Abstract
Glial fibrillary acidic protein and its breakdown products (GFAP-BDP) are brain-specific proteins released into serum as part of the pathophysiological response after traumatic brain injury (TBI). We performed a multi-center trial to validate and characterize the use of GFAP-BDP levels in the diagnosis of intracranial injury in a broad population of patients with a positive clinical screen for head injury. This multi-center, prospective, cohort study included patients 16–93 years of age presenting to three level 1 trauma centers with suspected TBI (loss of consciousness, post-trauma amnesia, and so on). Serum GFAP-BDP levels were drawn within 24 h and analyzed, in a blinded fashion, using sandwich enzyme-linked immunosorbent assay. The ability of GFAP-BDP to predict intracranial injury on admission computed tomography (CT) as well as delayed magnetic resonance imaging was analyzed by multiple regression and assessed by the area under the receiver operating characteristic curve (AUC). Utility of GFAP-BDP to predict injury and reduce unnecessary CT scans was assessed utilizing decision curve analysis. A total of 215 patients were included, of which 83% suffered mild TBI, 4% moderate, and 12% severe; mean age was 42.1±18 years. Evidence of intracranial injury was present in 51% of the sample (median Rotterdam Score, 2; interquartile range, 2). GFAP-BDP demonstrated very good predictive ability (AUC=0.87) and demonstrated significant discrimination of injury severity (odds ratio, 1.45; 95% confidence interval, 1.29–1.64). Use of GFAP-BDP yielded a net benefit above clinical screening alone and a net reduction in unnecessary scans by 12–30%. Used in conjunction with other clinical information, rapid measurement of GFAP-BDP is useful in establishing or excluding the diagnosis of radiographically apparent intracranial injury throughout the spectrum of TBI. As an adjunct to current screening practices, GFAP-BDP may help avoid unnecessary CT scans without sacrificing sensitivity (Registry: ClinicalTrials.gov Identifier: NCT01565551).
Key words: : biomarkers, imaging, traumatic brain injury
Introduction
Clinical care and research in traumatic brain injury (TBI) rely on classification systems, such as the Glasgow Coma Scale (GCS), that are not adequately calibrated for injury assessment across mild and moderate TBI.1 Radiographic evaluation is central to the initial stratification of injury severity and to monitor for acute changes; however, its use is limited by cost and perceived risk of ionizing radiation.
Simpler, sensitive, and specific tests for identifying and stratifying TBI would provide more rapid and tailored diagnosis of TBI while minimizing the time, risk, and cost associated with current standards. To this end, there has been increasing investigation into serum proteins as biomarkers of TBI; however, none have yet been validated for routine use. Potential biomarkers under investigation include glial protein S-100 beta (S100B), neuron-specific enolase (NSE), myelin basic protein, ubiquitin c-terminal hydrolase, and glial fibrillary acid protein (GFAP).2,3 GFAP, initially investigated in the 1970s, has emerged as a promising biomarker candidate to improve diagnosis, triage, and targeted treatment of TBI patients.4 GFAP is an intermediate filament protein component of the astrocyte cytoskeleton expressed almost exclusively in the central nervous system (CNS). While insoluble in intact astrocytes, overactivation of calpain after initial injury and gliolysis produce soluble GFAP polymers (or breakdown products) that are released into interstitial fluid.5 These GFAP breakdown products (GFAP-BDP) can be measured in serum in association with a number of CNS disorders, including TBI.1,2 Previous studies have correlated elevated GFAP-BDP with the presence of clinical and radiographic injury as well as worse outcome and need for neurosurgical intervention.2,3 To date, previous work has focused primarily on the severe TBI population or compared TBI patients against either uninjured patients or those not meeting clinical criteria for head injury. Our previous study was one of the first to prospectively assess GFAP-BDP with regard to presence and severity of radiographic injury on computed tomography (CT) across the entire spectrum of disease after TBI.4,6
The aim of this study was to evaluate and validate the utility of GFAP-BDP for the diagnosis of intracranial injury in patients with a positive clinical screen for head injury across the spectrum of TBI typically presenting to a level 1 trauma center. We expand on our previous analysis of the utility of GFAP-BDP to identify TBI, including injury evaluation by MRI, cut-off values for GFAP-BDP specifically in the mild and moderate TBI groups, and analysis of the potential reduction of CT scans by utilizing the biomarker for injury detection.6
Methods
Study population
Recruitment of subjects was part of the TRACK-TBI (Transforming Research and Clinical Knowledge in Traumatic Brain Injury) Pilot Study, a National Institute of Neurological Disorders and Stroke–funded, multi-center, prospective collaboration among three U.S. level 1 trauma centers enrolling acute TBI patients (University of Pittsburgh Medical Center [UPMC]; University Medical Center Brackenridge [UMCB]; and University of California, San Francisco [UCSF]) and one rehabilitation center (Mount Sinai Rehabilitation Center) enrolling late-presenting TBI patients to develop, test, and refine TBI common data elements (TBI-CDEs) for research across four major domains: demographics, neuroimaging, biomarkers, and outcome measures.7 The TBI population under investigation spanned the entire injury spectrum, from severe to mild. Both patients with negative imaging and those discharged from the emergency department (ED) are also included in the total population. Institutional review boards of participating centers approved all study protocols. All participants or their legal authorized representatives gave written informed consent. At follow-up, participants previously consented by legal authorized representative, if neurologically improved to be cognizant, were consented for continuation in the study.
