Abstract
Background
Lymphedema is a chronic debilitating condition and curative treatment is yet to be found. Tissue engineering approach, which combines cellular components, scaffold, and molecular signals hold great potential in the treatment of secondary lymphedema with the advent of lymphatic graft to reconstruct damaged collecting lymphatic vessel. This review highlights the ideal characteristics of lymphatic graft, the limitation and challenges faced, and the approaches in developing tissue-engineered lymphatic graft.
Methods
Literature on tissue engineering of lymphatic system and lymphatic tissue biology was reviewed.
Results
The prime challenge in the design and manufacturing of this graft is producing endothelialized conduit with intraluminal valves. Suitable scaffold material is needed to ensure stability and functionality of the construct. Endothelialization of the construct can be enhanced via biofunctionalization and nanotopography, which mimics extracellular matrix. Nanocomposite polymers with improved performance over existing biomaterials are likely to benefit the development of lymphatic graft.
Conclusions
With the in-depth understanding of tissue engineering, nanotechnology, and improved knowledge on the biology of lymphatic regeneration, the aspiration to develop successful lymphatic graft is well achievable.
Keywords: Lymphedema, Tissue engineering, Lymphatic graft, Nanotechnology, Lymphatic regeneration
1. Introduction
Lymphedema is a debilitating condition resulting from dysfunction of lymphatic system characterized by progressive soft tissue swelling, which commonly involves the limbs and genitalia. Lymphedema is categorized into primary and secondary lymphedema. Primary lymphedema is rare and caused by genetic defects. Secondary lymphedema is common and caused by acquired damage secondary to surgical resection (lymph node clearance), tumor infiltration, parasitic infection, and radiation-induced fibrosis.
In the United Kingdom alone, there are estimated 240,000 people living with lymphedema [1]. Despite the wide practice of breast conserving surgery and minimal lymphatic intervention, the world wide incidence of secondary lymphedema remains high with an estimate of 295,320 patients developing upper-limb lymphedema yearly after breast cancer surgery [2]. This huge number highlights the critical clinical need to treat surgery-induced secondary lymphedema.
The current armamentarium of therapeutic intervention involves nonsurgical and surgical treatment. Nonsurgical treatment includes bandaging and physiotherapy, which does not prevent progression of disease and results in poor quality of life. The surgical treatment of lymphedema has evolved tremendously over the years. In 1990, Baumeister [3] reported the use of lymphatic (lymphaticolymphatic) bypass graft to bridge damaged lymphatic vessels using autologous lymphatic graft harvested from ventromedial part of the thigh. Although reporting a volume reduction of 80% compared with preoperative conditions, this technique did not gain popularity because of the high risk of donor site morbidity. Following the same principle, Campisi [4] used vein graft (lymphatic-venous-lymphatic shunt) and reported volume reduction of 75% in almost half of his patients. Several other form of surgical treatment such as liposuction (suction-assisted lipectomy), subcutaneous excision, and ablative surgery with skin grafting were practiced but with poor outcomes. In the recent years, surgical treatment of lymphedema has evolved into supermicrosurgical approach. The techniques undertaken are lymphatic bypass surgery (lymphaticovenular anastomosis) and vascularized lymph node transfer. Most authors reported a modest improvement in limb volumes (decrease of 30%–50%), although some patients experienced more significant improvement [5]. However, none of these techniques are curative and they are exclusively for patients in early stages of lymphedema.
With these challenges in mind, tissue-engineered lymphatic graft is an ideal alternative that should be explored for the treatment of secondary lymphedema as it is likely to offer greater versatility and therapeutic power. The engineered lymphatic graft can be used to bridge defects involving collecting lymphatic vessels because of surgical resection or as a bypass for congenital cause of blockade to lymphatic circulation. Furthermore, it could also benefit patients with venous impairment in the same lymphedema limb that are not suitable candidate for lymphovenous shunt. To match the properties of native lymphatic vessel, an ideal lymphatic graft should be durable, able to maintain patency, easy to sterilize, nontoxic, nonallergenic, and noncarcinogenic. In terms of handling, it should be kink resistant, easy to suture, and have adequate fatigue strength. The goal of tissue-engineered lymphatic graft is to reproduce the structure, function, and cellular organization of a native collecting lymphatic vessel.
This article aims to discuss the tissue engineering approaches in developing lymphatic graft and the unique limitations and challenges involved. This review also features the advancement in the knowledge of lymphatic biology, with emphasis on lymphatic regeneration and the molecular signals involved.
2. Tissue engineering of lymphatic graft
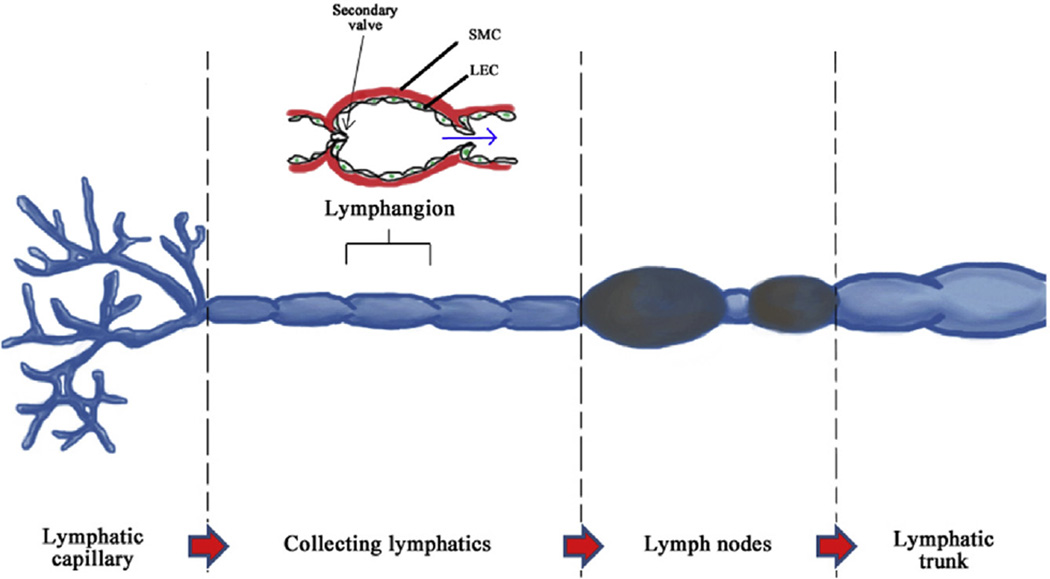
The lymphatic system can be partitioned into five sections: lymphatic capillary, collecting lymphatic, lymph nodes, lymphatic trunk, and lymphatic duct (Fig. 1). The collecting lymphatic vessels contain intraluminal (secondary) valves and endothelial cells that are spindle shaped with continuous basement membrane, pericytes, and associated smooth muscle cell cover enabling intrinsic contractility of the vessels [6]. Collecting lymphatic vessels are made up of functional subunits known as lymphangion, which propels lymph in a peristalsis manner. Lymph flow is driven by local forces and affected by neighboring musculoskeletal movement and vasomotion. The collecting lymphatics have similar wall as of blood vessels and its smooth muscle tissue contains specialized pacemaker cells that regulate spontaneous electrical activity (Table 1) [7].
Fig. 1.
Organization of lymphatic system. SMC, smooth muscle cell. (The color version of the figure is available online.)
Table 1.
