Abstract
Background: Klebsiella pneumoniae is an important cause of nosocomial infections, but its role in severe acute pancreatitis (SAP) is not well defined. Few cases of K. pneumoniae associated SAP have been reported. Due to the emergence of extended-spectrum beta-lactamases (ESBLs) and carbapenemases, treatment of multidrug-resistant (MDR) K. pneumoniae presents a challenge. Tigecycline and colistin have gained recent attention for their broad-spectrum antimicrobial activity.
Methods: We describe a case of SAP due to K. pneumoniae bearing K. pneumoniae carbapenemase (KPC) treated successfully with colistin plus tigecycline and offer a review of similar experiences published in the literature.
Results: The case reported herein required surgical drainage of multiple pancreatic abscesses and treatment with tigecycline and colistin. Our comparative analysis revealed a number of unique features associated with SAP due to K. pneumoniae: 1) underlying pancreatic injury, 2) multiple drug resistance determinants and virulence factors that complicate treatment, and 3) surgical debridement as a requirement for cure.
Conclusion: As the prevalence of K. pneumoniae bearing KPC continues to increase in the healthcare setting, SAP caused by this MDR pathogen will become more common. Tigecycline plus colistin was a successful antibiotic regimen for the treatment of SAP due to K. pneumoniae bearing KPC.
The emergence of antibiotic resistance in Enterobacteriaceae mediated by extended-spectrum β-lactamases (ESBLs), first recognized in the 1980s and more common after the 1990s, led to the establishment of carbapenems for the treatment of serious infections caused by these organisms. However, during the past decade, carbapenem resistance has emerged in Enterobacteriaceae. For instance, the retrospective analysis of approximately one-half million isolates from almost three hundred clinical laboratories throughout the United States revealed that the proportion of carbapenem-resistant Klebsiella pneumoniae increased from less than 0.1% in 2002 to 4.5% in 2010 [1]. Klebsiella pneumoniae carbapenemase (KPC) is the most common mechanism of carbapenem resistance in the United States, and clonally related strains carrying blaKPC are identified throughout the world [2]. The evolution and spread of KPC is a worrisome harbinger of increased mortality and longer-duration and higher-cost hospitalizations, and underscores the exhaustion of our current antimicrobial armamentarium [3].
Necrotizing pancreatitis is complicated frequently with infection caused by gram-negative bacteria. However, KPC-producing K. pneumoniae infection has been associated only rarely with necrotizing pancreatitis [4,5]. Acute pancreatitis results in gland necrosis in 10–20% of patients and is associated with mortality rates of 10–25%. Secondary bacterial infection of necrotizing pancreatitis confers even higher mortality (40–70%), emphasizing the need for early effective antibiotic therapy and appropriate surgical debridement [6,7]. Although the benefit of antibiotic prophylaxis in necrotizing pancreatitis is dubious, it remains common practice to implement empiric carbapenem therapy (imipenem-cilastatin or meropenem) until culture results are available from fine-needle aspiration biopsy or open debridement of the pancreas [8]. Regrettably, the emergence of multi-drug-resistant (MDR) organisms has become an impediment to effective empiric antibiotic therapy in this syndrome.
Here we describe a case of necrotizing pancreatitis infected with KPC-producing K. pneumoniae associated with failure of antibiotic therapy with imipenem-cilastatin. We compare it to other reported cases in the literature, and introduce the use of colistin and tigecycline as a useful tactic in patients with infected necrotizing pancreatitis who fail empiric antibiotic treatment due to the presence of MDR organisms.