To be eligible for this analysis, patients must have presented to an ED within 24 h of their injury and had a positive clinical screen for acute TBI necessitating a noncontrast head CT according to American College of Emergency Physicians/Centers for Disease Control and Prevention (ACEP/CDC) evidence-based joint practice guidelines.8 These guidelines represent an amalgam of the Canadian CT Head Rule and the New Orleans Criteria (Haydel, Indications for computed tomography in patients with minor head injury; Stiell, The Canadian CT Head Rule for patients with minor head injury). GCS score was assessed by a neurosurgeon at admission and was reconfirmed by study personnel at the time of biomarker collection. TBI severity was broadly defined by GCS, with mild between 13 and 15, moderate between 9 and 12, and severe between 3 and 8. Patients were excluded if they were younger than 16 or greater than 95 years of age, suffered penetrating head injury, or had a premorbid neurologic condition.
Sample collection and measurement of glial fibrillary acidic protein and its breakdown products
Data from the three level 1 trauma centers were used for this analysis. Serum samples were collected within 24 h of injury and were dated and time stamped to compare with time of injury. The TBI-CDE Biospecimens and Biomarkers Working Group Guidelines for sample preparation were followed.9 Samples were centrifuged and serum aliquots stored at −80°C for future batch processing. UPMC and UMCB batch-shipped samples, overnight on dry ice, to UCSF. All deidentified samples were then stored with a unique study number specific to site and subject. A central database was maintained by the coordinating center (UCSF) with each site entering site-specific data for final statistical reporting. Blinded sample analysis occurred in a single laboratory (Banyan Biomarkers, Alachua, FL) using a sandwich enzyme-linked immunosorbent assay (ELISA) to GFAP-BDP. The GFAP ELISA utilized a proprietary mouse monoclonal antibody for solid-phase immobilization, and a proprietary polyclonal rabbit antibody for detection.10,11 Testing procedure and detection of GFAP was carried out as previously described.6 Both whole GFAP molecules as well as GFAP-BDPs are detected by the assay, potentially resulting in a more complete measure of overall GFAP released into circulation. All samples were analyzed in duplicate concomitantly with calibrators prepared in compatible matrix, as described previously.6 From high concentration to low, the previously reported intraassay coefficient of variance for the ELISA is 4.3–7.8% and the interassay coefficient of variance is 7.8–14.3%. The estimated limit of detection for GFAP is ∼0.01 ng/mL.11
Evaluation of endpoints
All patients underwent CT imaging of the brain at the time of initial presentation to the ED. Patients were offered a follow-up, out-patient MRI upon enrollment in the TRACK-TBI study. The MRI was on a voluntary, opt-in basis to be performed 1–2 weeks postinjury. Radiographic images were deidentified, uploaded to a central imaging database, and reviewed by a blinded central reader. Imaging features were extracted and entered into the TRACK-TBI database. Each patient's head CT and magnetic resonance image (MRI) were characterized using the recommendations of the TBI-CDE Neuroimaging Working Group regarding specific radiologic features, data definitions needed to characterize injuries, and best practices needed to optimize and harmonize imaging data acquisition for TBI research during data collection.12,13 Specifically, the presence of cisternal effacement, mid-line shift, epidural hematoma, subarachnoid hemorrhage, and intraventricular hemorrhage were recorded to determine the Rotterdam score for all scans (assessment of TBI severity based on noncontrast head CT). The presence of any intracranial abnormalities on MRI was considered a positive scan. Imaging studies were performed at the discretion of each study site using their standard equipment and protocols.
The primary endpoint for analysis was intracranial injury, as identified on CT scan at time of presentation. Secondary endpoints included severity of intracranial injury, as measured by the Rotterdam score, and presence of intracranial injury, as identified by delayed MRI.
Statistical analysis
Continuous demographic characteristics were assessed for normality using the Kolmogorov-Smirnov's test; normally distributed data were analyzed by t-test, whereas the remainders were compared using the Wilcoxon's rank-sum test. Categorical data were analyzed by Pearson's chi-squared or Fisher's exact test. Differences between groups in multi-level ordinal measurements (i.e., Rotterdam score, GCS, and Glasgow Outcome Scale) were tested using Kruskal-Wallis' test. Univariable regression analysis was performed to assess the association between GFAP-BDP level and radiographic presence of intracranial injury. Multi-variate regression models were later built to evaluate the predictive capabilities GFAP-BDP after adjustment for known factors associated with severity of intracranial injury (age, pupillary reactivity, GCS, and Injury Severity Score [ISS]). The ability of GFAP-BDP to predict severity of intracranial injury was assessed using ordered logistic regression modeling.
The ability of GFAP-BDP to predict the presence of intracranial injury was analyzed apropos of accuracy, discrimination, calibration, and clinical utility. Discrimination was assessed using the area under the receiver operating characteristic (ROC) curve (AUC). Using current statistical consensus, AUCs of 0.8–0.9 are considered very good, 0.7–0.8 as adequate, and below 0.7 as poor. Calibration was tested with the Hosmer-Lemeshow's goodness-of-fit test. Cut-off values for GFAP-BDP were assessed both for the highest accuracy and for the highest specificity, specifically in the mild to moderate injury groups. Values were determined utilizing ROC curves and AUC and Brier scores were calculated. Clinical utility was evaluated by decision curve analysis.14 Statistical significance was set at p<0.05. All data were analyzed using STATA statistical software (12; StataCorp LP, College Station, TX).
