Abstract
Purpose
To analyze the prognostic factors for survivals and to evaluate the impact of postoperative whole pelvic radiotherapy (WPRT) on pelvic failure in patients with uterine sarcoma treated with radical surgery.
Materials and Methods
We retrospectively analyzed 75 patients with uterine sarcoma who underwent radical surgery with (n = 22) or without (n = 53) radiotherapy between 1990 and 2010. There were 23 and 52 patients with carcinosarcoma and non-carcinosarcoma (leiomyosarcoma, 22; endometrial stromal sarcoma, 25; others, 5), respectively. The median follow-up period was 64 months (range, 17 to 269 months).
Results
The 5-year overall survival (OS) and pelvic failure-free survival (PFFS) of total patients was 64.2% and 83.4%, respectively. Multivariate analysis revealed that mitotic count (p = 0.006) was a significant predictor of OS. However, factors were not found to be associated with PFFS. On analyzing each of the histologic subtypes separately, postoperative WPRT significantly reduced pelvic failure in patients with carcinosarcoma (10.0% vs. 53.7%; p = 0.046), but not in patients with non-carcinosarcoma (12.5% vs. 9.9%; p = 0.866). Among the patients with carcinosarcoma, 4 patients (17%) had recurrence within the pelvis and 3 patients (13%) had recurrence in other sites as an initial failure, whereas among the patients with non-carcinosarcoma, 3 patients (6%) experienced pelvic failure and 13 patients (25%) experienced distant failure.
Conclusion
The most significant predictor of OS was mitotic count. Based on the improved PFFS after postoperative WPRT only in patients with carcinosarcoma and the difference in patterns of failure between histologic subtypes, optimal adjuvant treatment options should be offered to patients based on the risk of recurrence patterns.
Keywords: Uterus, Sarcoma, Neoplasms by histology type, Pelvic failure, Adjuvant radiotherapy
Introduction
Uterine sarcoma is a rare gynecologic malignancy, which accounts for only 2%-8% of all uterine malignancies [1]. Its clinical behavior is more aggressive than that of other tumors arising at the same site, and the 5-year overall survival (OS) rates for the tumor are reported to range from 31%-64% [2,3,4,5]. Due to its low incidence, the standard treatment scheme has not been well established until now. The role of adjuvant radiotherapy (RT) has been debated for many years, but there is no consensus as yet. Only one phase III randomized trial evaluating the effect of RT has been published, but the study was limited by the small number of patients [6].
The aim of this study is to evaluate treatment outcomes of uterine sarcoma at a single institute and to validate the role of adjuvant whole pelvic radiotherapy (WPRT).
Materials and Methods
1. Patients
This study was designed as a retrospective study of the patients with uterine sarcoma who were treated at this hospital. Ninety-eight patients were diagnosed with uterine sarcoma and treated from January 1990 to April 2010 according to the pathology database and radiation oncology database of this institute.
We excluded the patients who initially had any distant metastatic lesion (15 patients), no follow-up data after surgery (7 patients), or received only conservative treatments (1 patient). The remaining 75 patients were analyzed as the study population.
The stages of all patients were re-assessed by the investigator according to FIGO staging 7th edition updated in 2010. Since histologic grade was reported with inconsistent ways in a few patients, we used mitotic count for the analysis instead.
Endodermal stromal sarcoma (ESS) included only low-grade ESS and high-grade ESS was considered as undifferentiated sarcoma which was categorized into the 'other' group.
2. Definitions of variable
OS was measured from the date of the first operation to death or last follow-up for censored patients. Pelvic failure was defined as local or regional recurrence within the pelvis and was measured from the date of the first operation to the date of the first local or regional recurrence. Recurrence events were classified as local, regional or distant failure. Recurrence was defined as the diagnosis of the first recurrence.
3. Statistical analysis
Analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA). OS and pelvic failure-free survival (PFFS) were estimated using the Kaplan-Meier method with two-sided log-rank test. Multivariate analyses were performed using the Cox proportional hazard model. A p-value <0.05 indicated statistical significance for all tests.
Results
1. Patient and treatment characteristics
The patient's clinical characteristics according to conducting RT or not are summarized in Table 1. The median follow-up time for 52 surviving patients was 64 months (range, 17 to 269 months). The number of patients with carcinosarcoma (CS, n = 23), leiomyosarcoma (LMS, n = 22), and ESS (n = 25) was similar.
Table 1. Clinical characteristics of patients.
