Abstract
Genetic counseling and testing for hereditary breast and ovarian cancer now includes practitioners from multiple healthcare professions, specialties, and settings. This study examined whether non-genetics professionals (NGPs), perform guideline-based patient intake and informed consent before genetic testing. NGPs offering BRCA testing services in Florida (n = 386) were surveyed about clinical practices. Among 81 respondents (response rate = 22%), approximately half reported: sometimes scheduling a separate session for pretest counseling lasting 11–30 minutes prior to testing, discussing familial implications of testing, benefits and limitations of risk management options, and discussing the potential psychological impact and insurance related issues. Few constructed a three-generation pedigree, discussed alternative hereditary cancer syndromes, or the meaning of a variant result. This lack of adherence to guideline based practice may result in direct harm to the patients and their family members. NGPs that are unable to deliver guideline adherent cancer genetics services should focus on identification and referral of at-risk patients to in person or telephone services provided by genetics professionals.
Keywords: BRCA, Genet Counseling, Genet Testing, Hereditary Breast and Ovarian Cancer, Non-Genetics Professionals
Introduction
Clinical BRCA1 and BRCA2 (BRCA) testing enables the identification of individuals at greatly elevated risk for hereditary breast and ovarian cancer (HBOC) (1). Individuals at risk for HBOC (2) may present in primary or oncology care; thus, genetic counseling (GC) and testing for HBOC is increasingly being delivered by non-genetics professionals (NGPs) (3–5). In the current study, NGPs are defined as health care professionals from multiple disciplines (e.g., nursing, medicine), specialties (e.g., gynecology, medical oncology, surgery, family medicine) and practice settings (e.g., hospitals, ambulatory clinics, surgical center) without advanced academic and/or clinical training in genetics.
Professional organization guidelines strongly endorse counseling prior to genetic testing (6–10) and provide guidelines for the conduct of GC including: 1) patient intake of personal and family medical history to facilitate risk assessment; and 2) informed consent prior to genetic testing (6–8, 10, 11). For patient intake, some organizations specify the use of a three-generation pedigree (7), and the majority detail information that should be incorporated in the informed consent process including: information about genetic testing, why it is indicated, possible test outcomes, testing alternatives, implications of test results for family, economic wellbeing, psychosocial wellbeing, and cancer surveillance and prevention options (7, 11). As part of a larger research initiative regarding delivery of BRCA counseling and testing services in Florida, we examined the extent to which NGPs deliver pretest GC, perform patient intake, and address components of informed consent.
Materials and Methods
Between November 2011 and March 2012, healthcare providers utilizing BRCA genetic testing were surveyed regarding knowledge, recommendations, clinical practices, and preferences for information related to genetic testing for HBOC. The Institutional Review Board approved this research and granted an informed consent waiver. The current study focused on pretest GC practices and the extent to which patient intake and informed consent processes are incorporated into the delivery of genetics services among a sample of Florida-based practitioners. The parent study methods are described in detail elsewhere (12) and summarized below.
Sample
Myriad Genetics held the patent for clinical BRCA testing in the U.S. at the time of this study. Their website was used to identify healthcare practitioners and/or healthcare facilities that provided BRCA testing services in Florida as of September 2011 (12). The list was supplemented with additional Florida-based practitioners known to provide BRCA testing services. Surveys were mailed to a total of 386 practitioners using Dillman’s (13) method.
Instrument
The survey covered: 1) pretest GC practices; 2) knowledge, clinical opinion, and recommendations; 3) information sources and preferences; and 4) demographic and practice characteristics. Previous studies reported on the second (12) and third areas (14); the current study focused on pretest GC practices. Participants reported the frequency with which they schedule specific pretest GC visits (four-point Likert scale: never, sometimes, usually, always), and the average total minutes spent counseling their patients prior to BRCA testing, whether in one or multiple visit(s). Participants also reported whether they constructed a three-generation pedigree as part of GC, and the frequency with which they discussed the following topics with their patients before genetic testing: the possibility of other hereditary cancer syndromes, meaning of a variant (i.e., uncertain) result, implications of test results to family members, psychological impact of testing, insurance-related issues (including life and disability), and the benefits and limitations of risk management options (e.g., screening, surgery, chemoprevention) (five-point Likert scale: ranging from never to always).