Results
Baseline demographics
A total of 215 patients were available for analysis. Demographic characteristics are shown in Table 1. Mean age was 42±18 years, with a minimum of 16 and maximum of 93 years. Approximately 73% of patients were male. Median GCS for the entire sample was 15 (interquartile range [IQR], 1), with mild TBI (GCS, 13–15) constituting 83% (GCS,13–15), moderate 4% (GCS, 9–12), and severe 13% (GCS, 3–8). Seventy percent of patients had a documented loss of consciousness (LOC), whereas 38% had documented post-traumatic amnesia (PTA). Median Injury Severity Score (ISS) was 10 (IQR, 17), with 36% suffering significant polytrauma (ISS, ≥16). Mean GFAP-BDP was 1.59±2.98 ng/mL, and minimum and maximum levels detected were 0.02 and 20.1 ng/mL, respectively. Pair-wise correlation between CT and MRI was 0.33 (p=0.0096). There was no significant correlation between MRI and Rotterdam score.
Table 1.
Baseline Demographic Characteristics at Time of Admission by Presence of Intracranial Injury on CT
| Baseline characteristics | All (n=215) | CT negative (n=105) | CT positive (n=110) | p value |
|---|---|---|---|---|
| Age, mean±SD (years) | 42±18 | 37±16 | 47±18 | <0.01 |
| Sex, % male | 73 (156) | 69 (72) | 76 (84) | 0.22 |
| GCS, median (IQR) | 15 (1) | 15 (0) | 15 (4) | <0.01 |
| Mild, % 13–15 | 83 (179) | 97 (102) | 70 (77) | |
| Moderate, % 9–12 | 4 (9) | 2 (2) | 6 (7) | |
| Severe, % 3–8 | 13 (27) | 1 (1) | 24 (26) | |
| Pupillary reactivity, % | <0.01 | |||
| Both | 94 (202) | 100 (105) | 88 (97) | |
| Anisocoria | 2 (4) | — | 4 (4) | |
| Unreactive | 4 (9) | — | 8 (9) | |
| ISS, median (IQR) | 10 (17) | 0 (4) | 17 (12) | <0.01 |
| Polytrauma, % ISS ≥16 (n) | 36 (78) | 5 (5) | 66 (73) | <0.01 |
| Rotterdam score, median (IQR) | — | 3 (1) | ||
| GFAP-BDP, mean±SD (ng/mL) | 1.59±2.98 | 0.26±0.41 | 2.86±3.74 | <0.01 |
CT, computed tomography; GCS, Glasgow Coma Score; ISS, Injury Severity Score; SD, standard deviation; IQR, interquartile range; GFAP-BDP, glial fibrillary acidic protein and its breakdown products.
Glial fibrillary acidic protein and its breakdown products and computed tomography outcomes
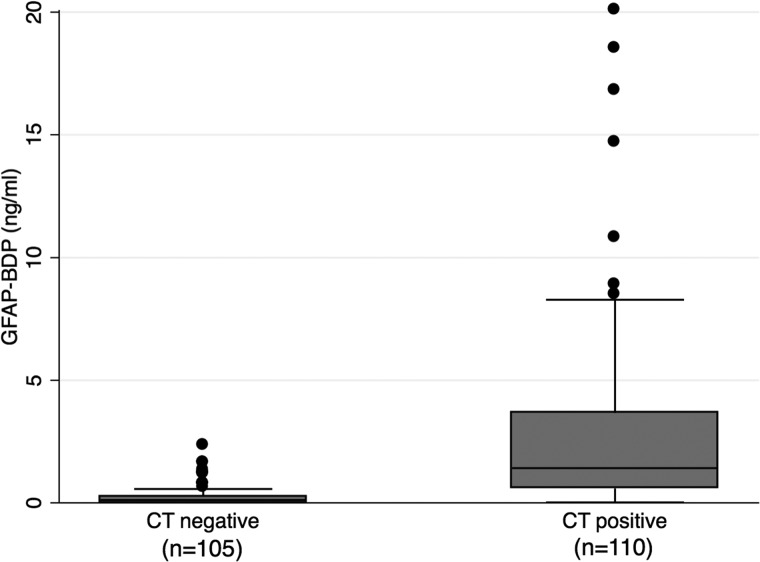
Fifty-one percent (n=110) of patients presenting with positive clinical screen for TBI had intracranial pathology demonstrated on admission CT. Median Rotterdam score of this cohort was 3 (IQR, 1). Serum level of GFAP-BDP was significantly higher in those with CT-positive intracranial injury, compared to those without (2.86±3.74 vs. 0.26±0.41 ng/mL, respectively; p<0.001). Figure 1 presents a box plot of GFAP-BDP values for the two patient cohorts. Univariable analysis demonstrated elevated GFAP-BDP level and conferred significant risk of intracranial injury on initial CT (odds ratio [OR], 8.9; 95% confidence interval [CI], 2.3–2.5; p<0.001), as also demonstrated in our previous study.6 Further, elevated GFAP-BDP remained a significant predictor after adjustment for known predictors of intracranial injury severity and functional outcome (i.e., age, pupillary activity, GCS, and ISS; OR, 5.5; 95% CI, 2.00–14.9; p<0.001).