Case Report
A 79-year-old Caucasian male with a history of coronary artery disease who had undergone four-vessel coronary artery bypass grafting, with a left ventricular ejection fraction of 60%, hypertension, moderate aortic stenosis, obstructive sleep apnea, and prostate cancer, presented to the hospital with abdominal pain. He was diagnosed with severe acute pancreatitis (SAP) secondary to gallstones and was managed with intravenous fluids and opiods for pain control. His early hospital course was complicated by respiratory distress requiring intensive care management, and ileus requiring nasogastrictube placement and total parenteral nutrition (TPN). On day 18 of hospitalization, he was transferred to a long-term acute care facility for rehabilitation while still on TPN. On day 40, he was readmitted for worsening abdominal pain and found to have leukocytosis (18,000 white blood cell count). Computed-tomography (CT) of the abdomen and pelvis revealed formation of two pancreatic pseudocysts in the mid-abdomen and inferior to the splenic flexure of the colon. Due to concern for infection, piperacillin-tazobactam was initiated. Leukocytosis initially improved to 12,000 white blood cell count; however, on day 51, the patient developed fever and tachycardia with worsening abdominal pain and leukocytosis to 29,000×109cells/L. A repeat CT of the abdomen (Fig. 1A) showed a 10×19 cm pancreatic pseudocyst and loculated fluid collections inferior to the splenic flexure (9.3×4.2 and 8×4.2 cm). The patient was transferred to the medical intensive care unit (MICU) where a central venous catheter was placed for aggressive fluid resuscitation; imipenem-cilastatin was started while piperacillin-tazobactam was discontinued. On day 52, he underwent CT-guided drainage of the pancreatic pseudocysts; cultures were sterile. He was stabilized and transferred to the medicine ward on day 57; due to persistence of the mid-abdominal pseudocyst, a retroperitoneal drain was left in place. On day 61, the patient again developed leukocytosis to 15,000×109cells/L, although transient; culture of a sample obtained on that day from the proximal aspect of the retroperitoneal drain grew K. pneumoniae resistant to all beta-lactams, including carbapenems, and to trimethoprim-sulfamethoxazole, fluoroquinolones, and amikacin. The isolate was only susceptible to tigecycline (minimum inhibitory concentration [MIC]=2 mcg/mL), colistin (MIC=2 mcg/mL), and gentamicin (MIC=2 mcg/mL). No additional bacterial isolates were identified. Fever and leukocytosis recurred on day 68, and treatment was initiated with tigecycline (100 mg IV initially, then 50 mg IV q 12 h) and colistin (75 mg IV q 12 h); carbapenem-resistant K. pneumoniae was isolated again from samples of abdominal fluid obtained from the retroperitoneal drain and CT imaging demonstrated enlargement of the pancreatic pseudocyst with formation of gas bubbles (Fig. 1B). This prompted surgical debridement on day 70; behind the stomach, a collection of purulent fluid was drained revealing a necrotic mass (Fig. 2). Cultures obtained from surgical specimens also grew carbapenem-resistant K. pneumoniae. Polymerase chain reaction (PCR) detected the presence of blaKPC in these MDR K. pneumoniae isolates, and DNA sequencing of the amplicon identified it further as blaKPC-3.
FIG. 1.
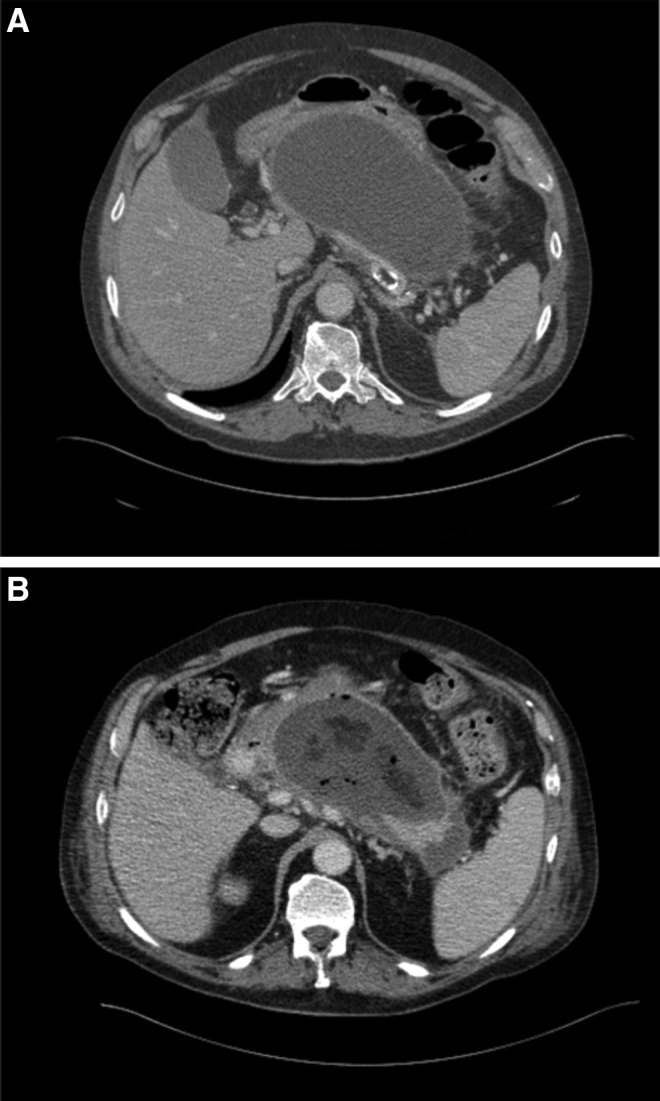
(A) Computed tomography of abdomen on day 50 after presentation showing a large cystic lesion replacing most of the pancreas and measuring approximately 19×10 cm in diameter. (B) Computed tomography of abdomen obtained on day 69 revealing fluid collection with presence of gas bubbles, suggesting infection.
