Abstract
AIM: To determine the relevance of the 10-mm size criterion of the generally accepted surgical indication for gallbladder polyps (GBPs).
METHODS: We collected data of patients who were confirmed to have GBPs through cholecystectomy at Samsung Medical Center between January 1997 and December 2012. Among the patients who underwent cholecystectomy for GBP, those with a definite evidence for malignancy such as adjacent organ invasion, metastasis on preoperative imaging studies, polyp larger than 20 mm, absence of preoperative imaging study results, and patients having gallstones were excluded. We retrospectively collected and analyzed information on patient’s clinical characteristics, symptoms, ultrasonographic findings, and blood laboratory tests.
RESULTS: A total of 836 patients who had undergone cholecystectomy were retrospectively analyzed. Seven hundred eighty patients (93%) had benign polyps, whereas 56 patients (7%) had malignant polyps. Of the 56 patients with malignancy, 4 patients (7%) had borderline GBP (10-12 mm) and a patient had small GBP (< 10 mm) with T2 stage. We conducted an ROC curve analysis to verify the 10-mm size criteria (AUC = 0.887, SD = 0.21, P < 0.001). In the ROC curve for polyp size and malignancy, sensitivity and specificity of the 10-mm size criterion was 98.2% and 19.6%, respectively. The specificity of the 11-mm and 12-mm size criteria was 44.6% and 56%, respectively, whereas the sensitivity of these two size criteria was similar. We defined the GBPs of 10 to 12 mm as a borderline-sized GBP, which were found in 411 patients (49%). In this group, there was a significant difference in age between patients with benign and malignant GBPs (47 years vs 60 years, P < 0.05).
CONCLUSION: GBPs larger than 13 mm need immediate excision whereas for borderline-sized GBPs detected in young patients, careful medical observation can be a rational decision.
Keywords: Gallbladder polyp, Gallbladder cancer, Cholecystectomy, Polyp size, Borderline-sized gallbladder polyp
Core tip: Gallbladder polyp (GBP) is a well-known pre-malignant lesion. The size of GBP, patient’s age and presence of symptoms are the risk factors for GB cancer. GBPs of 10 to 12 mm in diameter have lower risk of malignancy compared to that in GBPs larger than 13 mm, which is similar to the risk of malignancy in GBPs smaller than 10 mm. The use of this surgical indication (GBPs larger than 13 mm GBP) can prevent 50% of unnecessary cholecystectomies without the risk of missing malignant GBPs. Our findings suggest that GBPs with a diameter of 10 to 12 mm in patients younger than 45 years of age old can be observed carefully.
INTRODUCTION
Polypoid lesions of the gallbladder are becoming an increasingly common incidental finding. It is very important to distinguish between a benign polyp and a malignant polyp because of the poor prognosis of gallbladder (GB) cancer, but the radiologic tools such as abdominal ultrasound (US) and computed tomography (CT) are not sufficient to distinguish between a benign polyp and a malignant polyp[1]. Therefore, many researchers have attempted to identify the factors that can help in preoperative differentiation between a benign polyp and a malignant polyp[2,3].
Although the natural history of gallbladder polyps (GBPs) is not completely understood and most of the available studies are retrospective in nature[4,5], the well-known predictor of malignancy in GBPs is a size greater than 10 mm in diameter[6]. However, when we applied the 10-mm size criterion for performing surgery, many polyps were found to be benign in a clinical setting. Most of the benign polyps had a size of 10 or 11 mm, and the incidence of these polyps is increasing as general medical examination is being universalized. Previous studies have shown that polyp size of more than 10 mm is associated with higher risk of developing malignancy; however so far, none of the studies have tried to differentiate between polyps of more than 10 mm in size for determining the incidence of malignancy.
Therefore, we analyzed the pathologically proven GBPs after cholecystectomy for 16 years at Samsung Medical Center to determine the relevance of the 10-mm size criterion.
MATERIALS AND METHODS
Data source and patient population
We collected data of patients who were confirmed to have GBPs through cholecystectomy at Samsung Medical Center between January 1997 and December 2012. Among the patients who underwent cholecystectomy for GBP, those with a definite evidence for malignancy such as adjacent organ invasion, metastasis on preoperative imaging studies, polyp larger than 20 mm, absence of preoperative imaging study results, and patients having gallstones were excluded. A total of 836 patients were enrolled. Information on age, sex, symptoms, US findings, and blood laboratory tests was reviewed retrospectively.