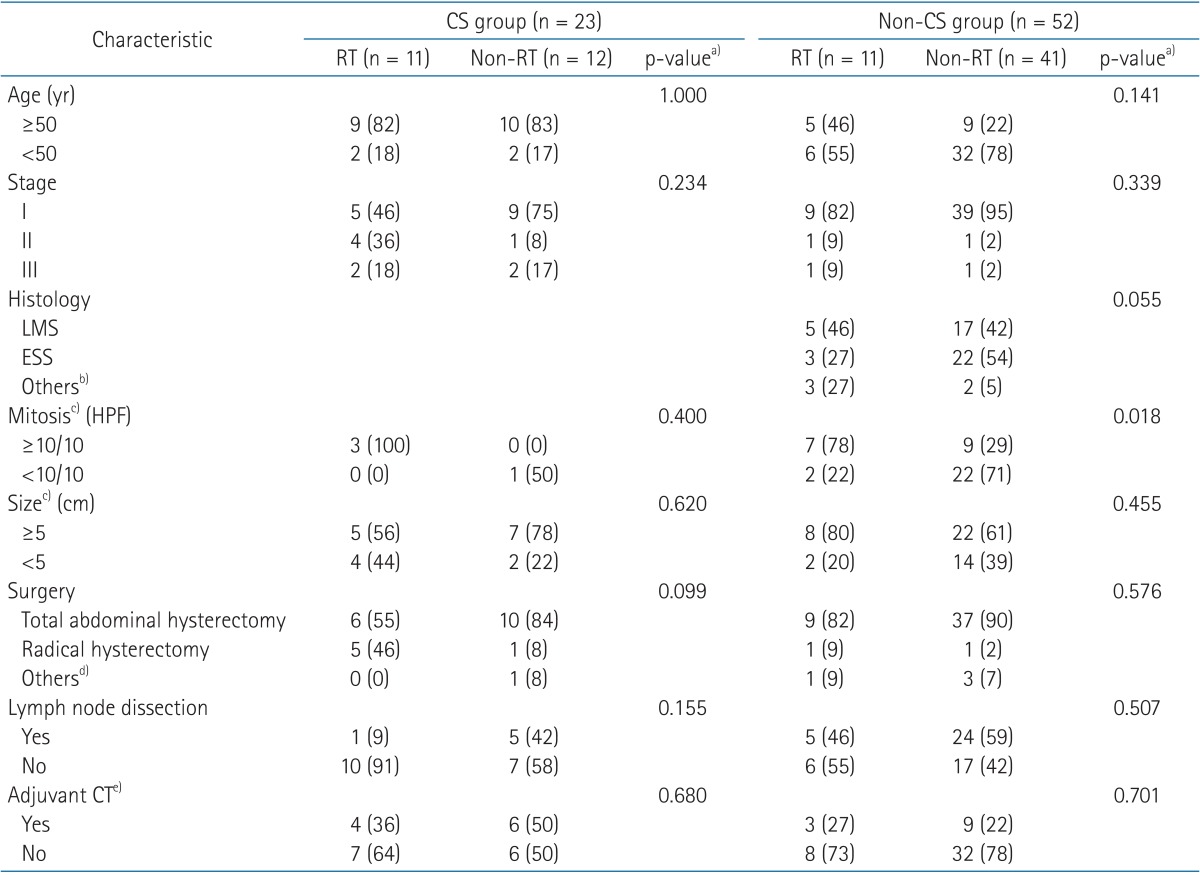
Values are presented as number of patients (%).
CS, carcinosarcoma; LMS, leiomyosarcoma; ESS, endometrial stromal sarcoma; HPF, high-power field; RT, radiotherapy; CT, chemotherapy.
a)Pearson chi-square. b)High grade endodermal stromal sarcoma (undifferentiated sarcoma) (n = 3), high grade spindle cell sarcoma (n = 1), adenosarcoma (n = 1). c)Available data only. d)Vaginal total hysterectomy, subtotal hysterectomy. e)VIP (etoposide, ifosfamide, cisplatin), IP (ifosfamide, cisplatin), CEP (cyclophosphamide, etoposide, cisplatin), CAP (cyclophosphamide, adriamycin, cisplatin), CYVADIC (cyclophosphamide, vincristine, Adriamycin, DTIC) or cisplatin alone.