Statistical analyses
Frequencies and percentages were calculated to characterize participants’ demographic and practice characteristics and responses to questions about pretest GC practices.
Results
The final sample size was 81 and the response rate was 22% (14). The majority (73%) of respondents was female and 67% were physicians. The largest proportion had no formal genetics training (56%), and had five to nine years of experience performing genetic testing (37%). Almost all practiced in a community or private practice setting, and 38% practiced with one or two other individuals providing genetic testing. Respondents reported seeing a predominately non-Hispanic White (86%), privately insured (58%) patient population. About 61% saw one to five new patients each week for GC. Additional descriptive information about respondents was previously reported (14).
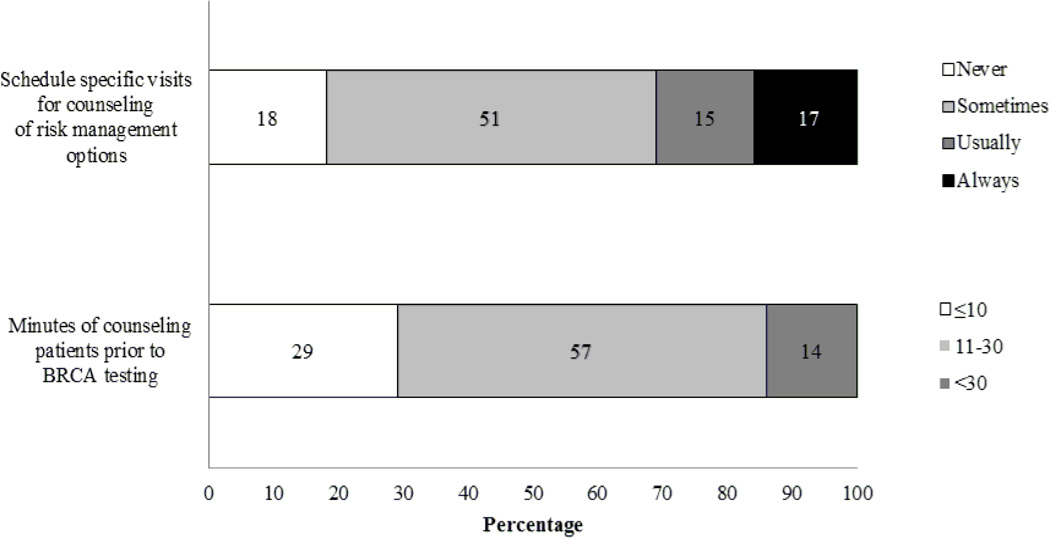
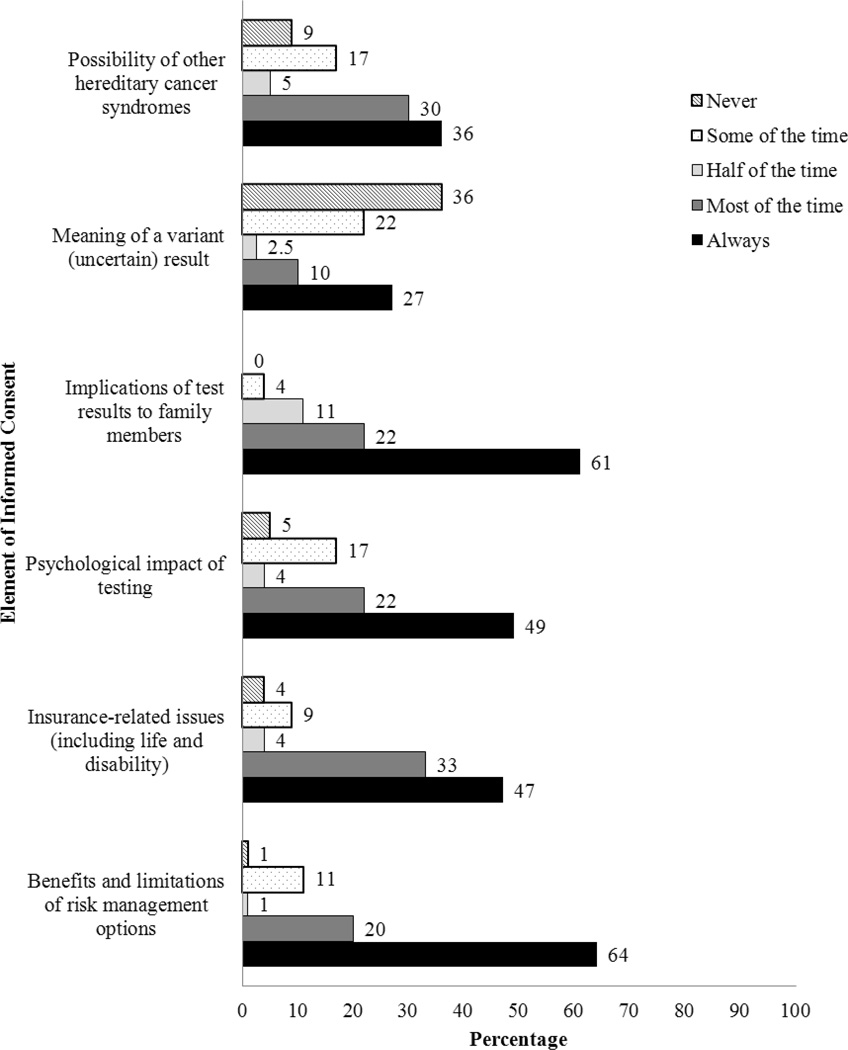
As shown in Figure 1, 51% reported sometimes scheduling a separate session for pretest counseling. Additionally, 57% reported spending an average of 11–30 minutes counseling patients prior to BRCA testing. The majority (73%) did not construct a three-generation pedigree. As shown in Figure 2, the majority reported always discussing implications of test results for family members (61%) and benefits and limitations of risk management options (64%). About half always discussed the psychological impact of testing (49%) and insurance-related issues (47%). Relatively few reported always discussing the possibility of another hereditary cancer syndrome (36%) or the meaning of a variant result (27%).
Figure 1.
Frequency with which Participants Schedule Specific Visits for and Average Number of Minutes Spent Counseling Patients (N = 81)
Note: Two participants did not respond to one or more of these questions.
Figure 2.
Frequency with which Participants Discuss Commonly Recommended Elements of Informed Consent for Genetic Testing with Patients (N = 81)
