pathway functions downstream of RAS (8), which is mutated in 90% of PDACs, and plays a key role in insulin/IGF receptor signaling. PDAC cells express insulin and IGF-1 receptors and over-express IRS-1 and IRS-2 (9–12) and PDAC (but not normal) tissue expresses activated IGF-1R (12) and IGF-1 (13). Mutation of p53, as seen during the progression of 50–75% of PDAC, has been recognized to up-regulate the insulin/IGF-1/mTORC1 pathway (14). Recently, individual gene variations in the IGF-1 signaling system have been associated with worse survival in PDAC (15). Crosstalk between insulin/IGF-1 receptors and G protein-coupled receptor (GPCR) signaling systems potently stimulate mTORC1, DNA synthesis and cell proliferation in a panel of PDAC cells (16,17). Consequently, mTORC1, downstream of PI3K/AKT, has emerged as an attractive therapeutic target in PDAC and other common malignancies.
In addition to growth-promoting signaling, the mTORC1/S6K axis also mediates negative feedback loops that restrict signaling via insulin/IGF receptor and other tyrosine kinase receptors (18). Indeed, suppression of mTORC1/S6K feedback loops unleashes over-activation of PI3K/AKT (7) that potentially counterbalances the anti-proliferative effects of mTOR inhibitors in many cancer cell types (19–22), including PDAC cells (23). In an effort to prevent PI3K/AKT over-activation in response to allosteric mTORC1 inhibition, dual PI3K and mTOR kinase inhibitors (PI3K/TOR-KIs), including NPV-BEZ235 (24,25), PKI-587 (26,27) GDC-0980 (28) have been developed. Although these inhibitors are well suited to prevent activation of PI3K/AKT caused by suppression of mTORC1/S6K, much less is known about negative feedback loops impinging on other pro-oncogenic pathways (e.g. MEK/ERK) and/or concerning mTORC2 instead mTORC1. Here, we show that clinically relevant PI3K/TOR-KIs, including NPV-BEZ235, PKI-587 and GDC-0980 induce MEK/ERK pathway over-activation in human PDAC cells. Based on our results, we propose that mTORC2 mediates a feedback loop that curtails the activity of the MEK/ERK pathway in PDAC cells.
Material and Methods
Cell culture
The human pancreatic cancer cell lines PANC-1, MiaPaCa-2, AsPC-1 and BxPC-3 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). PANC-1 and MiaPaCa-2 were chosen because they harbor mutations typical of human pancreatic cancer (29), including activating mutations in KRAS, TP53 (encoding the p53 protein) and CDKN2A (also known as p16 or p16INK4a). These cell lines, authenticated by ATCC by short-tandem repeat analysis, were used within 15 passages and cultured for less than 6 months after recovery from frozen stocks (no authentication was done by the authors). Cells were obtained from ATCC at the following dates: MiaPaca-2 (June 2012, August 2013 and October 2014); PANC-1 (January 2012 and October 2014); BxPC-3 (June 2013); AcPC-1 (December 2009). Cells were grown in Dulbecco’s modified Eagle Medium (DMEM) with 2 mM glutamine, 1 mM Na-pyruvate, 100 units/mL penicillin, and 100 μg/mL streptomycin and 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere containing 10% CO2.
Western blot analysis
Confluent cultures of PANC-1 or MiaPaCa-2 cells, grown on 35 mm tissue culture dishes, were washed and then incubated for 24 h in DMEM containing 5 mM glucose and 1% FBS. The cells were washed twice with DMEM containing 5 mM glucose and incubated in serum-free medium for 4 h and then treated as described in individual experiments. The cultures were then directly lysed in 2 × SDS-PAGE sample buffer [200 mM Tris-HCl (pH 6.8), 2 mM EDTA, 0.1 M Na3VO4, 6% SDS, 10% glycerol, and 4% 2-mercaptoethanol], followed by SDS-PAGE on 10% gels and transfer to Immobilon-P membranes (Millipore, Billerica, MA). Western blots were then performed on membranes incubated overnight with the specified antibodies in phosphate-buffered saline (PBS) containing 0.1% Tween-20. The immunoreactive bands were detected with ECL (enhanced chemiluminescence) reagents (GE Healthcare Bio-Sciences Corp, Piscataway, NJ). In most experiments, the antibodies used detected the phosphorylated state of S6 at Ser 240/244, S6K at Thr389, 4E-BP1 at Thr37/46, AKT at Ser473and at Thr308, MEK at Ser217/221 and ERK at Thr202 and Tyr204or the total levels of these proteins.
Cell transfection
MiaPaCa-2 cells were transfected with the plasmid containing a cDNA encoding a green fluorescent protein (GFP) tagged-AKT pleckstrin homology domain (AKT-PH-GFP) from Addgene (pcDNA3-AKT-PH-GFP cat # 18836) by using Lipofectamine 2000 (Invitrogen) as suggested by the manufacturer. Analysis of the cells transiently transfected was performed 24 h after transfection.
Real-time GFP-AKT-PH imaging in single live cells
Single live-cell imaging of the GFP tagged AKT-PH domain was achieved with a fluorescence microscope. The microscope used was an epifluorescence Zeiss Axioskop and a Zeiss water objective (Achroplan 40/.75W Carl Zeiss, Inc.). Images were captured as uncompressed 24-bit TIFF files with a cooled (-12°C) single CCD color digital camera (Pursuit, Diagostic Instruments) driven by SPOT version 4.7 software.
Quantitative analysis of the relative change in plasma membrane and cytosol fluorescence intensity of individual cells were performed by importing the TIF images into Zeiss LSM 510 software and performing profile scans with the largest line width. Five equally spaced line profiles were taken for each cell or cell pair. Intensities were background corrected, and the intensities at the membrane were divided by those in the immediately surrounding cytoplasm. We analyzed 30–45 cells in each experiment, and each experiment was performed in duplicate. The selected cells displayed in the figures were representative of 90% of the population of positive cells.
Knockdown of Rictor levels via siRNA transfection
Silencer Select siRNAs was purchased from Life Technologies (Grand Island, NY) and designed to target human Rictor. Cells were transfected using the reverse transfection method. Either Silencer Select non-targeting negative control or a 10 nM Rictor siRNA was mixed with Lipofectamine RNAi MAX (Life Technologies Grand Island, NY) according to the manufacturer’s protocol and added to 35mm tissue culture plates. MiaPaCa-2 cells were then plated on top of the siRNA/Lipofectamine RNAiMAX complex at a density of 105 cells/well in DMEM containing 5 mM glucose and 10% FBS. Three days after transfection, cells were used for experiments and subsequent Western blot analysis.
