Abstract
Ca2+ transfer from the endoplasmic reticulum (ER) to the mitochondria critically controls cell survival and cell death decisions. Different oncogenes and deregulation of tumor suppressors exploit this mechanism to favor the survival of altered, malignant cells. Two recent studies of the Pinton team revealed a novel, non-transcriptional function of cytosolic p53 in cell death. During cell stress, p53 is recruited to the ER and the ER-mitochondrial contact sites. This results in augmented ER Ca2+ levels by enhancing sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) activity, ultimately promoting mitochondrial Ca2+ overload. The boosting of “toxic” Ca2+ signaling by p53 appears to be a critical component of the cell death-inducing properties of chemotherapeutic agents and anti-cancer treatments, like photodynamic stress. Strikingly, the resistance of p53-deficient cancer cells to these treatments could be overcome by facilitating Ca2+ transfer between the ER and the mitochondria via overexpression of SERCA or of the mitochondrial Ca2+ uniporter (MCU). Importantly, these concepts have also been supported by in vivo Ca2+ measurements in tumor masses in mice. Collectively, these studies link for the first time the major tumor suppressor, p53, to Ca2+ signaling in dictating cell-death outcomes and by the success of anti-cancer treatments.
Keywords: p53, ER-mitochondrial Ca2+ signaling, in vivo Ca2+ imaging, anti-cancer treatments, cell death
Oncogenes and deregulation of tumor suppressors favor oncogenesis through signaling pathways that impact cell-death resistance, uncontrolled cell proliferation and altered energy production and requirements [1]. During the last decade, alterations in intracellular Ca2+ signaling have emerged as an important factor in the development of tumors and their invasive and metastatic properties [2-4]. Cancer cells display alterations in the expression and regulation of different Ca2+-transport systems at both the plasma membrane and the membranes of organelles, like the endoplasmic reticulum (ER) and the mitochondria, thereby impacting different hallmarks of cancer progression [3, 5].
Besides remodeling of Ca2+-flux pathways at the level of the plasma membrane [2, 6-8], deregulation of Ca2+ signaling from the ER, the main intracellular Ca2+-storage site, to the mitochondria, the main apoptosis-inducing organelle, serves as an important oncogenic mechanism for driving cancer progression [9]. The ER and the mitochondria are closely connected via contact sites (mitochondria-associated ER membranes, MAMs), containing Ca2+-transport systems, including the inositol 1,4,5-trisphosphate receptor (IP R) at the ER and the voltage-dependent anion channel 1 (VDAC1) at the mitochondrial outer membrane [10, 11]. The mitochondrial Ca2+ uniporter (MCU) ensures Ca2+ transport across the mitochondrial inner membrane into the mitochondrial matrix [12, 13]. MCU mediates the rate-limiting step for the transfer of Ca2+ signals from the ER into the mitochondrial matrix.
Hence, Ca2+ signals that arise from the ER directly impact mitochondrial processes in a “dual” manner, promoting survival processes like ATP production and basal autophagy as well as cell-death processes like apoptosis [14, 15]. On the one hand, low-level Ca2+ signaling (Ca2+ oscillations) increases the activity of pyruvate, isocitrate and α-ketoglutarate dehydrogenases and thus drives mitochondrial ATP production and bio-energetics [14]. Interfering with these basal Ca2+ fluxes into the mitochondria results in impaired ATP production and in the engagement of AMPK signaling and autophagy as an adaptive survival pathway [16, 17]. On the other hand, excessive Ca2+ transients trigger apoptosis by causing the opening of the mitochondrial permeability transition pore [18-20]. This will result in mitochondrial swelling and rupture of the outer mitochondrial membrane, causing the release of pro-apoptotic factors, including cytochrome c, in the cytosol and subsequent activation of caspases and apoptosis [21].