The patients were categorized as having a benign polyp or a malignant polyp according to their histopathologic results. Benign GBPs were subcategorized as benign tumorous polyps if the pathological finding indicated that the polyps had a potential for malignant transformation, whereas benign non-tumorous polyps were not regarded as precancerous lesions. The benign tumorous polyps included adenomas, lipomas, neurofibromas, leiomyomas, and carcinoid tumors. The benign non-tumorous polyps included cholesterol polyps, inflammatory polyps, fibromas, and adenomyomatosis. Malignant GBPs were defined as GB cancer. This study was approved by the Institutional Review Board of the Samsung Medical Center (SMC 2013-12-063).
Definition of GBPs and imaging study
The following standardized US criteria were used to identify polyps on US: immobile, hyperechoic compared to the surrounding bile, non-shadowing, and attached to the GB wall[7]. US examinations were performed by an experienced certified radiologist by using 3.5-MHz transducers (iU-22, Philips Healthcare, Bothell, Washington; ATL 5000, Philips Healthcare, Acuson 128, Siemens, Mountain View, California). The US examinations were interpreted by board certified radiologists who were trained in abdominal imaging, delineation of the number and size of GBPs.
Statistical analysis
Statistical analysis of the data was performed by utilizing SPSS 11.0 (Chicago, IL, United States). Continuous variables were presented as mean ± SD. Intergroup comparisons were conducted with the χ2 test. In order to identify the risk factors for gallbladder cancer, the odds ratio was obtained using multiple logistic regression analysis. The area under the curve (AUC) was calculated using the receiver-operating characteristic curve (ROC) to determine the sensitivity and specificity of the 10-mm size criterion for predicting malignant polyps. If the criterion was not considered sufficient, we tried to determine an optimal cut-off point of polyp size to predict malignant polyps. Differences were considered significant when the P value was less than 0.05.
RESULTS
Demographic findings and clinical characteristics
Among the 836 patients who underwent cholecystectomy, 780 patients (93%) had benign polyps, and 56 patients (7%) had malignant polyps, which were adenocarcinomas. Benign tumorous polyps were adenomas, and 165 patients (20%) had adenomas. Among all of the polyps, the cholesterol polyp was the most common type, and it was found in 559 patients (67%). The demographic and clinical characteristics of all the 836 patients are listed in Table 1. The mean age of the patients and the mean size of polyps were 47 ± 12.3 years and 11.6 ± 3.5 mm, respectively. The sex ratio was 0.86:1 (male:female = 387:449). Among the 836 patients, 464 patients (55%) had solitary polyps, and 372 patients (45%) had multiple polyps. Indications for surgery were collected while allowing for repetition. The majority (695 patients, 83%) of patients underwent cholecystectomy because they had a polyp of greater than 10 mm; this indicated that the 10-mm size criterion is the most important factor in making a decision regarding surgery in a clinical setting. Fifty-four patients had symptoms; some of the patients had specific symptoms such as right upper quadrant pain or epigastric pain, but the other patients complained of a vague abdominal pain, dyspepsia, fatigue, or loss of body weight.
Table 1.
Demographic and clinical characteristics of 836 patients n (%)
| Characteristics | n = 836 |
| Female | 449 (54) |
| Age (yr) | 47 ± 12.3 |
| Indication for surgery1 | |
| Size ≥ 10 mm | 695 (83) |
| Increased size2 | 184 (22) |
| Abnormal imaging3 | 59 (7) |
| Size of the polyp (mm) | 11.6 ± 3.5 |
| Number of polyps | |
| 1 | 460 (55.0) |
| 2 | 128 (15.3) |
| ≥ 3 | 248 (29.6) |
| BMI (kg/m2) | 26.7 ± 32.1 |
| Total cholesterol (mg/dL) | 172.9 ± 33.2 |
| Total bilirubin (mg/dL) | 0.8 ± 0.6 |
| ALT (U/L) | 32.1 ± 31.3 |
| ALP (U/L) | 61.8 ± 25.9 |
| Fasting glucose (mg/dL) | 110.3 ± 30.4 |
| CA 19-9 (U/mL) | 12.4 ± 38.0 |
| HBsAg positivity | 67 (8.0) |
Repetition was allowed;
If the polyp size was increased during the follow-up period compared to that in the initial imaging study;
Gallbladder wall thickness, irregular margin of the polyp, enhancing nodule. GB: Gallbladder; BMI: Body mass index; ALT: Alanine transaminase; ALP: Alkaline phosphatase; CA19-9: Carbohydrate antigen 19-9; HBsAg: Hepatitis B surface antigen.