Assay of cell proliferation
Cells (105) were plated on 35 mm tissue culture dishes in DMEM containing 10% FBS. After 24 h of incubation at 37°C, cultures were incubated with DMEM containing 5% FBS in the absence or presence of NPV-BEZ235, PD0325901 (30) or the combination of both drugs. In other experiments, PKI-587 and GDC-0980 were tested instead of NPV-BEZ235. The concentrations of PD0325901 used in the experiments reflected that we found MiaPaCa-2 cells more sensitive to this inhibitor than PANC-1 cells. After 72 h, cell count was determined from a minimum of six dishes per condition using a Coulter counter, after cell clumps were disaggregated by passing the cell suspension 10 times through a 19-gauge, and subsequently, a 21-gauge needle.
For cell colony formation, 300 MiaPaCa-2 cells were plated into 35 mm tissue culture dishes in DMEM containing 10% FBS. After 24 h of incubation at 37°C, cultures were incubated with DMEM containing 5% FBS either in the absence or presence of 5 nM of NPV-BEZ235, 5 nM of PD0325901 or the combination of both drugs. A colony consisted of at least 50 cells (31). Cell colony numbers from 3 dishes per condition were determined after 8 days of incubation.
Materials
DMEM was obtained from Invitrogen (Carlsbad, CA). Neurotensin and insulin were obtained from Sigma Chemical (St. Louis, MO). NPV-BEZ235, PKI-578 and GCD-0980 were from Selleck Chemicals (Houston, TX). PD0325901 and U0126 were from Tocris BioScience (Minneapolis, MN). The structure of these inhibitors is shown in Fig. S1. All antibodies were purchased from Cell Signaling Technology (Danvers, MA). Horseradish peroxidase–conjugated anti-rabbit IgG and anti-mouse IgG were from GE Healthcare Bio-Sciences Corp (Piscataway, NJ). All other reagents were of the highest grade available.
RESULTS
NPV-BEZ235 causes over-activation of the ERK pathway in human PDAC cells
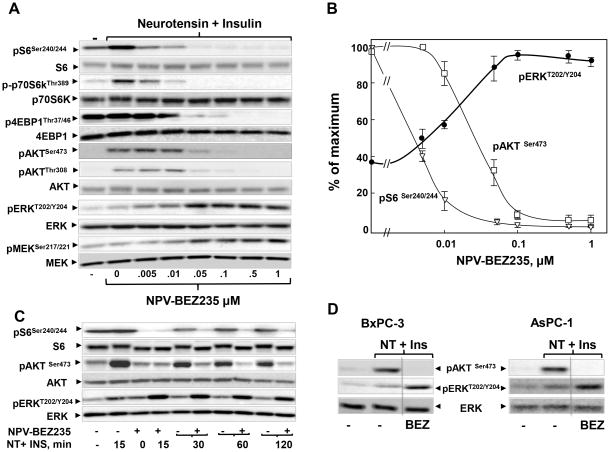
Initially, we determined the effect of the PI3K/TOR-KI NPV-BEZ235 (24,25) on the activity of mTORC1 and mTORC2 in MiaPaCa-2 cells, an extensively used model of ductal pancreatic adenocarcinoma cells. Serum-starved cultures of MiaPaCa-2 cells were incubated with increasing concentrations of NPV-BEZ235 (0.005 μM-1 μM) for 2 h. Then, the cells were stimulated with a combination of insulin and neurotensin to elicit potent mitogenic crosstalk signaling (16,17), including phosphorylation of S6K at Thr389, a site directly phosphorylated by mTORC1 and S6 at Ser240/244, a site directly targeted by S6K (Fig. 1A). Treatment with NPV-BEZ235, at the lowest concentration tested (0.005 μM), markedly inhibited phosphorylation of both S6K and S6 (Fig. 1A; quantification in Fig. 1B). The phosphorylation of these proteins was completely suppressed by higher doses of NPV-BEZ235 (> 0.01μM). NPV-BEZ235 also inhibited the phosphorylation of 4E-BP1 at Thr37/46 (Fig. 1A), sites that are sensitive to active-site mTOR inhibitors but not to rapamycin in PDAC cells (23).
Figure 1. NPV-BEZ235 induces over-activation of ERK phosphorylation in PDAC cells.
A: Cultures of MiaPaCa-2 cells were incubated in the absence or in the presence of NPV-BEZ235 (0,005–1 μM) for 2h. Then, the cells were stimulated for 30 min with 5 nM neurotensin (NT) and 10 ng/ml insulin (Ins) and lysed with SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with antibodies illustrated in the figure. B: Quantification of phosphorylated ERK at Thr202 and Tyr204, S6 at Ser 240/244 and AKT at Ser473 was performed using Multi Gauge V3.0. in 3 independent experiments similar to Fig 1A. C: Cultures of MiaPaCa-2 cells were incubated in the absence or in the presence of 1μM of NPV-BEZ235 for 2h and then stimulated with 5 nM neurotensin (NT) and 10 ng/ml insulin (Ins) for various times and lysed. Immunoblotting was performed as described in panel A. D: Cultures of BxPC-3 and AsPC-1 cells were treated with or without 1μM of NPV-BEZ235 (BEZ) for 2h and then stimulated with NT and Ins for 30 min and lysed. Immunoblotting was performed as in panel A. Image Editing: Irrelevant lanes were removed (indicated by a thin, vertical black line) from the acquired digital images and flanking lanes juxtaposed using Adobe Photoshop
Stimulation with neurotensin and insulin also induced phosphorylation of AKT on Ser473, a site directly phosphorylated by mTORC2 and at Thr308, a site phosphorylated by PDK1 in response to PI3K activation. AKT phosphorylation on both Ser473 and Thr308 was markedly decreased at 0.05 μM NPV-BEZ235 and it was completely abrogated at higher concentrations. These results indicate that NPV-BEZ235 inhibits the S6K arm of mTORC1 signaling at lower doses (< 0.01 μM) than those required to block mTORC1/4E-BP1, mTORC2 or PI3K/PDK1 (> 0.05 μM) in MiaPaCa-2 cells.
The salient feature in Fig. 1A is that NPV-BEZ235 induced a striking and dose-dependent stimulatory effect on ERK activity in MiaPaCa-2 cells, as monitored by ERK phosphorylation on Thr202 and Tyr204 (quantification in Fig. 1B). The maximal enhancement of ERK activation (3.1±0.2 fold; n=3) occurred at doses of NPV-BEZ235 that inhibited 4E-BP1 and mTORC2 (>0.05 μM). Treatment with NPV-BEZ235 also activated extracellular signal-regulated kinase kinase (MEK), upstream of ERK, as scored by phosphorylation of Ser217/221, residues in MEK directly phosphorylated by RAF kinases (Fig. 1A).
