Abstract
AIM: To investigate the bactericidal effects of Chenopodium ambrosioides L. (CAL) against Helicobacter pylori (H. pylori) both in vitro and in vivo.
METHODS: For in vitro experiments, the inhibitory activity of CAL was tested using an agar dilution method; H. pylori strain NCTC11637 was incubated on Columbia blood agar plates containing serial concentrations of CAL. The minimal inhibitory concentration (MIC) was determined by the absence of H. pylori colonies on the agar plate. Time-kill curves were used to evaluate bactericidal activity; the average number of colonies was calculated at 0, 2, 8 and 24 h after liquid incubation with concentrations of CAL at 0.5, 1, and 2 × MIC. For in vivo experiments, H. pylori-infected mice were randomly divided into CAL, triple therapy (lansoprazole, metronidazole, and clarithromycin), blank control, or H. pylori control groups. The eradication ratios were determined by positive findings from rapid urease tests (RUTs) and by histopathology.
RESULTS: In vitro, the MIC of CAL against H. pylori was 16 mg/L. The time-kill curves showed a stable and persistent decreasing tendency with increasing CAL concentration, and the intensity of the bactericidal effect was proportional to dose; the 1 and 2 × MIC completely inhibited the growth of H. pylori at 24 h. In vivo, the eradication ratios in the CAL group were 60% (6/10) by RUT and 50% (5/10) by histopathology. Ratios in the triple therapy group were both 70% (7/10), and there was no difference between the CAL and triple therapy groups. Histopathologic evaluation revealed massive bacterial colonization on the surface of gastric mucosa and slight infiltration of mononuclear cells after inoculation with H. pylori, but no obvious inflammation or other pathologic changes in gastric mucosa of mice from CAL and triple therapy groups.
CONCLUSION: CAL demonstrates effective bactericidal activity against H. pylori both in vitro and in vivo.
Keywords: Helicobacter pylori, Bactericidal activity, Chenopodium ambrosioides L., Phytotherapy
Core tip: The Helicobacter pylori (H. pylori) eradication rate of triple therapy has been markedly decreased due to increasing bacterial antibiotic resistance. Natural Chinese medicines, such as Chenopodium ambrosioides L. (a Chinese herb derived from Jinhua Weikang Capsule utilized for gastritis and peptic ulcers), represent complementary and collaborative therapies. This report demonstrates that C. ambrosioides has in vitro and in vivo bactericidal effects against H. pylori, and may be a good candidate for the treatment of H. pylori infection.
INTRODUCTION
Helicobacter pylori (H. pylori) is the etiologic agent associated with many gastrointestinal diseases, such as chronic gastritis, peptic ulcers, gastric carcinoma, and mucosa-associated lymphoid tissue lymphoma. The eradication of H. pylori has thus been utilized as the primary treatment strategy of these diseases for three decades. In the past, standard triple therapy consisting of a proton pump inhibitor and two broad-spectrum antibiotics (usually amoxicillin, clarithromycin or metronidazole) was able to achieve eradication rates > 90%[1]. However, recent studies have demonstrated that the eradication rates of triple therapy have declined below 80%[2-5]. Among the factors causing this decline, antibiotic resistance (particularly clarithromycin and metronidazole resistance) is the primary cause. In China, the eradication rate decreased from 88.9% in 1996[6] to 73.5% in 2012[7], while the prevalence of antibiotic resistance to clarithromycin and metronidazole increased to 65.4% and 78.8%[8], 21.5% and 95.4%[9], and 20.7% and 42.2%[10], respectively, in various regions. Hence, alternative strategies, including sequential, concomitant, and bismuth-based quadruple therapies, have been proposed to counteract increasing antibiotic resistance rates. The updated Chinese guidance for H. pylori infection has recommended 10-14 d of bismuth-based quadruple therapy as the initial treatment[11]. However, the additional drugs and prolonged duration result in poorer compliance, severer adverse reactions, and higher costs for sufferers.