Thus, the impact of oncogenes and tumor suppressors on cell-death resistance may at least in part be mediated by their respective and opposing effects on Ca2+ transfer between the ER and the mitochondria [9]. In healthy cells, the proper oncogene/tumor suppressor balance allows for cell survival with an adequate ability to induce cell death in response to various forms of cell stress, thereby preventing the survival of damaged or altered cells (Fig. 1A). There is now a growing list of oncogenes and tumor suppressors that appear to be present at the ER and/or MAMs, thereby directly targeting and controlling IP Rs, VDAC1 and the sarco/endoplasmic reticulum Ca2+ ATPase (SERCA), responsible for pumping Ca2+ into the ER [9, 22]. The functional impact of oncogenes like Bcl-2 [23-26], Bcl-XL [27, 28] and PKB/Akt [29, 30] is to limit the “cytotoxic” Ca2+ transfer from ER to mitochondria, while tumor suppressors like PTEN [31], PML [32], FHIT [33] and BRCA1 [34] promote this “cytotoxic” Ca2+ transfer. Furthermore, these proteins can execute their function by a concerted action on different Ca2+-transport systems. For instance, Bcl-2 proteins have been reported to suppress excessive Ca2+ signaling by i) inhibiting SERCA activity [35] or increasing Ca2+ leak from the ER through sensitized IP3Rs [25], which both reduce the loading of the ER Ca2+ stores, ii) dampening Ca2+ release from ER by directly inhibiting IP3Rs [36], and iii) limiting mitochondrial Ca2+ uptake by inhibiting VDAC1 [26]. Of note, other Bcl-2-family members, like Bcl-XL and Mcl-1, can also favor cell survival by promoting basal pro-survival Ca2+ oscillations by enhancing IP3R [37, 38] or VDAC1 activity [39, 40]. Indeed, basal VDAC1 activity appears to be essential for the cell growth and proliferation of a variety of cancer cells [41]. Besides oncogenic and tumor suppressor proteins, also microRNAs implicated in cancer can modulate mitochondrial Ca2+ uptake [42]. For instance, MCU expression is dynamically controlled by a cancer-related miR-25 [43]. Downregulation of MCU by miR-25 suppresses mitochondrial Ca2+ uptake, causing apoptotic resistance and favoring cancer cell survival [43]. In any case, a major impact of the deregulated balance between oncogenes and tumor suppressors in tumor cells is a strong dampening of ER-mitochondrial Ca2+ transfers. This will thereby contribute to the excessive cell-death resistance of cancer cells despite on-going pro-death signaling due to oncogenic stress.
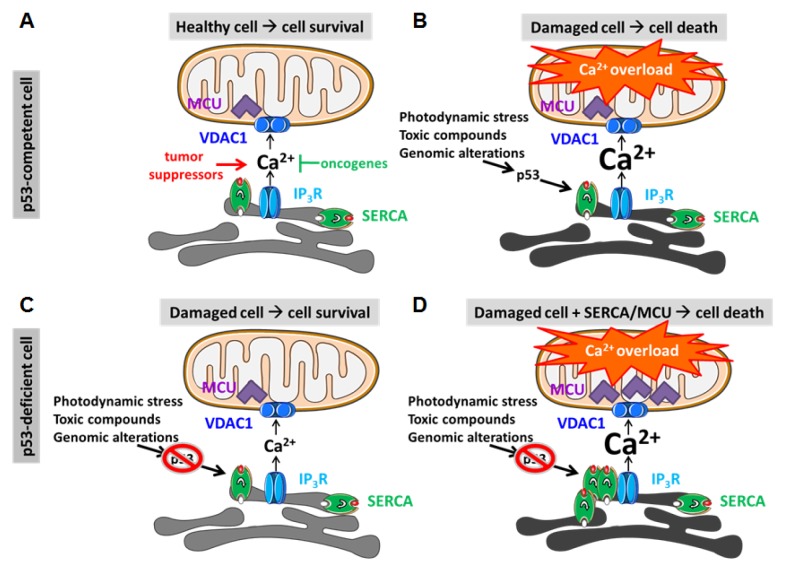
Fig.1. The interplay between p53 and Ca2+ signaling for cell death in response to oncogenic stress or anti-cancer treatments.