Characteristics of lymphatic vessels and blood vessels.
| Characteristic | Lymphatic capillary | Lymphatic collector | Blood vessel |
|---|---|---|---|
| Diameter | 10–60 µm | 50–400 µm | Wide range |
| Inner lining (intima) | LEC | LEC | BEC |
| Basement membrane | Patchy or absent. Lacks pericytes. | Continuous, multilayered with pericytes | Continuous, multilayered with pericytes |
| Smooth muscle wall (media) | None | Present | Present |
| Valves | Primary valve | Secondary valve | Present in vein |
| Subunit | None | Lymphangion | None |
Lymphatic system regulates extracellular fluid homeostasis by preserving tissue fluid balance, involved in immune response and absorption of dietary fat. The amount of lymph formation and flow rate is dependent on the characteristics of extracellular matrix (ECM), type of tissue, and the degree of swelling [8].
The lymphatic network is lined by specialized endothelial cells known as lymphatic endothelial cells (LECs). The LECs embryologically originate from out pouching of blood endothelial cells from cardinal vein, and they both share close structural similarities. About 300 genes are expressed differentially between blood endothelial cells and LECs, which includes LEC-specific genes (lymphatic vessel hyaluronan receptor 1, vascular endothelial growth factor receptor 3, prox1, podoplanin, and β-chemokine receptor D6), integrins, cadherins, proinflammatory cytokines, and chemokines [9].
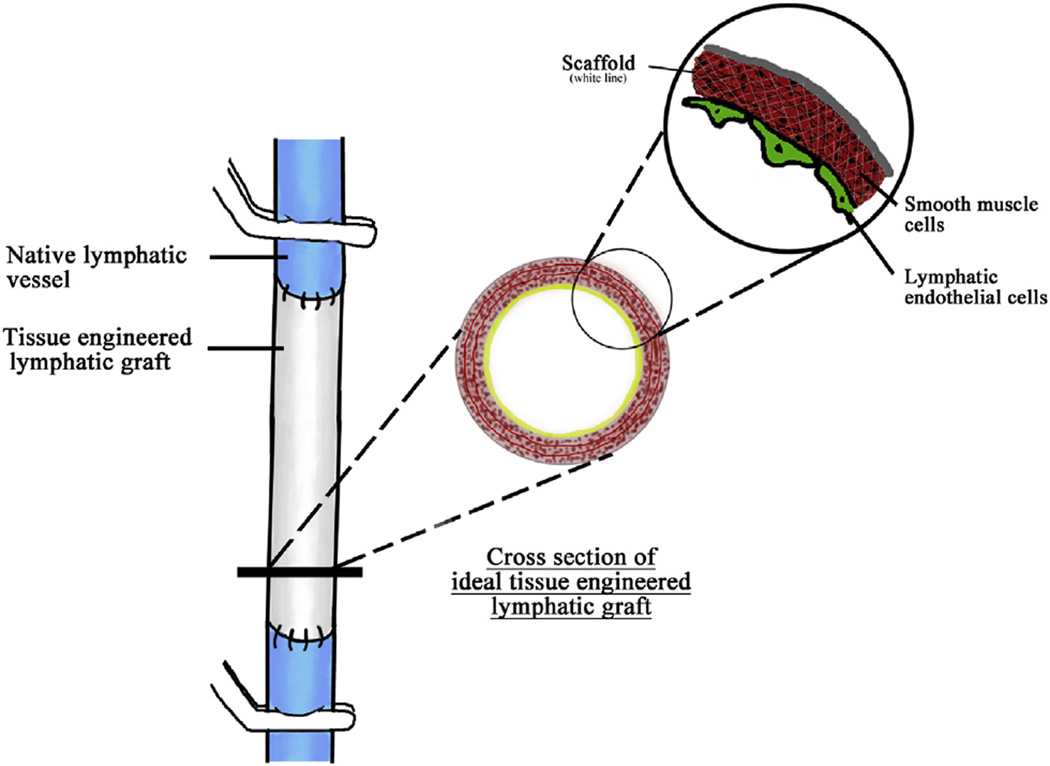
Three key aspects that tissue engineering could benefit lymphatic system are development of artificial lymphatic graft (Fig. 2), regeneration of lymphatic network via lym-phangiogenesis, and engineering artificial lymph node.
Fig. 2.
Illustration of tissue-engineered lymphatic graft bridging damaged lymphatic vessel. (The color version of the figure is available online.)
Developing tissue-engineered lymphatic graft involves proper material selection and fabrication of scaffold to create an environment conducive for LEC growth and for lymphatic regeneration. Although artificial lymphatic graft has yet to be successful, it holds great potential as there is possibility of translating technology and knowledge from tissue-engineered vascular graft. Regenerating lymphatic network is a long-standing challenge for tissue engineers as it required self-organization of endothelial cells into a network of conduits in vivo. A possible solution is with the use of stem cell technology and growth factors to form an extensive network of lymphatic capillary, which drains into existing or neighboring collecting lymphatic. Artificial lymph node that is immunologically functional was successfully engineered by Watanabe et al. [10]. Although this artificial organ does not enhance lymphatic circulatory function, nevertheless this is a step forward in achieving alternative treatment for lymphedema in future.
The challenges involved in developing lymphatic graft differ from vascular graft. The major differences are the mechanical property and hydrostatic pressure. Lymphatic system is a low pressure and pulseless system compared with vascular system. This allows lymphatic graft to be built with lower strength and elasticity; however, intraluminal valve is an essential feature to be included to ensure its functionality. The following section discusses on the strategies involved in developing lymphatic graft.
3. Strategies in development of lymphatic graft
3.1. Lymphatic pressure
Two primary mechanical forces that require special attention in tissue engineering of lymphatic graft are the transaxial pressure gradient, which governs fluid shear stress; and average transmural pressure, which governs circumferential stress [11]. These two intrinsic pressures work in tandem with extrinsic pressures such as internal tissue pressure, tissue osmotic pressure, and venous pressure, which determine the flow rate of lymphatic fluid [12]. Intrinsic contraction of lymphatic vessel ranges between 12 and 70 mm Hg in healthy human. The extrinsic pressure in healthy human ranges between 0 and 60 mm Hg [13]. In pathologic condition, the intrinsic lymphatic pressure is elevated and the accumulation of fibrotic tissue and adipocytes due to lymph stasis drastically alters the surrounding mechanical environment [14,15]. This condition may eventually overwhelm the lymphatic vessels and result in decreased contractile tone and frequency of collecting lymphatics as seen in rat thoracic duct model (negative chronotropic and inotropic effect) [16]. However, this is not seen in the mesenteric lymphatic vessels, which indicated that the amount of reduction varies in different location. The pressure gradient at which flow cessation takes place in human is yet to be known. It is crucial to quantify these values in diseased human lymphatic system as the ability to simulate these pressures ex vivo is paramount in determining the success of the lymphatic graft.
The advancement of imaging modalities coupled with computational power and image processing algorithms has enabled reliable quantification and correlation between lymph flow rate and intrinsic contractile activity [17,18]. The use of noninvasive imaging modalities, the near-infrared fluorescence imaging in combination with the clinically approved indocyanine green dye has enabled researchers to better understand the draining velocity of initial lymphatics and contractile physiology of collecting lymphatics in human as most previous studies were mostly limited to animal models [19–21]. This advancement in imaging has been particularly useful in understanding the changes that takes place in diseased lymphatic system, which is a step closer to unraveling ways to manipulate the biological and mechanical changes taking place in lymphedema.
