Abstract
Background: Congenital hearing loss is one of the most common sensory disorders, with 50–70% of cases attributable to genetic causes. Although recent advances in the identification of deafness genes have resulted in more accurate molecular diagnosis, leading to the better determination of suitable clinical interventions, difficulties remain with regard to clinical applications due to the extreme genetic heterogeneity of deafness. Aim: Toward more effective genetic testing, we adopted Massively Parallel DNA Sequencing (MPS) of target genes using an Ion PGM™ system and an Ion AmpliSeq™ panel to diagnose common mutations responsible for deafness and discover rare causative gene mutations. Before its clinical application, we investigated the accuracy of MPS-based genetic testing. Results: We compared the results of Invader assay-based genetic screening, the accuracy of which has already been verified in previous studies, with those of MPS-based genetic testing for a large population of Japanese deafness patients and revealed that over 99.98% of the results were the same for each genetic testing system. Conclusion: The Ion Personal Genome Machine system had sufficient uniformity and accuracy for application to the clinical diagnosis of common causative mutations and efficiently identified rare causative mutations and/or mutation candidates.
Introduction
Congenital hearing loss is one of the most common sensory disorders. It appears in one of 1000 newborns, with 50–70% of cases attributable to genetic causes (Morton and Nance, 2006). Approximately 100 genes are estimated to be involved in hereditary hearing loss, so there is a great need for effective genetic testing (Hereditary Hearing Homepage; http://webh01.ua.ac.be/hhh/). One-by-one gene screening is, however, time-consuming. By focusing on frequently recurring mutations with ethnic origin that are most likely to be encountered in a clinical setting, we developed the Invader assay-based genetic screening test for 46 mutations in 13 genes, which can identify ∼30–40% of hearing loss patients (Abe et al., 2007; Usami et al., 2012). From 2012, genetic testing for hearing loss patients using the Invader assay has been covered by social health insurance in Japan. To improve the diagnostic rate of this genetic testing, additional genetic analysis for many rare genes was nevertheless required.
Massively Parallel DNA Sequencing (MPS) of target genes offers a useful method of identifying rare causative gene mutations and, thereby, improving the diagnostic rate. In our previous study, MPS analysis using an Ion PGM™ system and Ion AmpliSeq™ for the known 63 deafness-causing genes was able to identify rare gene mutations responsible for hearing loss in patients with cochlea implantation (Miyagawa et al., 2013).
In the current study, we compared the results of Invader assay-based genetic screening with MPS-based genetic testing for a large population of Japanese hearing loss patients to investigate the accuracy of the MPS-based genetic test and its potential clinical application.
Subjects and Methods
Subjects
Three hundred eighty-four Japanese patients with bilateral sensorineural hearing loss from 53 ENT departments nationwide participated in this study. Informed written consent was obtained from all subjects, their next of kin, caretakers, or guardians (in the case of minors) before participation in the project. This study was approved by the Shinshu University Ethics Committee as well as the ethical committees of each of the other participating institutions listed in Acknowledgements.
Genetic analysis
We performed the Invader assay to screen for 46 known pathogenic mutations of 13 genes as a standard genetic test. This was followed by TaqMan genotyping assays for 55 known mutations of six genes and the direct sequencing of the GJB2 gene for all cases. Direct sequencing of the SLC26A4 gene was also performed for patients with enlarged vestibular aqueduct (EVA). We also performed MPS analysis, as described below, for all cases and compared the results obtained from the Invader assay, TaqMan genotyping, and direct sequencing with the MPS results.
Invader assay
We first applied the Invader assay to screen for 46 known mutations of 13 known deafness genes listed previously (Usami et al., 2012). These mutations were selected on the basis of a mutation/gene database established for the Japanese deafness population. The detailed protocol was described elsewhere (Usami et al., 2012).
Direct sequencing
Direct sequencing of the GJB2 gene was performed for all subjects, and the SLC26A4 gene was analyzed for the subjects with EVA and for the patients with heterozygous SLC26A4 mutations identified by the Invader assay. DNA fragments containing the entire coding region and splicing region were amplified and sequenced, as described elsewhere (Tsukada et al., 2010; Miyagawa et al., 2014).
TaqMan genotyping assay
For additional screening, TaqMan genotyping assays for 55 known mutations of six deafness genes were applied for all subjects using a custom TaqMan SNP Genotyping Assay (Applied Biosystems, Life Technologies), TaqMan genotyping master mix (Applied Biosystems, Life Technologies), and a StepOne Plus real-time PCR system (Applied Biosystems, Life Technologies) according to the manufacturer's instructions.
Amplicon library preparation
An Amplicon library of the target exons was prepared with an Ion AmpliSeq Custom Panel (Applied Biosystems, Life Technologies) and designed with an Ion AmpliSeq Designer (http://ampliseq.com) for 63 genes reported to cause nonsyndromic hearing loss (Hereditary Hearing loss Homepage; http://hereditaryhearingloss.org/) using an Ion AmpliSeq Library Kit 2.0 (Applied Biosystems, Life Technologies) and Ion Xpress™ Barcode Adapter 1–96 Kit (Applied Biosystems, Life Technologies) according to the manufacturer's instructions. The detailed protocol was described elsewhere (Miyagawa et al., 2013).
Emulsion PCR and sequencing
The emulsion PCR was performed with the Ion OneTouch™ System and Ion OneTouch 200 Template Kit v2 (Applied Biosystems, Life Technologies) according to the manufacturer's instructions. After the emulsion PCR, template-positive Ion Sphere™ Particles were enriched with the Dynabeads® MyOne™ Streptavidin C1 Beads (Applied Biosystems, Life Technologies) and washed with the Ion OneTouch Wash Solution included in the kit. This process was performed using an Ion OneTouch ES system (Life Technologies).
After the Ion Sphere Particle preparation, MPS was performed with an Ion Torrent Personal Genome Machine (PGM) system using the Ion PGM 200 Sequencing Kit and Ion 318™ Chip (Life Technologies) according to the manufacturer's instructions.