Note: Two to three participants did not respond to one or more of these questions and frequencies will not add to 100%
Discussion
The lack of adherence to clinical guidelines in the delivery of genetics services among this sample is concerning. Failure to obtain a complete risk assessment and provide complete informed consent for genetic testing by NGPs has resulted in undesirable outcomes including: ordering the wrong test, negative emotional effects, incorrect medical management guidelines, misinterpreting test results, leading to wasted health care resources (e.g., provider time, money), late stage cancer diagnosis, and unnecessary prophylactic surgeries (15–18). Selective or partial application of clinical guidelines when providing genetic services may also lead to medicolegal issues for NGPs (12, 19).
Policy, provider, and patient level factors may contribute to study findings. First, the lack of state or federal policy results in ambiguity about who should provide genetic services, possibly leading to uncertainty among NGPs about the appropriate provider to deliver genetic services. Fifteen states require licensure and include title protections which may help NGPs identify individuals who meet training standards to provide genetics services (19). In addition to increasing the number of states requiring licensure for genetics professionals, policies from accrediting bodies such as the 2012 Commission on Cancer Standards (20) or major insurers that specify genetics services be provided by qualified genetics professionals can serve as a model for national policies. These efforts should occur in tandem with policies that increase the avaialblity of genetics professionals such as pipeline training programs to support entry into gentics health professions, increase funding to agument the number of educational and clinical training sites, offer targeted loan forgiveness programs for genetics professionals, and third-party payer reimbursement.