3.2. Mechanical property
The elastic modulus, tensile stress and strain at failure and the burst strength of human collecting lymphatic vessel are not as well studied and documented as for arteries and veins [22]. Several studies have been conducted on animal models however these values might not precisely reflect that of human due to difference in size and capacity of the vessel [23–25]. An ideal tissue-engineered lymphatic graft should mimic the mechanical properties of native collecting lymphatic vessel. Features of an ideal tissue-engineered lymphatic graft are high strength and flexibility, good hydraulic conductivity, kink resistant and good suture retention. This will ensure that the conduit and anastomosis remain intact especially in regions with great range of motion such as the axilla and groin. In terms of achieving a reliable standard and to ensure patient’s safety, the international standard for cardiovascular implant—tubular vascular prostheses BS ISO 7198:1998—is an excellent guideline to adhere to at the moment.
3.3. Lymphatic valve
The main challenge in the design of artificial lymphatic graft is the inclusion of valves. Lymphatic valves are bileaflet and made up of connective tissue lined by LECs, which are anchored to the base by elastin and collagen that are responsible to resist inversion [26]. Valves are present at intervals of every few millimeters and usually found in vessel bifurcations and branch points [27,28]. Valves function to prevent backflow and are the anatomical sites of fluid shear sensation for the production of nitric oxide to regulate lymphatic contractions [29]. Valvular dysfunction would exacerbate lymphedema, which is witnessed in genetic conditions involving lymphatic valve malformation such as in FOXC2 mutation [30]. Valves also play an important role in sectioning the conduit into shorter segments to assist capillary action.
Capillary action is described as [31] follows:
| (a) |
where h is the height of the meniscus, θ is the contact angle, γ is the liquid-air surface tension (force/unit length), g is the gravitational acceleration (length/square of time), p is the density of liquid (mass/volume), and r is the radius of conduit (length).
Equation (a) implies that height of capillary action is inversely proportional to the radius of the conduit. A simplified assumption using properties of water suggests that the height of capillary action is 3.6 cm if the r is 0.4 mm. However, knowing that the density of lymph is greater than that of water, the height of meniscus is expected to be much lower and hence capillary action alone is insufficient to drive the lymph through the whole length of lymphatic vessels if valves were not present to segment the columns.
3.4. Scaffold
Tissue engineering is aimed at creating a construct, which allows harmonious interaction between scaffold, cells, and appropriate growth factors [32]. An ideal scaffold should bridge the gap between lymphatic vessels and guide regeneration of functional lymphatic tissue. The importance of designing a conduit with intraluminal valves exclude the possibility of using nonscaffold-based tissue engineering approach such as “sheet-based” method [22]. Scaffold for lymphatic graft can be broadly divided into synthetic and natural (decellularized).
3.4.1. Biodegradable synthetic scaffold
Synthetic scaffold has the advantage of being custom designed and shaped with well-defined physical and biochemical property at production. Biodegradable synthetic scaffold acts as temporary scaffold before being substituted by cellular matrix that is balanced by the polymer degradation rate. Polyglycolic acid scaffold was used by Dai et al. [33] to engineer lymphatic vessel and reported successful endothelialization by LEC. Polyglycolic acid was used because of its well-documented biocompatibility and the ease in manipulating and design. This is the sole reported work on biodegradable lymphatic conduit and despite being a preliminary work; it showed the ability of LEC to attach onto polymer scaffold.
3.4.2. Nonbiodegradable synthetic scaffold
This scaffold has stable mechanical strength and able to maintain its shape. The main issues with most of these materials used in vascular grafts are maintaining patency of the graft and high infection rate especially when the diameter is <5 mm [34,35]. This is due to the absence of adaptive tissue that is responsive to local environment [36]. These limitations have driven researches to develop vascular graft with endothelial cell lining as well as hybrid graft, which has the combination of mechanical strength and biological cues [37,38].
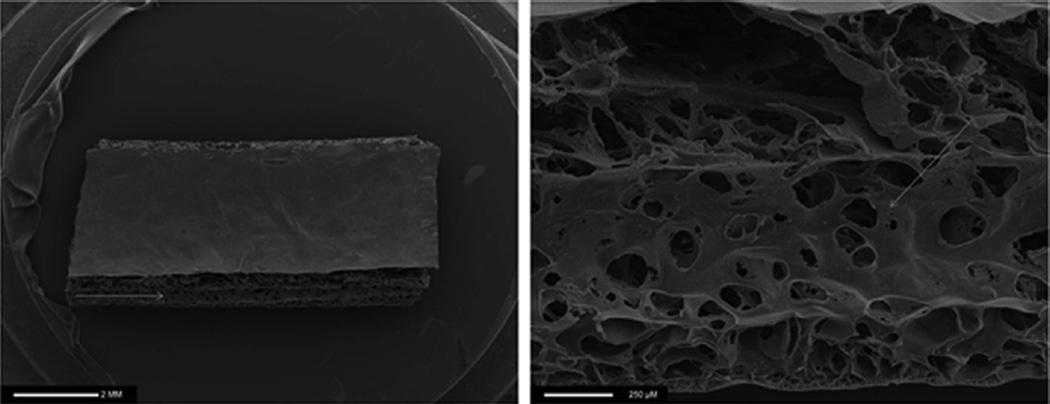
A particular nanocomposite polymer, the UCL-Nano (polyhedral oligomeric silsesquioxane poly(carbonate-urea) urethane [POSS-PCU]) is a novel nanocomposite developed in our laboratory and has great potential in lymphatic graft engineering [39]. This polymer has shown several superior properties over present synthetic polymer contributed by its surface nanotopography and its viscoelastic properties (Fig. 3). It has been successful in cardiovascular applications such as heart valves and bypass graft because of its ideal mechanical and biochemical properties, which include biocompatibility, durability, ability for biofunctionalization and endothelialization, and antithrombogenic property [40–42]. These merits are expected to be favorable in the engineering of lymphatic graft. This nanomaterial has been used in the development of synthetic trachea, lacrimal drainage conduit, and vascular bypass graft [43]. POSS-PCU has also been incorporated with a second polymer polycaprolactone (UCL Nano-Bio) to produce a degradable nanocomposite polymer [44].
Fig. 3.
SEM image of porous POSS-PCU polymer scaffold extruded with sodium bicarbonate (100 µm particle size) porogen. Images are of a longitudinal section. Note the highly porous structure similar to structure of extracellular matrix indicative of possible better tissue engineering properties (highlighted by the arrow); ×10 magnification (left), ×80 magnification (right).
The superior qualities of nanocomposites are contributed by the interaction at nanoscale level resulting in the unique quantal properties instead of bulk properties of conventional material [45]. The general advantages offered by nanocomposites are high surface area-to-mass ratio, thermodynamic stability, biostability, amphiphilicity, and enhanced mechanical property compared with conventional materials for graft. These properties can be enhanced by nanofibers and nanotubes as fillers [22]. Carbon nanotubes are able to reduce macrophage adhesion and proliferation, which could prevent blockage of lymph flow [46]. However, the potential toxicity of carbon nanotube limits its translation potential [47].