The patients who had GBPs showed a high BMI and fasting glucose level, but total cholesterol, total bilirubin, ALT, ALP, and CA 19-9 levels were normal. Interestingly, higher proportion of patients with GB polyps showed positivity of hepatitis B surface antigen compared to healthy controls aged from 40 to 49 years in South Korea.
Risk factors for malignant GBPs
The size of GBPs was a significant risk factor for malignant GBPs (P < 0.001, OR = 1.516; 95%CI: 1.356-1.694). Old age and presence of symptoms were associated with a higher risk of malignant GBPs (P < 0.001, OR = 1.120, 95%CI: 1.078-1.164; P = 0.005, OR = 5.019, 95%CI: 1.649-15.276). Number of polyps, ALT, ALP, and fasting glucose levels did not increase the risk of malignancy (Table 2).
Table 2.
Predictors of gallbladder cancer (multiple logistic regression analysis)
| Variables |
GB cancer |
|
| OR (95%CI) | P value | |
| Female | 0.615 (0.276-1.370) | 0.234 |
| Size | 1.516 (1.356-1.694) | < 0.001 |
| Number of polyps | 0.812 (0.531-1.244) | 0.339 |
| Age | 1.120 (1.078-1.164) | < 0.001 |
| Symptoms1 | 5.019 (1.649-15.276) | 0.005 |
| BMI | 1.004 (0.995-1.014) | 0.383 |
| Total cholesterol | 0.991 (0.980-1.003) | 0.139 |
| Total bilirubin | 1.534 (0.604-3.894) | 0.368 |
| ALT | 1.007 (0.999-1.015) | 0.079 |
| ALP | 1.001 (0.991-1.012) | 0.813 |
| Fasting glucose | 1.002 (0.991-1.013) | 0.771 |
| CA19-9 | 1.022 (0.005-1.049) | 0.116 |
| HBsAg positivity | 2.461 (0.587-10.327) | 0.218 |
Right upper quadrant pain, epigastric pain, vague abdominal discomfort, dyspepsia, fatigue, body weight loss. GB: Gallbladder; BMI: Body mass index; ALT: Alanine transaminase; ALP: Alkaline phosphatase; CA19-9: Carbohydrate antigen 19-9; HBsAg: Hepatitis B surface antigen.
Optimal size to predict malignant GBPs
Of 56 patients with malignant pathologic results, only a patient (1.8%) had GBP lesser than 10 mm (intramural, 8 mm). In case of 230 patients with GBP of 10 to 11 mm size, no malignancy was reported. Among 104 patients with GBP of 12 mm, 4 patients (3.8%) have been confirmed to have malignancy. Two of them (50%) were intraepithelial tumors and the other two malignant polyps were intramural tumors.
We calculated the AUC using the ROC curve to test the conventional size criteria for predicting the risk of malignancy (Figure 1, Table 3). When the size cut-off point was set at 10 mm, sensitivity and specificity for predicting malignant polyps was 98.2% and 19.6%, respectively; but when the size cut-off point was set at 11 mm and 12 mm, the sensitivity was the same as that when the size cut-off point was set at 10 mm, but the specificity was increased as the size increased (44.6% and 56.0%, respectively). The sensitivity and the specificity for predicting a polyp of 13 mm was 91.0% and 71.8%, respectively. The sensitivity fell sharply from a size cut-off point of 14 mm. Therefore, after considering the sensitivity and specificity for predicting malignant polyps, 13 mm might be the best cut-off point.
Figure 1.
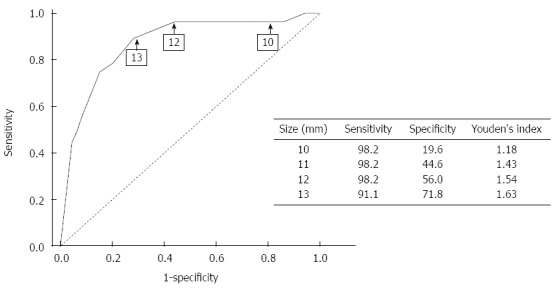
Receiver operating characteristic curves for the size of gallbladder polyp are shown. The area under the curve is 0.887 (95%CI: 0.846-0.927; P < 0.001) for the polyp size. The sensitivity and specificity of each size is presented.