The Chinese guidance also describes the application of natural medicine as a complementary and collaborative therapy. The anti-H. pylori effects of dozens of herbs and formulas have been investigated, but the findings are limited by varying drug quality and sources, as well as the numerous and complicated components of formulas. Thus, a drug utilized clinically with simple ingredients, reliable and stable quality, and traceable sources of raw materials is an ideal choice. Jinghua Weikang Capsule (JWC) is a popular Chinese patent drug that consists of two ingredients, Chenopodium ambrosioides L. (CAL) and Adina pilulifera (AP). Our previous works demonstrated the inhibitory, bactericidal, and synergistic effects (with clarithromycin and metronidazole) of CAL against H. pylori strain 26695 and antibiotic-resistant strains isolated from patients suffering from H. pylori-associated gastric ulcers[12-14]. In addition, multicenter, randomized, controlled clinical trials demonstrated that H. pylori eradication of JWC-containing therapy was superior to standard triple therapy and equal to bismuth-based quadruple therapy[15,16]. These results indicate the synergistic effect of the drug, of which CAL is the major effective ingredient, with antibiotics. However, it is not known if CAL alone is capable of eradicating H. pylori in vivo. The intragastric environment differs dramatically from agar plates, thus the drug effect might be conflicting. This study investigated the bactericidal activities of CAL against H. pylori in vivo and in vitro to confirm the consistency effects in different environments.
MATERIALS AND METHODS
H. pylori strains and experimental animals
H. pylori strains (NCTC11637 and Sydney strain 1) were kindly provided by the Department of Gastroenterology of Peking University First Hospital and preserved in brain heart infusion broth (No. 783396; OXOID of Thermo Fisher Scientific, Waltham, MA, United States) at -80 °C.
Forty-four specific-pathogen-free (SPF) male Kunming mice (18-22 g) were purchased from Viral River Laboratories (Beijing, China; Certification No. SCXK 2012-0001). All the animals were housed in an SPF environment and had free access to sterile neutral water and standard mouse feed. The experimental procedures in this study were approved by the Experimental Animal Ethics Committee of Peking University First Hospital (Certification No. J201150).
Drugs and reagents
The volatile oil of CAL (No. 20110311; Tianjin Tasly Pharmaceutical Co., Ltd., Tianjin, China) was diluted in DMSO (Sinopharm Chemical Regent Co., Ltd., Shanghai, China). Lansoprazole (No. 20130205), metronidazole (No. 20120228), and clarithromycin (No. 20120704; Dalian Meilun Biology Technology Co., Ltd., Dalian, China) were used as the triple therapy control in animal experiments. Columbia blood agar plates with 5% sheep’s blood (No. 20130205; bioMérieux Industry Ltd., France), Columbia agar (No. 848706), Brucella broth (No. 8170491), fetal calf serum (No. NTMO133) and brain heart infusion broth (OXOID) were prepared for H. pylori liquid culture and collection.
Culture and collection of H. pylori
Frozen stocks of H. pylori were recovered at room temperature, inoculated on the Columbia blood agar base, and incubated under a microaerobic environment (15% CO2, 5% O2, 80% N2) at 37 °C with over 90% humidity for 48-72 h. The bacterial colonies were collected and prepared in Brucella broth with fetal calf serum for in vitro bactericidal tests, or in brain heart infusion broth for oral gavage of animals.
Inhibitory and bactericidal activities test
The inhibitory activity of CAL against the growth of H. pylori was assessed using an agar dilution method. Briefly, serial dilutions (1:2) of CAL were added to the Columbia blood agar for final concentrations starting from 512 mg/L down to 1 mg/L. The non-drug agar and DMSO agar served as negative controls. Agar plates were inoculated with H. pylori [NCTC11637; 3 × 108 colony forming units (CFU)/mL] and cultured for 72 h. The minimal inhibitory concentration (MIC) was defined as the minimal concentration of CAL required for complete inhibition of H. pylori growth.
Bactericidal activity was evaluated using time-kill curves with 0.5, 1.0 and 2.0 × MIC CAL, and blank and DMSO controls. H. pylori (NCTC11637; 0.1 mL at 1 × 106 CFU/mL) was added to 90 mm plates with the calculated concentrations of CAL or DMSO and Brucella broth containing fetal calf serum (final volume 10 mL) and cultured with gentle shaking at 37 °C in a microaerobic environment. At 0, 4, 8 and 24 h, 0.5 mL of liquid was removed, serially diluted, and plated on Columbia blood agar plates (n = 2 per group). The colonies were counted and averaged after 72 h incubation.