FIG. 1.
Box plots showing median levels of GFAP-BDP measured on admission in two groups of patients. Boxes show interquartile ranges, and I bars represent highest and lowest values. CT, computed tomography;GFAP-BDP, glial fibrillary acidic protein and its breakdown products.
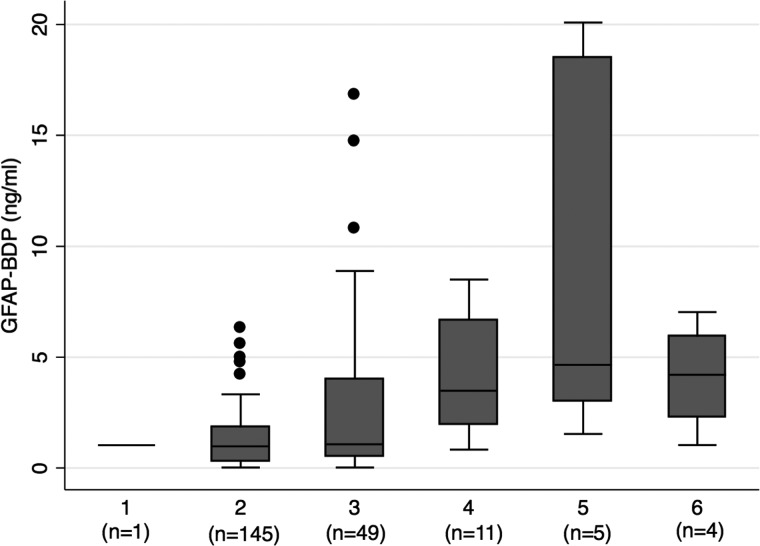
Figure 2 shows GFAP-BDP levels in relation to radiographic injury severity classification according to Rotterdam score. Level of GFAP-BDP differed significantly as a function of Rotterdam score (p<0.001). Ordinal regression analysis revealed that elevated GFAP-BDP level significantly predicted worse Rotterdam score, both independently (OR, 1.20; 95% CI 1.1–1.3) as well as after adjustment for age, GCS, and ISS (OR, 1.17 95% CI, 1.1–1.3; p<0.001).
FIG. 2.
Box plots showing median levels of GFAP-BDP measured on admission among patients in each of the Rotterdam classifications of injury on CT. Boxes show interquartile ranges, and I bars represent highest and lowest values. Overall, GFAP-BDP was significantly different across each level of Rotterdam score (p≤0.001). CT, computed tomography; GFAP-BDP, glial fibrillary acidic protein and its breakdown products.
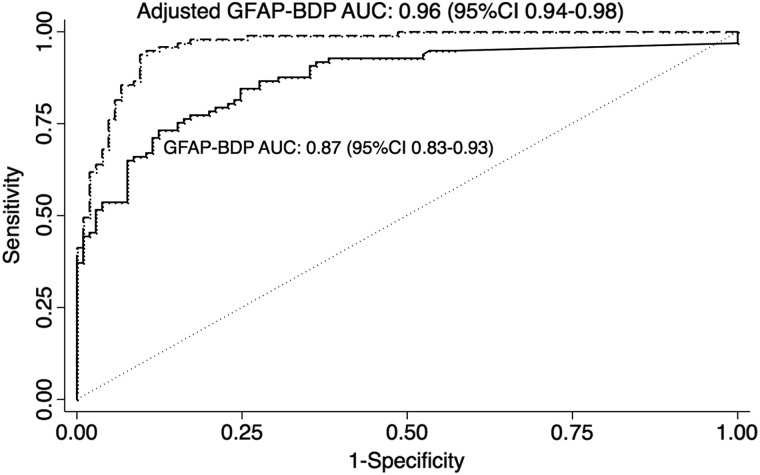
GFAP-BDP level was the most accurate predictor of the presence or absence of intracranial injury detected by radiographic imaging (accuracy, 81%), as compared with accepted clinical predictors of intracranial injury (age, 65%; GCS, 62%; LOC and/or PTA, 54%; pupillary status, 52%). In our sample, accuracy of GFAP-BDP for injury prediction was superior to the ACEP/CDC recommended criteria for neuroimaging in TBI (81% vs. 65%, respectively).8 Discriminatory analysis of GFAP-BDP resulted in an AUC of 0.88 (95% CI, 0.83–0.93), indicating very good discriminatory ability. Level of GFAP-BDP retained its discriminatory value after adjustment for age, pupillary exam, GCS, and ISS (AUC, 0.96; 95% CI, 0.7–0.91; Fig. 3). Calibration analysis did not show systematic error across risk deciles (p=0.15). Calculation of a cut-off value to maximize accuracy in the mild and moderate injury range specifically yielded a GFAP-BDP level of 0.6 ng/mL, with a sensitivity of 67%, a specificity of 89%, and a Brier score of 0.21. A cut-off value to maximize specificity was calculated at a GFAP-BDP concentration of 1.66 ng/mL, resulting in a sensitivity of 45%, specificity of 99%, and a Brier score of 0.29.
FIG. 3.