FIG. 2.
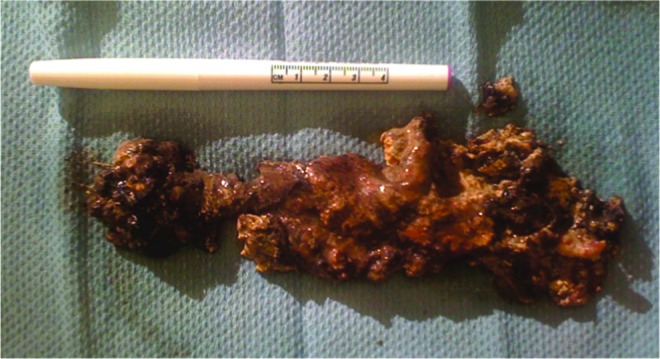
Necrotic pancreas removed surgically from our patient on day 70. Color images available online at www.liebertpub.com/sur
Discussion
The rate of infectious complications in SAP is 40–70% in the first three weeks of disease. Most cases are due to a bacterial species found frequently in the gastrointestinal tract including Escherichia coli, Pseudomonas aeruginosa, Enterococcus spp., and Staphylococcus aureus [9]. Bacterial infection is a late-stage complication of necrotizing pancreatitis that occurs generally after the second week of an acute episode, and is belived to be secondary to bacterial translocation from the small bowel and colon [6,7]. Infection of the necrotic pancreas with enteric organisms is the most common cause of death in patients with SAP. Mortality rates average 30% (range 14–62%), triple the rate of death in sterile SAP [8]. These complications are likely to increase further in patients infected with MDR bacteria, such as KPC-bearing K. pneumoniae, and in the presence of increasing age, mechanical ventilation, malignant disease, cardiac disease, and intensive care. Whereas the use of antimicrobial prophylaxis in SAP is debated, timely diagnosis of bacterial infection and identification of the infecting organism is necessary for targeted therapy [7]. Few reported cases of K. pneumoniae-infected pancreatitis are documented in the literature. We were prompted to perform a case review of the published literature to identify patients with K. pneumoniae-infected pancreatitis [5,10–12] (Table 1).
Table 1.
Review of Characteristics of Five Cases of Klebsiella pneumoniae-Associated Pancreatitis
| Case | Reference | Age | Sex | Comorbidities | Presentation | CT imaging | Diagnosis | Antibiotics used | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Chong [11] | 72 | Male | Poorly controlled type 2 diabetes mellitus, dyslipidemia, depression | Two months of abdominal pain, distension, anorexia, weight loss | Two-centimeter mass at pancreatic head | K. pneumoniae cultured from percutaneous aspiration biopsy | Imipenem-cilastatin | Clinical improvement |
| 2 | Orzechowska [12] | 49 | Male | Chronic alcoholism | Impaired consciousness, rigors and fever two weeks following hospitalization with fever, anorexia and abdominal pain | Pancreatic pseudocyst | K. pneumoniae (K2) cultured from blood, post-mortem pancreatic pseudocyst and meninges | Amoxicillin-clavulanic acid, metronidazole, gentamicin, followed by piperacillin-tazobactam | Death |
| 3 | Bhasin [10] | 35 | Male | Chronic alcoholism, chronic calcific pancreatitis, complete pancreas divisum | Abdominal pain and fever | Communicating intrasplenic pancreatic abscess | K. pneumoniae cultured from percutaneous aspiration biopsy | Cefotaxime and amikacin | Clinical improvement |
| 4 | Di Carlo [5] | 50 | Female | Chronic alcoholism, chronic pancreatitis, tobacco dependence | Fever and leukocytosis four months after hospitalization with abdominal pain anorexia, chills, abdominal distension and weight loss | Mass of pancreatic head | K. pneumoniae belonging to sequence type 258 and harboring blaKPC-3 cultured from percutaneous aspiration biopsy | Tigecycline and colistin | Clinical improvement |
| 5 | This article | 79 | Male | Gallstone pancreatitis, prostate cancer, coronary artery disease with coronary artery bypass grafts, hypertension, aortic stenosis | Abdominal pain | Two pancreatic pseudocysts | K. pneumoniae harboring blaKPC-3 cultured from pseudocyst drainage and surgical specimen two months after admission | Tigecycline and colistin | Clinical improvement |
Review of these cases suggests that pancreatic injury contributes to infection with K. pneumoniae. One of the features of the observed cases of K. pneumoniae pancreatitis is the prolonged duration of illness; in this patient series we observed an illness lasting greater than two weeks. Patient 1 reported two months of abdominal pain before his presentation and diagnosis of K. pneumoniae-infected pancreatitis. Patients 2 and 4 were hospitalized with pancreatitis for two weeks and four months, respectively, before diagnosis of K. pneumoniae-infected pancreatitis. Our patient had been hospitalized for 61 days at the time that K. pneumoniae was isolated from a pancreatic pseudocyst. In addition to acute injury, chronic pancreatic injury contributes to bacterial infection. Our search revealed three patients who suffered from chronic alcoholism; two of these patients (patients 3 and 4) had a history of chronic pancreatitis, one of whom (patient 4) had radiographic evidence thereof. This suggests that loss of pancreatic parenchymal integrity facilitates infection with K. pneumoniae, consistent with previous observations of secondary bacterial infection of the necrotic pancreas.