In vivo inoculation
Mice (n = 44) were randomly divided into four groups: blank control, H. pylori control, CAL, and triple therapy group. Except for the blank controls, all animals received a single intraperitoneal injection of cyclophosphamide (200 mg/kg), followed by a total of five gastric intubations (every other day) with 0.4 mL of H. pylori (Sydney strain 1; 12 × 108 CFU/mL). Animals were fasted 24 h before and 2 h after each inoculation. Two weeks after the last inoculation, one mouse was randomly selected from each group and sacrificed to test the bacterial colonization by rapid urease test (RUT) and hematoxylin and eosin (HE) staining of gastric tissue. Giemsa staining was performed to detect H. pylori colonization when HE results were unclear.
Drug administration
Drug doses used in in vivo experiments are equivalent to clinical administrations. The CAL group was treated with 49.32 mg/kg daily for 4 wk. The triple therapy group was treated daily with a suspension of lansoprazole (12.33 mg/kg), metronidazole (164.40 mg/kg), and clarithromycin (205.54 mg/kg) for 1 wk. The H. pylori group was given the same volume of sterile water, and the blank control group was free of any gavage. All drugs were suspended in sterile water and administrated by gastric intubation.
Determination of H. pylori colonization and gastric inflammation
Four weeks after inoculation, animals were deprived of feed but allowed free access to water for 24 h and then sacrificed. Stomachs were collected at the time of sacrifice, opened at the side of the greater curvature, and washed with 4 °C PBS. Half of the antral section was isolated for RUT determination at room temperature within 24 h to observe the change to pink color as positive. The second half of the antral section and partial gastric body tissue were fixed in formaldehyde for histopathology. Fixed tissues were embedded in paraffin, sectioned, and stained with HE or Giemsa to determine inflammation and degree of H. pylori colonization. Eradication success was determined by negative findings from both RUT and histopathology.
The degrees of H. pylori colonization and inflammation were scored by a pathologist blind to treatment groups using the visual analogue scale of the updated Sydney System[17]. H. pylori colonization was graded as: 0, no bacteria detected; +, occasional or several bacterial colonies distributed within 1/3 of the specimen length; ++, consecutive but thin bacterial colonization in the mucosal surface between 1/3 and 2/3 specimen length; +++, piles of bacterial colonies along the length of the specimen. Mononuclear infiltration in the gastric mucosa was graded as: 0, < 5 mononuclear cells in each high power field; +, few mononuclear cells restricted to superficial layer within 1/3 of the mucosa; ++, moderate mononuclear infiltration within 2/3 of the mucosa; +++, intensive mononuclear infiltration throughout the mucosa.
Statistical analysis
Eradication rates were compared among groups by a Fisher’s exact test using SPSS 20.0 software (IBM Corp., Armonk, NY, United States). P < 0.05 was considered as statistically significant.
RESULTS
In vitro
Results of the in vitro inhibitory activity test indicated that there was no visible growth of bacterial colonies after 72 h incubation on the plate containing 16 mg/L CAL, thus defined as the MIC of CAL. Construction of the time-kill curve reveals continual growth of H. pylori colonies in blank and DMSO control groups, but marked inhibition in a dose-dependent manner with CAL (Figure 1). No H. pylori colonies could be detected at 24 h in the 1 and 2 × MIC CAL groups.
Figure 1.

Time-kill curves of Chenopodium ambrosioides L. at different concentrations. MIC: Minimal inhibitory concentration; CAL: Chenopodium ambrosioides L.
In vivo
H. pylori eradication rates, as determined by RUT and histopathology, are presented in Table 1. There was no significant difference between CAL and the triple therapy groups (P = 0.650). H. pylori colonization and inflammation scores are presented in Table 2. Images of HE staining are shown in Figure 2.
Table 1.
Negative rapid urease test and histopathology results
| Group | RUT (n) | Histopathology (n) | Eradication ratio |
| Blank control | 10 | 10 | - |
| Helicobacter pylori | 0 | 0 | 0/10 |
| Chenopodium ambrosioides L. | 6 | 5 | 5/101 |
| Triple therapy | 7 | 7 | 7/10 |
The P value is 0.650, vs triple therapy. n = 10/group. RUT: Rapid urease test.
Table 2.