Receiver-operating-characteristic curves for various cut-off levels of GFAP-BDP in differentiating presence or absence of intracranial injury on CT. Curves for GFAP-BDP alone and after adjustment for known predictors of injury and severity (age, GCS, pupillary reactivity, and ISS). AUC, area under the receiver operating characteristic curve; CI, confidence intreval; CT, computed tomography; GCS, Glasgow Coma Scale; GFAP-BDP, glial fibrillary acidic protein and its breakdown products; ISS, Injury Severity Scale.
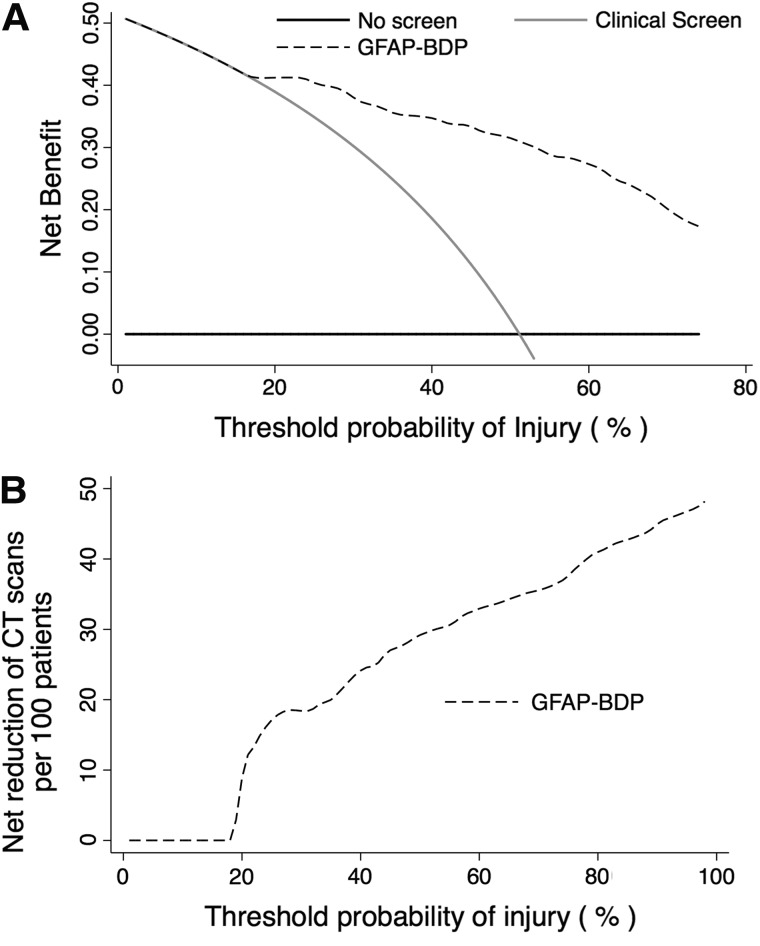
Clinical utility of GFAP-BDP was evaluated through decision curve analysis as an extension of currently established practice guidelines.15 Decision curves are displayed in Figure 4. Use of GFAP-BDP displayed superior net benefit, as compared to scanning all patients with a positive clinical screen for head injury beginning at a threshold probability (i.e., perceived risk of injury) of approximately 20% or higher. This correlated to a net reduction of 12 CT scans per 100 patients without missing a single injury (12% reduction in unnecessary imaging). Reduction of unnecessary scans increased to 18% when applied to patients with a perceived risk of injury of 25% and by more than 30% if the risk of injury was equivalent to the prevalence of injury in this sample (CT-positive after clinical screen, ∼51%).
FIG. 4.
(A) Decision curve analysis of the net benefit of GFAP-BDP to predict injury compared to current clinical screening method or scanning all patients regardless of screening across various probabilities of injury. (B) Decision curve analysis of the reduction of unnecessary CT scans per 100 patients using GFAP-BDP as an adjunct to predict injury compared to current clinical screening methods across various probabilities of injury. CT, computed tomography; GFAP-BDP, glial fibrillary acidic protein and its breakdown products.
Glial fibrillary acidic protein and its breakdown products and magnetic resonance imaging outcomes
Sixty patients underwent MRI in the subacute injury phase; of these, 35% (n=21) had positive scans (see Table 2). Of note, MRI revealed injuries in 13 patients who had had negative CT imaging on initial evaluation. Further, 4 patients with positive CT scans had negative follow-up findings on MRI. There was no significant difference between MRI-positive and -negative patients in age, gender, pupillary status, GCS, ISS, or functional outcome (Glasgow Outcome Scale Extended at 6 and 12 months). Admission GFAP-BDP values were significantly higher in MRI-positive patients (1.31±1.8 vs. 0.28±0.57 ng/mL, respectively; p=0.001). In univariable analysis, GFAP-BDPs significantly predicted the presence of intracranial pathology, as observed on MRI (OR, 2.7; 95% CI, 1.2–5.7). GFAP-BDP remained an independent predictor of injury on MRI after multivariate analysis, adjusting for age, pupillary status, GCS, and ISS (OR, 3.8; 95% CI, 1.3–11.3). Post-hoc, subgroup analysis performed on CT-negative, MRI-positive patients, in comparison with the remainder of the CT-negative cohort (35 patients), did not demonstrate significant differences in age, GCS, ISS, or GFAP-BDP levels.
Table 2.