Most of the patients underwent total abdominal hysterectomy with or without bilateral salpingo-oophorectomy as the initial surgical modality (n = 62), followed by radical hysterectomy (n = 8) and others (n = 5), which included vaginal total hysterectomy (n = 4) and subtotal hysterectomy (n = 1). Fifteen patients (20%) received systemic chemotherapy without RT and 7 patients (9%) received concurrent chemoradiotherapy. Twenty-two patients (29%) received WPRT postoperatively using linear accelerators, and median RT dose was 50.4 Gy in 1.8 Gy fractions (range, 48.6 to 50.4 Gy). None of the patients received brachytherapy.
2. Survival and pattern of failure
The 5-year OS and PFFS for all patients were 64.2% and 82.7%, respectively. The multivariate analysis showed that mitotic count was a significant prognostic factor for OS, but other factors, such as age, stage, histology, tumor size, WPRT and chemotherapy, did not affect OS (Table 2). Among factors including age, stage, histology, mitotic count, tumor size, adjuvant chemotherapy and RT, there was no significant prognostic factor for PFFS.
Table 2. Prognostic factors for overall survival (n = 75).
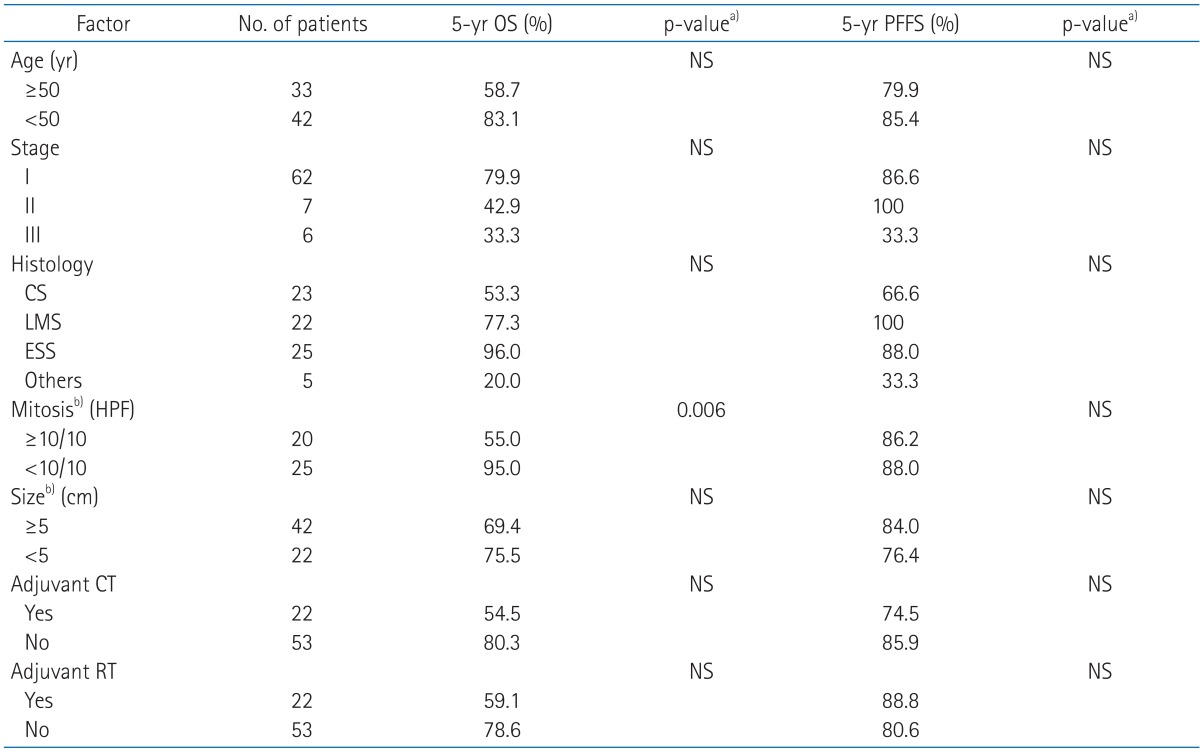
OS, overall survival; PFFS, pelvic failure-free survival; CS, carcinosarcoma; LMS, leiomyosarcoma; ESS, endometrial stromal sarcoma; HPF, high-power field; RT, radiotherapy; CT, chemotherapy; NS, no significant.
a)Cox regression analysis. b)Available data only.