At the provider level, studies over the last decade document NGPs interest in providing genetic services (5, 17, 21). However a lack of training (5, 21, 22, 23), time (19), and reimbursement (19, 24), compounded by new challenges such as ordering the appropriate test and interpreting results associated with panel testing (25) may exacerbate the problems NGPs already face in providing genetic services consistent with standard of care. Although individuals at risk for HBOC may present in primary and oncology clinics, the focus of NGP education should acknowledge the important role NGPs play an in the identification and referral (to genetics specialists) of patients at risk for hereditary cancer (18, 25 – 26). In addition, more direct NGP education about the role and importance of genetics professionals in delivering guideline adherent genetics services in an increasing complicated and rapidly changing field should be emphasized. Notably, in our study, ~30% and 11% of respondents reported referral to a medical geneticist or genetic counselor for genetic testing or risk assessment in the past 12 months, respectively (data not shown). Despite concerns about the limited availability of genetics professionals (27), “accessible models of care” (28) including telegentic counseling and “collaborative models” (29) provide effective methods for overcoming access barriers.
Finally, at the patient level, direct-to-consumer (DTC) advertising increased awareness of genetic testing (30). However, these advertisements fail to mention the benefits of consulting a genetics professional. Therefore, patients may request genetic testing from NGPs, rather than a referral from NGPs to a genetics professional (18). DTC advertisements should be required to mention the benefits of consultation with a genetics professional. Professional organizations must also continue public education about the importance of obtaining genetics care from genetics professionals. For example, our team has developed an intervention to promote uptake of GC (not testing) among breast cancer survivors at increased risk for HBOC (31).
There are several study limitations. First, 81 providers completed and returned the survey, resulting in a 22% response rate. Response bias is possible given more females than males, and more physicians than non-physicians, completed the survey. Additionally, respondents may have been more interested in genetics; thus, limiting generalizability. Furthermore, NGPs interested in delivering genetics services may have been more likely to respond to our survey and follow practice guidelines; therefore, the results may be a more positive representation than actual practice of guideline based GC among all NGPs. Finally, all commonly recommended elements of GC were not addressed. It is possible respondents focus their time on unexamined elements. Future research should entail a more comprehensive assessment including other recommended elements of pretest GC.
Table 1.
Demographics and Practice Characteristics of Respondents
| Variable | Respondents (N = 81) n (%) |
|---|---|
| Genetics traininga | |
| No formal training | 45 (56) |
| Myriad education only | 20 (25) |
| Clinical genetics course taken | 15 (19) |
| Physician (% yes) | 54 (67) |
| Community/Private Practice setting (% yes) | 80 (99) |
| Sex (% female) | 59 (73) |
| # of years performed genetic testinga | |
| 1–4 | 28 (35) |
| 5–9 | 30 (37) |
| ≥10 | 18 (22) |
| # of individuals providing genetic testing in practice setting (including participant)a | |
| 0 | 2 (3) |
| 1 | 23 (28) |
| 2–3 | 31 (38) |
| ≥4 | 22 (27) |
| # of new patients seen each week for genetic counseling in consideration of testing for hereditary breast/ovarian cancera | |
| 0 | 25 (31) |
| 1–5 | 49 (61) |
| ≥6 | 5 (6) |
| Majority of patients are Non-Hispanic White (% yes) | 70 (86) |
| Over half of patient population has private insurance/HMO (% yes) | 47 (58) |
Percentages may not add to 100% due to rounding and/or participant non-response ranging from 1 to 5 participants, depending on the question.