Baseline Demographic Characteristics at Time of Admission by Presence of Intracranial Injury on MRI
| Baseline characteristics | MRI negative (n=39) | MRI positive (n=21) | p value |
|---|---|---|---|
| Age, mean±SD (years) | 39±17 | 42±15 | 0.32 |
| Sex, % male | 64 (25) | 76 (16) | 0.33 |
| GCS, median (IQR) | 15 (0) | 15 (0) | 0.68 |
| ISS, median (IQR) | 0 (0) | 0 (10) | 0.12 |
| GFAP-BDP, mean±SD (ng/mL) | 0.28±0.57 | 1.31±1.77 | <0.01 |
MRI, magnetic resonance imaging; GCS, Glasgow Coma Score; ISS, Injury Severity Score; SD, standard deviation; IQR, interquartile range; GFAP-BDP, glial fibrillary acidic protein and its breakdown products.
Analysis of GFAP-BDP for the prediction of injury on MRI demonstrated an accuracy of 72%, adequate discrimination of 0.70 (AUC; 95% CI, 0.55–0.85), and adequate calibration (p=0.41). Decision curve analysis demonstrated that GFAP-BDP contributes a net benefit above an injury-risk threshold of 25%, with a 13% reduction in unnecessary scans. Utilization of the cut-off value of 0.6 ng/mL in the mild-to-moderate range of injury was calculated to have a net benefit at an injury threshold of 24% and an overall net reduction in CT scans of 30 per 100 patients in this group.
Discussion
This multi-center, prospective study demonstrates that serum measurement of GFAP-BDP as a biomarker possesses the necessary characteristics (accuracy, discrimination, calibration, and clinical utility) for improved prediction of radiographically evident injury across the spectrum of TBI. Additionally, GFAP-BDP levels were able to discriminate severity of intracranial injury independent of other classic injury predictors. GFAP-BDP also accurately predicted persistence of intracranial injury on imaging performed in the subacute period, again independent of other markers of injury risk. These data expand upon our previous study demonstrating a correlation between injuries observed on CT scan and elevated levels of GFAP-BDP.6 Taken together, these results indicate that GFAP-BDP is a viable early indicator of intracranial injury and represents a useful adjunct to current diagnostic methods for TBI.
Numerous serum biomarker candidates for the diagnosis of TBI have come under intense scrutiny; however, none to this point have demonstrated sufficient utility to justify routine clinical use. Studies have reported a consistent correlation between elevated serum levels of S-100B and GCS, radiographic findings, and outcome.16 Despite its sensitivity, S-100B has been shown to be elevated in trauma patients without head injury, as well as after hemorrhagic shock and in normal pediatric patients.16 This lack of specificity limits its possible diagnostic practicality. Similarly, NSE, although rapidly elevated post-TBI, is also found in states of hemolysis.17 GFAP-BDP is a product of astrocyte cytoskeleton degradation by calpain protease activation and therefore considered specific to the CNS. This has already been corroborated by a number of studies evaluating levels after TBI, compared to noninjured controls, as well as those suffering only traumatic extracranial injuries.11,18 This study further supports the specificity of GFAP-BDP to detect radiographically evident injury given that predictive ability was evaluated among patients with similar clinical scenarios and presenting neurological exams. Against this clinically relevant sample, GFAP-BDP remained a sensitive and specific predictor of injury even after adjustment for the presence of polytrauma (i.e., ISS).
Previous evaluations of GFAP-BDP, largely focusing on severe TBI, have demonstrated a correlation between elevated marker levels and injury severity, number of lesions, and mortality.19 More recently, Papa and colleagues specifically studied GFAP-BDP within the mild-to-moderate TBI population and found that GFAP-BDP adequately predicted presence of injury, severity of injury, and need for neurosurgical intervention.11 The current study evaluates GFAP-BDP across the entire spectrum of TBI, in the context of all patients who screen positive for intracranial injury using established guidelines. Alone, GFAP-BDP demonstrated the highest accuracy among predictors and very good discrimination (AUC, 0.88). Importantly, despite varied injury states and severity, calibration did not demonstrate systematic errors, further supporting the use of GFAP-BDP across severity cohorts. Importantly, GFAP-BDP also independently predicted the degree of radiographic injury throughout the spectrum of presenting neurological exams. This correlation supports the idea that GFAP release, breakdown, and translocation to serum mirrors radiographic evidence of parenchymal injury and disruption of the blood–brain barrier.