The failure pattern was different according to the histologic type. In CS, initial failure occurred more frequently within the pelvis than distant metastasis (Table 3). Recurrence in pelvic lymph nodes (4 patients) is more common than stump failure (2 patients) in CS. Contrarily, in LMS, ESS, and other subtypes, distant metastasis was the main cause of initial failure. The most common site of distant metastasis in non-CS was lung and/or mediastinum (9 patients), followed by peritoneal seeding (5 patients). Because the influence of adjuvant therapy can be different according to the failure pattern, subset analysis was performed in patients with CS and non-CS, respectively.
Table 3. Failure patterns according to the histologic type.
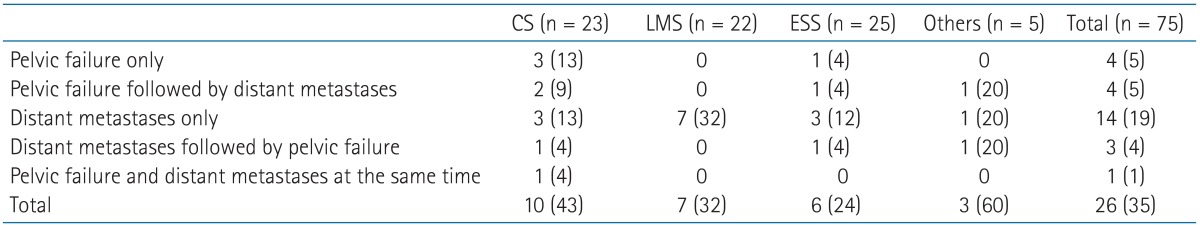
Values are presented as number of patients (%).
CS, carcinosarcoma; LMS, leiomyosarcoma; ESS, endometrial stromal sarcoma.
3. Subgroup analysis
1) Carcinosarcoma
The 5-year OS in patients with CS was 53.3%. There was no difference in factors, such as age, stage, histology, mitosis, and size, between the WPRT group (n = 11) and the non-WPRT group (n = 12) by Pearson chi-square test (Table 1).
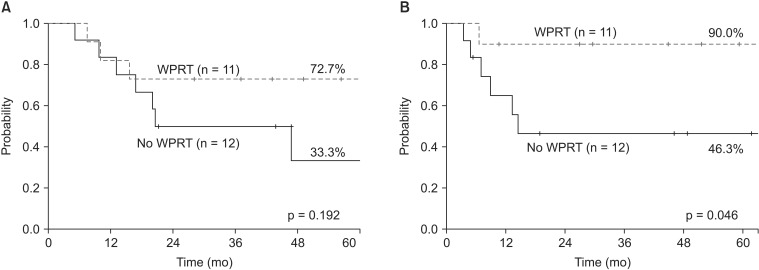
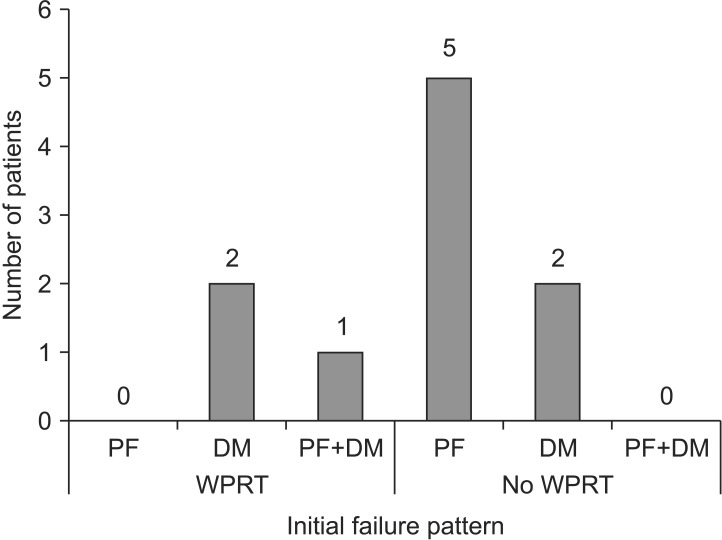
The OS was not significantly different between the WPRT group and the non-WPRT group (72.7% vs. 33.3%; p = 0.192) (Fig. 1A). Adjuvant WPRT was the only factor associated with the 5-year PFFS (90.0% vs. 46.3%; p = 0.046) (Fig. 1B). While initial failure occurred primarily in the pelvis for the 5 patients that did not receive WPRT, pelvis-only failure, without signs of distant metastasis, was not seen in patients that underwent WPRT (Fig. 2).