Acknowledgements
This work was supported by a grant through Florida Biomedical (IBG09-34198). Dr. Courtney L. Scherr is supported by the Behavioral Oncology Education & Career Development Grant R25 CA 090314. We acknowledge the Survey Methods Core Facility at the H. Lee Moffitt Cancer Center and Research Institute for developing versions of the survey that could be scanned into an electronic data file.
Footnotes
Conflict of Interest:
This study was supported by a grant through Florida Biomedical (IBG09-34198). However Florida Biomedical played no role in the study design, data collection, data analysis, or the decision to publish this research. The authors declare that they have no conflict of interests
References
- 1.Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Genetic/Familial High-risk Assessment: Breast and Ovarian. NCCN Practice Guidelines. V.1.2014 ed. Fort Washington, PA: National Comprehensive Cancer Network; 2014. [Google Scholar]
- 3.Cohen SA, Gustafson SL, Marvin ML, et al. Report from the National Society of Genetic Counselors service delivery model task force: a proposal to define models, components, and modes of referral. J Genet Couns. 2012;21:645–651. doi: 10.1007/s10897-012-9505-y. [DOI] [PubMed] [Google Scholar]
- 4.Keating NL, Stoeckert KA, Regan MM, et al. Physicians’ experiences with BRCA1/2 testing in community settings. J Clin Onc. 2008;26:5789–5796. doi: 10.1200/JCO.2008.17.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wideroff L, Freedman AN, Olson L, et al. Physician use of genetic testing for cancer susceptibility results of a national survey. Cancer Epidemiol Biomarkers Prev. 2003;12:295–303. [PubMed] [Google Scholar]
- 6.Robson ME, Storm CD, Weitzel J, et al. American Society of Clinical Oncology policy statement update: genetic and genomic testing for cancer susceptibility. J Clin Oncol. 2010;28:893–901. doi: 10.1200/JCO.2009.27.0660. [DOI] [PubMed] [Google Scholar]
- 7.Riley BD, Culver JO, Skrzynia C, et al. Essential elements of genetic cancer risk assessment, counseling, and testing: updated recommendations of the National Society of Genetic Counselors. J Genet Couns. 2012;21:151–161. doi: 10.1007/s10897-011-9462-x. [DOI] [PubMed] [Google Scholar]
- 8.National Research Counsil. Cancer-related genetic testing and counseling: workshop proceedings. Washington, DC: The National Academies Press; 2007. [Google Scholar]
- 9.Role of the Oncology Nurse in Cancer Genetic Counseling. Oncology Nursing Society Positions. Pittsburgh, PA: Oncology Nursing Society; 2009. [Google Scholar]
- 10.American College of Obstetricians and Gynecologists, ACOG Committee on Practice Bulletins--Gynecology, ACOG Committee on Genetics et al. ACOG practice bulletin no 103: hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2009;113:957–966. doi: 10.1097/AOG.0b013e3181a106d4. [DOI] [PubMed] [Google Scholar]
- 11.American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol. 2003;21:2397–2406. doi: 10.1200/JCO.2003.03.189. [DOI] [PubMed] [Google Scholar]
- 12.Pal T, Cragun D, Lewis C, et al. A statewide survey of practitioners to assess knowledge and clinical practices regarding hereditary breast and ovarian cancer. Genetic Testing and Molecular Biomarkers. 2013;17:367–375. doi: 10.1089/gtmb.2012.0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dillman D. Mail and internet surveys: the tailored design method. New York: Wiley; 2000. [Google Scholar]
- 14.Cragun D, Besharat A, Lewis C, et al. Educational needs and preferred methods of learning among Florida practitioners who order genetic testing for hereditary breast and ovarian cancer. J Canc Educ. 2013;28:690–697. doi: 10.1007/s13187-013-0525-6. [DOI] [PubMed] [Google Scholar]