3.4.3. Decellularized scaffold
Decellularized scaffold has been widely used in tissue-engineered vascular graft as it overcomes the challenge of design and fabrication [48,49]. The preformed scaffold carries the ECM architecture of native vessel and biochemical cues to support cell viability while preserving the bulk mechanical feature of native tissue [50]. Wong et al. [51] showed that decellularized scaffold can provide necessary extracellular signaling molecules for lymphatic tissue regeneration by evaluating lymphatic vessel ingrowth in acellular dermal matrices. However, decellularized collecting lymphatic vessel has not been used to engineer lymphatic graft thus far. In view of the successful clinical application of autologous lymphatic graft by Baumeister, the application of decellularized collecting lymphatic as scaffold should be tested. One of the challenges with decellularized scaffold is the extreme density of the matrix, which delays cell infiltration on seeding resulting in excess culture time [52]. In terms of translational potential, decellularized scaffold are often more expensive, limited in resource, and difficult for surgical manipulation.
3.5. Endothelialization of lymphatic graft
Lymphatic endothelium is essential in modulating the fluid flow and dendritic cell transport function [53]. Compared with vascular graft, lymphatic graft does not hold risk of thrombosis; however, in view of the low flow rate and the content of lymphatic fluid, risk of coagulation in the lymphatic graft may be present. The main concern in producing an endothelialized construct is the attachment of seeded cells onto scaffold material [33].
To ensure a higher rate of endothelialization and to increase its potential, scaffolds could be biofunctionalized with appropriate ligands. Peptide arginine-glycine-aspartic acid (RGD), which is the minimal binding domain of fibronectin, has been studied comprehensively to increase cell adhesion on vascular grafts [42]. Peptide RGD forms receptor-ligand interactions with integrins on BEC. To identify the best form of RGD for integrins on LEC, polymer scaffold could be modified with different forms of RGD. This biofunctionalized scaffold would provide environment, which mimics ECM to help anchor LECs to the polymer scaffold.
Surface topography that is favorable for LEC has yet to be outlined but this feature has been extensively explored with regards to vascular graft engineering. Nanostructuralization increases total surface area, which in return facilitates protein interaction and promotes cell adhesion [54].
3.6. Interstitial flow
Swartz and Rutkowski [55] have made great contribution in characterizing the importance of interstitial fluid flow in regulation of LEC proliferation, function, and migration. They found that BEC and LEC have differential responses to interstitial flow leading to variation in cellular morphology and branching patterns indicating that LEC differentiation is influenced by specialized responses from environmental stimuli [56]. Similar concept was demonstrated in a mouse model showing that interstitial fluid flow guides LEC organization along the direction of flow [57,58]. These suggest that scaffold that encourages interstitial flow is vital in engineering lymphatic graft.
3.7. Growth factor
Regenerative medicine research is likely to produce more effective and better tolerated treatment for lymphedema in the long term, and proper understanding of lymphangiogenesis is the essence of it. Lymphangiogenesis occurs physiologically during wound healing and pathologically in inflammation, tumors metastasis, and transplant rejection [59,60].
Tables 2 and 3 provide a comprehensive summary of the main molecular factors found to be important in normal lymphatic development, function, and maintenance.
Table 2.
List of molecular factors important in lymphatic system function described according to the embryologic developmental stage in which discovered.
| Developmental Step |
Molecular factor | Function | References |
|---|---|---|---|
| Competence | LYVE-1 (lymphatic vessel hyaluronan receptor-1) | The first proteins expressed by endothelial cells that differentiate into LEC. Likely function is to aid leukocyte migration via hyaluronan receptor. Is a useful LEC marker. | [82] |
| Bias and specification | Prox-1* (homeobox transcription factor) | Master control gene, required to maintain LEC phenotype. Expression can induce LEC-like phenotype in mature blood endothelial cells. | [83] |
| SOX-18*,† (sex determining region Y box 18) | A nuclear transcription factor binds to a proximal promoter of the Prox-1 gene. Not required for sustaining LEC phenotype. | [84] | |
| COUP-TFII* (chicken ovalbumin upstream promoter transcription factor II) | An orphan nuclear receptor that has a vital role in organogenesis generally. Acts as a regulator of transcriptional activity of Prox-1, notably by down regulating VEGFR-3 (effects cell migration) and cyclin El (cell proliferation gene) transcription. | [85] | |
| VEGFR-3*,† (vascular endothelial growth factor receptor 3) | Vascular endothelial growth factor receptor essential for lymphatic and blood vessel embryologic development. Becomes specifically expressed on LEC’s by birth. Not required for LEC phenotype maintenance, but is required or lymphangiogenesis. Useful LEC marker in adults. | [86,87] | |
| VEGF-C* and -D | Vascular endothelial growth factors-ligands for VEGFR-3. The major lymphoangiogenic factor. VEGF-D is redundant, but can rescue VEGF-C deficiency. Not required to maintain LEC phenotype, but is required for lymphangiogenesis. Both also bind to VEGFR-2 present on blood and lymphatic vessels. Therapeutic use in lymphedema and cancer under investigation. | [88,89] | |
| NRP-2 (Neuropilin 2) | A glycoprotein transmembrane protein binds to VEGFR-2 and −3 and has a modulating effect important for normal lymphatic development. | [90] | |
| Maturation | Syk and SLP-76* (tyrosine kinase and adaptor protein) | Hematopoietic signaling proteins essential in maintaining blood and lymphatic systems separation, preventing hemorrhage and organ failure. | [91,92] |
| Spred-1/2* , fasting-induced adipose factor (FIAF), or angiopoietin-like protein 4 (Angptl4) | Spred 1 and 2 are negative regulators for growth factors including the VEGF-C/VEGFR-3 signaling cascade. Have similar role to Syk or SLp-76. | [93] | |
| Phospholipase Cγ2 (PLCγ2). | PLCy2 like Syk/SLP-76 and Spreds has a role in the separation of the lymphatic from blood system. | [94] | |
| Foxc2† (previously known as mesenchyme forkhead 1 (MFH1) | A transcription factor expressed in mesenchymal tissue including the heart and endothelial cells. Foxc2 deficiency leads to severe modeling defects resulting in abnormal lymphatic vessels lacking valves. | [95] | |
| NAFTc1 (Nuclear factor of activated T cells 1) | A transcription factor that interacts with Foxc2 in remodeling the lymphatic vasculature, in particular maturation of collecting vessels. | [96] | |
| Integrins (α9)* | Integrin α9β1 is involved in LEC migration toward VEGF-C. Has a crucial role in organizing fibronectin matrix for normal valve development. | [97] | |
| Ang2/Tie2 (angiopoietin growth factor binds to TIE receptors) | Essential in recruiting smooth muscle cells to collecting lymphatic vessels and patterning of the lymphatic capillaries. Ang1 can rescue loss of Ang2, TIE 1 may have a modulatory role. | [98] | |
| Ephrin B2 | Ephrin B2 acts through Eph B4 receptor to regulate maturing lymphatic vessels. Mutation of the Ephrin B2 PDZ interaction site causes failure of hierarchal organization, failure of valve formation, and abnormal smooth muscle recruitment on lymphatic capillaries. | [99] | |
| Podoplanin T1α | A mucin-type transmembrane glycoprotein. Probable function in cell adhesion, migration, and tube formation. Deficiency results in lymphedema secondary to abnormal patterning. | [100] | |
| Adrenomedullin* (AM) | Multifunction peptide hormone acts via its receptor calcitonin receptor—like receptor (CALCRL,) and receptor activity—modifying protein (RAMP2). It is a potent inducer of endothelial cell proliferation, migration, and tube formation essential in blood and lymphatic development. Under investigation for the treatment of lymphedema. | [101,102] |
Gene loss lethal.