Base call and data analysis
The sequence data were processed with standard Ion Torrent Suite™ Software ver 4.0 and the Torrent Server was used to successively map the human genome sequence (build GRCh37/hg19) with a Torrent Mapping Alignment Program optimized to Ion Torrent™ data. After the sequence mapping, the DNA variant regions were piled up with Torrent Variant Caller plug-in software set to run at high stringency. Selected variant positions were detected with the Hot Spot BED option. In conventional variant detection processes, only the mutation position is called; however, using the Hot Spot BED option, the variant positions specified in the BED file are always genotyped into wild type, heterozygous, or homozygous. After variant detection, variant effects were analyzed using the wANNOVAR website (Wang et al., 2010; Chang and Wang, 2012).
Results
Uniformity of the MPS-based comprehensive mutation screening test
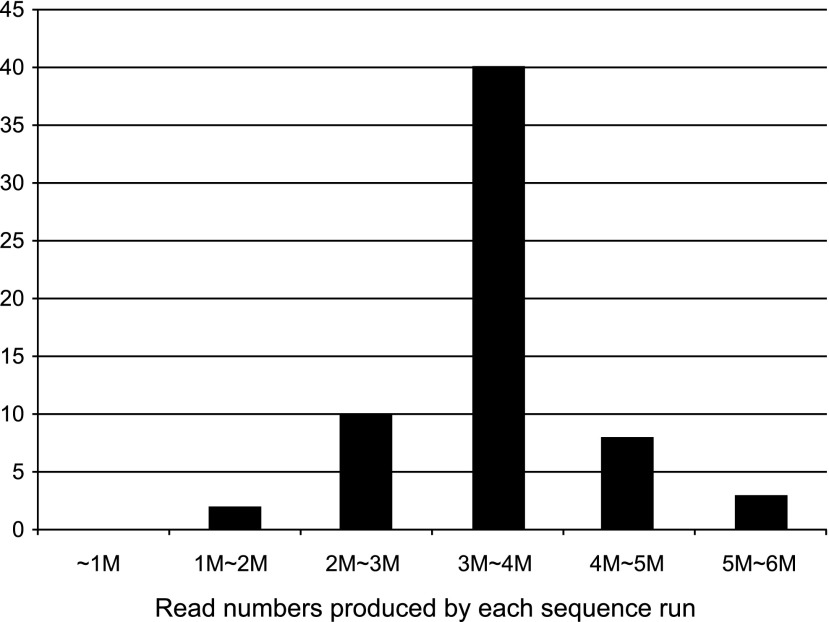
We first analyzed the uniformity of each MPS run and sample. In 64 sequence runs using the Ion torrent PGM sequencer with Ion 318-chips, the mean number (±standard deviation) of reads was 3.56±0.75 M. The distribution of the read numbers produced by each sequence run is shown in Figure 1. The uniformity of the read number for each MPS run was sufficiently high, with 41 of the 64 MPS runs (64%) providing 3–4 M reads. The mean number of sequenced bases of sufficient quality (>Q17) produced by each sequence run was 461±120 M.
FIG. 1.
The distribution of read numbers produced by each sequence run. In the 64 sequence runs, the average read number for each sequence was 3.56 M reads, and 41 massively parallel DNA sequencing (MPS) runs (64%) providing 3–4 M reads.
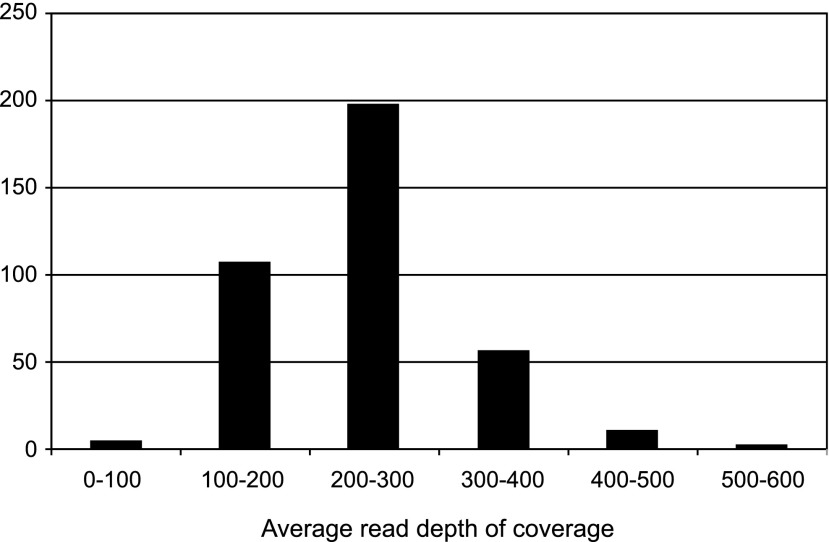
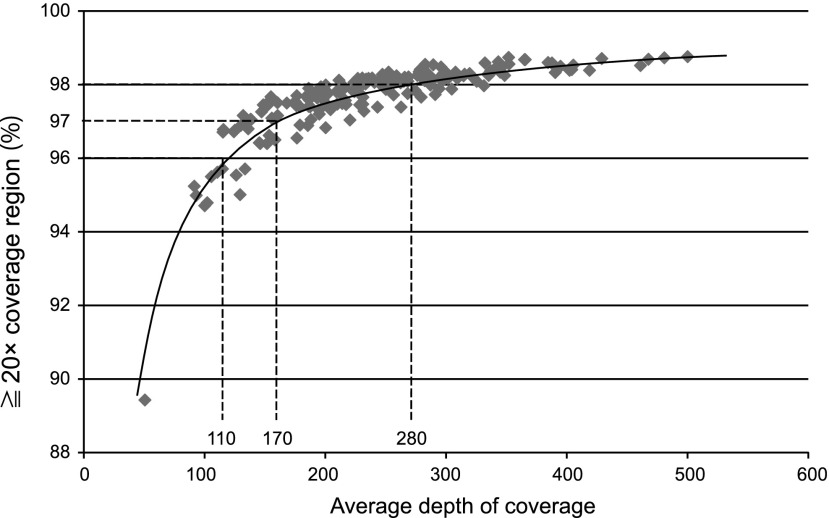
The mean number of reads of the 384 samples analyzed by the 64 sequence runs was 580±168 thousand reads for each sample. The distribution of the average depth of coverage of the target region is shown in Figure 2. The mean depth of coverage of the target region of each of the 384 samples was 241±76×. Among the 384 samples, only five samples (1.3%) showed an average depth of coverage under 100×, with the other 379 samples (98.7%) all over 100×. The distribution of the average depth of coverage of the target region and the percentage of each region with over 20×coverage (indicating the percentage of each region sequenced 20 times or more by MPS) are shown in Figure 3. An average of 97.72±0.90% of each target region was sequenced with over 20×coverage. These data revealed that the MPS-based genetic testing has sufficient uniformity for clinical use. To reduce instances of incorrect genotyping and missed single-nucleotide polymorphism in poor coverage regions, we employed a minimum average depth of coverage of 100 and a minimum percentage of over 20× region coverage of 96%. Among the 384 samples, 14 samples (3.6%) did not fulfill these criteria, so we analyzed these samples again. After reanalysis, all of the samples fulfilled the above criteria.