Fig. 1. Overall survival (A) and pelvic failure-free survival (B) in carcinosarcoma patients with or without WPRT (n = 23). WPRT, whole pelvic radiotherapy.
Fig. 2. Initial failure patterns in carcinosarcoma patients. PF, pelvic failure; DM, distant metastases; PF+DM, pelvic failure and distant metastases at the same time; WPRT, whole pelvic radiotherapy.
2) Non-carcinosarcoma
Among the 52 patients with non-CS, the 5-year OS was 80.5%. Mitotic counts were significantly higher in the WPRT group than in the non-WPRT group (p = 0.018) (Table 1). The OS was significantly worse after adjuvant WPRT (45.5% vs. 90.0%; p = 0.001), but in the multivariate analysis, WPRT did not significantly influence OS.
Among the 16 patients who failed initial treatment, crude initial pelvic failure occurred in 3 patients (4%). Of the 3 patients with initial pelvic failure, 2 patients developed subsequent distant recurrence after 12 and 25 months. The PFFSs were similar in groups with and without adjuvant WPRT both in the univariate analysis (87.5% vs. 90.1%; p = 0.866) and in the multivariate analysis (p = 0.951).
3) Toxicity
Among the 22 patients who received WPRT, 13 patients (59%) experienced grade 1 or 2 acute gastrointestinal (GI) toxicity, and 5 patients (23%) experienced grade 1 or 2 acute genitourinary toxicity. However, none of the patients developed grade 3 or 4 acute toxicity or late GI toxicity.
Discussion and Conclusion
The standard initial therapy for uterine sarcoma is considered to be radical surgery but the need for adjuvant therapy is widely debated. OS benefit with adjuvant RT has been described in several retrospective series [1,5,7,8,9,10], but other studies showed lack of OS benefit with adjuvant RT [2,4,11,12]. However, uterine sarcomas are a heterogeneous group of tumors, and it was observed that the outcomes or patterns of failure vary according to the histologic type [1,4,6,7,13,14,15,16,17]. Therefore, this contradictory finding of RT efficacy may be due to the various subtypes of uterine sarcoma.
Sampath et al. [3] reported 53% risk reduction in locoregional failure at 5 years with adjuvant RT compared to surgery alone in a database of 3,650 uterine sarcoma patients. The rate of locoregional failure at 5 years was relatively high in patients with CS (16%) compared to patients with LMS (13%) and ESS (7%). Likewise, in other retrospective studies, the rates of pelvic failure and distant metastases in patients with CS were reported to range from 25% to 55% and from 29% to 53%, respectively, when they were treated without RT [4,7,14,15,16,17]. In contrast, lower pelvic failure rates (17%-20%) and higher distant metastases rates (33%-61%) were seen in patients with LMS [4,16,18]. These patterns of failure were also reported in the EORTC study which is the only phase III randomized trial comparing RT and observation after surgery [6]. The number of CS patients who experienced pelvic failure (33%) as an initial recurrence was higher than that of CS patients with distant metastases (27%), whereas the number of LMS patients with distant metastases (80%) was higher than that of LMS patients with pelvic failure (41%) [6].
Since higher pelvic failure rates in CS were also seen in our study, we performed subgroup analysis of CS patients and could determine the benefit of WPRT in achieving pelvic control in CS patients. Several other studies also report the benefit of WPRT in achieving local control for CS patients [7,16,19], which is consistent with our study. MD Anderson Cancer Center reviewed 300 patients with stage I to III CS and found that adjuvant pelvic RT decreased the risk of pelvic recurrence and delayed distant metastasis [7].
The RT fields for patients in our study were limited to whole pelvis, with total doses of 48.6-50.4 Gy in 1.8 Gy per fraction without vaginal brachytherapy. The phase III randomized trial by gynecologic oncology group with carcinosarcoma patients used whole abdominal irradiation (WAI) of 30 Gy in 1 Gy per fraction, and found no benefit of WAI over combined chemotherapy [14]. Among the patients received WAI, 10 patients (10%) had grade 2-4 late GI toxicities and 2 patients (2%) died of radiation hepatitis. We did not use WAI due to our concern of its toxicity and insufficient evidence of its benefit over WPRT. Several other studies included patients who received vaginal brachytherapy with or without WPRT [3,15,20]. In a retrospective study by Chi et al. [15], there was no benefit of brachytherapy boost to pelvic control (p = 0.94), and the author commented that isolated vaginal failure may be a rare event, and therefore the necessity of additional brachytherapy may need to be reconsidered. Another study using vaginal brachytherapy without WPRT reported high rate of pelvic failure (17%), and concluded that the addition of WPRT would be needed despite early-stage disease [20]. Among 11 patients who experienced pelvic failure in our study, recurrences at stump occurred only in non-RT group. Therefore, the addition of brachytherapy to WPRT may not be necessary.