Pressure to deliver cost-effective care and concern over the potential effects of unnecessary ionizing radiation have prompted more judicious use of CT imaging for the evaluation of head injury. Despite the implementation of the Canadian CT Head Rule and/or New Orleans Criteria to stratify patients, approximately 60–90% of patients imaged for head injury will have a negative CT.20 Biomarkers, ideally, could act as adjuncts to these validated approaches, to better and more cost-efficiently classify at-risk patients. To assess clinical utility in this context, we analyzed GFAP-BDP utilizing decision curve analyses to determine the probability of injury above which GFAP-BDP benefits diagnosis without increasing unnecessary scans. This study found that use of GFAP-BDP has a superior net benefit from a threshold probability of injury of 20% and greater. This suggests that measuring serum GFAP-BDP, in conjunction with current practice guidelines, would lead to a 12% reduction in unnecessary imaging at this relatively low-risk threshold for injury (common probability thresholds for cancer and cardiac screening are 10–20%). Specifically in the mild to moderate groups, where there is the most potential benefit from a reduction in CT scans, we calculated that, at a concentration of 0.6 ng/mL, there is a net benefit at an injury probability threshold of 24% with a potential reduction in scans of 30 per 100 patients. When used as an adjunct to ACEP Guidelines, GFAP-BDP would reduce unnecessary CT scans by greater than 20% at a risk threshold of 25%, and by more than 30% in a population with a prevalence of injury similar to our sample (∼51%).8 Currently only 6–10% of patients with GCS 13–15 have lesions detected on CT scan, and only 0.4–1% of these require neurosurgical intervention, indicating that many patients may not need imaging if other reliable and accurate options for injury detection are available.21 With approximately 1.5 million patients diagnosed as sustaining a mild TBI, estimating 80% receive a CT scan, and an average cost of $216 per CT scan, a reduction in scans of 30% could yield a potential savings of $77.8 million dollars per year in this population.22,23
There are several limitations to our study. GFAP-BDP was only measured at initial presentation and thus levels were unable to be trended to evaluate whether decreasing GFAP-BDP correlates with injury resolution or to track the trend in concentration over time. This precluded analysis of changes in concentration of GFAP-BDP over time as compared to evolution of injury on imaging. Our analysis included only those patients who received a head CT as part of enrollment in the TRACK-TBI study, and we therefore had a relatively high number of mild TBI patients with positive findings on CT scan. This may have excluded the less severely injured patients from GFAP-BDP measurement. Additionally, our analysis was limited to the clinical indicators of injury as defined by the TRACK-TBI study, and we were unable to compare GFAP-BDP against the numerous indicators of intracranial injury that may otherwise be used. We also were unable to include cost data on serum analysis for GFAP-BDP concentrations given that the data are publicly not available and remain confidential owing to the fact that the test is not yet U.S. Food and Drug Administration approved for clinical use. Therefore, we were unable to provide further analysis as to potential cost savings compared to CT scans. This is the first study, to our knowledge, to evaluate the performance of GFAP-BDP against the Rotterdam score and against positive findings on MRI. However, MRI data were collected on an opt-in basis at up to 2 weeks postinjury, potentially biasing this cohort to include patients with more-severe or persistent symptoms. This may help to account for the lower discriminatory ability of GFAP-BDP among MRI patients; nonetheless, GFAP-BDP remained a significant predictor after adjustment.
This analysis demonstrates that GFAP-BDP can reliably detect the presence of injury on radiographic imaging as well as predict injury severity across the spectrum of TBI. Early measurement of GFAP-BDP can contribute to more-accurate diagnosis and triage of TBI patients, decreasing the number of unnecessary CT scans and allowing more tailored management of the brain injury.
Contributor Information
Collaborators: TRACK-TBI investigators including:
Acknowledgments
This work was funded by the National Institutes of Health (grant no.: 1RC2 NS069409).
Author Disclosure Statement
No competing financial interests exist. No conflicts of interest.
References
- 1.Stocchetti N., Pagan F., Calappi E., Canavesi K., Beretta L., Citerio G., Cormio M., Colombo A. (2004). Inaccurate early assessment of neurological severity in head injury. J. Neurotrauma 21, 1131–1140 [DOI] [PubMed] [Google Scholar]
- 2.Vos P.E., Lamers K.J., Hendriks J.C., van Haaren M., Beems T., Zimmerman C., van Geel W., de Reus H., Biert J., and Verbeek M.M. (2004). Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology 62, 1303–1310 [DOI] [PubMed] [Google Scholar]
- 3.Papa L., Lewis L.M., Silvestri S., Falk J.L., Giordano P., Brophy G.M., Demery J.A., Liu M.C., Mo J., Akinyi L., Mondello S., Schmid K., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J. Trauma Acute Care Surg. 72, 1335–1344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng L.F., Ghirnikar R.S., and Lee Y.L. (2000). Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 25, 1439–1451 [DOI] [PubMed] [Google Scholar]
- 5.Lee Y.B., Du S., Rhim H., Lee E.B., Markelonis G.J., and Oh T.H. (2000). Rapid increase in immunoreactivity to GFAP in astrocytes in vitro induced by acidic pH is mediated by calcium influx and calpain I. Brain Res. 864, 220–229 [DOI] [PubMed] [Google Scholar]