FIG. 2.
The distribution of the average read depth of coverage of the target regions for the 384 samples. Among the 384 samples, only five samples (1.3%) had a depth of coverage of under 100×, with the other 379 samples (98.7%) showing a depth over 100×.
FIG. 3.
The distribution of the average depth of coverage of the target regions and the percentage of regions with greater than 20× coverage. Diamond shapes indicate the average coverage depth of each sample and the ratio of regions with coverage depth over 20×. The results indicate that sufficient coverage was obtained for 96% of the target region.
Comparison of the Invader assay-based mutation screening and MPS-based comprehensive screening of deafness genes
To investigate the accuracy of the MPS-based comprehensive genetic screening, we compared the results of MPS-based genetic screening with those of Invader assay-based mutation screening and direct sequencing (Table 1).
Table 1.
Comparison of the Invader Assay-Based Mutation Screening and Massively Parallel DNA Sequencing-Based Comprehensive Screening of Deafness Genes
| Mutations | Number of patients with mutations detected by Invader screening (n=384) | Variant alleles detected by Invader screening (n=768) | Variant alleles detected by MPS (n=768) | Variant alleles detected by direct sequencing (n=768) |
|---|---|---|---|---|
| GJB2:NM_004004:c235delC:p.L79fs | 42 (10.9%) | 52 (6.8%) | 52 | 52 |
| GJB2:NM_004004:c.109G>A:p.V37I | 19 (4.9%) | 21 (2.7%) | 21 | 21 |
| GJB2:NM_004004:c.[134G>A; 408C>A]:p.[G45E; Y136X] | 16 (4.2%) | 17 (2.2%) | 18b | 17 |
| GJB2:NM_004004:c.427C>T:p.R143W | 13 (3.4%)a | 13 (1.7%)a | 15b | 14 |
| GJB2:NM_004004:c.176_191del16:p.59_64del | 9 (2.3%) | 10 (1.3%) | 10 | 10 |
| GJB2:NM_004004:c.257C>G:p.T86R | 5 (1.3%) | 6 (0.8%) | 6 | 6 |
| GJB2:NM_004004:c.299_300del:p.100_100del | 6 (1.6%) | 6 (0.8%) | 6 | 6 |
| SLC26A4:NM_000441:c.2168A>G:p.H723R | 15 (3.9%) | 20 (2.6%) | 20 | 20 |
| SLC26A4:NM_000441:c.1229C>T:p.T410M | 4 (1.0%) | 6 (0.8%) | 6 | 6 |
| SLC26A4:NM_000441:c.1174A>T:p.N392Y | 1 (0.3%) | 1 (0.1%) | 1 | 1 |
| SLC26A4:NM_000441:c.367C>T:p.P123S | 1 (0.3%) | 1 (0.1%) | 1 | 1 |
| SLC26A4:NM_000441:c.2162C>T:p.T721M | 1 (0.3%) | 1 (0.1%) | 1 | 1 |
| SLC26A4:NM_000441:c.601-1G>A:Splicing | 1 (0.3%) | 1 (0.1%) | 1 | 1 |
| SLC26A4:NM_000441:c.916dupG:p.I305fs | 1 (0.3%) | 1 (0.1%) | 1 | 1 |
| SLC26A4:NM_000441:c.1648dupT:p.R549fs | 1 (0.3%) | 1 (0.1%) | 1 | 1 |
| SLC26A4:NM_000441:c.919-2A>G:Splicing | 1 (0.3%) | 1 (0.1%) | 0c | 1 |
| CRYM:NM_001888:c.941A>C:p.K314T | 1 (0.3%) | 1 (0.1%) | 1 | 1 |
| Mitochondria 1555A>G | 5 (1.3%) | — | — | — |
| Mitochondria 3243A>G | 8 (2.1%) | — | — | — |
| Mitochondria 8296A>G | 1 (0.3%) | — | — | — |
c.427C>T mutation was not detected by Invader screening in one case (reason unknown).
MPS misgenotyped heterozygous as homozygous mutations in one case each because of the other mutations located in the AmpliSeq primer region (see details in main text).
c.919-2A>G mutation was located in the region not covered by AmpliSeq primers.
MPS, massively parallel DNA sequencing.