A potent over-activation of ERK induced in response to NPV-BEZ235 was also demonstrated when MiaPaCa-2 cells were stimulated with neurotensin and insulin for 2 h instead of 30 min (Fig. S2A). Exposure to NPV-BEZ235 markedly enhanced the level of phosphorylated ERK in MiaPaCa-2 cells stimulated with neurotensin and insulin for as little as 15 min and persisted for 120 min (Fig. 1C). Treatment with NPV-BEZ235 also enhanced ERK activation in other PDAC cells, including AsPC-1, BxPC-3 cells (Fig. 1D) and PANC -1 cells, another extensively used model of PDAC cells (Fig. S2B). Collectively, these results show that the dual PI3K/TOR-KI NPV-BEZ235 profoundly inhibits mTORC1, mTORC2 and PI3K but induces rapid, striking and dose-dependent activation of the MEK/ERK pathway in human PDAC cells.
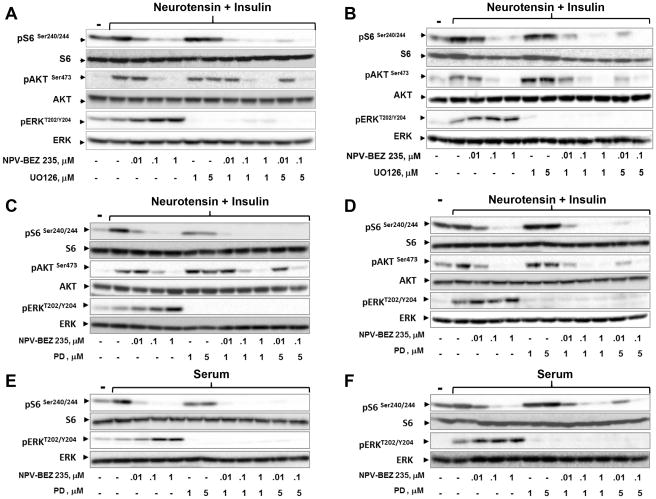
Treatment with MEK inhibitors abolishes over-activation of the ERK pathway induced by NVP-BEZ235
We next determined whether cell exposure to MEK inhibitors prevents ERK over-activation in response to PI3K/mTOR inhibition. Treatment of MiaPaCa-2 cells with U0126 (32), a preferential inhibitor of MEK, abrogated ERK over-activation induced by NPV-BEZ235 (Fig. 2A). Similarly, enhanced ERK phosphorylation induced by NPV-BEZ235 was blunted by U0126 in PANC-1 cells (Fig. 2B). PD0325901, a potent and specific allosteric inhibitor of MEK (30,33), also abrogated ERK over-activation induced by increasing doses of NPV-BEZ235 (Fig. 2C and Fig 2D). An inhibitory effect was elicited by PD0325901 at a dose as low as 5 nM (Fig. S3).
Figure 2. MEK inhibitors suppresses ERK over-activation induced by NPV-BEZ235.
A and B: Cultures of MiaPaCa-2 cells (A) and PANC-1 (B) cells were incubated for 2 h in the absence or presence of increasing doses of NPV-BEZ235 with or without U0126 at 1μM or 5μM. C and D: Cultures of MiaPaCa-2 cells (C) and PANC-1 (D) cells were incubated in the presence of increasing doses of NPV-BEZ235 with or without PD0325901 (PD) at 1μM or 5μM for 2 h. Then, for panels A–D, the cells were stimulated for 2 h with 5 nM neurotensin and 10 ng/ml insulin and lysed with SDS–PAGE sample buffer. E and F: Cultures of MiaPaCa-2 (E) and PANC-1 (F) cells were incubated for 2 h in the absence or presence of increasing doses of NPV-BEZ235 with or without the addition of PD0325901 (PD) at 1μM and 5μM. Then, the cells were stimulated for 2 h with 2% fetal bovine serum (serum) and lysed with SDS–PAGE sample buffer. All samples were analyzed by SDS-PAGE and immunoblotting with the antibodies that detect phosphorylated or total proteins, as described in each panel.
To extend further these findings, we also examined the effects of NPV-BEZ235 without or with PD0325901 in PDAC cells stimulated with fetal bovine serum (FBS). Exposure to NPV-BEZ235 over-activated ERK phosphorylation on Thr202 and Tyr204 in serum-stimulated cells, an effect abolished by PD-0325901 (Fig. 2E and 2F). The results indicate that enhanced ERK activation induced by treatment with NPV-BEZ235 can be prevented by co-targeting MEK in PDAC cells.
The intensity and duration of ERK activation are tightly regulated by negative feedback loops within the pathway, including inhibitory phosphorylations of SOS and RAF mediated by active by ERK (18). Negative feedback regulation of the ERK pathway has been recently shown in cancer cells with RAS mutation (34). Accordingly, treatment with PD0325901 released feedback inhibition as revealed by over-phosphorylation of MEK in either MiaPaCa-2 or PANC-1 cells (Fig. S4). Interestingly, NVP-BEZ235 further augmented MEK phosphorylation in PDAC cells treated with PD0325901, implying that the dual PI3K/mTOR inhibitor enhanced RAF/MEK activity in cells without ERK-mediated negative feedbacks loops.
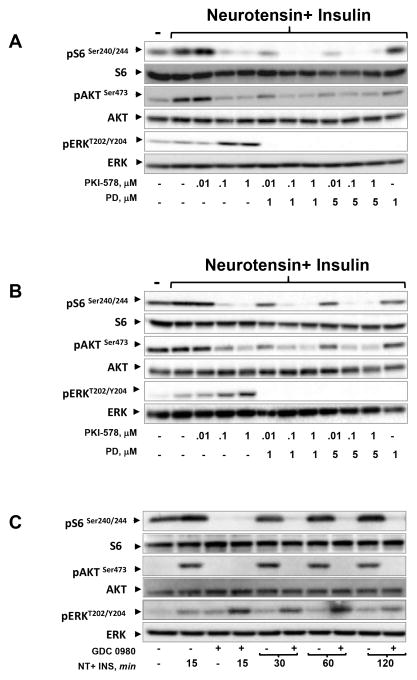
Enhanced ERK activation is also elicited by the mTOR/PI3K inhibitors PKI-587 and GDC-0980
Reflecting the intense interest in targeting the PI3K/mTOR pathway, a number of dual mTOR/PI3K inhibitors, other than NPV-BEZ235, have been developed, including PKI-587 (26,27) and GDC-0980 (28), the structure of which is displayed in Fig. S1. Next, we determined whether PKI-587 and GDC-0980 also enhance ERK activation in PDAC cells. As shown in Fig. 3A, phosphorylation of S6 on Ser240/244 and AKT on Ser473, monitoring mTORC1 and mTORC2 activity respectively, was inhibited by treatment with 0.1 μM and 1 μM of PKI-587. Exposure to PKI-587 also caused a striking increase in ERK activation, an effect completely blocked by concomitant exposure to the MEK inhibitor PD0325901. Similar effects were elicited by PKI-587 and PD0325901 in PANC-1 cells (Fig. 3B).