Table 3.
Receiver operating characteristic curve summary statistics
| Size, mm (patients) | Sensitivity | Specificity | Youden’s index | ppv | npv |
| 8 (54) | 1.000 | 0.103 | 1.103 | 0.074 | 1.000 |
| 9 (87) | 0.982 | 0.144 | 1.126 | 0.076 | 0.991 |
| 10 (105) | 0.982 | 0.196 | 1.178 | 0.081 | 0.994 |
| 11 (89) | 0.982 | 0.446 | 1.428 | 0.113 | 0.997 |
| 12 (127) | 0.982 | 0.560 | 1.542 | 0.138 | 0.998 |
| 13 (69) | 0.911 | 0.718 | 1.629 | 0.188 | 0.991 |
| 14 (41) | 0.804 | 0.799 | 1.603 | 0.223 | 0.983 |
Ppv: Positive predictive value; npv: Negative predictive value.
When the size cut-off point was set at 8 mm, the sensitivity was 100%. This result implied that the use of the 10-mm size criterion might lead to unnecessary cholecystectomies, and on the other hand, the 10-mm size criterion might be insufficient to completely exclude malignancy.
Borderline-sized GBPs
We defined GBPs that were more than 10 mm and less than 13 mm in size while maintaining the sensitivity above 90% as “borderline-sized GBPs”. Among the 836 cases, there were 411 cases (49%) of borderline-sized GBPs (Figure 2). In this group, benign non-tumorous polyps accounted for 81% (334 patients) of polyps, which was higher than that in all of the patients. Adenomas were detected in 73 patients. Four patients had malignant polyps, and these patients had polyps measuring 12 mm in size on preoperative imaging studies (Table 4). The patients with borderline GBPs would not have undergone unnecessary surgeries if the criterion for performing surgery was more specific.
Figure 2.
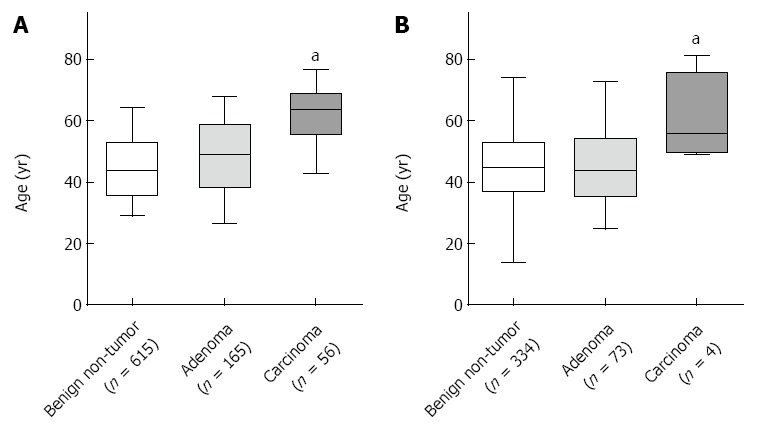
Patient’s age was positively correlated with the malignancy risk. A: All of the patients; B: Patients with borderline-sized gallbladder polyps. aP < 0.05 vs benign non-tumor group.
Table 4.
Characteristics of patients with malignant borderline-sized gallbladder polyps
| Case | Sex/age (yr) | Symptom | Underlying disease | Imaging finding | Operation | TNM stage | Prognosis |
| 1 | F/49 | Dyspepsia | - | 12 mm, polyp | Lap.chole | T2N0M0 | 63 mo, alive |
| 2 | F/52 | - | Hypothyroidism | 12 mm, enhancing nodule | Lap.chole | T1aN0M0 | 30 mo, alive |
| 3 | F/60 | - | - | 12 mm, enhancing nodule | Lap.chole | T1aN0M0 | 24 mo, alive |
| 4 | F/81 | - | CHF, A fib | 12 mm, enhancing sessile nodule | Lap.chole | T2N0M0 | 13 mo, alive |
Lap.chole: Laparoscopic cholecystectomy.