Helicobacter pylori colonization and gastric inflammation scores
| Group |
H. pylori colonization |
Gastric inflammation |
||||||
| 0 | + | ++ | +++ | 0 | + | ++ | +++ | |
| Blank control | 10 | 0 | 0 | 0 | 10 | 0 | 0 | 0 |
| H. pylori | 4 | 5 | 1 | 0 | 7 | 3 | 0 | 0 |
| CAL | 5 | 5 | 0 | 0 | 8 | 2 | 0 | 0 |
| Triple therapy | 7 | 3 | 0 | 0 | 10 | 0 | 0 | 0 |
n = 10/group. CAL: Chenopodium ambrosioides L.; H. pylori: Helicobacter pylori.
Figure 2.
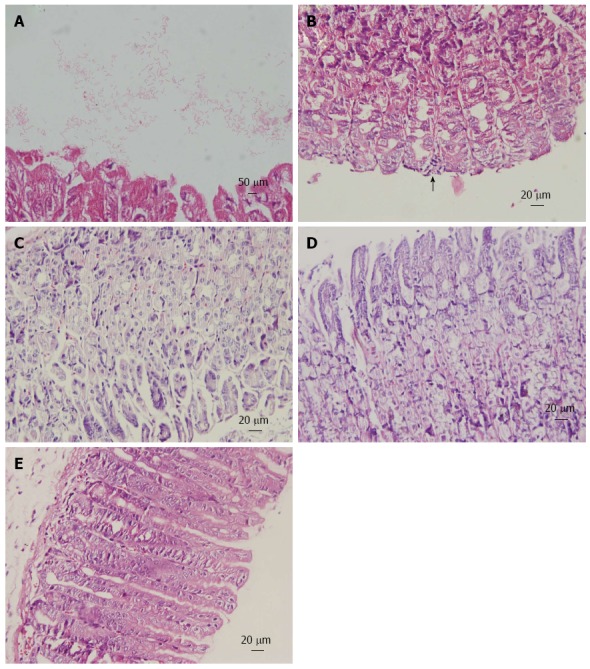
Histopathology of gastric mucosa. Hematoxylin and eosin staining of gastric mucosa from mice inoculated with Helicobacter pylori. A: Helicobacter pylori (H. pylori) colonization on the surface of gastric mucosa (magnification × 100); B: Mononuclear cell infiltrates (arrow; magnification × 40); C: Uninfected mice exhibit a normal gastric mucosa (magnification × 40); D: Treatment with Chenopodium ambrosioides L. for 4 wk revealed no obvious inflammation (magnification × 40); E: Treatment with lansoprazole, metronidazole and clarithromycin for 1 wk showed no pathologic changes (magnification × 40).
DISCUSSION
In China, the prevalence of H. pylori infection is 56.22%[18], which is higher than the global level. Bismuth-based quadruple therapy has become the preferred therapeutic regimen for H. pylori eradication because of increasing antibiotic resistance. However, adverse effects and poor compliance necessitate the development of alternative therapies. Traditional Chinese herbs can promote relief of symptoms as well as producing anti-H. pylori activities. However, the active ingredient is not always obvious, and the in vitro efficacy does not always correlate with eradication in animal models[19].
JWC has been utilized for gastritis and peptic ulcers, with effects of regulating qi, dissipating cold, clearing heat, and resolving stasis according to Chinese medicine theory. Our previous work indicated that CAL was the active ingredient in JWC[12], which was confirmed by the in vitro experiments in the present study. To eliminate possible effects of Tween-80 used in the previous study, we utilized DMSO, which showed no effect on H. pylori growth. The MIC of 16 mg/L was sufficient to completely eliminate H. pylori after 24 h. The in vivo experiments demonstrate the clinical potential of CAL for H. pylori eradication, with efficacy equivalent to the triple therapy.
Despite the promising results, the eradication with CAL was not ideal, possibly be due to the acidic environment of the mucous layer of stomach, or an insufficient dose converted from clinical usage. Furthermore, the conspicuous and classic mucosal inflammation was not observed in this study, which might be ascribed to the short infection period and the application of cyclophosphamide. The conclusion that the effect of CAL rivals triple therapy should be made prudently due to the small sample size. Furthermore, the mice may have had other bacterial species present, as H. hepaticus has been detected in 19.2%-29.5% of SPF mice in China[20,21]. In order to eliminate the interference of other Helicobacter spp., a PCR test should be added in future studies.
In our previous random clinical trial, the therapy containing JWC, a proton pump inhibitor, amoxicillin, and clarithromycin achieved a higher eradication ratio than triple therapy[15]. CAL is a complex compound, for which the effective composition remains undiscovered. Hence, further research to identify the active ingredients would be helpful to evaluate and improve the intensity of the effect. Nevertheless, this study confirms the in vitro and in vivo anti-H. pylori effects of CAL, providing experimental support for future human trials.