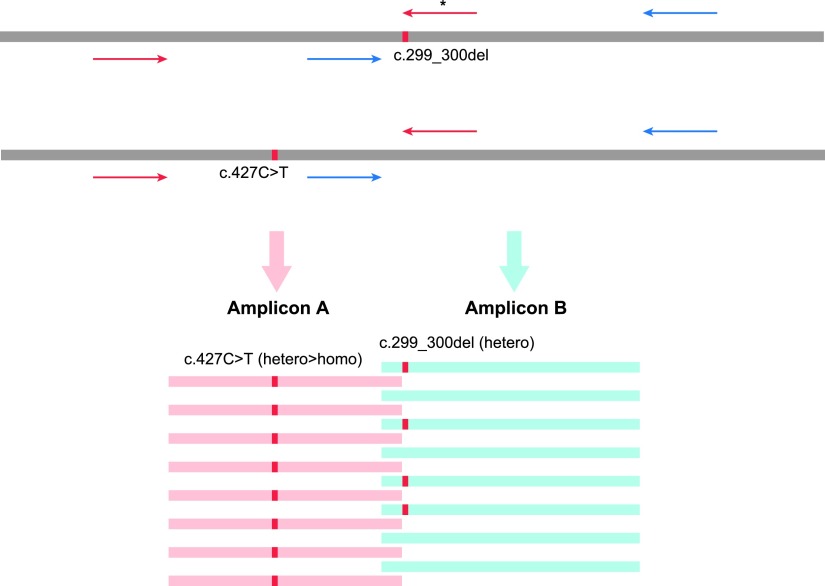
From 384 patients, the Invader assay-based genetic screening detected 174 mutations (Table 1). According to our previous report, about 30% of patients (112 patients) carry one or more mutations, with GJB2 mutations being the most frequent, followed by SLC26A4 and Mitochondrial 1555A>G mutations. Among the invader assay results, one c.427C>T mutation was not detected in one case due to an unknown technical error (Usami et al., 2012). The Invader assay was performed for the 46 variants in 384 samples with only one mutation not detected in the 17,664 SNVs examined, indicating that the accuracy of the Invader assay was over 99.99% (17,663/17,664). In the MPS-based screening, c.919-2A>G mutations of SLC26A4 gene and mitochondrial mutations were not detected because these mutations are located in regions not covered by the AmpliSeq library primers. Misgenotyping of GJB2 c.408C>A and c.427C>T heterozygous mutations as homozygous mutations was also observed in two cases (Table 1). This misgenotyping was caused by combined c.299_300del mutations located at the 3′ end of the AmpliSeq primer (Fig. 4). On the other hand, there were no false-positive results for the target mutations observed in the Invader assay. In this comparison, the MPS covered the 41 variants in the Invader assay in 384 samples, with only two mutations misgenotyped among the 15,744 SNVs, indicating that the accuracy of the MPS-based genetic screening test was 99.98% (15,742/15,744).
FIG. 4.
Heterozygous c.427C>T (p.R143W) mutations were misgenotyped as homozygous by MPS because the c.299_300del mutations were located at the 3′ end of the amplicon. Upper figure indicated the position of c.299_300del, c.427C>T mutations and AmpliSeq primers. c.299_300del mutations were located in 3′ end of PCR primer of Amplicon A marked by asterisk. As a result, all of Amplicon A was produced from the allele with c.427C>T mutation and misgenotyped as a homozygous mutation illustrated in lower figure.
Comparison of the TaqMan genotyping assay-based mutation screening and direct sequencing with the MPS-based comprehensive screening of deafness genes
The TaqMan genotyping assay was performed, with the 58 mutations listed in Table 2 identified from the 384 patients. The c.211delC mutation of the KCNQ4 gene and the c.2229_2301delGAA mutation of the SLC26A4 mutation were not detected by the MPS-based genetic screening as these mutations were located in regions not covered by the AmpliSeq primers. The c.211delC mutation of KCNQ4 was located in a GC-rich region with a GC content of about 80%, and we also found it difficult to detect this mutation by direct sequencing. In addition, CDH23 c.4877A>C heterozygous mutations were not detected by MPS in one case. In this patient, the c.4877A>C mutation region had a depth of coverage of only 7×, which did not meet the filtering threshold of the variant caller software, resulting in a no call status. No false-positive cases were observed among the TaqMan genotyping assay target mutations.
Table 2.
Comparison of the TaqMan Assay-Based Mutation Screening and Massively Parallel DNA Sequencing-Based Comprehensive Screening of Deafness Genes
| Mutations | Number of patients with mutations detected by TaqMan genotyping (n=384) | Variant alleles detected by TaqMan genotyping (n=768) | Variant alleles detected by MPS (n=768) |
|---|---|---|---|
| CDH23:NM_001171930:c.719C>T:p.P240L | 15 (3.9%) | 18 (2.3%) | 18 |
| CDH23:NM_022124:c.4762C>T:p.R1588W | 6 (1.6%) | 6 (0.8%) | 6 |
| CDH23:NM_022124:c.6085C>T:p.R2029W | 4 (1.0%) | 5 (0.7%) | 5 |
| CDH23:NM_022124:c.4249C>T:p.R1417W | 1 (0.3%) | 2 (0.3%) | 2 |
| CDH23:NM_022124:c.5147A>C:p.Q1716P | 2 (0.5%) | 2 (0.3%) | 2 |
| CDH23:NM_022124:c.5627G>A:p.S1876N | 2 (0.5%) | 2 (0.3%) | 2 |
| CDH23:NM_022124:c.5722G>A:p.V1908I | 2 (0.5%) | 2 (0.3%) | 2 |
| CDH23:NM_022124:c.4877A>C:p.D1626A | 1 (0.3%) | 1 (0.1%) | 0a |
| CDH23:NM_001171933:c.141T>G:p.N47K | 1 (0.3%) | 1 (0.1%) | 1 |
| CDH23:NM_022124:c.5131G>A:p.V1711I | 1 (0.3%) | 1 (0.1%) | 1 |
| KCNQ4:NM_004700:c.211delC:p.Q71fs | 6 (1.6%) | 6 (0.8%) | 0b |
| MYO15A:NM_016239:c.9478C>T:p.L3160F | 7 (0.9%) | 7 (0.9%) | 7 |
| OTOF:NM_194323:c.3515G>A:p.R1172Q | 2 (0.5%) | 2 (0.3%) | 2 |
| OTOF:NM_194248:c.1422T>A:p.Y474X | 1 (0.3%) | 1 (0.1%) | 1 |
| SLC26A4:NM00441:c.2229_2301delGAA | 1 (0.3%) | 1 (0.1%) | 0b |
| SLC26A4:NM_000441:c.1315G>A:p.G439R | 1 (0.3%) | 1 (0.1%) | 1 |
c.4877A>C mutation did not call by variant calling program (low depth).
These mutations were located in the region not covered by AmpliSeq primers.
Direct sequencing of the GJB2 gene was performed for all patients and that of the SLC26A4 gene for patients with EVA. As a result, a total of 27 mutations not identified by the Invader or TaqMan genotyping assays were detected (Table 3). Direct sequencing did not detect GJB2 c.257C>T or c.511G>A mutations in one case each due to the low signal intensity of these nucleotide positions. Our comparison of results showed that these mutations in the GJB2 gene were identified by MPS. We, therefore, reanalyzed the direct sequencing data and finally confirmed these mutations by direct sequencing. On the other hand, c.107_120del and c.147C>G mutations of the SLC26A4 gene (one case each) were not detected by MPS analysis. These results indicate that the accuracy of the MPS was equivalent to that of direct sequencing.