To characterize the patients with borderline-sized GBPs, we examined the patient’s age, which had a relationship with malignant GBPs on the multivariate analysis (Figure 2). The average age of patients with benign polyps and malignant polyps was 47 years and 60 years, respectively. This difference was statistically significant. Especially all four patients who had malignant polyps were more than 45 years of age.
DISCUSSION
GBPs represent a wide spectrum from pseudo lesions to gall bladder cancer. After Boulton and colleagues reported the strategy for managing GBPs using the risk factors of gallbladder cancer which were identified in previous studies, many reports on the management of GBPs have been published[2]. The risk factors suggested in these studies were symptoms, size greater than 10 mm, age more than 50 years, presence of gallstones etc. The risk factors for malignant polyps in our study were polyp size, patient’s age, and presence of symptoms. This result was similar to that in previous reports[8-11].
Polyp size has long been considered to be an important factor[12,13]. Current guidelines recommend cholecystectomy for polyps measuring 10 mm or larger. The use of this strategy may result in a large number of unnecessary cholecystectomies, because the detection rate of relatively small GBPs has been increasing since 2004, which was the year due to the expanded use of abdominal US in South Korea.
In South Korea, the prevalence of GBPs considering this criterion has been increasing steadily since 2004, which was the year in which increased ultrasound surveillance of asymptomatic persons was performed.
Corwin et al[7] presented a study in 2011. This study provides further directions for managing incidentally diagnosed polyps in adults. They monitored 346 incidentally detected GBPs, and there were no cases of GB cancer and there were 3 cases of adenomas. In their study, the average size of polyps was 5 mm (range: 1-18 mm), and the proportion of polyps greater than 10mm was only 3.5% (n = 12). This study showed that incidentally discovered polyps were usually small in size and the risk of malignancy was low.
We defined the polyps having a size of 10 to 12 mm as borderline-sized GBPs. These polyps had a low malignant potential, and they were mostly benign non-tumorous polyps. In particular, the polyps were not malignant in any of the patients who had a 10-11 mm sized polyp, thus suggesting that cholecystectomy was an inappropriate management strategy in these patients. The use of this surgical indication (GBPs larger than 13 mm GBP) can prevent 50% of unnecessary cholecystectomies without the risk of missing malignant GBPs.
The role of gallbladder adenoma in the pathogenesis of gallbladder carcinoma is controversial[14]. It is thought that adenoma may play a role in some cases of gallbladder cancer. The adenoma-carcinoma sequence was first suggested by Kozuka et al[15], who conducted a study of 1605 resected gallbladder specimens and found 7 adenomas with malignant changes and evidence of adenomatous residue in 15 of 79 (19%) invasive carcinomas[15]. However, the incidence of the combined lesion is low and varies between 0.14% and 1.1% in different series[16-18]. Wistuba et al[19] performed molecular studies on tissue from gallbladder adenoma and detected no mutations in the TP53 gene, a frequent finding in dysplasia, carcinoma in situ, and invasive cancer, which led the researchers to conclude that adenomas are not precursors of invasive gallbladder carcinoma. Similarly, Roa et al[20] found no evidence of adenoma residue in their study of completely mapped early carcinomas of the gallbladder. These reports indicate that the adenoma-carcinoma sequence is less important in the gallbladder than in the other organs of the digestive tract[21]. The dysplasia-carcinoma sequence has been considered as the main route of carcinogenesis in the gallbladder[22-24]. We performed this study with more emphasis on GB carcinoma than adenoma. This could be a limitation of our study.
Many studies have demonstrated that malignant GBPs are significantly more common in patients aged more than 50 years[25-27]. Our study confirmed that patient’s age was associated with the risk of developing malignant polyps. Besides, there was a significant difference in the mean age of patients with malignant borderline-sized GBPs and those with benign borderline-sized GBPs. The mean age of patients with malignant polyps was 60 years, whereas that of patients with benign polyps was 47 years, and all of the malignant polyps were detected in patients aged 45 years and older. This finding indicates that there is a low possibility of GB carcinoma in patients having borderline-sized GBPs and who are less than 49 years of age, and there is a high possibility of GB carcinoma in patients having borderline-sized GBPs and who are more than 60 years of age.