In this study, there was benefit in achieving local control in CS patients, but not in patients with other histologic subtypes. This is probably due to predominance of distant failure in patients with other subtypes. Especially among the 22 LMS patients, none experienced pelvic failure but 7 patients (32%) developed distant failure. Twelve among the 52 non-CS patients received systemic chemotherapy initially (3 patients received with RT concurrently) to prevent distant metastases, but the outcome was disappointing. Neither OS nor distant metastasis-free survival (DMFS) improved after adjuvant chemotherapy (5-yr OS: 66.7% vs. 84.6%, p = 0.242; 5-yr DMFS: 72.7% vs. 71.1%, p = 0.737) in the non-CS patients. The chemotherapy regimens were inconsistent among these patients (Table 1).
In earlier studies of soft-tissue sarcoma, doxorubicin and ifosfamide were the most active single agents with reported objective responses in about 25% of patients with metastatic soft-tissue sarcoma [21,22]. The combination of doxorubicin and ifosfamide in treatment of unresectable or metastatic soft-tissue sarcoma has been reported to achieve response rates of about 35%, which was superior compared to that achieved with other combination regimens [23,24,25]. The SARCGYN study which was published recently, investigated the role of adjuvant systemic therapy with doxorubicin, ifosfamide, and cisplatin which are shown to be effective in soft tissue sarcoma of uterus, in addition to RT [26]. The disease-free survival (DFS) was significantly better in the chemotherapy arm than in the RT alone arm (3-yr DFS: 55% vs. 41%, p = 0.048), but the OS was not different (3-yr OS: 81% vs. 69%, p = 0.41) [26].
This study was a retrospective study; hence, there could be a selection bias that we performed RT in patients who had a high probability of recurrence in the non-CS group. The chi-square test in the non-CS group showed differences in age and mitotic counts, which were prognostic factors for earlier development of distant failure and higher mortality rates in other studies [16,27,28,29,30,31]. Several studies demonstrated benefit of adjuvant RT in achieving pelvic control [3,9,10,12,18,32,33], but it was not demonstrated in this study. The reason why the outcome of this study did not show the benefit in achieving pelvic control might be because the patients who were selected to receive RT tended to have more factors for earlier development of distant failure and higher mortality rates. There was not specified protocol for RT; hence, RT was performed at the discretion of the physicians. Among the non-CS patients treated at our institute, none received the combination regimen including both ifosfamide and doxorubicin. If the systemic disease is controlled more successfully with an effective chemotherapy regimen, the pelvic failure may also be an important issue in these patients. We cautiously assume that the patients who received adjuvant RT and did not experience pelvic failure still suffered from distant failure, which is a major cause of recurrence in non-CS. Therefore, before achieving a higher control rate of distant failure by the use of a more appropriate systemic modality, pelvic control by adjuvant therapy may not be beneficial in patients with uterine sarcoma.
In conclusion, the most significant predictor of OS was mitotic count. Since failure patterns of uterine sarcoma vary according to the histologic types, the treatment approach should be different. In CS, WPRT after radical surgery could reduce pelvic failure. In other subtypes of uterine sarcoma, achieving more pelvic control via WPRT may not be beneficial for distant control or survival. Adjuvant chemotherapy should be the mainstay after surgery, but an efficient regimen has not yet been established.