Table 3.
Comparison of the Direct Sequencing Analysis of the Selected Genes and Massively Parallel DNA Sequencing-Based Comprehensive Screening
| Number of patients with mutations detected by direct sequencing (n=384) | Variant alleles detected by direct sequencing (n=768) | Variant alleles detected by MPS (n=768) | |
|---|---|---|---|
| GJB2:NM_004004:c.95G>A:p.R32H | 2 (0.5%) | 2 (0.3%) | 2 |
| GJB2:NM_004004:c.11G>A:p.G4D | 1 (0.3%) | 1 (0.1%) | 1 |
| GJB2:NM_004004:c.257C>T:p.T86M | 0a | 0a | 1 |
| GJB2:NM_004004:c.511_512insAACG:p.A171fs | 4 (1.0%) | 4 (0.5%) | 4 |
| GJB2NM_004004:c.595T>C:p.S199P | 1 (0.3%) | 1 (0.1%) | 1 |
| GJB2:NM_004004:c.558_559ins46:p.E187_K188delinsEKTVFTVFMIAVSGIX | 2 (0.5%) | 2 (0.3%) | 2 |
| GJB2:NM_004004:c.583A>G:p.M195V | 2 (0.5%) | 2 (0.3%) | 2 |
| GJB2:NM_004004:c.53C>G:p.T18S | 1 (0.3%) | 1 (0.1%) | 1 |
| GJB2:NM_004004:c.379C>T:p.R127C | 1 (0.3%) | 1 (0.1%) | 1 |
| GJB2:NM_004004:c.511G>A:p.A171T | 0a | 0a | 1 |
| GJB2:NM_004004:c.334_335del:p.112_112del | 1 (0.3%) | 1 (0.1%) | 1 |
| GJB2:NM_004004:c.318C>A:p.F106L | 1 (0.3%) | 1 (0.1%) | 1 |
| GJB2:NM_004004:c.637T>A:p.L213M | 1 (0.3%) | 1 (0.1%) | 1 |
| GJB2:NM_004004:c.223C>T:p.R75W | 1 (0.3%) | 1 (0.1%) | 1 |
| SLC26A4:NM_000441:c.945T>A:p.Y315X | 1 (0.3%) | 1 (0.1%) | 1 |
| SLC26A4:NM_000441:c.2123T>C:p.F708S | 1 (0.3%) | 1 (0.1%) | 1 |
| SLC26A4:NM_000441:c.641A>G:p.Y214C | 1 (0.3%) | 1 (0.1%) | 1 |
| SLC26A4:NM_000441:c.863T>A:p.L288X | 2 (0.5%) | 2 (0.3%) | 2 |
| SLC26A4:NM_000441:c.1264-2A>G:Splicing | 1 (0.3%) | 1 (0.1%) | 1 |
| SLC26A4:NM_000441:c.918+1G>A:Splicing | 1 (0.3%) | 1 (0.1%) | 1 |
| SLC26A4:NM_000441:c.107_120del13ins16 | 1 (0.3%) | 1 (0.1%) | 0b |
| SLC26A4:NM_000441:c.147C>G:p.S49R | 1 (0.3%) | 1 (0.1%) | 0b |
These mutations were not detected by direct sequencing in one case each (low signal intensity).
These mutations were not detected by MPS (reason unknown).
Advantage of the MPS-based comprehensive sequencing of deafness genes
The advantage of the MPS-based comprehensive sequencing of deafness genes lay in the improved diagnostic rate. When heterozygous pathogenic mutations are identified as autosomal recessive deafness causative genes by the Invader assay, it is possible that other mutations might exist in the coding region of the same genes, but the Invader assay did not detect these mutations. Among the 384 patients, 36 heterozygous mutations of autosomal recessive deafness genes were detected by the Invader assay (27 GJB2 heterozygous and nine SLC26A4 heterozygous mutations). Among these 36 patients, MPS detected an additional 16 mutations in the same genes, leading to a final diagnosis of compound heterozygous mutations (10 GJB2 and seven SLC26A4 mutations, Table 4). A similar situation was observed for TaqMan genotyping assay target mutations. Among the 384 patients, 34 heterozygous mutations of autosomal recessive deafness genes were detected by TaqMan genotyping assay (24 CDH23, seven MYO15A, two SLC26A4, and one OTOF mutation). Among these 34 patients, MPS detected eight additional mutations in the same genes, leading to a final diagnosis of compound heterozygous mutations (six CDH23, one MYO15A, and one OTOF mutation, Table 4). MPS, therefore, improved the diagnostic rate in 24 cases (6.3%). In addition, MPS-based genetic testing was able to identify previously reported pathogenic mutations, also contributing to an improved diagnostic rate. Among the 384 patients, MPS found 20 previously reported pathogenic mutations not identified in the Invader or TaqMan genotyping assays listed in Table 5. Of course, it was difficult to distinguish whether the variants detected by MPS were really pathogenic or benign, so most of the mutations identified by MPS were considered to be variations of uncertain significance, and further examination is needed to elucidate the pathogenicity of the variants found in this study.
Table 4.