GBPs generally do not cause any symptoms[26], although most of the prevalence studies did not assess the symptoms. Terzi et al[28] reported that in a series of 74 patients undergoing cholecystectomy for GBPs, 91% of patients had symptoms, most commonly right upper quadrant pain, nausea, dyspepsia, and jaundice. However, about 60% of the patients also had gallstones, and therefore it is unclear whether the polyps were primarily driving the symptoms. The symptoms were related to malignancy in our research, nevertheless, we excluded the patients with gallstones. Kwon et al[14] suggested that symptoms may be associated with the size of the polyp rather than the association of gallstone. Therefore, patient’s symptoms should be considered as the red flag for malignancy.
Patients with GBPs were classified as over-weight according to BMI and had a high fasting glucose level. The researchers suggested that metabolic syndrome contributes to the formation of cholesterol polyps in the gallbladder[20,29]. However, because of the absence of a similar finding in overt diabetic patients in their study[29] and in other prevalence studies[16,30], the validity of this association is questionable. Also, hepatitis B surface antigen positivity was greater in patients with GBPs compared to the general population. However, in contrast to the findings presented by Lin et al[31], hepatitis B surface antigen positivity was not a predictive factor for GB cancer in this study.
Our study indicates that the natural history of borderline-sized GBPs is benign, although most of the borderline-sized GBPs were removed. Hence, it is necessary to redefine the surgical indications for GBPs, which are increasingly being detected on surveillance imaging. Our study is a retrospective study from a single center despite large study subjects. So, prospective multicenter study will be needed to validate our study.
In conclusion, the need for performing immediate surgery for GBPs measuring more than 13 mm in size is undebatable, whereas borderline-sized GBPs, especially in asymptomatic young patients (less than 45 years old), have low risk of malignancy, and therefore, a careful “wait and see” strategy is appropriate. Further studies are needed to define the characteristics of borderline-sized GBPs and to demonstrate the natural history of borderline-sized GBPs.
COMMENTS
Background
Gallbladder polyp (GBP) is a well-known pre-malignant lesion, especially the size of GBP, age and presence of symptoms are risk factors for gallbladder (GB) cancer. The well-known predictor of malignancy in GBPs is a size greater than 10 mm in diameter. However, when we applied the 10-mm size criterion for performing surgery, many polyps were found to be benign in a clinical setting. Most of the benign polyps had a size of 10 or 11 mm, and the incidence of these polyps is increasing as general medical examination is being universalized.
Research frontiers
Some recent studies have shown that incidentally discovered polyps were usually small in size and the risk of malignancy was low.
Innovations and breakthroughs
Previous studies have shown that polyp size of more than 10 mm is associated with higher risk of developing malignancy; however so far, none of the studies have tried to differentiate between polyps of more than 10 mm in size for determining the incidence of malignancy. Therefore, we analyzed the pathologically proven GBPs after cholecystectomy to determine the relevance of the 10-mm size criterion.
Applications
This study aimed to determine the relevance of the 10-mm size criterion of the generally accepted surgical indication for GBPs, and to provide a new surgical indication clues for decrease unnecessary cholecystectomies.
Terminology
GBPs of 10 to 12 mm in diameter (borderline-sized GBPs) have lower risk of malignancy compared to that in GB polyps larger than 13 mm, which is similar to the risk of malignancy in GB polyps smaller than 10 mm. The use of this surgical indication (GBPs larger than 13 mm GBP) can prevent 50% of unnecessary cholecystectomies without the risk of missing malignant GBPs. Also, this study confirmed that patient’s age was associated with the risk of developing malignant polyps. This finding indicates that there is a low possibility of GB carcinoma in patients having borderline-sized GBPs and who are less than 49 years of age.