A, In normal, p53-competent cells (upper row), oncogenes (including PKB/Akt and Bcl-2-family members) and tumor suppressors (PTEN, PML, FHIT, BRCA1 and p53) are in balance, thereby providing the proper flux of Ca2+ from the ER into the mitochondria. This delicate ratio allows the production of ATP for survival, while maintaining normal cell death sensitivity. B, Upon stress conditions, including photodynamic therapy, toxic compounds or oncogenic stress due to genomic instability and alterations, tumor suppressors, like p53, are activated. This can provoke increased ER-mitochondrial Ca2+ transfer, mitochondrial Ca2+ overload and the elimination of altered or damaged cells, preventing oncogenesis and cancer initiation/progression. In the presence of p53, SERCA pumps become hyperactive due to p53 recruitment, causing Ca2+ overload in the ER and increased sensitivity towards IP3 receptor-mediated Ca2+ release and subsequent Ca2+ uptake into the mitochondria. C, In malignant, p53-deficient cells (lower row), excessive cell-death resistance prevails, allowing the survival of damaged or altered cells. These cells fail to engage increased ER-mitochondrial Ca2+ fluxes and thus cell death in response to cell stress, toxic compounds or genomic alterations, favoring oncogenesis and cancer progression. These cells are also resistant to anti-cancer therapies, like photodynamic therapy. In part, this is due to their failure to increase SERCA activity due to the absence of p53. D, Excitingly, p53-deficient cells can be re-sensitized to photodynamic therapy by promoting ER-mitochondrial Ca2+ fluxes via overexpression of SERCA, thereby increasing ER Ca2+ levels, or via overexpression of MCU. These strategies facilitate mitochondrial Ca2+ uptake in the mitochondrial matrix, thereby promoting mitochondrial Ca2+ overload in response to cell stress.
Yet, until now, the role of Ca2+ signaling for the pro-apoptotic function of p53, a major tumor suppressor critical for cell death in response to cell stress and chemo-/phototherapy but mutated in >50% of all human cancers [44], remained elusive. Also, the in vivo relevance of Ca2+ signaling from ER to mitochondria for cell-death therapies in cancers and how this is impacted by p53 was not known. Two recent studies by Pinton and co-workers [45, 46] addressed these key points and revealed groundbreaking insights in the p53/Ca2+ signaling connection for inducing cell death in cancer cells in response to anti-cancer treatments. These studies reveal a novel, non-transcriptional role for cytosolic p53 at the level of the ER Ca2+ stores (Fig. 1B-D). First, Pinton and co-workers demonstrated that photodynamic therapy prominently elevated cytosolic and mitochondrial Ca2+ levels in mutant Ras-transformed cells in vitro, contributing to the photo-induced stress and cell death. Strikingly, transformed cells lacking p53 displayed a reduced [Ca2+] rise from the ER, preventing mitochondrial Ca2+ overload and subsequent cell death. These findings revealed that i) the exacerbated ER Ca2+ release in response to photodynamic stress is a novel “non-transcriptional” function of extra-nuclear p53; and ii) the cell-death resistance of p53-deficient cancer cells to photodynamic therapy is due to a failure to induce “toxic” Ca2+ signaling events. Excitingly, in another recent study [46], Pinton's team showed that cytosolic p53 accumulated at ER-mitochondrial contact sites in response to chemotherapeutic agents/cell-death stimuli. This event promoted ER-mitochondrial Ca2+ transfer and mitochondrial Ca2+ overload and thus apoptotic cell death. Cells lacking p53 failed to display these responses upon chemotherapy treatment. This could be restored by p53 lacking its nuclear localization sequence or by p53 targeted to the ER, but not by naturally occurring oncogenic p53 mutants. The effects of wild-type p53 on mitochondrial Ca2+ signaling in cells exposed to chemotherapy were found to be caused by a direct interaction of ER-localized p53 with SERCAs, lowering their oxidation state, accelerating Ca2+-pump activity and provoking ER Ca2+ overload. Oncogenic p53 mutants failed to promote SERCA activity and thus lacked the boosting of ER-mitochondrial transfers of “cytotoxic” Ca2+ signals. Consistent with these findings, an apoptotic response of p53-deficient cancer cells to photodynamic therapy could also be restored by overexpressing either SERCA or the MCU, thereby enhancing “toxic” Ca2+-signaling events. Next, the team performed in vivo Ca2+ imaging in three-dimensional tumor masses in mouse models using a skinfold chamber technique, visualizing for the first time intracellular Ca2+ dynamics in tumors exposed to photodynamic therapy in living animals. In these tumor masses, photodynamic stress prominently elevated cytosolic and mitochondrial [Ca2+] in vivo, resulting in tumor cell death. Buffering intracellular Ca2+ prevented this in vivo photodynamic stress-induced tumor cell death. These events critically depended on p53, since p53-deficient tumor cells failed to display photodynamic stress-induced [Ca2+] rise and cell death.