Figure 3. MEK inhibition blunts the enhancement of ERK activity induced by PKI-587 or GDC-0980.
A and B: Cultures of MiaPaCa-2 cells (A) and PANC-1 (B) cells were incubated for 2 h in the absence or presence of increasing doses of PKI-587 with or without PD0325901 (PD) at 1μM or 5μM. Then, the cells were stimulated for 90 min with 5 nM neurotensin and 10 ng/ml insulin and lysed with SDS–PAGE sample buffer. All samples were analyzed by SDS-PAGE and immunoblotting using the antibodies that detect phosphorylated or total proteins, as described in each panel. C: Cultures of MiaPaCa-2 cells were incubated in the absence or presence of 1μM of GDC-0980 for 2 h, and then the cells were stimulated for 15, 30, 60 or 120 min with 5 nM neurotensin (NT) and 10 ng/ml insulin (Ins) and lysed with SDS–PAGE sample buffer. The extracts were then analyzed as in Panels A and B.
GDC-0980 has also recently identified as a selective, potent and orally bio-available inhibitor of PI3K and mTOR (28). To examine the effects of GDC-0980, MiaPaCa-2 cells were incubated with or without this PI3K/TOR-KI and then stimulated for various times (Fig. 3C), as shown before with NVP-BEZ235 in Fig. 1C. GDC-0980 completely inhibited phosphorylation of S6 on Ser240/244 and AKT on Ser473 but produced a prominent ERK over-activation at all times examined (Fig. 3C). Thus, multiple clinically relevant dual PI3K/mTOR inhibitors induce ERK over-activation in PDAC cells.
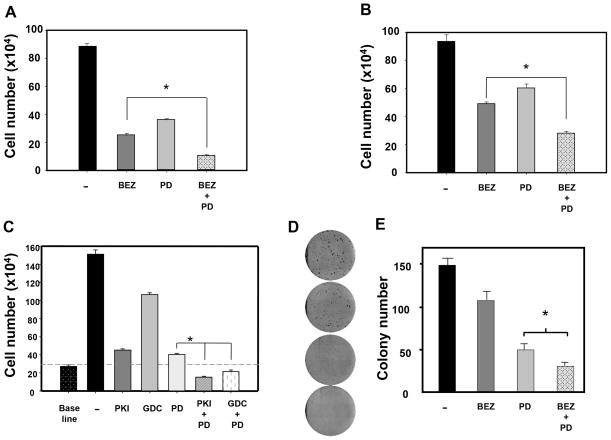
Effect of NPV-BEZ235, PD0325901 and their combination on PDAC cell proliferation and colony formation
In order to examine whether the over-activation of the ERK pathway counterbalances the growth-suppressive effect of mTOR/PI3K inhibitors, we determined the proliferation of MiaPaCa-2 cells treated with NPV-BEZ235, PD0325901 or a combination of NPV-BEZ235 and PD0325901 (Fig. 4A). Each inhibitor reduced cell proliferation but the combination of NPV-BEZ235 and PD0325901 produced a further inhibitory effect on MiaPaCa-2 cell proliferation. Importantly, the difference between PD0325901 and the combination of NPV-BEZ with PD0325901 was statistically significant. Similar results were obtained using PANC-1 cells (Fig. 4B). Similarly, the inhibitory effect of PKI-587 and GDC-0980 on MiaPaCa-2 proliferation was markedly enhanced by PD0325901 (Fig. 4C). The results indicate that co-targeting the PI3K/mTOR and MEK induces profound inhibition of PDAC cell proliferation. To test further this conclusion, we determined the effect of long exposure to low concentrations of NPV-BEZ, PD0325901 or their combination on the colony-forming ability of MiaPaCa-2 cells. Treatment with either NPV-BEZ or PD0325901, each at a concentration as low as 5 nM, markedly reduced the number of colonies formed by MiaPaCa-2 cells (Figs. 4D and 4E). Exposure to the combination of NPV-BEZ and PD0325901 further inhibited the number of colonies formed by MiaPaCa-2 cells (Figs. 4D and 4E).
Figure 4. Dual PI3K/mTOR kinase inhibitors and PD0325901 inhibit the proliferation of PDAC cells.
A, Single-cell suspensions of MiaPaCa-2 cells were plated at a density of 105 cells per dish. After 24 h, the cultures were shifted to media containing fetal bovine serum (FBS) with 100n NPV-BEZ235 (BEZ), 100 nM PD0325901 (PD) or combination of both drugs as indicated. After 72 h, cell numbers were determined from 6 plates per condition. Results are presented as means ± SEM. B, Single-cell suspensions of PANC-1 cells were plated at a density of 105 cells per dish. After 24 h, the cultures were shifted to media containing FBS with 100 nM NPV-BEZ235 (BEZ), 500 nM PD0325901 (PD) or combination of both drugs as indicated. After 72 h, cell numbers were determined from 6 plates per condition. Results are presented as means ± SEM. C: Single-cell suspensions of MiaPaCa-2 cells were plated at a density of 105 cells per dish. After 24 h, the cultures were shifted to media containing FBS with 100 nM PKI-587 (PKI), 100 nM of GDC-0980 (GDC), 100 nM PD0325901 (PD) and combinations of either PKI-587 or GDC-0980 with PD0325901, as indicated. After 72 h, cell numbers were determined from 6 plates per condition. Results are presented as means ± SEM. D: Cell colony formation was performed as described in the methods section. MiaPaCa-2 cells were incubated for 8 days with 5 nM of NPV-BEZ235 (BEZ), 5 nM PD0325901 (PD) or with a combination of both drugs. E: The bars represent the number of colonies (means ± SEM; n=3 dishes per condition). * T-test p values comparing the indicated 2 groups were < 0.001.