Peer-review
Authors made a retrospective revision of their database regarding gallbladder polyps, in order to find a potential best predictor of malignancies rather than 10 mm size. They concluded that 13 mm size was a better cut-off value to indicate immediately surgery, whereas 11 and 12 mm size polyps, in younger patients could be strictly followed up before undergoing surgery. The paper certainly brings new information on the subject and may represent an interesting option for the readers.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: July 22, 2014
First decision: August 27, 2014
Article in press: December 1, 2014
P- Reviewer: Li YM, Nagem RG, Tolone S S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.Konstantinidis IT, Bajpai S, Kambadakone AR, Tanabe KK, Berger DL, Zheng H, Sahani DV, Lauwers GY, Fernandez-del Castillo C, Warshaw AL, et al. Gallbladder lesions identified on ultrasound. Lessons from the last 10 years. J Gastrointest Surg. 2012;16:549–553. doi: 10.1007/s11605-011-1696-2. [DOI] [PubMed] [Google Scholar]
- 2.Boulton RA, Adams DH. Gallbladder polyps: when to wait and when to act. Lancet. 1997;349:817. doi: 10.1016/S0140-6736(05)61744-8. [DOI] [PubMed] [Google Scholar]
- 3.Okamoto M, Okamoto H, Kitahara F, Kobayashi K, Karikome K, Miura K, Matsumoto Y, Fujino MA. Ultrasonographic evidence of association of polyps and stones with gallbladder cancer. Am J Gastroenterol. 1999;94:446–450. doi: 10.1111/j.1572-0241.1999.875_d.x. [DOI] [PubMed] [Google Scholar]
- 4.Gurusamy KS, Abu-Amara M, Farouk M, Davidson BR. Cholecystectomy for gallbladder polyp. Cochrane Database Syst Rev. 2009;(1):CD007052. doi: 10.1002/14651858.CD007052.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mainprize KS, Gould SW, Gilbert JM. Surgical management of polypoid lesions of the gallbladder. Br J Surg. 2000;87:414–417. doi: 10.1046/j.1365-2168.2000.01363.x. [DOI] [PubMed] [Google Scholar]
- 6.Koga A, Watanabe K, Fukuyama T, Takiguchi S, Nakayama F. Diagnosis and operative indications for polypoid lesions of the gallbladder. Arch Surg. 1988;123:26–29. doi: 10.1001/archsurg.1988.01400250028003. [DOI] [PubMed] [Google Scholar]
- 7.Corwin MT, Siewert B, Sheiman RG, Kane RA. Incidentally detected gallbladder polyps: is follow-up necessary?--Long-term clinical and US analysis of 346 patients. Radiology. 2011;258:277–282. doi: 10.1148/radiol.10100273. [DOI] [PubMed] [Google Scholar]
- 8.Andrén-Sandberg A. Diagnosis and management of gallbladder polyps. N Am J Med Sci. 2012;4:203–211. doi: 10.4103/1947-2714.95897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JK, Yoon YB, Kim YT, Ryu JK, Yoon WJ, Lee SH, Yu SJ, Kang HY, Lee JY, Park MJ. Management strategies for gallbladder polyps: is it possible to predict malignant gallbladder polyps? Gut Liver. 2008;2:88–94. doi: 10.5009/gnl.2008.2.2.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park JY, Hong SP, Kim YJ, Kim HJ, Kim HM, Cho JH, Park SW, Song SY, Chung JB, Bang S. Long-term follow up of gallbladder polyps. J Gastroenterol Hepatol. 2009;24:219–222. doi: 10.1111/j.1440-1746.2008.05689.x. [DOI] [PubMed] [Google Scholar]
- 11.Shin SR, Lee JK, Lee KH, Lee KT, Rhee JC, Jang KT, Kim SH, Choi DW. Can the growth rate of a gallbladder polyp predict a neoplastic polyp? J Clin Gastroenterol. 2009;43:865–868. doi: 10.1097/MCG.0b013e31819359aa. [DOI] [PubMed] [Google Scholar]
- 12.Chattopadhyay D, Lochan R, Balupuri S, Gopinath BR, Wynne KS. Outcome of gall bladder polypoidal lesions detected by transabdominal ultrasound scanning: a nine year experience. World J Gastroenterol. 2005;11:2171–2173. doi: 10.3748/wjg.v11.i14.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee KF, Wong J, Li JC, Lai PB. Polypoid lesions of the gallbladder. Am J Surg. 2004;188:186–190. doi: 10.1016/j.amjsurg.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Kwon W, Jang JY, Lee SE, Hwang DW, Kim SW. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009;24:481–487. doi: 10.3346/jkms.2009.24.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozuka S, Tsubone N, Yasui A, Hachisuka K. Relation of adenoma to carcinoma in the gallbladder. Cancer. 1982;50:2226–2234. doi: 10.1002/1097-0142(19821115)50:10<2226::aid-cncr2820501043>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Collett JA, Allan RB, Chisholm RJ, Wilson IR, Burt MJ, Chapman BA. Gallbladder polyps: prospective study. J Ultrasound Med. 1998;17:207–211. doi: 10.7863/jum.1998.17.4.207. [DOI] [PubMed] [Google Scholar]