Pathogenic Mutation Candidates Combined with One Known Pathogenic Variant Detected by the Invader Assay or TaqMan Genotyping Assay of the Same Genes
| Gene | Pathogenic mutations detected by Invader assay or TaqMan genotyping assays as heterozygous | MPS detected mutations found in the same gene |
|---|---|---|
| GJB2 | NM_004004:c.235delC:p.L79fs | NM_004004:c.511_512insAACG:p.A171fs |
| GJB2 | NM_004004:c.235delC:p.L79fs | NM_004004:c.511_512insAACG:p.A171fs |
| GJB2 | NM_004004:c.235delC:p.L79fs | NM_004004:c.C257T:p.T86M |
| GJB2 | NM_004004:c.235delC:p.L79fs | NM_004004:c.T595C:p.S199P |
| GJB2 | NM_004004:c.235delC:p.L79fs | NM_004004:c.558_559ins46:p.E187_K188delins |
| GJB2 | NM_004004:c.C427T:p.R143W | NM_004004:c.A583G:p.M195V |
| GJB2 | NM_004004:c.G109A:p.V37I | NM_004004:c.C379T:p.R127C |
| GJB2 | NM_004004:c.C408A:p.Y136X | NM_004004:c.558_559ins46:p.E187_K188delins |
| GJB2 | NM_004004:c.C257G:p.T86R | NM_004004:c.C53G:p.T18S |
| GJB2 | NM_004004:c.176_191del:p.59_64del | NM_004004:c.511_512insAACG:p.A171fs |
| SLC26A4 | NM_000441:c.A2168G:p.H723R | NM_000441:c.A641G:p.Y214C |
| SLC26A4 | NM_000441:c.A2168G:p.H723R | NM_000441:c.T863A:p.L288X |
| SLC26A4 | NM_000441:c.A2168G:p.H723R | NM_000441:c.T863A:p.L288X |
| SLC26A4 | NM_000441:c.A2168G:p.H723R | NM_000441:c.T945A:p.Y315X |
| SLC26A4 | NM_000441:c.A2168G:p.H723R | NM_000441:c.T2123C:p.F708S |
| SLC26A4 | NM_000441:c.C2162T:p.T721M | NM_000441:exon7:c.918+1G>A |
| SLC26A4 | NM_000441:c.C1229T:p.T410M | NM_000441:exon11:c.1264-2A>G) |
| CDH23 | NM_001171930:c.C719T:p.P240L | NM_001171930:c.G1282A:p.D428N |
| CDH23 | NM_001171930:c.C719T:p.P240L | NM_001171933:c.2079_2085del:p.693_695del |
| CDH23 | NM_001171930:c.C719T:p.P240L | NM_001171933:c.2265dupT:p.H755fs |
| CDH23 | NM_001171930:c.C719T:p.P240L | NM_022124:c.G4672A:p.G1558R |
| CDH23 | NM_022124:c.C4762T:p.R1588W | NM_022124:c.G5419A:p.V1807M |
| CDH23 | NM_022124:c.C4762T:p.R1588W | NM_001171933:c.G746A:p.R249H |
| MYO15A | NM_016239:c.C9478T:p.L3160F | NM_016239:c.A9938C:p.H3313P |
| OTOF | NM_194323:c.G3515A:p.R1172Q | NM_194322:c.G1186A:p.G396R |
Table 5.
Previously Reported Pathogenic Variants Detected by Massively Parallel DNA Sequencing, Which Were Not Identified in the Invader and TaqMan Genotyping Assays
| Gene name | Reported pathogenic mutation | Reference | |
|---|---|---|---|
| Autosomal dominant inheritance mutations | |||
| ACTG1 | NM_001199954:c.A353T:p.K118M | Zhu et al. (2003) | |
| ACTG1 | NM_001199954:c.G721A:p.E241K | Morín et al. (2009) | |
| KCNQ4 | NM_004700:c.C546G:p.F182L | Su et al. (2007) | |
| KCNQ4 | NM_004700:c.C546G:p.F182L | Su et al. (2007) | |
| KCNQ4 | NM_004700:c.C546G:p.F182L | Su et al. (2007) | |
| MYH9 | NM_002473:c.G2114A:p.R705H | Dong et al. (2005) | |
| TECTA | NM_005422:c.C5597T:p.T1866M | Sagong et al. (2010) | |
| WFS1 | NM_001145853:c.G1846T:p.A616S | Liu et al. (2005) | |
| WFS1 | NM_001145853:c.G2185A:p.D729N | Domènech et al. (2002) | |
| WFS1 | NM_001145853:c.G2590A:p.E864K | Eiberg et al. (2006) | |
| Gene name | Reported pathogenic mutation | Novel mutation found by MPS | Reference |
|---|---|---|---|
| Autosomal recessive inheritance mutations | |||
| CDH23 | NM_001171930:c.C805T:p.R269W | NM_001171933:c.C2407T:p.R803W | Oshima et al. (2006) |
| MYO7A | NM_000260:c.G635A:p.R212H | NM_000260:c.G3475A:p.G1159S | Weil et al. (1997) |
| MYO15A | NM_016239:c.G6731A:p.G2244E | NM_016239:c.6457delG:p.A2153fs | Nal et al. (2007) |
| SLC26A4 | NM_000441:c.T2228A:p.L743X | NM_000441:c.C1208A:p.A403D | Yuan et al. (2009) |
Among the autosomal recessive causative genes, only the reported pathogenic variants with other mutation candidates in the same genes detected by MPS were listed.
Discussion
In our previous study, MPS analysis of 63 genes known to cause deafness using an Ion PGM system and Ion AmpliSeq was able to identify rare gene mutations responsible for hearing loss in patients with cochlea implantation (Miyagawa et al., 2013).
Before the clinical application of such new diagnostic tools, the uniformity of the results and the reliability/accuracy of the method should be confirmed in a clinical setting, but most of the previous reports regarding MPS focused mainly on the detection of novel gene mutations or rare causative mutations (Rehman et al., 2010; Shearer et al., 2010; Walsh et al., 2010; Brownstein et al., 2011; Lin et al., 2012). In this study, we focused on the uniformity and the accuracy of the MPS-based genetic test in comparison with the results of Invader assay-based genetic screening, TaqMan genotyping assays, and direct sequencing.
With regard to uniformity, most of the samples were sequenced deeply enough for accurate genotyping (average depth of coverage 241×) and the percentage samples with greater than 20×was also sufficient (97.72% of the target region was sequenced with an average depth of coverage of over 20×). Furthermore, only 14 (3.6%) of the 384 samples did not fulfill the minimum coverage (average coverage of over 100×) or minimum depth of coverage (over 96% of the target region must be sequenced at a depth of over 20×) criteria. However, all of these 14 samples could be analyzed by another sequence run to fulfill the minimum criteria. Therefore, all samples could be analyzed by the MPS-based genetic analysis used in this study. One of the advantages of Ion AmpliSeq library preparation is thought to be this high assay success rate. The Ion AmpliSeq library preparation used in this study required only 20 ng DNA samples, and the quality of the DNA samples did not affect the sequence results. This robustness with regard to DNA quality was also found to apply to the MPS analysis of fragmented DNA samples obtained from Formalin-Fixed Paraffin-Embedded (FFPE) samples (Tsongalis et al., 2014).