These elegant in vivo experiments underpin the central role of ER-mitochondrial Ca2+ transfers not only in cell death but also in therapeutic responses to anti-cancer strategies. In addition, they underscore the potential of potentiating Ca2+-transport systems at the level of the ER and the mitochondria to overcome cell-death resistance of tumors (like p53-deficient cancers) to therapeutic treatments. These studies also highlight the importance of considering and investing in the future application of Ca2+-signaling-based therapies to increase the therapeutic success of anti-cancer treatments. In particular, treatments based on inducing tumor cell death by phototherapy and likely also chemotherapy, could be boosted by activation of toxic Ca2+-signaling. The search for molecules that could improve ER-mitochondrial Ca2+ fluxes, being it activators of SERCA, IP Rs, VDAC1 or MCU, may turn out to be promising tools in patients that suffer from tumors that poorly respond to therapeutic regimens. Given the central role of Ca2+ signaling in a variety of physiological processes, an important challenge will be to avoid toxic effects in unaltered, healthy cells. Strategies could involve the local release of compounds using coated nanoparticles or their coupling via a protease-sensitive linker sequence to a chemical moiety. This would allow the compound to enter cancer, but not healthy, cells expressing the particular protease. This strategy has been successfully used for analogues of thapsigargin, a high-affinity, irreversible inhibitor of SERCA [47]. Further opportunities will also lie in mapping and understanding the altered “Ca2+-signaling” context of cancer cells due to deranged expression of Ca2+ channels and pumps or deranged signaling pathways involved in the activation of these channels, like arachidonic acid signaling towards plasmalemmal Orai1/Orai3 channels in prostate cancers [48] and IP3 signaling towards type 2 IP3Rs in B-cell malignancies [49]. Indeed, normal and some B-cell cancer cells appear to withstand the disruption of IP R/Bcl-2 complexes, while other B-cell cancer cells are very sensitive to this treatment [49, 50]. This indicates a critical difference between normal versus cancer cells on the one hand and between different subsets of cancer cells on the other hand in their addiction to Bcl-2 at level of their ER/IP Rs [51]. These insights may lead to the development of innovative “Ca2+-signaling drugs” that could enhance Ca2+ fluxes in (a subset of) cancer, but not in healthy, cells, thereby killing or sensitizing these cancers, including resistant p53-deficient cancers, to treatments.
In summary, these recent findings by Pinton and co-workers elucidate that the ability of chemo-/photo-therapies to trigger pro-apoptotic Ca2+ signaling from the ER into the mitochondria, critically depends on the presence of p53. These data reveal that pro-apoptotic Ca2+ signaling is an important factor that determines the success of these strategies to kill cancer cells, including those related to oncogenic p53 mutants. This work also provides good hope that promoting Ca2+ signaling from the ER into the mitochondria could be beneficial for the targeting of a variety of tumors, including tumors resistant to chemotherapy, and re-establishing sensitivity of malignant cells to anti-cancer therapies.