Gene mutation related to known cases of human primary lymphedema.
Table 3.
Other factors important in lymphatic function not clearly related to embryologic development.
| Molecular factor | Function | References |
|---|---|---|
| Emilin-1 (elastic extracellular matrix microfibril associated glycoprotein) | Patterning in relation to anchoring filament attachment to the ECM | [103] |
| CCBE1* (collagen and calcium-binding EGF domain-1) | Essential in lymphatic development in a zebra fish, recent link to human primary lymphedema. Has not been found to be expressed in lymphatic endothelium and is more likely to be found within the ECM. | [104] |
| HGF (hepatocyte growth factor) | Lymphangiogenic growth factor, investigated for the treatment of lymphedema. | [105] |
| FGF-2 (fibroblast growth factor-2) | Lymphangiogenic growth factor | [106] |
| PDGF-BB (platelet-derived growth factor BB) | Lymphangiogenic growth factor | [106] |
| IGF-1/2 (insulin-like growth factors) | Lymphangiogenic growth factor | [106] |
Gene mutation related to known cases of human primary lymphedema.
In pathologic condition such as lymphedema, the vascular endothelial growth factor-C (VEGF-C) expression or even overexpression is insufficient to promote lymphatic regeneration [61,62]. In such cases, lymphatic function and regeneration could be improved by inhibition of transforming growth factor beta 1 [63]. The transforming growth factor beta 1 has potent antilymphangiogenic activity leading to decreased LEC proliferation and in vivo LEC migration, impaired lymphatic tubule formation, and down regulation of lymphatic-specific gene expression [63]. Furthermore, the uses of antilymphangiogenic factors are also clinically relevant in cases where VEGF-C is contraindicated such as in immediate postcancer resection because VEGF-C expression could contribute to tumor growth and metastasis [64]. Hence tissue engineering approach could augment lymphangiogenesis by regulating the equilibrium between lymphangiogenic and antilymphangiogenic forces.
3.8. Gene therapy
Genetic and recombinant protein therapeutic strategies are likely to enhance lymphangiogenesis and treat both primary and secondary lymphedema. Hepatocyte growth factor gene therapy has successfully stimulated the growth of lymphatic vascular system in mouse model [65]. Hepatocyte growth factor plasmid DNA was used in vascular system, heart, and lung, and also reported to be safe in patients with critical limb ischemia [66,67]. Besides that, VEGF-C activation using viral vectors also reported to have potential in lymphedema treatment [68]. However, VEGF-C therapy was reported to exacerbate edema by causing poor functioning and hyperplastic lymphatic vessels [62]. Nevertheless, gene therapy and growth factors have the potential to be an adjunct to engineered biomaterials, which can be administered locally or systemically to enhance collateral lymphatic vessel formation and also to provide greater biocompatibility for the biomaterial.
3.9. Cell source
Common source of LEC isolation is the skin dermis, and its isolation from human dermis has been well established [69–71]. An alternative source of LEC is from pluripotent stem cells such as embryonic stem cells that have been reported to differentiate into LECs in vitro in the presence of VEGF-C and Ang1 [72]. Besides that, mesenchymal stem cells derived from bone marrow or adipose tissue also show good potential to differentiate into LECs in the company of recombinant VEGF-C [73]. Adipose-derived mesenchymal stem cells have been reported to improve lymphatic regeneration and restore function on direct injection into rat hind limb and mouse tail models [74]. The huge potential of adipose derived stem cells in the field of lymphedema should be acknowledged and explored further in view of its abundant resource and easy availability [32].
3.10. Animal model
The first step in developing an animal model to evaluate the effectiveness of the tissue-engineered lymphatic graft is producing a sustainable model of secondary lymphedema. The animal model should permit quantification of lymphatic function over time and allow assessment of practical effectiveness of the tissue-engineered lymphatic graft. Animal models that have been used include dog hind limb models, rabbit ear models, and rodent tail and hind limb models [75–78]. The main limitations of these models are difficulty in quantifying the lymphatic function, size that is much smaller than that of human, and unsustainable effect of lymphedema. On lymph node resection, some degree of lymphangiogenesis may take place; however, transport properties exhibited by the newly formed lymphatics are insufficient to restore flow parameters to its original state [79]. Combination of radiotherapy with surgical ablation has shown to induce sustained lymphedema after surgery [80].
Tobbia et al. [81] outlined the advantage of using sheep as lymphedema model because of the anatomical dimension of the lymphatic vessel, which allows quantification of lymphatic function and therapeutic outcome. In their study, the popliteal lymph node was excised because of the ease in identification and sufficient size for cannulation. Furthermore, there is just one postnodal duct despite having about six to 12 prenodal ducts, which is ideal for the application of the tissue-engineered lymphatic graft. Multiple prenodal ducts can be anastomosed to the lymphatic graft distally as performed by Campisi [4] with the use of venous graft. Objective quantification of lymphatic function before and after administration of the tissue-engineered lymphatic graft can be performed with the usage of radiolabeled human serum albumin [81].
A robust preclinical study design is crucial in determining the right time to administer the tissue-engineered lymphatic graft. Comparison should be made on the benefit of early application of the lymphatic graft on surgical resection of lymph nodes over application on onset of clinical symptoms. Such robust study carries a huge potential to revolutionize the current clinical practice in the management of secondary lymphedema.
4. Conclusions and future prospective
Treatment of lymphedema is evolving positively of late because of the growing knowledge in lymphatic biology, which has enhanced our understanding on lymphatic growth and repair. In conjunction with this, more studies need to be carried out to further characterize the human lymphatic system to bridge the current limitations that exit alongside the promise that new technological advancement holds. Tissue-engineered lymphatic graft is a tool that could benefit many around the world by reducing the morbidity related to lymphedema. Furthermore, tissue-engineered lymphatic tissue model would be valuable for lymphatic biology and cancer research. This could reduce the usage of animal models for lymphatic and cancer research. With the in-depth understanding of tissue engineering, nanotechnology and improved knowledge on biology of lymphatic regeneration, the aspiration to develop successful lymphatic graft is highly achievable. A successful lymphatic graft requires design, which is functional and scaffold, which encourages and maintains endothelialization and mechanical strength. The next step on creation of successful lymphatic graft is regenerating lymphatic network to channel lymphatic fluid from the distal part of the body to functional host lymphatic vessels.
Footnotes
Authors’ contributions: M.K. contributed to manuscript writing and made the literature review. N.M.P. contributed to literature review. D.M.K., A.M. and A.M.S. reviewed the manuscript. B.J.M. was research collaborator. A.M.S. provided the outline for study and writing.
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
REFERENCES
- 1.Information for Doctors and Health Care Professionals. [Accessed 06.01.2014];The lymphoedema support network. 2014 Available from: http://www.lymphoedema.org/Menu4/Index.asp.