With regard to the accuracy of MPS-based genetic screening, we confirmed that it was sufficient for clinical diagnosis by comparison of the test results of the MPS-based genetic test to the Invader assay or direct sequencing. Another advantage of this MPS genetic test is thought to be in its potential for the efficient detection of short insertion and deletion mutations such as GJB2 c.176_191del16, c.511_512insAACG, and c.558_559ins46. As the IonPGM sequencer had a longer read length (200 bp for Amplicon resequencing), this might assist the mapping process of the read fragments of such insertion and deletion mutations.
With regard to the improvement in the diagnostic rate, MPS improved the diagnostic rate by 11.5% (MPS identified an additional mutation in the same gene in 24 cases of heterozygous mutations detected by the Invader or TaqMan genotyping assays, and 20 cases of previously reported pathogenic mutations were found by MPS) over those for the Invader assay and TaqMan genotyping assays in the most conservative setting (this improvement did not include any novel mutations without clues identified by the Invader or TaqMan genotyping assays or in previous reports). Of course, various novel candidate causative variants as well as the previously reported variants were found by MPS analysis, but it is difficult to determine the pathogenicity of these mutations. We are now analyzing family samples for such candidate causative mutations and intend to report our results at a later date.
In conclusion, the MPS-based comprehensive mutation screening for deafness genes had high uniformity, high assay success rate, and sufficient accuracy for clinical use. In addition, this screening method affords an improved diagnostic rate among hearing loss patients. This genetic analysis system is expected to facilitate more precise clinical genetic diagnosis, appropriate genetic counseling, and proper medical management for auditory disorders.
Acknowledgments
The authors thank the participants of the Deafness Gene Study Consortium: Drs. Noriko Ogasawara and Tetsuo Himi (Sapporo Medical University), Drs. Teruyuki Sato and Kazuo Ishikawa (Akita University), Drs. Yumiko Kobayashi and Hiroaki Sato (Iwate Medical University), Drs. Tetsuaki Kawase and Toshimitsu Kobayashi (Tohoku University), Dr. Kenji Ohyama (Tohoku Rosai Hospital), Drs. Tomoo Watanabe, Tsukasa Ito, and Seiji Kakigi (Yamagata University), Drs. Hiroshi Ogawa and Koichi Omori (Fukushima Medical University), Drs. Kenichi Nakamura and Keiichi Ichimura (Jichi Medical University), Drs. Takaaki Murata, Kyoko Nagai, and Ichiro Chikamatu (Gunma University), Drs. Misato Kasai and Katsuhisa Ikeda (Jyuntendo University), Drs. Masahiro Takahashi and Naoko Sakuma (Yokohama City University), Dr. Hideaki Sakata (Mejiro University), Dr. Kotaro Ishikawa (National Rehabilitation Center), Drs. Shuntaro Shigihara, Yasuyuki Nomura, and Minoru Ikeda (Nihon University School), Drs. Tetsuo Ikezono (Saitama Medical University), Drs. Nobuhiro Nishiyama and Mamoru Suzuki (Tokyo Medical University), Drs. Hiromi Kojima and Yuika Sakurai (Jikei University), Dr. Satoko Abe (Abe ENT clinic), Dr. Kozo Kumakawa (Toranomon Hospital), Drs. Hajime Sano and Makito Okamoto (Kitasato University), Drs. Tatuo Matunaga and Kimitaka Kaga (Tokyo Medical Center Institute of Sensory Organs), Dr. Satoshi Iwasaki (International University Health and Welfare Mita Hospital), Drs. Akihiro Shinnabe and Yukiko Iino (Jichi University Saitama Medical Center), Drs. Tomoko Esaki and Taku Hattori (Aichi Children's Health Medical Center), Dr. Eisuke Sato (Chubu Rosai Hospital), Dr. Sawako Masuda (Mie Hospital), Drs. Mirei Taniguchi, Shinichiro Kitajiri, and Juichi Itoh (Kyoto University), Drs. Hirofumi Sakaguchi and Yasuo Hisa (Kyoto Prefectural University), Dr. Kazuhiko Takeuchi (Mie University), Dr. Masako Nakai, Rie Horie (Shiga Medical Center for Children), Drs. Jun Nakayama and Takeshi Shimizu (Shiga Medical University), Drs. Yumi Ohta and Hidenori Inohara (Osaka University), Drs. Masaya Konishi and Kouichi Tomoda (Kansai Medical University), Drs. Daisuke Yamashita and Kenichi Nibu (Kobe University), Dr. Hiroshi Nishimura (Osaka Medical Center and Research Institute for Maternal and Children Health), Drs. Yuko Saito and Masafumi Sakagami (Hyogo College of Medicine), Dr. Yasushi Naito (Kobe City Medical Center General Hospital), Drs. Keiji Fujihara, Akihiro Sakai, and Noboru Yamanaka (Wakayama Medical University), Drs. Taisuke Kobayahi and Masamitsu Hyodo (Kouchi University), Drs. Takeshi Ishino and Katsuhiro Hirakawa (Hiroshima University), Dr. Ikuo Inokuchi (Hiroshima City Hiroshima Citizen Hospital), Drs. Kazuma Sugahara and Hiroshi Yamashita (Yamaguchi University), Dr. Naoto Hato (Ehime University), Drs. Chie Oshikawa and Shizuo Komune (Kyushu University), Drs. Mayumi Sugamura and Takashi Nakagawa (Fukuoka University), Drs. Yoshihisa Ueda and Tadashi Nakashima (Kurume University), Dr. Haruo Takahashi (Nagasaki University), Dr. Yukihiko Kanda (Kanda ENT Clinic), Drs. Keiji Matsuda and Tetsuya Tono (Miyazaki Medical College), Drs. Ikuyo Miyanohara and Yuichi Kurono (Kagoshima University), and Drs. Akira Ganaha and Mikio Suzuki (Ryukyus University), for providing samples from their patients.