- 6.Okonkwo D.O., Yue J.K., Puccio A.M., Panczykowski D., Inoue T., McMahon P.J., Sorani M.D., Yuh E.L., Lingsma H., Maas A., Valadka A. and Manley G.T.; Transforming Research and Clinical Knowledge in Traumatic Brain Injury (TRACK-TBI) Investigators. (2013). GFAP-BDP as an acute diagnostic marker in traumatic brain injury: results from the prospective TRACK-TBI Study. J. Neurotrauma 30, 1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yue J.K., Vassar M.J., Lingsma H.F., Cooper S.R., Okonkwo D.O., Valadka A.B., Gordon W.A., Maas A.I., Mukherjee P., Yuh E.L., Puccio A.M., Schnyer D.M., Manley G.T., Track-Tbi I., Casey S.S., Cheong M., Dams-O'Connor K., Hricik A.J., Knight E.E., Kulubya E.S., Menon D.K., Morabito D.J., Pacheco J.L., and Sinha T.K. (2013). Transforming research and clinical knowledge in traumatic brain injury pilot: multicenter implementation of the common data elements for traumatic brain injury. J. Neurotrauma 30, 1831–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jagoda A.S., Bazarian J.J., Bruns J.J., Jr., Cantrill S.V., Gean A.D., Howard P.K., Ghajar J., Riggio S., Wright D.W., Wears R.L., Bakshy A., Burgess P., Wald M.M., and Whitson R.R.; American College of Emergency Physicians, Centers for Disease Control and Prevention. (2008). Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann. Emerg. Med. 52, 714–748 [DOI] [PubMed] [Google Scholar]
- 9.Manley G.T., Diaz-Arrastia R., Brophy M., Engel D., Goodman C., Gwinn K., Veenstra T.D., Ling G., Ottens A.K., Tortella F., and Hayes R.L. (2010). Common data elements for traumatic brain injury: recommendations from the biospecimens and biomarkers working group. Arch. Phys. Med. Rehabil. 91, 1667–1672 [DOI] [PubMed] [Google Scholar]
- 10.Zoltewicz J.S., Scharf D., Yang B., Chawla A., Newsom K.J., and Fang L. (2012). Characterization of antibodies that detect human GFAP after traumatic brain injury. Biomark. Insights 7, 71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papa L., Lewis L.M., Falk J.L., Zhang Z., Silvestri S., Giordano P., Brophy G.M., Demery J.A., Dixit N.K., Ferguson I., Liu M.C., Mo J., Akinyi L., Schmid K., Mondello S., Robertson C.S., Tortella F.C., Hayes R.L., and Wang K.K. (2012). Elevated levels of serum glial fibrillary acidic protein breakdown products in mild and moderate traumatic brain injury are associated with intracranial lesions and neurosurgical intervention. Ann. Emerg. Med. 59, 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duhaime A.C., Gean A.D., Haacke E.M., Hicks R., Wintermark M., Mukherjee P., Brody D., Latour L., and Riedy G.; Common Data Elements Neuroimaging Working Group Members, Pediatric Working Group Members. (2010). Common data elements in radiologic imaging of traumatic brain injury. Arch. Phys. Med. Rehabil. 91, 1661–1666 [DOI] [PubMed] [Google Scholar]
- 13.Whyte J., Vasterling J., and Manley G.T. (2010). Common data elements for research on traumatic brain injury and psychological health: current status and future development. Arch. Phys. Med. Rehabil. 91, 1692–1696 [DOI] [PubMed] [Google Scholar]
- 14.Vickers A.J., and Elkin E.B. (2006). Decision curve analysis: a novel method for evaluating prediction models. Med. Decis. Making 26, 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papa L., Stiell I.G., Clement C.M., Pawlowicz A., Wolfram A., Braga C., Draviam S., and Wells G.A. (2012). Performance of the Canadian CT Head Rule and the New Orleans Criteria for predicting any traumatic intracranial injury on computed tomography in a United States Level I trauma center. Acad. Emerg. Med. 19, 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mondello S., Muller U., Jeromin A., Streeter J., Hayes R.L., and Wang K.K. (2011). Blood-based diagnostics of traumatic brain injuries. Exp. Rev. Mol. Diagn. 11, 65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda M., Tsuruta R., Kaneko T., Kasaoka S., Yagi T., Todani M., Fujita M., Izumi T., and Maekawa T. (2010). Serum glial fibrillary acidic protein is a highly specific biomarker for traumatic brain injury in humans compared with S-100B and neuron-specific enolase. J. Trauma 69, 104–109 [DOI] [PubMed] [Google Scholar]
- 18.Pelinka L.E., Kroepfl A., Leixnering M., Buchinger W., Raabe A., and Redl H. (2004). GFAP versus S100B in serum after traumatic brain injury: relationship to brain damage and outcome. J. Neurotrauma 21, 1553–1561 [DOI] [PubMed] [Google Scholar]
- 19.Mondello S., Papa L., Buki A., Bullock M.R., Czeiter E., Tortella F.C., Wang K.K., and Hayes R.L. (2011). Neuronal and glial markers are differently associated with computed tomography findings and outcome in patients with severe traumatic brain injury: a case control study. Crit. Care 15, R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stiell I.G., Clement C.M., Rowe B.H., Schull M.J., Brison R., Cass D., Eisenhauer M.A., McKnight R.D., Bandiera G., Holroyd B., Lee J.S., Dreyer J., Worthington J.R., Reardon M., Greenberg G., Lesiuk H., MacPhail I., and Wells G.A. (2005). Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA 294, 1511–1518 [DOI] [PubMed] [Google Scholar]
- 21.Smits M., Dippel D.W., Nederkoorn P.J., Dekker H.M., Vos P.E., Kool D.R., van Rijssel D.A., Hofman P.A., Twijnstra A., Tanghe H.L., and Hunink M.G. (2010). Minor head injury: CT-based strategies for management—a cost-effectiveness analysis. Radiology 254, 532–540 [DOI] [PubMed] [Google Scholar]
- 22.Ruan S., Noyes K., and Bazarian J.J. (2009). The economic impact of S-100B as a pre-head CT screening test on emergency department management of adult patients with mild traumatic brain injury. J. Neurotrauma 26, 1655–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunink M.G. (2005). Decision making in the face of uncertainty and resource constraints: examples from trauma imaging. Radiology 235, 375–383 [DOI] [PubMed] [Google Scholar]