- 2.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: a systematic review and meta-analysis. The Lancet Oncol. 2013;14:500. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 3.Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg. 1990;85:64. doi: 10.1097/00006534-199001000-00012. discussion 75—66. [DOI] [PubMed] [Google Scholar]
- 4.Campisi C, Boccardo F, Zilli A, Maccio A, Napoli F. The use of vein grafts in the treatment of peripheral lymphedemas: long-term results. Microsurgery. 2001;21:143. doi: 10.1002/micr.1027. [DOI] [PubMed] [Google Scholar]
- 5.Mehrara BJ, Zampell JC, Suami H, Chang DW. Surgical management of lymphedema: past, present, and future. Lymphatic Res Biol. 2011;9:159. doi: 10.1089/lrb.2011.0011. [DOI] [PubMed] [Google Scholar]
- 6.Schmid-Schonbein GW. The second valve system in lymphatics. Lymphatic Res Biol. 2003;1:25. doi: 10.1089/15396850360495664. discussion 29–31. [DOI] [PubMed] [Google Scholar]
- 7.McHale N, Hollywood M, Sergeant G, Thornbury K. Origin of spontaneous rhythmicity in smooth muscle. The J Physiol. 2006;570:23. doi: 10.1113/jphysiol.2005.098376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swartz MA, Kaipainen A, Netti PA, et al. Mechanics of interstitial-lymphatic fluid transport: theoretical foundation and experimental validation. J Biomech. 1999;32:1297. doi: 10.1016/s0021-9290(99)00125-6. [DOI] [PubMed] [Google Scholar]
- 9.Hitchcock T, Niklason L. Lymphatic tissue engineering: progress and prospects. Ann New York Acad Sci. 2008;1131:44. doi: 10.1196/annals.1413.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okamoto N, Chihara R, Shimizu C, Nishimoto S, Watanabe T. Artificial lymph nodes induce potent secondary immune responses in naive and immunodeficient mice. The J Clin Invest. 2007;117:997. doi: 10.1172/JCI30379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornuta JA, Brandon Dixon J. Ex vivo lymphatic perfusion system for independently controlling pressure gradient and transmural pressure in isolated vessels. Ann Biomed Eng. 2014 doi: 10.1007/s10439-014-1024-6. http://dx.doi.org/10.1007/sl0439-014-1024-6. [DOI] [PMC free article] [PubMed]
- 12.Gashev AA. Lymphatic vessels: pressure- and flow-dependent regulatory reactions. Ann N Y Acad Sci. 2008;1131:100. doi: 10.1196/annals.1413.009. [DOI] [PubMed] [Google Scholar]
- 13.Olszewski WL, Engeset A. Intrinsic contractility of prenodal lymph vessels and lymph flow in human leg. Am. J. Phys. 1980;239:H775. doi: 10.1152/ajpheart.1980.239.6.H775. [DOI] [PubMed] [Google Scholar]
- 14.Gretener SB, Lauchli S, Leu AJ, Koppensteiner R, Franzeck UK. Effect of venous and lymphatic congestion on lymph capillary pressure of the skin in healthy volunteers and patients with lymph edema. J Vase Res. 2000;37:61. doi: 10.1159/000025714. [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski JM, Markhus CE, Gyenge CC, Alitalo K, Wiig H, Swartz MA. Dermal collagen and lipid deposition correlate with tissue swelling and hydraulic conductivity in murine primary lymphedema. Am J Pathol. 2010;176:1122. doi: 10.2353/ajpath.2010.090733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- 17.Kassis T, Kohan AB, Weiler MJ, et al. Dual-channel in-situ optical imaging system for quantifying lipid uptake and lymphatic pump function. J Biomed Opt. 2012;17:086005. doi: 10.1117/1.JBO.17.8.086005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nipper ME, Dixon JB. Engineering the lymphatic system. Cardiovasc Eng Technol. 2011;2:296. doi: 10.1007/s13239-011-0054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;127:1979. doi: 10.1097/PRS.0b013e31820cf5df. [DOI] [PubMed] [Google Scholar]
- 20.Proulx ST, Luciani P, Derzsi S, et al. Quantitative imaging of lymphatic function with liposomal indocyanine green. Cancer Res. 2010;70:7053. doi: 10.1158/0008-5472.CAN-10-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Unno N, Nishiyama M, Suzuki M, et al. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vase Endovasc Surg. 2008;36:230. doi: 10.1016/j.ejvs.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 22.de Mel A, Bolvin C, Edirisinghe M, Hamilton G, Seifalian AM. Development of cardiovascular bypass grafts: endothelialization and applications of nanotechnology. Expert Rev Cardiovasc Ther. 2008;6:1259. doi: 10.1586/14779072.6.9.1259. [DOI] [PubMed] [Google Scholar]
- 23.Moriondo A, Boschetti F, Bianchin F, Lattanzio S, Marcozzi C, Negrini D. Tissue contribution to the mechanical features of diaphragmatic initial lymphatics. The J Physiol. 2010;588:3957. doi: 10.1113/jphysiol.2010.196204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohhashi T, Azuma T, Sakaguchi M. Active and passive mechanical characteristics of bovine mesenteric lymphatics. The Am J Physiol. 1980;239:H88. doi: 10.1152/ajpheart.1980.239.1.H88. [DOI] [PubMed] [Google Scholar]
- 25.Rahbar E, Weimer J, Gibbs H, et al. Passive pressure-diameter relationship and structural composition of rat mesenteric lymphangions. Lymphatic Res Biol. 2012;10:152. doi: 10.1089/lrb.2011.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauweryns J, Boussauw L. The ultrastructure of lymphatic valves in the adult rabbit lung. Z.Zellforsch. 1973;143:149. doi: 10.1007/BF00307476. [DOI] [PubMed] [Google Scholar]
- 27.Sabine A, Agalarov Y, Maby-El Hajjami H, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Developmental cell. 2012;22:430. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Alexander JS, Orr AW. Integrins and their extracellular matrix ligands in lymphangiogenesis and lymph node metastasis. International journal of cell biology. 2012;2012:853703. doi: 10.1155/2012/853703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zawieja DC. Contractile physiology of lymphatics. Lymphatic Res Biol. 2009;7:87. doi: 10.1089/lrb.2009.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brice G, Mansour S, Bell R, et al. Analysis of the phenotypic abnormalities in lymphoedema-distichiasis syndrome in 74 patients with FOXC2 mutations or linkage to 16q24. Journal of medical genetics. 2002;39:478. doi: 10.1136/jmg.39.7.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Batchelor GK. An introduction to fluid dynamics. Cambridge: Cambridge University Press; 1967. [Google Scholar]
- 32.Weitman E, Cuzzone D, Mehrara BJ. Tissue engineering and regeneration of lymphatic structures. Future oncology (London, England) 2013;9:1365. doi: 10.2217/fon.13.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai T, Jiang Z, Li S, et al. Reconstruction of lymph vessel by lymphatic endothelial cells combined with polyglycolic acid scaffolds: a pilot study. Journal of biotechnology. 2010;150:182. doi: 10.1016/j.jbiotec.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 34.Greenwald SE, Berry CL. Improving vascular grafts: the importance of mechanical and haemodynamic properties. The Journal of pathology. 2000;190:292. doi: 10.1002/(SICI)1096-9896(200002)190:3<292::AID-PATH528>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 35.Salacinski HJ, Goldner S, Giudiceandrea A, et al. The mechanical behavior of vascular grafts: a review. Journal of biomaterials applications. 2001;15:241. doi: 10.1106/NA5T-J57A-JTDD-FD04. [DOI] [PubMed] [Google Scholar]
- 36.Parikh SA, Edelman ER. Endothelial cell delivery for cardiovascular therapy. Advanced drug delivery reviews. 2000;42:139. doi: 10.1016/s0169-409x(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 37.Rashid ST, Fuller B, Hamilton G, Seifalian AM. Tissue engineering of a hybrid bypass graft for coronary and lower limb bypass surgery. FASEB. 2008;22:2084. doi: 10.1096/fj.07-096586. [DOI] [PubMed] [Google Scholar]