All of these patients, including our patient (Fig. 1), demonstrated CT evidence of pancreatic mass(es), which drained purulent material on aspiration biopsy. The degree of necrosis correlates with the risk for bacterial infection; >50% necrosis of the pancreas is associated with an eight-fold increase in infection rate compared to less severe involvement [13]. The CT severity index created by Balthazar et al. combines the percentage of necrosis with a grade of its severity to predict mortality and morbidity; these scores are comparable to the Ranson criteria and the APACHE II score [7,14]. As expected, survival is best when the focus of infection is removed via catheter-guided drainage (in selected cases), or surgical debridement and open drainage. In the absence of drainage, the mortality of patients with infected pancreatic necrosis approaches 100% [13]. Before surgical drainage, empiric antibiotics are employed to treat the expected pathogen. Antimicrobial agents including carbapenems, fluoroquinolones, and nitroimidazoles are used often. Although their use for prophylaxis is not of benefit, empiric therapy is appropriate for patients who have a clinical picture of infection suggested by fever, leukocytosis, and hemodynamic instability [6].
Multi-drug resistant strains of K. pneumoniae often carry plasmids that confer resistance to multiple classes of antibiotics, including cephalosporins, aminoglycosides, tetracyclines and trimethoprim-sulfamethaxazole. Interestingly, independent of ESBL-containing plasmids, resistance to beta-lactam/beta-lactamase inhibitor combinations and extended-spectrum cephalosporins has also been described. Fluoroquinolone resistance is well established with a 10–20% overall prevalence, and 50% in ESBL-containing strains [15]. Whereas carbapenems have been the agents of choice for ESBL-producing organisms, K. pneumoniae resistant to carbapenems has unfortunately emerged, mediated frequently by the presence of blaKPC, a widespread carbapenemase. Patient 4 was infected with KPC carrying the blaKPC-3 gene, therefore necessitating treatment with alternate classes of antimicrobials. As was our patient, she was treated with colistin and tigecycline.
The spread of MDR organisms is a formidable threat for patients and emphasizes the need for new antimicrobial agents. As a result of this peril, tigecycline and colistin have gained attention for their antimicrobial activity against MDR organisms. Shortly before Di Carlo et al. first described the treatment of K. pneumoniae pancreatitis with tigecycline and colistin, we treated our patient successfully with this regimen [5]. Further experience in intra-abdominal infections, and recent studies of bacteremia due to K. pneumonia containing blaKPC, also demonstrated successful therapy with a combination of colistin and tigecycline [3,16–18]
Tigecycline is a bacteriostatic antimicrobial agent that interferes with protein synthesis by binding to the 30S ribosomal subunit. Tigecycline is excreted mainly in feces via bile, which may have been a unique advantage in this case. However, little tigecycline, is excreted in the urine. The pharmocokinetics and pharmacodynamics (PK/PD) of this antibiotic have been evaluated mainly in complicated skin and skin structure infections (cSSSI) and complicated intra-abdominal infections (cIAI) in both the community and nosocomial setting; these studies demonstrate that tigecycline has a large volume of distribution, a long elimination half-life, and a low total clearance. It is recognized that tigecycline elimination is greater in males and correlates with weight and creatinine clearance; overweight patients and patients with renal dysfunction have reduced tigecycline clearance. Despite differences in drug clearance, the modest variations do not compel adjustment in drug dosing [19–22].