Author Disclosure Statement
This study was supported by a Health and Labour Sciences Research Grant for Comprehensive Research on Disability Health and Welfare from the Ministry of Health, Labour and Welfare of Japan (http://mhlw.go.jp/english/) (S.-I.U.) and by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (http://mext.go.jp/english/) (S.-I.U.). This study was also supported by Life Technologies Japan Ltd. as collaborative study (S.-I.U.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
References
- Abe S, Yamaguchi T, Usami S. (2007) Application of deafness diagnostic screening panel based on deafness mutation/gene database using invader assay. Genet Test 11:333–340 [DOI] [PubMed] [Google Scholar]
- Brownstein Z, Friedman LM, Shahin H, et al. (2011) Targeted genomic capture and massively parallel sequencing to identify genes for hereditary hearing loss in middle eastern families. Genome Biol 12:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Wang K. (2012) wANNOVAR: annotating genetic variants for personal genomes via the web. J Med Genet 49:433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domènech E, Gómez-Zaera M, Nunes V. (2002) WFS1 mutations in Spanish patients with diabetes mellitus and deafness. Eur J Hum Genet 10:421–426 [DOI] [PubMed] [Google Scholar]
- Dong F, Li S, Pujol-Moix N, et al. (2005) Genotype-phenotype correlation in MYH9-related thrombocytopenia. Br J Haematol 130:620–627 [DOI] [PubMed] [Google Scholar]
- Eiberg H, Hansen L, Kjer B, et al. (2006) Autosomal dominant optic atrophy associated with hearing impairment and impaired glucose regulation caused by a missense mutation in the WFS1 gene. J Med Genet 43:435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X, Tang W, Ahmad S, et al. (2012) Applications of targeted gene capture and next-generation sequencing technologies in studies of human deafness and other genetic disabilities. Hear Res 288:67–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YH, Ke XM, Xiao SF. (2005) Heterogeneous mutations of Wolfram syndrome I gene responsible for low frequency nonsyndromic hearing loss. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 40:764–768 [article in Chinese] [PubMed] [Google Scholar]
- Miyagawa M, Nishio SY, Ikeda T, et al. (2013) Massively parallel DNA sequencing successfully identifies new causative mutations in deafness genes in patients with cochlear implantation and EAS. PLoS One 8:e75793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa M, Nishio SY, Usami S, Deafness Gene Study Consortium (2014) Mutation spectrum and genotype-phenotype correlation of hearing loss patients caused by SLC26A4 mutations in the Japanese: a large cohort study. J Hum Genet 59:262–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morín M, Bryan KE, Mayo-Merino F, et al. (2009) In vivo and in vitro effects of two novel gamma-actin (ACTG1) mutations that cause DFNA20/26 hearing impairment. Hum Mol Genet 18: 3075–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton CC, Nance WE. (2006) Newborn hearing screening: a silent revolution. N Engl J Med 354:2151–2164 [DOI] [PubMed] [Google Scholar]
- Nal N, Ahmed ZM, Erkal E, et al. (2007) Mutational spectrum of MYO15A: the large N-terminal extension of myosin XVA is required for hearing. Hum Mutat 28:1014–1019 [DOI] [PubMed] [Google Scholar]
- Oshima A, Jaijo T, Aller E, et al. (2006) Mutation profile of the CDH23 gene in 56 probands with Usher syndrome type I. Hum Mutat 29:E37–E46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman AU, Morell RJ, Belyantseva IA, et al. (2010) Targeted capture and next-generation sequencing identifies C9orf75, encoding taperin, as the mutated gene in nonsyndromic deafness DFNB79. Am J Hum Genet 86:378–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagong B, Park R, Kim YH, et al. (2010) Two novel missense mutations in the TECTA gene in Korean families with autosomal dominant nonsyndromic hearing loss. Ann Clin Lab Sci 40:380–385 [PubMed] [Google Scholar]
- Shearer AE, DeLuca AP, Hildebrand MS, et al. (2010) Comprehensive genetic testing for hereditary hearing loss using massively parallel sequencing. Proc Natl Acad Sci U S A 107:21104–21109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su CC, Yang JJ, Shieh JC, et al. (2007) Identification of novel mutations in the KCNQ4 gene of patients with nonsyndromic deafness from Taiwan. Audiol Neurootol 12:20–26 [DOI] [PubMed] [Google Scholar]
- Tsongalis GJ, Peterson JD, de Abreu FB, et al. (2014) Routine use of the Ion Torrent AmpliSeq™ Cancer Hotspot Panel for identification of clinically actionable somatic mutations. Clin Chem Lab Med 52:707–714 [DOI] [PubMed] [Google Scholar]
- Tsukada K, Nishio S, Usami S, Deafness Gene Study Consortium (2010) A large cohort study of GJB2 mutations in Japanese hearing loss patients. Clin Genet 78:464–470 [DOI] [PubMed] [Google Scholar]
- Usami S, Nishio SY, Nagano M, et al. (2012) Simultaneous screening of multiple mutations by invader assay improves molecular diagnosis of hereditary hearing loss: a multicenter study. PLoS One 7:e31276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, Shahin H, Elkan-Miller T, et al. (2010) Whole exome sequencing and homozygosity mapping identify mutation in the cell polarity protein GPSM2 as the cause of nonsyndromic hearing loss DFNB82. Am J Hum Genet 87:90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H. (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil D, Küssel P, Blanchard S, et al. (1997) The autosomal recessive isolated deafness, DFNB2, and the Usher 1B syndrome are allelic defects of the myosin-VIIA gene. Nat Genet 16:191–193 [DOI] [PubMed] [Google Scholar]
- Yuan YY, Dai P, Zhu QW, et al. (2009) Sequencing analysis of whole SLC26A4 gene related to IVS7-2A>G mutation in 1552 moderate to profound sensorineural hearing loss patients in China. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 44:449–454 [article in Chinese] [PubMed] [Google Scholar]
- Zhu M, Yang T, Wei S, et al. (2003) Mutations in the gamma-actin gene (ACTG1) are associated with dominant progressive deafness (DFNA20/26). Am J Hum Genet 73:1082–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]