Acknowledgments
Work in the authors' laboratory is supported by grants from the Research Council of the KU Leuven (OT14/101) and the Research Foundation-Flanders (G.0819.13N and G.0C91.14). The authors thank all lab members for fruitful discussions and Humbert De Smedt and Jan B. Parys for critically reading the manuscript.
Footnotes
CONFLICT OF INTEREST
The authors declare no competing interests.
REFERENCES
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: new roles for known actors. Nat Rev Cancer. 2011;11(8):609–618. doi: 10.1038/nrc3105. [DOI] [PubMed] [Google Scholar]
- 3.Stewart TA, Yapa KT, Monteith GR. Altered calcium signaling in cancer cells. Biochim Biophys Acata - Biomembr. 2014 doi: 10.1016/j.bbamem.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer. 2008;8(5):361–375. doi: 10.1038/nrc2374. [DOI] [PubMed] [Google Scholar]
- 5.Monteith GR, Davis FM, Roberts-Thomson SJ. Calcium channels and pumps in cancer: changes and consequences. J Biol Chem. 2012;287(38):31666–31673. doi: 10.1074/jbc.R112.343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motiani RK, Stolwijk JA, Newton RL, Zhang X, Trebak M. Emerging roles of Orai3 in pathophysiology. Channels (Austin) 2013;7(5):392–401. doi: 10.4161/chan.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteith GR. Prostate cancer cells alter the nature of their calcium influx to promote growth and acquire apoptotic resistance. Cancer Cell. 2014;26(1):1–2. doi: 10.1016/j.ccr.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Kondratskyi A, Kondratska K, Skryma R, Prevarskaya N. Ion channels in the regulation of apoptosis. Biochim Biophys Acata - Biomembr. 2014 doi: 10.1016/j.bbamem.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 9.Marchi S, Giorgi C, Oparka M, Duszynski J, Wieckowski MR, Pinton P. Oncogenic and oncosuppressive signal transduction at mitochondria-associated endoplasmic reticulum membranes. Molecular & Cellular Oncology. 2014;1(2) doi: 10.4161/23723548.2014.956469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochim Biophys Acta - Bioenerg. 2014;1837(4):461–469. doi: 10.1016/j.bbabio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Pinton P, Giorgi C, Missiroli S, Patergnani S, Duszynski J, Wieckowski M. Mitochondria-associated Membranes (MAMs): Composition, Molecular Mechanisms and Physiopathological Implications. Antioxid Redox Signal. 2015 doi: 10.1089/ars.2014.6223. [DOI] [PubMed] [Google Scholar]
- 12.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476(7360):336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476(7360):341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rizzuto R, De Stefani D, Raffaello A, Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol. 2012;13(9):566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 15.Patron M, Raffaello A, Granatiero V, Tosatto A, Merli G, De Stefani D, Wright L, Pallafacchina G, Terrin A, Mammucari C, Rizzuto R. The mitochondrial calcium uniporter (MCU): molecular identity and physiological roles. J Biol Chem. 2013;288(15):10750–10758. doi: 10.1074/jbc.R112.420752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142(2):270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cardenas C, Foskett JK. Mitochondrial Ca2+ signals in autophagy. Cell Calcium. 2012;52(1):44–51. doi: 10.1016/j.ceca.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giorgi C, Baldassari F, Bononi A, Bonora M, De Marchi E, Marchi S, Missiroli S, Patergnani S, Rimessi A, Suski JM, Wieckowski MR, Pinton P. Mitochondrial Ca2+ and apoptosis. Cell Calcium. 2012;52(1):36–43. doi: 10.1016/j.ceca.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its adaptive responses in tumor cells. Cell Calcium. 2014;56(6):437–445. doi: 10.1016/j.ceca.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonora M, Bravo-San Pedro JM, Kroemer G, Galluzzi L, Pinton P. Novel insights into the mitochondrial permeability transition. Cell Cycle. 