Acknowledgments
This work was supported by National R&D Program for Cancer Control (No. 1320220) from National Cancer Center Korea.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol Oncol. 2004;93:204–208. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 2.Olah KS, Gee H, Blunt S, Dunn JA, Kelly K, Chan KK. Retrospective analysis of 318 cases of uterine sarcoma. Eur J Cancer. 1991;27:1095–1099. doi: 10.1016/0277-5379(91)90300-3. [DOI] [PubMed] [Google Scholar]
- 3.Sampath S, Schultheiss TE, Ryu JK, Wong JY. The role of adjuvant radiation in uterine sarcomas. Int J Radiat Oncol Biol Phys. 2010;76:728–734. doi: 10.1016/j.ijrobp.2009.02.077. [DOI] [PubMed] [Google Scholar]
- 4.Sorbe B, Johansson B. Prophylactic pelvic irradiation as part of primary therapy in uterine sarcomas. Int J Oncol. 2008;32:1111–1117. [PubMed] [Google Scholar]
- 5.Ferrer F, Sabater S, Farrus B, et al. Impact of radiotherapy on local control and survival in uterine sarcomas: a retrospective study from the Grup Oncologic Català-Occità. Int J Radiat Oncol Biol Phys. 1999;44:47–52. doi: 10.1016/s0360-3016(98)00515-x. [DOI] [PubMed] [Google Scholar]
- 6.Reed NS, Mangioni C, Malmstrom H, et al. Phase III randomised study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II: an European Organisation for Research and Treatment of Cancer Gynaecological Cancer Group Study (protocol 55874) Eur J Cancer. 2008;44:808–818. doi: 10.1016/j.ejca.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ. Malignant mixed Müllerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. Int J Radiat Oncol Biol Phys. 2004;58:786–796. doi: 10.1016/S0360-3016(03)01561-X. [DOI] [PubMed] [Google Scholar]
- 8.Denschlag D, Masoud I, Stanimir G, Gilbert L. Prognostic factors and outcome in women with uterine sarcoma. Eur J Surg Oncol. 2007;33:91–95. doi: 10.1016/j.ejso.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Vongtama V, Karlen JR, Piver SM, Tsukada Y, Moore RH. Treatment, results and prognostic factors in stage I and II sarcomas of the corpus uteri. AJR Am J Roentgenol. 1976;126:139–147. doi: 10.2214/ajr.126.1.139. [DOI] [PubMed] [Google Scholar]
- 10.Moskovic E, MacSweeney E, Law M, Price A. Survival, patterns of spread and prognostic factors in uterine sarcoma: a study of 76 patients. Br J Radiol. 1993;66:1009–1015. doi: 10.1259/0007-1285-66-791-1009. [DOI] [PubMed] [Google Scholar]
- 11.Tinkler SD, Cowie VJ. Uterine sarcomas: a review of the Edinburgh experience from 1974 to 1992. Br J Radiol. 1993;66:998–1001. doi: 10.1259/0007-1285-66-791-998. [DOI] [PubMed] [Google Scholar]
- 12.Livi L, Andreopoulou E, Shah N, et al. Treatment of uterine sarcoma at the Royal Marsden Hospital from 1974 to 1998. Clin Oncol (R Coll Radiol) 2004;16:261–268. doi: 10.1016/j.clon.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Sampath S, Gaffney DK. Role of radiotherapy treatment of uterine sarcoma. Best Pract Res Clin Obstet Gynaecol. 2011;25:761–772. doi: 10.1016/j.bpobgyn.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Wolfson AH, Brady MF, Rocereto T, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin-ifosfamide and mesna (CIM) as post-surgical therapy in stage I-IV carcinosarcoma (CS) of the uterus. Gynecol Oncol. 2007;107:177–185. doi: 10.1016/j.ygyno.2007.07.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chi DS, Mychalczak B, Saigo PE, Rescigno J, Brown CL. The role of whole-pelvic irradiation in the treatment of early-stage uterine carcinosarcoma. Gynecol Oncol. 1997;65:493–498. doi: 10.1006/gyno.1997.4676. [DOI] [PubMed] [Google Scholar]
- 16.Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early-stage uterine sarcoma: a Gynecologic Oncology Group study. Cancer. 1993;71(4 Suppl):1702–1709. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 17.Gerszten K, Faul C, Kounelis S, Huang Q, Kelley J, Jones MW. The impact of adjuvant radiotherapy on carcinosarcoma of the uterus. Gynecol Oncol. 1998;68:8–13. doi: 10.1006/gyno.1997.4901. [DOI] [PubMed] [Google Scholar]