- 17.Nakajo S, Yamamoto M, Tahara E. Morphometrical analysis of gall-bladder adenoma and adenocarcinoma with reference to histogenesis and adenoma-carcinoma sequence. Virchows Arch A Pathol Anat Histopathol. 1990;417:49–56. doi: 10.1007/BF01600109. [DOI] [PubMed] [Google Scholar]
- 18.Roa I, de Aretxabala X, Morgan R, Molina R, Araya JC, Roa J, Ibacahe G. [Clinicopathological features of gallbladder polyps and adenomas] Rev Med Chil. 2004;132:673–679. [PubMed] [Google Scholar]
- 19.Wistuba II, Miquel JF, Gazdar AF, Albores-Saavedra J. Gallbladder adenomas have molecular abnormalities different from those present in gallbladder carcinomas. Hum Pathol. 1999;30:21–25. doi: 10.1016/s0046-8177(99)90295-2. [DOI] [PubMed] [Google Scholar]
- 20.Roa I, de Aretxabala X, Araya JC, Roa J. Preneoplastic lesions in gallbladder cancer. J Surg Oncol. 2006;93:615–623. doi: 10.1002/jso.20527. [DOI] [PubMed] [Google Scholar]
- 21.Goldin RD, Roa JC. Gallbladder cancer: a morphological and molecular update. Histopathology. 2009;55:218–229. doi: 10.1111/j.1365-2559.2008.03192.x. [DOI] [PubMed] [Google Scholar]
- 22.Ajiki T, Fujimori T, Onoyama H, Yamamoto M, Kitazawa S, Maeda S, Saitoh Y. K-ras gene mutation in gall bladder carcinomas and dysplasia. Gut. 1996;38:426–429. doi: 10.1136/gut.38.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roa I, Araya JC, Villaseca M, De Aretxabala X, Riedemann P, Endoh K, Roa J. Preneoplastic lesions and gallbladder cancer: an estimate of the period required for progression. Gastroenterology. 1996;111:232–236. doi: 10.1053/gast.1996.v111.pm8698204. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto M, Nakajo S, Tahara E. Dysplasia of the gallbladder. Its histogenesis and correlation to gallbladder adenocarcinoma. Pathol Res Pract. 1989;185:454–460. doi: 10.1016/S0344-0338(89)80062-7. [DOI] [PubMed] [Google Scholar]
- 25.Cha BH, Hwang JH, Lee SH, Kim JE, Cho JY, Kim H, Kim SY. Pre-operative factors that can predict neoplastic polypoid lesions of the gallbladder. World J Gastroenterol. 2011;17:2216–2222. doi: 10.3748/wjg.v17.i17.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gallahan WC, Conway JD. Diagnosis and management of gallbladder polyps. Gastroenterol Clin North Am. 2010;39:359–367, x. doi: 10.1016/j.gtc.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Kianmanesh R, Scaringi S, Castel B, Flamant Y, Msika S. [Precancerous lesions of the gallbladder] J Chir (Paris) 2007;144:278–286. doi: 10.1016/s0021-7697(07)91953-5. [DOI] [PubMed] [Google Scholar]
- 28.Terzi C, Sökmen S, Seçkin S, Albayrak L, Uğurlu M. Polypoid lesions of the gallbladder: report of 100 cases with special reference to operative indications. Surgery. 2000;127:622–627. doi: 10.1067/msy.2000.105870. [DOI] [PubMed] [Google Scholar]
- 29.Segawa K, Arisawa T, Niwa Y, Suzuki T, Tsukamoto Y, Goto H, Hamajima E, Shimodaira M, Ohmiya N. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol. 1992;87:630–633. [PubMed] [Google Scholar]
- 30.Shinchi K, Kono S, Honjo S, Imanishi K, Hirohata T. Epidemiology of gallbladder polyps: an ultrasonographic study of male self-defense officials in Japan. Scand J Gastroenterol. 1994;29:7–10. doi: 10.3109/00365529409090429. [DOI] [PubMed] [Google Scholar]
- 31.Lin WR, Lin DY, Tai DI, Hsieh SY, Lin CY, Sheen IS, Chiu CT. Prevalence of and risk factors for gallbladder polyps detected by ultrasonography among healthy Chinese: analysis of 34 669 cases. J Gastroenterol Hepatol. 2008;23:965–969. doi: 10.1111/j.1440-1746.2007.05071.x. [DOI] [PubMed] [Google Scholar]