2014;13(17):2666–2670. doi: 10.4161/15384101.2014.949082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morciano G, Giorgi C, Bonora M, Punzetti S, Pavasini R, Wieckowski MR, Campo G, Pinton P. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J Mol Cell Cardiol. 2015;78:142–153. doi: 10.1016/j.yjmcc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Akl H, Bultynck G. Altered Ca2+ signaling in cancer cells: proto-oncogenes and tumor suppressors targeting IP3 receptors. Biochim Biophys Acta - Rev Cancer. 2013;1835(2):180–193. doi: 10.1016/j.bbcan.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol. 2000;148(5):857–862. doi: 10.1083/jcb.148.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol. 2004;166(2):193–203. doi: 10.1083/jcb.200309146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2005;102(1):105–110. doi: 10.1073/pnas.0408352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arbel N, Shoshan-Barmatz V. Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J Biol Chem. 2010;285(9):6053–6062. doi: 10.1074/jbc.M109.082990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbel N, Ben-Hail D, Shoshan-Barmatz V. Mediation of the antiapoptotic activity of Bcl-xL protein upon interaction with VDAC1 protein. J Biol Chem. 2012;287(27):23152–23161. doi: 10.1074/jbc.M112.345918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monaco G, Decrock E, Arbel N, van Vliet AR, La Rovere R, De Smedt H, Parys JB, Agostinis P, Leybaert L, Shoshan-Barmatz V, Bultynck G. The BH4 domain of anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the voltage-dependent anion channel 1 (VDAC1)-mediated transfer of pro-apoptotic Ca2+ signals to mitochondria. J Biol Chem. 2015 doi: 10.1074/jbc.M114.622514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Soderberg O, Bootman MD, Roderick HL. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci U S A. 2008;105(7):2427–2432. doi: 10.1073/pnas.0711324105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchi S, Marinello M, Bononi A, Bonora M, Giorgi C, Rimessi A, Pinton P. Selective modulation of subtype III IP3R by Akt regulates ER Ca2+ release and apoptosis. Cell Death Dis. 2012;3:e304. doi: 10.1038/cddis.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP, Pinton P. Identification of PTEN at the ER and MAMs and its regulation of Ca2+ signaling and apoptosis in a protein phosphatase-dependent manner. Cell Death Differ. 2013;20(12):1631–1643. doi: 10.1038/cdd.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giorgi C, Ito K, Lin HK, Santangelo C, Wieckowski MR, Lebiedzinska M, Bononi A, Bonora M, Duszynski J, Bernardi R, Rizzuto R, Tacchetti C, Pinton P, et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science. 2010;330(6008):1247–1251. doi: 10.1126/science.1189157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rimessi A, Marchi S, Fotino C, Romagnoli A, Huebner K, Croce CM, Pinton P, Rizzuto R. Intramitochondrial calcium regulation by the FHIT gene product sensitizes to apoptosis. Proc Natl Acad Sci U S A. 2009;106(31):12753–12758. doi: 10.1073/pnas.0906484106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedgepeth SC, Garcia MI, Wagner LE, 2nd, Rodriguez AM, Chintapalli SV, Snyder RR, Hankins GD, Henderson BR, Brodie KM, Yule DI, van Rossum DB, Boehning D. The BRCA1 Tumor Suppressor Binds to Inositol 1,4,5-Trisphosphate Receptors to Stimulate Apoptotic Calcium Release. J Biol Chem. 2015 doi: 10.1074/jbc.M114.611186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schoneich C. Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) Biochem J. 2004;383(Pt 2):361–370. doi: 10.1042/BJ20040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rong YP, Aromolaran AS, Bultynck G, Zhong F, Li X, McColl K, Matsuyama S, Herlitze S, Roderick HL, Bootman MD, Mignery GA, Parys JB, De Smedt H, et al. Targeting Bcl-2-IP receptor interaction to reverse Bcl-2's inhibition of apoptotic calcium signals. Mol Cell. 2008;31(2):255–265. doi: 10.1016/j.molcel.