Dual mTOR/PI3K inhibitors induce ERK over-activation through a PI3K-independent pathway
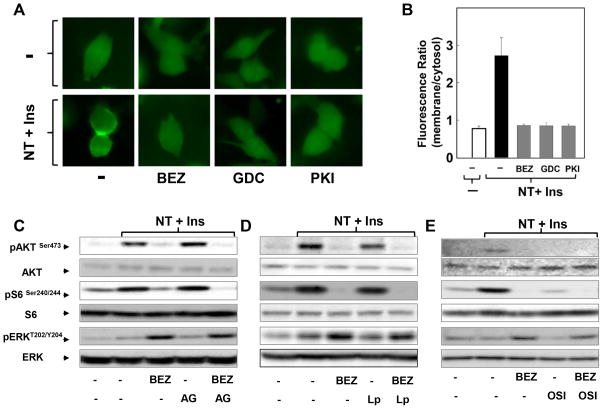
Having established that dual PI3K/mTOR inhibitors lead to enhanced MEK/ERK activation in PDAC cells, we next examined the mechanism(s) involved. Previous studies with prostate and breast cancer cells identified a feedback loop that mediates ERK over-activation in response to rapamycin analogs through a PI3K-dependent pathway (35). In order to evaluate PI3K activity, we determined the effect of NVP-BEZ235, PKI-587 and GDC-0980 on PI3K-generated accumulation of phosphatidylinositol 3,4,5-trisphosphate (PIP3) in the plasma membrane of individual PDAC cells. MiaPaCa-2 cells were transiently transfected with a plasmid encoding a fusion protein between green fluorescent protein (GFP) and the PH domain of AKT (AKT-PH-GFP), an in vivo reporter of PIP3 (36,37). In unstimulated cells, the PIP3 sensor did not display any detectable accumulation at the plasma membrane (Fig. 5A and Fig. S5). Stimulation with neurotensin and insulin induced a rapid and striking translocation of AKT-PH-GFP to the plasma membrane, indicative of robust PI3K activation (Fig. 5A; quantification in Fig. 5B). Prior exposure to NPV-BEZ235, PKI-587 or GDC-0980 completely prevented the translocation of the PIP3 sensor to the plasma membrane (5A; quantification in Fig. 5B). Similar results were obtained after different times of stimulation (Fig. S5). The results presented in Figs.5 and S5 indicate that dual PI3K/mTOR inhibitors induce MEK/ERK activation in PDAC cells through a PI3K-independent pathway.
Figure 5. NPV-BEZ235 enhances ERK pathway activation through a pathway that does not require PI3K, EGFR, HER2, insulin receptor or IGF-1R.
A: MiaPaCa-2 cells were transiently transfected with a plasmid encoding a fusion protein between GFP and the PH domain of AKT (AKT-PH-GFP). After 24h, the cultures were incubated in DMEM without or with NVP-BEZ235 (BEZ), PKI-587 (PKI) or GDC-0980 (GDC) each at 1μM for 1h prior to stimulation with 5 nM neurotensin and 10 ng/ml insulin. The intracellular distribution of AKT-PH-GFP was monitored under a fluorescence microscope. The selected cells displayed in the figures were representative of 90% of the population of GFP-positive cells. B: Graphic represents quantification from panel A (ratio of membrane/cytoplasm fluorescence). C, D and E: Cultures of MiaPaCa-2 cells were incubated for 2 h in the absence or presence of 1 μM NPV-BEZ235 (BEZ) with or without 1 μM of AG-1478 (AG, panel C), 1 μM of Lapatinib (Lp, panel D) or 1 μM of OSI-906 (OSI, panel E). Then, the cells were stimulated with 5 nM neurotensin and 10 ng/ml insulin and lysed with SDS–PAGE sample buffer. All samples were analyzed by SDS-PAGE and immunoblotting with the antibodies that detect phosphorylated or total S6, AKT and ERK proteins, as indicated in each panel.
NPV-BEZ235 enhances ERK activation independently of EGFR, HER2, insulin receptor and IGF-1 receptor
Chronic suppression of PI3K/mTORC1 stimulates FOXO-dependent expression of several tyrosine kinase receptors, including, IGF/insulin receptors and HER3 in tumor cells, thereby enhancing ERK activity (38,39). This mechanism is unlikely to explain our results, given the rapidity of the effects shown here with PDAC cells. To test this possibility directly, we determined whether inhibitors of EGFR (AG1438), EGFR and HER2 (lapatinib) or insulin/IGF-1 receptors (OSI-906) prevent enhanced ERK activation in response to NPV-BEZ235. As a control, we verified that the inhibitors, at the concentrations used, abrogated ERK activation induced by EGF or IGF-1 in MiaPaCa-2 cells (Fig. S6A). Neither AG1438 (EGFR tyrosine kinase inhibitor) nor lapatinib (inhibitor of EGFR and HER2) prevented enhanced ERK activation by NPV-BEZ235 (Fig. 5C and Fig. 5D; quantification in Fig. S6B and Fig. S6C).
We also examined the involvement of the insulin/IGF-1 receptors in mediating ERK activation in response to NPV-BEZ235. Exposure to the insulin/IGF-1 receptor inhibitor OSI-906 reduced baseline levels of ERK phosphorylation but did not prevent the ERK activation induced by NPV-BEZ235 (Fig. 5E). Indeed, the dual PI3K/mTOR inhibitor induced a similar relative enhancement of ERK phosphorylation either in the absence or presence of OSI-906 (Fig. S6D). Thus, NPV-BEZ235 enhances ERK activation through a pathway that does not require EGFR, HER2 or insulin/IGF-1 receptors.
Knockdown of Rictor prevents enhancement of ERK activation by NPV-BEZ235 independently of AKT
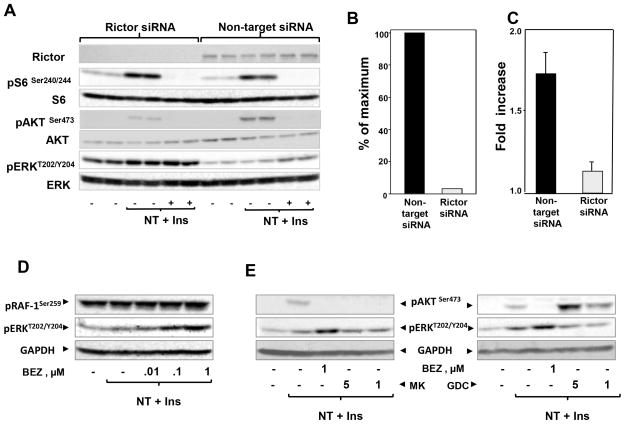
As shown throughout this study, the doses of NPV-BEZ235 that enhanced MEK/ERK activation coincided with those that inhibited AKT phosphorylation on Ser473, prompting us to hypothesize that NPV-BEZ235 suppresses a negative feedback mediated by mTORC2. In order to test this possibility, we used RNA interference to silence Rictor, an essential and specific component of mTORC2. Transfection of MiaPaCa-2 cells with siRNA targeting Rictor caused a striking decrease in the expression of Rictor protein but did not alter the expression of S6, AKT or ERK (Fig. 6A; quantification in Fig. 6B). As expected, knockdown of Rictor did not prevent mTORC1/S6K activation, scored by S6 phosphorylation but abolished AKT phosphorylation on Ser473, a function mediated by mTORC2. Surprisingly, knockdown of Rictor markedly increased baseline levels of ERK phosphorylation and treatment with NPV-BEZ235 failed to produce a significant further enhancement of ERK activation (Fig. 6A; quantification of 3 independent experiments shown in Fig. 6C) whereas NPV-BEZ235 enhanced ERK activation in cells transfected with non-targeting siRNA. Transfection with a siRNA directed to a different region of Rictor also attenuated the enhancement of ERK activity induced by NVP-BEZ235.