ACKNOWLEDGMENTS
We would like to thank Institute of Clinical Pharmacology Peking University for kindly providing facilities.
COMMENTS
Background
Helicobacter pylori (H. pylori) infects more than half of the human population, causing a global public health issue associated with gastritis, peptic ulcer, gastric carcinoma, mucosa-associated lymphoid tissue lymphoma, and other diseases. Successful eradication of bacteria is the effective approach to cure related diseases or to improve prognosis. Antibiotic resistance has become the leading cause of treatment failure, thus new regimens or medicines are required.
Research frontiers
Alternative and complementary treatment approaches including phytomedicine have been focused on eradicating H. pylori or enhancing the bactericidal effect of antibiotics. Various basic and clinical studies have been performed to explore the anti-H. pylori effect of herbs and patent medicines, and some of these traditional therapies have enormous potential. Testing the bactericidal activity or the synergistic effect with antibiotics in vitro and in vivo will provide additional evidence.
Innovations and breakthroughs
This study demonstrates the in vitro and in vivo anti-H. pylori effect of Chenopodium ambrosioides L. (CAL), derived from the Chinese patent medicine, Jinghua Weikang Capsule. The in vivo bactericidal activity was reported for the first time. The effect on H. pylori eradication was equivalent to that of a triple therapy.
Applications
Chenopodium ambrosioides L. has been utilized for gastritis and peptic ulcers under the guidance of traditional Chinese theory. The authors’ research provides experimental evidence for further clinical study of this compound, and the possible application for treating H. pylori infection.
Terminology
The agar dilution method provides the minimal inhibitory concentration of an antimicrobial agent. The time-kill curve method indicates the bactericidal effect of a testing agent.
Peer-review
This study investigated in vitro and in vivo the bactericidal activities against H. pylori strains using a Chinese patent drug containing the volatile oil of CAL. Currently, there is interest in alternative treatments for H. pylori infection due to the high resistance to antibiotics used as the gold standard.
Footnotes
Supported by National Natural Science Foundation Project of China, No. 81072952.
Ethics approval: The study was reviewed and approved by the Peking University First Hospital Institutional Review Board.
Institutional animal care and use committee: All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Peking University First Hospital (IACUC protocol number: J201150).
Conflict-of-interest: We declare that we have no competing financial or personal relationships with other people or organizations that can inappropriately influence our work.
Data sharing: Technical appendix, statistical code, and dataset available from the corresponding author at zhang.xuezhi@263.net. Participants gave informed consent for data sharing.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 31, 2014
First decision: November 14, 2014
Article in press: January 16, 2015
P- Reviewer: Chmiela M, Servin AL S- Editor: Ma YJ L- Editor: O’Neill M E- Editor: Ma S
References
- 1.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 2.Qua CS, Manikam J, Goh KL. Efficacy of 1-week proton pump inhibitor triple therapy as first-line Helicobacter pylori eradication regime in Asian patients: is it still effective 10 years on? J Dig Dis. 2010;11:244–248. doi: 10.1111/j.1751-2980.2010.00445.x. [DOI] [PubMed] [Google Scholar]
- 3.Sasaki M, Ogasawara N, Utsumi K, Kawamura N, Kamiya T, Kataoka H, Tanida S, Mizoshita T, Kasugai K, Joh T. Changes in 12-Year First-Line Eradication Rate of Helicobacter pylori Based on Triple Therapy with Proton Pump Inhibitor, Amoxicillin and Clarithromycin. J Clin Biochem Nutr. 2010;47:53–58. doi: 10.3164/jcbn.10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokwala A, Shah MV, Devani S, Yonga G. Helicobacter pylori eradication: A randomised comparative trial of 7-day versus 14-day triple therapy. S Afr Med J. 2012;102:368–371. doi: 10.7196/samj.5302. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L, Zhang J, Chen M, Hou X, Li Z, Song Z, He L, Lin S. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: a randomized multicenter trial. Am J Gastroenterol. 2014;109:535–541. doi: 10.1038/ajg.2014.26. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Sung JJ, Lin S, Jin Z, Ding S, Huang X, Xia Z, Guo H, Liu J, Chao W. A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chin Med J (Engl) 2003;116:11–14. [PubMed] [Google Scholar]