- 38.Kawazoe N, Inoue C, Tateishi T, Chen G. A cell leakproof PLGA-collagen hybrid scaffold for cartilage tissue engineering. Biotechnology. 2010;26:819. doi: 10.1002/btpr.375. [DOI] [PubMed] [Google Scholar]
- 39.Seifalian A, Hancock S, Salacinski H. Polymer for use in conduits and medical devices. Patent WO2005070998. 2005 [Google Scholar]
- 40.Kannan RY, Salacinski HJ, Sales KM, Butler PE, Seifalian AM. The endothelialization of polyhedral oligomeric silsesquioxane nanocomposites: an in vitro study. Cellule. 2006;45:129. doi: 10.1385/cbb:45:2:129. [DOI] [PubMed] [Google Scholar]
- 41.Kannan RY, Salacinski HJ, Odlyha M, Butler PE, Seifalian AM. The degradative resistance of polyhedral oligomeric silsesquioxane nanocore integrated polyurethanes: an in vitro study. Biomaterials. 2006;27:1971. doi: 10.1016/j.biomaterials.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 42.de Mel A, Punshon G, Ramesh B, et al. In situ endothelialization potential of a biofunctionalised nanocomposite biomaterial-based small diameter bypass graft. Bio-medical materials and engineering. 2009;19:317. doi: 10.3233/BME-2009-0597. [DOI] [PubMed] [Google Scholar]
- 43.Guasti L, Vagaska B, Bulstrode NW, Seifalian AM, Ferretti P. Chondrogenic differentiation of adipose tissue-derived stem cells within nanocaged POSS-PCU scaffolds: a new tool for nanomedicine. Nanomedicine: Nanotechnology, Biology and Medicine. doi: 10.1016/j.nano.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 44.Raghunath J, Zhang H, Edirisinghe MJ, Darbyshire A, Butler PE, Seifalian AM. A new biodegradable nanocomposite based on polyhedral oligomeric silsesquioxane nanocages: cytocompatibility and investigation into electrohydrodynamic jet fabrication techniques for tissue-engineered scaffolds. Biotechnology. 2009;52:1. doi: 10.1042/BA20070256. [DOI] [PubMed] [Google Scholar]
- 45.Kannan RY, Salacinski HJ, Butler PE, Seifalian AM. Polyhedral oligomeric silsesquioxane nanocomposites: the next generation material for biomedical applications. Accounts of chemical research. 2005;38:879. doi: 10.1021/ar050055b. [DOI] [PubMed] [Google Scholar]
- 46.Kim JY, Khang D, Lee JE, Webster TJ. Decreased macrophage density on carbon nanotube patterns on polycarbonate urethane. Journal of biomedical materials research. Part A. 2009;88:419. doi: 10.1002/jbm.a.31799. [DOI] [PubMed] [Google Scholar]
- 47.Hurt RH, Monthioux M, Kane A. Toxicology of carbon nanomaterials: Status, trends, and perspectives on the special issue. Toxicology of Carbon Nanomaterials. 2006;44:1028. [Google Scholar]
- 48.Schaner PJ, Martin ND, Tulenko TN, et al. Decellularized vein as a potential scaffold for vascular tissue engineering. Journal of vascular surgery. 2004;40:146. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 49.Amiel GE, Komura M, Shapira O, et al. Engineering of blood vessels from a cellular collagen matrices coated with human endothelial cells. Tissue engineering. 2006;12:2355. doi: 10.1089/ten.2006.12.2355. [DOI] [PubMed] [Google Scholar]
- 50.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Wong AK, Schonmeyr B, Singh P, Carlson DL, Li S, Mehrara BJ. Histologic analysis of angiogenesis and lymphangiogenesis in acellular human dermis. Plast Reconstr Surg. 2008;121:1144. doi: 10.1097/01.prs.0000302505.43942.07. [DOI] [PubMed] [Google Scholar]
- 52.Sheridan WS, Duffy GP, Murphy BP. Mechanical characterization of a customized decellularized scaffold for vascular tissue engineering. Journal of the mechanical behavior of biomedical materials. 2012;8:58. doi: 10.1016/j.jmbbm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Miteva DO, Rutkowski JM, Dixon JB, Kilarski W, Shields JD, Swartz MA. Transmural flow modulates cell and fluid transport functions of lymphatic endothelium. Circulation. 2010;106:920. doi: 10.1161/CIRCRESAHA.109.207274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miller DC, Thapa A, Haberstroh KM, Webster TJ. Endothelial and vascular smooth muscle cell function on poly(lactic-coglycolic acid) with nano-structured surface features. Biomaterials. 2004;25:53. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- 55.Rutkowski JM, Swartz MA. A driving force for change: interstitial flow as a morphoregulator. Trends. Cell. Biol. 2007;17:44. doi: 10.1016/j.tcb.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 56.Ng CP, Helm CL, Swartz MA. Interstitial flow differentially stimulates blood and lymphatic endothelial cell morphogenesis in vitro. Microvascular research. 2004;68:258. doi: 10.1016/j.mvr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 57.Boardman KC, Swartz MA. Interstitial flow as a guide for lymphangiogenesis. Circulation research. 2003;92:801. doi: 10.1161/01.RES.0000065621.69843.49. [DOI] [PubMed] [Google Scholar]
- 58.Helm CL, Zisch A, Swartz MA. Engineered blood and lymphatic capillaries in 3-D VEGF-fibrin-collagen matrices with interstitial flow. Biotechnology. 2007;96:167. doi: 10.1002/bit.21185. [DOI] [PubMed] [Google Scholar]
- 59.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 60.Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer. 2005;7:121. doi: 10.1016/j.ccr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 61.Rutkowski JM, Moya M, Johannes J, Goldman J, Swartz MA. Secondary lymphedema in the mouse tail: lymphatic hyperplasia, VEGF-C upregulation, and the protective role of MMP-9. Microvascular research. 2006;72:161. doi: 10.1016/j.mvr.2006.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldman J, Le TX, Skobe M, Swartz MA. Overexpression of VEGF-C causes transient lymphatic hyperplasia but not increased lymphangiogenesis in regenerating skin. Circulation research. 2005;96:1193. doi: 10.1161/01.RES.0000168918.27576.78. [DOI] [PubMed] [Google Scholar]
- 63.Avraham T, Daluvoy S, Zampell J, et al. Blockade of transforming growth factor-beta 1 accelerates lymphatic regeneration during wound repair. Am. J. Pathol. 2010;177:3202. doi: 10.2353/ajpath.2010.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. Journal of clinical oncology. 2005;23:1011. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 65.Saito Y, Nakagami H, Kaneda Y, Morishita R. Lymphedema and therapeutic lymphangiogenesis. BioMed research international. 2013;2013:804675. doi: 10.1155/2013/804675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Watanabe M, Ebina M, Orson FM, et al. Hepatocyte growth factor gene transfer to alveolar septa for effective suppression of lung fibrosis. Molecular therapy. 2005;12:58. doi: 10.1016/j.ymthe.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 67.Morishita R, Aoki M, Hashiya N, et al. Safety evaluation of clinical gene therapy using hepatocyte growth factor to treat peripheral arterial disease. Hypertension. 2004;44:203. doi: 10.1161/01.HYP.0000136394.08900.ed. [DOI] [PubMed] [Google Scholar]
- 68.Yoon YS, Murayama T, Gravereaux E, et al. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. The J Clin Invest. 2003;111:717. doi: 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu X, Jiang Z, Liu N. A novel approach for harvesting lymphatic endothelial cells from human foreskin dermis. Lymphatic Res Biol. 2006;4:191. doi: 10.1089/lrb.2006.4403. [DOI] [PubMed] [Google Scholar]