Figure 6. NPV-BEZ235 enhances ERK activation through a Rictor(mTORC2)-dependent but AKT- independent pathway.
A: MiaPaCa-2 cells were transfected with siRNA targeting Rictor or non-targeted siRNA. After 3 days, cells were incubated with 1μM NPV-BEZ235 (BEZ) for 2h. Then, the cells were stimulated for 60 min with 5 nM neurotensin and 10 ng/ml insulin and lysed with SDS–PAGE sample buffer. The samples were analyzed by SDS-PAGE and immunoblotting with the indicated antibodies. B: Representation of quantification of levels of Rictor protein after transfection with non-targeted siRNA or siRNA targeting Rictor. C: Fold increase of phosphorylated ERK. Quantification of phosphorylated ERK at Thr202 and Tyr204 from 3 independent experiments was performed using Multi Gauge V3.0. D: MiaPaCa-2 cells were incubated for 2 h in the absence or presence of increasing doses of NVP-BEZ235 (BEZ) and then stimulated for 90 min with 5 nM neurotensin (NT) and 10 ng/ml insulin (Ins) and lysed with SDS–PAGE sample buffer. All samples were analyzed by SDS-PAGE and immunoblotting using the antibodies that detect phosphorylated Raf-1 (at Ser259) and ERK or total glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control. E MiaPaCa-2 cells were incubated for 2 h in the absence or presence of 1μM NVP-BEZ235 (BEZ) and either MK2206 (MK) at 1μM or 5μM or GDC-0068 (GDC) at 1μM or 5μM, as indicated. The cultures were then stimulated for 60 min with 5 nM neurotensin (NT) and 10 ng/ml insulin (Ins) and lysed with SDS–PAGE sample buffer. All samples were analyzed by SDS-PAGE and immunoblotting using the antibodies that detect phosphorylated AKT and ERK or total GAPDH as a loading control. Note that MK2206 (allosteric inhibitor) inhibited AKT phosphorylation whereas GDC0068 (active-site inhibitor) increased AKT phosphorylation, presumably by stabilizing a conformation that prevents AKT dephosphorylation.
AKT has been proposed to inhibit RAF-1 activity by direct phosphorylation at Ser259 (40), but this mechanism of negative crosstalk was disputed in subsequent studies (41). Here, we tested whether the enhancement of ERK activation induced by NVP-BEZ235 is mediated by down-regulation of AKT-mediated RAF-1 phosphorylation at Ser259. As shown in Fig. 6D, exposure of MiaPaCa-2 cells to NVP-BEZ235 did not produce any detectable decrease in the high level of RAF-1 phosphorlation at Ser259, even at concentrations hat produced robust enhancement of ERK activation. Furthermore, treatment with allosteric (MK-2206) or active-site (GDC-0068) inhibitors of AKT did not replicate the increase in ERK activation produced by NVP-BEZ235 (Fig. 6E). These results indicate that treatment with the dual PI3K/mTOR inhibitor suppresses a novel negative feedback loop mediated by mTORC2, thereby leading to enhanced MEK/ERK pathway activity in pancreatic cancer cells.
DISCUSSION
While augmented PI3K/AKT activity in response to mTORC1/S6K inhibition by rapamycin and its analogs is well documented in a variety of cell types (18–21), including PDAC (23), over-activation of the MEK/ERK pathway by mTOR inhibitors has been less explored (18). Recently, we reported that active-site mTOR inhibitors (KU63794 and PP242) induce a marked increase of MEK/ERK pathway activity in PDAC cells (23). Here, we demonstrate that the structurally unrelated dual PI3K/mTOR inhibitors NPV-BEZ235 (24,25), PKI-587 (26,27) and GDC-0980 (28) promote a striking, dose-dependent increase in ERK activation in PDAC cells stimulated with cross-talking mitogens such as insulin and neurotensin or serum factors. The dual PI3K/mTOR inhibitors also induced MEK over-activation and MEK inhibitors, including U126 and PD0325901, abrogated the over-activation of MEK. Our findings show, for the first time, that dual PI3K/mTOR inhibitors induce rapid over-activation of the MEK/ERK pathway, a pivotal pathway in PDAC cells and other malignancies.
In order to understand the mechanism by which dual PI3K/mTOR inhibitors promoted ERK activation, we determined the role of a feedback loop involving mTORC1/S6K/PI3K/ERK, proposed to mediate ERK activation in prostate and breast cancer cells in response to rapamycin analogs (35). In detailed dose-response studies, we found that low doses of NPV-BEZ235 profoundly reduced mTORC1/S6K activity but produced small enhancement of the ERK pathway. Accordingly, neither rapamycin nor everolimus, at concentrations that completely blocked the mTORC1/S6K axis, produced any detectable enhancement of ERK activation in PDAC cells (23). These results indicate that ERK over-activation in response to dual PI3K/mTOR inhibitors can be dissociated from feedback loops mediated through the mTORC1/S6K axis in PDAC cells.
Further evidence supporting that PI3K/TOR-KIs enhance ERK over-activation through a PI3K-independent feedback loop was obtained by showing that these agents suppressed PI3K activity at concentrations that enhanced ERK. Specifically, we evaluated the effect of NPV-BEZ235, PKI-587 or GDC-0980 on PI3K activity in single cells, as monitored by the distribution of AKT-PH-GFP, an in vivo reporter of PIP3 (36,37). We found that dual PI3K/mTOR inhibitors blunted the translocation of AKT-PH-GFP from the cytosol to the plasma membrane, indicating that these agents prevented PIP3 accumulation at the plasma membrane. Collectively, the results with dual PI3K/mTOR catalytic kinase inhibitors identify a novel PI3K-independent feedback mechanism that restrains the activity of the MEK/ERK pathway which is different from the loop previously identified with rapamycin (35).
Treatment of a variety of tumor cells with inhibitors that block the PI3K/AKT/mTOR pathway induces a transcriptional response mediated, at least in part by FoxO family members that lead to the over-expression of tyrosine kinase receptors or adaptor proteins, including insulin/IGF-1 receptor and HER3 thereby leading to enhancement of ERK (38,39,42–45). This gene expression loop should be distinguished from the effects induced by dual PI3K/mTOR inhibitors in this study since the enhancement of MEK/ERK activation occurred rapidly (within minutes) and was not prevented by inhibitors of insulin/IGF-1 receptor (OSI-906) or EGFR and HER2 (lapatinib). Our results with PDAC cells with KRAS mutations also contrast with a recent study demonstrating that acute inhibition of PI3K transiently inhibits ERK in breast cancer cells harboring HER-2 amplification or lacking PTEN but expressing wild type RAS (46). We conclude that the impact of suppressing feedback loops mediated by the PI3K/AKT/mTOR pathway depends on cell context and leads to different MEK/ERK outcomes in cancer cells harboring mutations in different pro-oncogenic pathways.