- 18.Hornback NB, Omura G, Major FJ. Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys. 1986;12:2127–2130. doi: 10.1016/0360-3016(86)90011-8. [DOI] [PubMed] [Google Scholar]
- 19.Park HJ, Kim HJ, Wu HG, et al. The influence of adjuvant radiotherapy on patterns of failure and survivals in uterine carcinosarcoma. Radiat Oncol J. 2011;29:228–235. doi: 10.3857/roj.2011.29.4.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown L, Petersen I, Haddock M, Lee LJ, Cimbak N, Viswanathan A. Vaginal brachytherapy as the sole radiotherapeutic treatment for stage I carcinosarcoma of the uterus. Brachytherapy. 2014;13(Suppl 1):S76–S77. doi: 10.1016/j.brachy.2015.02.194. [DOI] [PubMed] [Google Scholar]
- 21.Borden EC, Amato DA, Rosenbaum C, et al. Randomized comparison of three adriamycin regimens for metastatic soft tissue sarcomas. J Clin Oncol. 1987;5:840–850. doi: 10.1200/JCO.1987.5.6.840. [DOI] [PubMed] [Google Scholar]
- 22.Mouridsen HT, Bastholt L, Somers R, et al. Adriamycin versus epirubicin in advanced soft tissue sarcomas: a randomized phase II/phase III study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer Clin Oncol. 1987;23:1477–1483. doi: 10.1016/0277-5379(87)90089-7. [DOI] [PubMed] [Google Scholar]
- 23.Loehrer PJ, Sr, Sledge GW, Jr, Nicaise C, et al. Ifosfamide plus doxorubicin in metastatic adult sarcomas: a multi-institutional phase II trial. J Clin Oncol. 1989;7:1655–1659. doi: 10.1200/JCO.1989.7.11.1655. [DOI] [PubMed] [Google Scholar]
- 24.Edmonson JH, Ryan LM, Blum RH, et al. Randomized comparison of doxorubicin alone versus ifosfamide plus doxorubicin or mitomycin, doxorubicin, and cisplatin against advanced soft tissue sarcomas. J Clin Oncol. 1993;11:1269–1275. doi: 10.1200/JCO.1993.11.7.1269. [DOI] [PubMed] [Google Scholar]
- 25.Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13:1537–1545. doi: 10.1200/JCO.1995.13.7.1537. [DOI] [PubMed] [Google Scholar]
- 26.Pautier P, Floquet A, Gladieff L, et al. A randomized clinical trial of adjuvant chemotherapy with doxorubicin, ifosfamide, and cisplatin followed by radiotherapy versus radiotherapy alone in patients with localized uterine sarcomas (SARCGYN study): a study of the French Sarcoma Group. Ann Oncol. 2013;24:1099–1104. doi: 10.1093/annonc/mds545. [DOI] [PubMed] [Google Scholar]
- 27.Gadducci A, Landoni F, Sartori E, et al. Uterine leiomyosarcoma: analysis of treatment failures and survival. Gynecol Oncol. 1996;62:25–32. doi: 10.1006/gyno.1996.0185. [DOI] [PubMed] [Google Scholar]
- 28.Abeler VM, Røyne O, Thoresen S, Danielsen HE, Nesland JM, Kristensen GB. Uterine sarcomas in Norway: a histopathological and prognostic survey of a total population from 1970 to 2000 including 419 patients. Histopathology. 2009;54:355–364. doi: 10.1111/j.1365-2559.2009.03231.x. [DOI] [PubMed] [Google Scholar]
- 29.Blom R, Guerrieri C, Stal O, Malmstrom H, Simonsen E. Leiomyosarcoma of the uterus: a clinicopathologic, DNA flow cytometric, p53, and mdm-2 analysis of 49 cases. Gynecol Oncol. 1998;68:54–61. doi: 10.1006/gyno.1997.4889. [DOI] [PubMed] [Google Scholar]
- 30.Ayhan A, Aksan G, Gultekin M, et al. Prognosticators and the role of lymphadenectomy in uterine leiomyosarcomas. Arch Gynecol Obstet. 2009;280:79–85. doi: 10.1007/s00404-008-0876-0. [DOI] [PubMed] [Google Scholar]
- 31.Wu TI, Chang TC, Hsueh S, et al. Prognostic factors and impact of adjuvant chemotherapy for uterine leiomyosarcoma. Gynecol Oncol. 2006;100:166–172. doi: 10.1016/j.ygyno.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Sorbe B. Radiotherapy and/or chemotherapy as adjuvant treatment of uterine sarcomas. Gynecol Oncol. 1985;20:281–289. doi: 10.1016/0090-8258(85)90209-4. [DOI] [PubMed] [Google Scholar]
- 33.Salazar OM, Dunne ME. The role of radiation therapy in the management of uterine sarcomas. Int J Radiat Oncol Biol Phys. 1980;6:899–902. doi: 10.1016/0360-3016(80)90334-x. [DOI] [PubMed] [Google Scholar]