- 15.Brierley KL, Blouch E, Cogswell W, et al. Adverse events in cancer genetic testing: medical, ethical, legal, and financial implications. Cancer J. 2012;18:303–309. doi: 10.1097/PPO.0b013e3182609490. [DOI] [PubMed] [Google Scholar]
- 16.Bensend TA, Veach PM, Niendorf KB. What’s the harm? Genetic counselor perceptions of adverse effects of genetics service provision by non-genetics professionals. J Genet Couns. 2013:1–16. doi: 10.1007/s10897-013-9605-3. [DOI] [PubMed] [Google Scholar]
- 17.Wideroff L, Vadaparampil ST, Greene MH, et al. Hereditary breast/ovarian and colorectal cancer genetics knowledge in a national sample of US physicians. J Med Genet. 2005;42:749–755. doi: 10.1136/jmg.2004.030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brierley KL, Campfield D, Ducaine W. Errors in delivery of cancer genetics services: implications for pratice. Conneticut Med. 2010;74:413–423. [PubMed] [Google Scholar]
- 19.Pal T, Radford C, Vadaparampil ST, et al. Practical considerations in the delivery of genetic counseling and testing services for inherited cancer predisposition. Community Oncology. 2013;10:147–153. [Google Scholar]
- 20.Commission on Cancer. Cancer program standards 2012: ensuring patient-centered care V 1.2.1. Chicago, IL: American College of Surgeons; 2012. [Google Scholar]
- 21.Marzuillo C, De Vito C, Boccia S, et al. Knowledge, attitudes and behavior of physicians regarding predictive genetic tests for breast and colorectal cancer. Preventive Medicine. 2013;57:477–482. doi: 10.1016/j.ypmed.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Blazer KR, MacDonald DJ, Ricker C, et al. Outcomes from intensive training in genetic cancer risk counseling for clinicians. Genet Med. 2005;7:40–47. doi: 10.1097/01.gim.0000151154.27612.49. [DOI] [PubMed] [Google Scholar]
- 23.Bankhead C, Emery J, Qureshi N, et al. New developments in genetics-knowledge, attitudes and information needs of practice nurses. Family Practice. 2001;18:475–486. doi: 10.1093/fampra/18.5.475. [DOI] [PubMed] [Google Scholar]
- 24.Cummings S. The genetic testing process: how much counseling is needed? J Clin Oncol. 2000;18:60s–64s. [PubMed] [Google Scholar]
- 25.Nagy R, Sturm A. Personalized medicine: impact on patient care in genetic counseling. Curr Genet Med Rep. 2013;1:129–134. [Google Scholar]
- 26.Esserman L, Kaklamani V. Lessons learned from genetic testing. JAMA. 2010;304:1011–1012. doi: 10.1001/jama.2010.1263. [DOI] [PubMed] [Google Scholar]
- 27.Weitzel JN, Blazer KR, MacDonald DJ, et al. Fenetics, genomics, and cancer risk assessment: state of the art and future directions in the era of personalized medicine. CA: A Cancer Journal for Clinicians. 2011;61:327–359. doi: 10.3322/caac.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald E, Lamb A, Grillo B, et al. Acceptibility of telemedicine and other cancer genetic counseling models of service delivery in geographically remote settings. J Genet Counsel. 2014;23:221–228. doi: 10.1007/s10897-013-9652-9. [DOI] [PubMed] [Google Scholar]
- 29.Cohen SA, McIlvried DE. Improving access with a collaborative approach to cancer genetic counseling services: a pilot study. Community Oncology. 2013;10:227–234. [Google Scholar]
- 30.Centers for Disease Control and Prevention. Genetic testing for breast and ovarian cancer susceptibility: evaluating direct-to-consumer marketing—Atlanta, Denver, Raleigh-Duram, and Seattle, 2003. Morb Mortal Wkly Rep. 2004;27:603–606. [PubMed] [Google Scholar]
- 31.Vadaparampil ST, Malo T, Nam K, Nelson A, de la Cruz C, Quinn GP. From observation to intervention: development of a psychoeducational intervention to increase uptake of BRCA genetic counseling among high-risk breast cancer survivors. Journal of Cancer Education. doi: 10.1007/s13187-014-0643-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]