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nat Cell Biol. 2005;7(10):1021–1028. doi: 10.1038/ncb1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckenrode EF, Yang J, Velmurugan GV, Foskett JK, White C. Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J Biol Chem. 2010;285(18):13678–13684. doi: 10.1074/jbc.M109.096040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang H, Hu X, Eno CO, Zhao G, Li C, White C. An interaction between Bcl-xL and the voltage-dependent anion channel (VDAC) promotes mitochondrial Ca2+ uptake. J Biol Chem. 2013;288(27):19870–19881. doi: 10.1074/jbc.M112.448290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang H, Shah K, Bradbury NA, Li C, White C. Mcl-1 promotes lung cancer cell migration by directly interacting with VDAC to increase mitochondrial Ca2+ uptake and reactive oxygen species generation. Cell Death Dis. 2014;5:e1482. doi: 10.1038/cddis.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arif T, Vasilkovsky L, Refaely Y, Konson A, Shoshan-Barmatz V. Silencing VDAC1 Expression by siRNA Inhibits Cancer Cell Proliferation and Tumor Growth In Vivo. Mol Ther Nucleic Acids. 2014;3:e159. doi: 10.1038/mtna.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchi S, Pinton P. Mitochondrial calcium uniporter, MiRNA and cancer: Live and let die. Commun Integr Biol. 2013;6(3):e23818. doi: 10.4161/cib.23818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchi S, Lupini L, Patergnani S, Rimessi A, Missiroli S, Bonora M, Bononi A, Corra F, Giorgi C, De Marchi E, Poletti F, Gafa R, Lanza G, et al. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr Biol. 2013;23(1):58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8(4):275–283. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 45.Giorgi C, Bonora M, Missiroli S, Poletti F, Ramirez FG, Morciano G, Morganti C, Pandolfi PP, Mammano F, Pinton P. Intravital imaging reveals p53-dependent cancer cell death induced by phototherapy via calcium signaling. Oncotarget. 2015;6(3):1435–1445. doi: 10.18632/oncotarget.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giorgi C, Bonora M, Sorrentino G, Missiroli S, Poletti F, Suski JM, Galindo Ramirez F, Rizzuto R, Di Virgilio F, Zito E, Pandolfi PP, Wieckowski MR, Mammano F, et al. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc Natl Acad Sci U S A. 2015;112(6):1779–1784. doi: 10.1073/pnas.1410723112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doan NT, Paulsen ES, Sehgal P, Moller JV, Nissen P, Denmeade SR, Isaacs JT, Dionne CA, Christensen SB. Targeting thapsigargin towards tumors. Steroids. 2014 doi: 10.1016/j.steroids.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubois C, Vanden Abeele F, Lehen'kyi V, Gkika D, Guarmit B, Lepage G, Slomianny C, Borowiec AS, Bidaux G, Benahmed M, Shuba Y, Prevarskaya N. Remodeling of channel-forming ORAI proteins determines an oncogenic switch in prostate cancer. Cancer Cell. 2014;26(1):19–32. doi: 10.1016/j.ccr.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 49.Akl H, Monaco G, La Rovere R, Welkenhuyzen K, Kiviluoto S, Vervliet T, Molgo J, Distelhorst CW, Missiaen L, Mikoshiba K, Parys JB, De Smedt H, Bultynck G. IP R2 levels dictate the apoptotic sensitivity of diffuse large B-cell lymphoma cells to an IP R-derived peptide targeting the BH4 domain of Bcl-2. Cell Death Dis. 2013;4:e632. doi: 10.1038/cddis.2013.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong F, Harr MW, Bultynck G, Monaco G, Parys JB, De Smedt H, Rong YP, Molitoris JK, Lam M, Ryder C, Matsuyama S, Distelhorst CW. Induction of Ca2+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP receptor interaction. Blood. 2011;117(10):2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Akl H, Vervloessem T, Kiviluoto S, Bittremieux M, Parys JB, De Smedt H, Bultynck G. A dual role for the anti-apoptotic Bcl-2 protein in cancer: mitochondria versus endoplasmic reticulum. Biochim Biophys Acata - Biomembr. 2014;1843(10):2240–2252. doi: 10.1016/j.bbamcr.2014.04.017. [DOI] [PubMed] [Google Scholar]