- 7.Xue Y, Feng H, Zhou LY, Yang XL, Lin SR, Wang YC. Analysis of related factors of H.pylori eradication therapy efficiency. Zhongguo Shiyong Neike Zazhi. 2012;32:693–695. [Google Scholar]
- 8.Gao W, Cheng H, Hu F, Li J, Wang L, Yang G, Xu L, Zheng X. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460–466. doi: 10.1111/j.1523-5378.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 9.Su P, Li Y, Li H, Zhang J, Lin L, Wang Q, Guo F, Ji Z, Mao J, Tang W, et al. Antibiotic resistance of Helicobacter pylori isolated in the Southeast Coastal Region of China. Helicobacter. 2013;18:274–279. doi: 10.1111/hel.12046. [DOI] [PubMed] [Google Scholar]
- 10.Sun QJ, Liang X, Zheng Q, Gu WQ, Liu WZ, Xiao SD, Lu H. Resistance of Helicobacter pylori to antibiotics from 2000 to 2009 in Shanghai. World J Gastroenterol. 2010;16:5118–5121. doi: 10.3748/wjg.v16.i40.5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu WZ, Xie Y, Cheng H, Lu NH, Hu FL, Zhang WD, Zhou LY, Chen Y, Zeng ZR, Wang CW, et al. Fourth Chinese National Consensus Report on the management of Helicobacter pylori infection. J Dig Dis. 2013;14:211–221. doi: 10.1111/1751-2980.12034. [DOI] [PubMed] [Google Scholar]
- 12.Huang XT, Zhang XZ, Li N, Cheng H. In vitro activity of Jinghuaweikang capsules against clinical isolates of antibiotic-resistant Helicobacter pylori. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2010;18:290–293. [Google Scholar]
- 13.Liu W, Zhang XZ, Li N, Li J. Bactericidal action of chenopodium ambrosioides combined with clarithromycin and metronidazole for Helicobacter pylori. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2011;19:281–284. [Google Scholar]
- 14.Liu W, Liu Y, Zhang XZ, Li N, Cheng H. In vitro bactericidal activity of Jinghua Weikang Capsule and its individual herb Chenopodium ambrosioides L. against antibiotic-resistant Helicobacter pylori. Chin J Integr Med. 2013;19:54–57. doi: 10.1007/s11655-012-1248-y. [DOI] [PubMed] [Google Scholar]
- 15.Hu FL, Cheng H, Zhang XZ, An HJ, Sheng JQ, Lü NH, Xie Y, Chen ZS, Xu JM, Hu NZ, et al. [Jinghuaweikang capsules combined with triple therapy in the treatment of Helicobacter pylori associated gastritis and duodenal ulcer and analysis of antibiotic resistance: a multicenter, randomized, controlled, clinical study] Zhonghua Yixue Zazhi. 2012;92:679–684. [PubMed] [Google Scholar]
- 16.Wang TT, Zhang YM, Zhang XZ, Cheng H, Hu FL, Han HX, Chen XW, Li JX, Lai YL, Liu Y. [Jinghuaweikang gelatin pearls plus proton pump inhibitor-based triple regimen in the treatment of chronic atrophic gastritis with Helicobacter pylori infection: a multicenter, randomized, controlled clinical study] Zhonghua Yixue Zazhi. 2013;93:3491–3495. [PubMed] [Google Scholar]
- 17.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 18.The Team of Collaboration of Helicobacter pylori research in China. Prevalence of Helicobacter pylori infection in China. Xiandai Xiaohua and Jieru Zhenliao. 2010;15:265–270. [Google Scholar]
- 19.Vale FF, Oleastro M. Overview of the phytomedicine approaches against Helicobacter pylori. World J Gastroenterol. 2014;20:5594–5609. doi: 10.3748/wjg.v20.i19.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding C, Feng J, Xie JY, Gao C, Hu JH. An Epidemiological Survey of Helicobacter spp. in Laboratory Mice and Rats in the Area around Shanghai by Two Detection Methods. Zhongguo Bijiao Yixue Zazhi. 2011;21:66–69, 78. [Google Scholar]
- 21.Ji SW, Wang S, Wang JB, Zhang YG. Investigation of Helicobacter hepaticus infection in various species of mice in China. Zhonghua Xiaohua Zazhi. 2010;30:597–601. [Google Scholar]