While many studies demonstrated negative feedback regulation by mTORC1 (18), a similar role for mTORC2 is only emerging. For example, a recent study revealed that mTORC2 can also regulate the cellular level of IRS-1 and concluded that mTORC1 and mTORC2 cooperate in promoting IRS-1 degradation (47). We noted that the doses of NPV-BEZ235 that enhanced MEK/ERK activation coincided with those that inhibited mTORC2-mediated AKT phosphorylation on Ser473 raising the possibility that NPV-BEZ235 suppresses a negative feedback loop operated through mTORC2. In order to test this possibility, we used RNA interference to silence Rictor, an essential and specific component of mTORC2. Knockdown of Rictor increased baseline levels of ERK phosphorylation and treatment with NPV-BEZ235 failed to produce a significant further enhancement of ERK activation. Based on our results, we propose that dual PI3K/mTOR inhibitors suppress a novel negative feedback loop mediated by mTORC2 thereby leading to enhanced MEK/ERK pathway activity in pancreatic cancer cells. We also show that the negative loop mediated by mTORC2 is through an AKT-independent pathway.
Given the role of the RAS/MEK/ERK pathway in PDAC initiation, development and maintenance (48), we hypothesized that inhibition of over-activated MEK/ERK should increase the growth-suppressive effects of dual PI3K/mTOR inhibitors in these cells. In line with this hypothesis, we found that the potent and highly specific MEK1/2 inhibitor PD0325901 suppressed enhanced ERK activation induced by PI3K/TOR-KIs and enhanced the growth-inhibitory effects of these agents in PDAC cells. Given that dual PI3K/mTOR inhibitors are increasingly considered for clinical use, the findings presented here suggest that suppression of cell-specific feedback loops by these inhibitors leading to MEK/ERK over-activation should be considered in their potential use for therapy of PDAC and other malignancies.
Supplementary Material
Acknowledgments
Financial support:
This study was supported by National Institutes of Health Grants P30DK41301, P01CA163200, R01DK100405, and Department of Veterans Affair Grant 1I01BX001473 all to E. Rozengurt. Funds from the Ronald S. Hirschberg Endowed Chair of Pancreatic Cancer Research to E. Rozengurt and a Ronald S. Hirschberg Foundation Seed Grant to H.P. Soares and a CSC scholarship to L. Han
Footnotes
Conflict of interest:
The authors declare no conflicts of interest
References
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Shimobayashi M, Hall MN. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat Rev Mol Cell Biol. 2014;15:155–62. doi: 10.1038/nrm3757. [DOI] [PubMed] [Google Scholar]
- 3.Asano T, Yao Y, Shin S, McCubrey J, Abbruzzese JL, Reddy SA. Insulin receptor substrate is a mediator of phosphoinositide 3-kinase activation in quiescent pancreatic cancer cells. Cancer Res. 2005;65:9164–8. doi: 10.1158/0008-5472.CAN-05-0779. [DOI] [PubMed] [Google Scholar]
- 4.Eser S, Reiff N, Messer M, Seidler B, Gottschalk K, Dobler M, et al. Selective requirement of PI3K/PDK1 signaling for Kras oncogene-driven pancreatic cell plasticity and cancer. Cancer Cell. 2013;23:406–20. doi: 10.1016/j.ccr.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL, Reddy SA. The rapamycin analog CCI-779 is a potent inhibitor of pancreatic cancer cell proliferation. Biochem Biophys Res Commun. 2005;331:295–302. doi: 10.1016/j.bbrc.2005.03.166. [DOI] [PubMed] [Google Scholar]
- 6.Pham NA, Schwock J, Iakovlev V, Pond G, Hedley DW, Tsao MS. Immunohistochemical analysis of changes in signaling pathway activation downstream of growth factor receptors in pancreatic duct cell carcinogenesis. BMC Cancer. 2008;8:43. doi: 10.1186/1471-2407-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castellano E, Downward J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes Cancer. 2011;2:261–74. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kornmann M, Maruyama H, Bergmann U, Tangvoranuntakul P, Beger HG, White MF, et al. Enhanced expression of the insulin receptor substrate-2 docking protein in human pancreatic cancer. Cancer Res. 1998;58:4250–4. [PubMed] [Google Scholar]
- 10.Kolb S, Fritsch R, Saur D, Reichert M, Schmid RM, Schneider G. HMGA1 controls transcription of insulin receptor to regulate cyclin D1 translation in pancreatic cancer cells. Cancer Res. 2007;67:4679–86. doi: 10.1158/0008-5472.CAN-06-3308. [DOI] [PubMed] [Google Scholar]
- 11.Kwon J, Stephan S, Mukhopadhyay A, Muders MH, Dutta SK, Lau JS, et al. Insulin receptor substrate-2 mediated insulin-like growth factor-I receptor overexpression in pancreatic adenocarcinoma through protein kinase C delta. Cancer Res. 2009;69:1350–7. doi: 10.1158/0008-5472.CAN-08-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoeltzing O, Liu W, Reinmuth N, Fan F, Parikh AA, Bucana CD, et al. Regulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by an insulin-like growth factor-I receptor autocrine loop in human pancreatic cancer. Am J Pathol. 2003;163:1001–11. doi: 10.1016/s0002-9440(10)63460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi WD, Meng ZQ, Chen Z, Lin JH, Zhou ZH, Liu LM. Identification of liver metastasis-related genes in a novel human pancreatic carcinoma cell model by microarray analysis. Cancer Lett. 2009;283:84–91. doi: 10.1016/j.canlet.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 14.Feng Z, Levine AJ. The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol. 2010;20:427–34. doi: 10.1016/j.tcb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong X, Javle M, Hess KR, Shroff R, Abbruzzese JL, Li D. Insulin-Like Growth Factor Axis Gene Polymorphisms and Clinical Outcomes in Pancreatic Cancer. Gastroenterology. 2010;139:464–73. doi: 10.1053/j.gastro.2010.