- 70.Hirakawa S, Hong YK, Harvey N, et al. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. The American journal of pathology. 2003;162:575. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kriehuber E, Breiteneder-Geleff S, Groeger M, et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. The Journal of experimental medicine. 2001;194:797. doi: 10.1084/jem.194.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kono T, Kubo H, Shimazu C, et al. Differentiation of lymphatic endothelial cells from embryonic stem cells on OP9 stromal cells. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:2070. doi: 10.1161/01.ATV.0000225770.57219.b0. [DOI] [PubMed] [Google Scholar]
- 73.Yan A, Avraham T, Zampell JC, Haviv YS, Weitman E, Mehrara BJ. Adipose-derived stem cells promote lymphangiogenesis in response to VEGF-C stimulation or TGF-betal inhibition. Future oncology (London, England) 2011;7:1457. doi: 10.2217/fon.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Conrad C, Niess H, Huss R, et al. Multipotent mesenchymal stem cells acquire a lymphendothelial phenotype and enhance lymphatic regeneration in vivo. Circulation. 2009;119:281. doi: 10.1161/CIRCULATIONAHA.108.793208. [DOI] [PubMed] [Google Scholar]
- 75.Huang GK, Hsin YP. An experimental model for lymphedema in rabbit ear. Microsurgery. 1983;4:236. doi: 10.1002/micr.1920040408. [DOI] [PubMed] [Google Scholar]
- 76.Olszewski W. [Induction of experimental lymphatic edema] Polski przeglad chirurgiczny. 1967;39:926. [PubMed] [Google Scholar]
- 77.Liu Y, Fang Y, Dong P, et al. Effect of vascular endothelial growth factor C (VEGF-C) gene transfer in rat model of secondary lymphedema. Vascular pharmacology. 2008;49:44. doi: 10.1016/j.vph.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 78.Swartz MA, Boardman KC., Jr The role of interstitial stress in lymphatic function and lymphangiogenesis. Ann. N. Y. Acad. Sci. 2002;979:197. doi: 10.1111/j.1749-6632.2002.tb04880.x. discussion 229134. [DOI] [PubMed] [Google Scholar]
- 79.Kim C, Li B, Papaiconomou C, Zakharov A, Johnston M. Functional impact of lymphangiogenesis on fluid transport after lymph node excision. Lymphology. 2003;36:111. [PubMed] [Google Scholar]
- 80.Park HS, Jung IM, Choi GH, Hahn S, Yoo YS, Lee T. Modification of a rodent hindlimb model of secondary lymphedema: surgical radicality versus radiotherapeutic ablation. BioMed research international. 2013;2013:208912. doi: 10.1155/2013/208912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tobbia D, Semple J, Baker A, Dumont D, Semple A, Johnston M. Lymphedema development and lymphatic function following lymph node excision in sheep. Journal of vascular research. 2009;46:426. doi: 10.1159/000194273. [DOI] [PubMed] [Google Scholar]
- 82.Luong MX, Tam J, Lin Q, et al. Lack of lymphatic vessel phenotype in LYVE-1/CD44 double knockout mice. Journal of cellular physiology. 2009;219:430. doi: 10.1002/jcp.21686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johnson NC, Dillard ME, Baluk P, et al. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes & development. 2008;22:3282. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Francois M, Caprini A, Hosking B, et al. Soxl8 induces development of the lymphatic vasculature in mice. Nature. 2008;456:643. doi: 10.1038/nature07391. [DOI] [PubMed] [Google Scholar]
- 85.Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes to cells : devoted to molecular & cellular mechanisms. 2009;14:425. doi: 10.1111/j.1365-2443.2008.01279.x. [DOI] [PubMed] [Google Scholar]
- 86.Makinen T, Jussila L, Veikkola T, et al. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat. Med. 2001;7:199. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- 87.Goldman J, Rutkowski JM, Shields JD, et al. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB. 2007;21:1003. doi: 10.1096/fj.06-6656com. [DOI] [PubMed] [Google Scholar]
- 88.Baldwin ME, Halford MM, Roufail S, et al. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Molecular and cellular biology. 2005;25:2441. doi: 10.1128/MCB.25.6.2441-2449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haiko P, Makinen T, Keskitalo S, et al. Deletion of vascular endothelial growth factor C (VEGF-C) and VEGF-D is not equivalent to VEGF receptor 3 deletion in mouse embryos. Molecular and cellular biology. 2008;28:4843. doi: 10.1128/MCB.02214-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yuan L, Moyon D, Pardanaud L, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development (Cambridge, England) 2002;129:4797. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- 91.Abtahian F, Guerriero A, Sebzda E, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sebzda E, Hibbard C, Sweeney S, et al. Syk and Slp-76 mutant mice reveal a cell-autonomous hematopoietic cell contribution to vascular development. Developmental cell. 2006;11:349. doi: 10.1016/j.devcel.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 93.Taniguchi K, Kohno R, Ayada T, et al. Spreads are essential for embryonic lymphangiogenesis by regulating vascular endothelial growth factor receptor 3 signaling. Molecular and cellular biology. 2007;27:4541. doi: 10.1128/MCB.01600-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ichise H, Ichise T, Ohtani O, Yoshida N. Phospholipase Cgamma2 is necessary for separation of blood and lymphatic vasculature in mice. Development (Cambridge, England) 2009;136:191. doi: 10.1242/dev.025353. [DOI] [PubMed] [Google Scholar]
- 95.Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Development. 2006;294:458. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 96.Kulkarni RM, Greenberg JM, Akeson AL. NFATc1 regulates lymphatic endothelial development. Mech. Dev. 2009;126:350. doi: 10.1016/j.mod.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bazigou E, Xie S, Chen C, et al. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Development. 2009;17:175. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Developmental cell. 2002;3:411. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 99.Makinen T, Adams RH, Bailey J, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genesis. 2005;19:397. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schacht V, Ramirez MI, Hong YK, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. The EMBO journal. 2003;22:3546. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. The J Clin Invest. 2008;118:40. doi: 10.1172/JCI33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jin D, Harada K, Ohnishi S, Yamahara K, Kangawa K, Nagaya N. Adrenomedullin induces lymphangiogenesis and ameliorates secondary lymphoedema. Cardiovascular research. 2008;80:339. doi: 10.1093/cvr/cvn228. [DOI] [PubMed] [Google Scholar]
- 103.Danussi C, Spessotto P, Petrucco A, et al. Emilin1 deficiency causes structural and functional defects of lymphatic vasculature. Molecular and cellular biology. 2008;28:4026. doi: 10.1128/MCB.02062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hogan BM, Bos FL, Bussmann J, et al. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009;41:396. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- 105.Saito Y, Nakagami H, Morishita R, et al. Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis. Circulation. 2006;114:1177. doi: 10.1161/CIRCULATIONAHA.105.602953. [DOI] [PubMed] [Google Scholar]
- 106.Maby-El Hajjami H, Petrova TV. Developmental and pathological lymphangiogenesis: from models to human disease. Histochemistry. 2008;130:1063. doi: 10.1007/s00418-008-0525-5. [DOI] [PubMed] [Google Scholar]