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kisfalvi K, Eibl G, Sinnett-Smith J, Rozengurt E. Metformin disrupts crosstalk between G protein-coupled receptor and insulin receptor signaling systems and inhibits pancreatic cancer growth. Cancer Res. 2009;69:6539–45. doi: 10.1158/0008-5472.CAN-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between Insulin/Insulin-like Growth Factor-1 Receptors and G Protein-Coupled Receptor Signaling Systems: A Novel Target for the Antidiabetic Drug Metformin in Pancreatic Cancer. Clin Cancer Res. 2010;16:2505–11. doi: 10.1158/1078-0432.CCR-09-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rozengurt E, Soares HP, Sinnet-Smith J. Suppression of Feedback Loops Mediated by PI3K/mTOR Induces Multiple Overactivation of Compensatory Pathways: An Unintended Consequence Leading to Drug Resistance. Mol Cancer Ther. 2014;13:2477–88. doi: 10.1158/1535-7163.MCT-14-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane HA. Breuleux Optimal targeting of the mTORC1 kinase in human cancer. Curr Opin Cell Biol. 2009;21:219–29. doi: 10.1016/j.ceb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 22.Figlin RA, Kaufmann I, Brechbiel J. Targeting PI3K and mTORC2 in metastatic renal cell carcinoma: New strategies for overcoming resistance to VEGFR and mTORC1 inhibitors. Int J Cancer. 2013;133:788–96. doi: 10.1002/ijc.28023. [DOI] [PubMed] [Google Scholar]
- 23.Soares HP, Ni Y, Kisfalvi K, Sinnett-Smith J, Rozengurt E. Different Patterns of Akt and ERK Feedback Activation in Response to Rapamycin, Active-Site mTOR Inhibitors and Metformin in Pancreatic Cancer Cells. PLoS ONE. 2013;8:e57289. doi: 10.1371/journal.pone.0057289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–63. doi: 10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 25.Yang F, Qian XJ, Qin W, Deng R, Wu XQ, Qin J, et al. Dual Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Inhibitor NVP-BEZ235 Has a Therapeutic Potential and Sensitizes Cisplatin in Nasopharyngeal Carcinoma. PLoS ONE. 2013;8:e59879. doi: 10.1371/journal.pone.0059879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallon R, Feldberg LR, Lucas J, Chaudhary I, Dehnhardt C, Santos ED, et al. Antitumor Efficacy of PKI-587, a Highly Potent Dual PI3K/mTOR Kinase Inhibitor. Clin Cancer Res. 2011;17:3193–203. doi: 10.1158/1078-0432.CCR-10-1694. [DOI] [PubMed] [Google Scholar]
- 27.Venkatesan AM, Dehnhardt CM, Delos Santos E, Chen Z, Dos Santos O, Ayral-Kaloustian S, et al. Bis(morpholino-1,3,5-triazine) Derivatives: Potent Adenosine 5′-Triphosphate Competitive Phosphatidylinositol-3-kinase/Mammalian Target of Rapamycin Inhibitors: Discovery of Compound 26 (PKI-587), a Highly Efficacious Dual Inhibitor. J Med Chem. 2010;53:2636–45. doi: 10.1021/jm901830p. [DOI] [PubMed] [Google Scholar]
- 28.Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, et al. GDC-0980 Is a Novel Class I PI3K/mTOR Kinase Inhibitor with Robust Activity in Cancer Models Driven by the PI3K Pathway. Mol Cancer Ther. 2011;10:2426–36. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- 29.Deer EL, Gonzalez-Hernandez J, Coursen JD, Shea JE, Ngatia J, Scaife CL, et al. Phenotype and genotype of pancreatic cancer cell lines. Pancreas. 2010;39:425–35. doi: 10.1097/MPA.0b013e3181c15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sebolt-Leopold JS, Herrera R. Targeting the mitogen-activated protein kinase cascade to treat cancer. Nat Rev Cancer. 2004;4:937–47. doi: 10.1038/nrc1503. [DOI] [PubMed] [Google Scholar]
- 31.Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protocols. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 32.Favata MF, Horiuchi KY, Manos EJ, Dauleri AJ, Stradley DA, Feeser WS, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–32. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 33.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishii N, Harada N, Joseph EW, Ohara K, Miura T, Sakamoto H, et al. Enhanced inhibition of ERK signaling by a novel allosteric MEK inhibitor, CH5126766, that suppresses feedback reactivation of RAF activity. Cancer Res. 2013;73:4050–60. doi: 10.1158/0008-5472.CAN-12-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–74. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon Y, Hofmann T, Montell C. Integration of Phosphoinositide- and Calmodulin-Mediated Regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni Y, Sinnett-Smith J, Young SH, Rozengurt E. PKD1 Mediates Negative Feedback of PI3K/Akt Activation in Response to G Protein-Coupled Receptors. PLoS One. 2013;8:e73149. doi: 10.1371/journal.pone.0073149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serra V, Scaltriti M, Prudkin L, Eichhorn PJA, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimmermann S, Moelling K. Phosphorylation and Regulation of Raf by Akt (Protein Kinase B) Science. 1999;286:1741–4. doi: 10.1126/science.286.5445.1741. [DOI] [PubMed] [Google Scholar]
- 41.Romano D, Nguyen LK, Matallanas D, Halasz M, Doherty C, Kholodenko BN, et al. Protein interaction switches coordinate Raf-1 and MST2/Hippo signalling. Nat Cell Biol. 2014;16:673–84. doi: 10.1038/ncb2986. [DOI] [PubMed] [Google Scholar]
- 42.Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012;109:2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21:227–39. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cen B, Mahajan S, Wang W, Kraft AS. Elevation of Receptor Tyrosine Kinases by Small Molecule AKT Inhibitors in Prostate Cancer Is Mediated by Pim-1. Cancer Res. 2013;73:3402–11. doi: 10.1158/0008-5472.CAN-12-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan Y, Serra V, Prudkin L, Scaltriti M, Murli S, Rodriguez O, et al. Evaluation and Clinical Analyses of Downstream Targets of the Akt Inhibitor GDC-0068. Clin Cancer Res. 2013;19:6976–86. doi: 10.1158/1078-0432.CCR-13-0978. [DOI] [PubMed] [Google Scholar]
- 46.Will M, Qin AC, Toy W, Yao Z, Rodrik-Outmezguine V, Schneider C, et al. Rapid induction of apoptosis by PI3K inhibitors is dependent upon their transient inhibition of RAS-ERK signaling. Cancer Discov. 2014;4:334–47. doi: 10.1158/2159-8290.CD-13-0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SJ, DeStefano MA, Oh WJ, Wu CC, Vega-Cotto NM, Finlan M, et al. mTOR complex 2 regulates proper turnover of insulin receptor substrate-1 via the ubiquitin ligase subunit Fbw8. Mol Cell. 2012;48:875–87. doi: 10.1016/j.molcel.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji B, Tsou L, Wang H, Gaiser S, Chang DZ, Daniluk J, et al. Ras Activity Levels Control the Development of Pancreatic Diseases. Gastroenterology. 2009;137:1072–82. doi: 10.1053/j.gastro.2009.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.