Presently, clinicians are concerned with reports of a higher risk of death among patients receiving tigecycline compared to other antibacterial drugs [23]. Based on those reports, the U.S. Food and Drug Administration issued a new “black box” warning recommending restriction of tigecycline to situations where suitable alternative treatments are not available [24]. Yet therapy with this drug may still be the only option in cases of MDR infections. Furthermore, in some reports of serious blood stream infection, regimens containing tigecycline have proved superior to other regimens; as mentioned, tigecycline may play an important role in the treatment of carbapenem-resistant K. pneumoniae as part of combination regimens including colistin and carbapenems [3,17,18].
Why would tigecycline be effective in our patient? In cSSSI, an area-under-the-curve:mean inhibitory concentration ratio (AUC:MIC) of 20–25 predicts 0.95 probability of cure. In cIAI, an AUC:MIC of 20–25 anticipates 0.90 probability of cure. Recent analysis of two randomized, controlled trials by Rubino et al. assessed the PK/PD of tigecycline in community-acquired pneumonia and found that fAUC20–24:MIC>12.8 is associated with faster resolution of infection. Whereas area-under-the-curve and mean inhibitory concentrations ratios correlate well with microbial eradication, surgery is central to the management of cIAI [19–21]. Likely, we were able to achieve these important pharmacologic parameters in our patient due to the dosing we employed and penetration of tigecycline into abdominal and pancreatic tissues [21]. Of note, nausea and vomiting occur commonly in patients treated with tigecycline, approximately 40% and 20%, respectively, which may complicate the treatment of pancreatitis where these symptoms are prominent. Less common, but equally relevant, are reports of tigecycline-induced acute pancreatitis, typically occurring during the second or third week of therapy [25].
We also added colistin methanesulfonate (CMS) to our patient's antibiotic regimen; CMS, a polymyxin antibiotic, is administered as an inactive prodrug that is converted in vivo to the active form. Colistin methanesulfonate is used in the treatment of infections caused by MDR gram-negative bacteria including Pseudomonas aeruginosa, Acinetobacter baumannii, and K. pneumoniae, especially when carbapenems are not an option. A bactericidal antimicrobial agent, colistin acts as a “surface detergent” that damages cell membranes, promoting the leakage of cellular proteins and cell death. The conversion of CMS to colistin is relatively inefficient and much of the inactive prodrug is eliminated in urine; it is notable that <1% of colistin is excreted renally owing to tubular reabsorption. Nephrotoxicity is likely mediated by the complex handling of both forms of the drug in renal tubules. Neurotoxicity, although rare, has also been associated with its use [26]. We took advantage of colistin's unique PK/PD to dose it so that time (T)>MIC was achieved to further enhance the efficacy of tigecycline by allowing even greater bacterial penetration thereby avoiding the emergence of resistance to colistin. Importantly, in critically ill patients, renal insufficiency affects steady-state concentrations, making alterations in dosing important to achieve therapeutic success with minimal side effects.
The combination of a bactericidal cell wall active agent, such as colistin, which enhances the effect of a bacteriostatic drug that interferes with protein synthesis, such as tigecycline, may have been advantageous in the treatment of our patient. Although we do not advocate the empiric use of colistin and tigecycline in all cases of infected pancreatic necrosis, the judicious use of this regimen in patients with documented MDR infection may prove beneficial. As stated above, patients at risk for toxicity, particularly with colistin, should be monitored carefully or alternative regimens should be considered.
In summary, our patient illustrates the risk factors that contribute to the development of K. pneumoniae bearing blaKPC-3, including exposure to multiple antibiotics, intensive care stay, indwelling catheter placement (bladder catheter and central venous catheter), prolonged hospitalization, and poor functional status. Increasing rates of KPC-producing K. pneumoniae in North America and its potential for horizontal transmission should lead clinicians to consider the presence of this pathogen in patients with severe intra-abdominal infection, especially in the intensive care setting. Infection control procedures are imperative to prevent further nosocomial spread. Despite concerns about increased mortality with tigecycline and the toxicity of colistin, in intra-abdominal infections where surgical drainage and adequate tissue concentrations of antibiotics are essential, this combination may offer an advantage when other therapies are not effective.
Conclusion
Far less common than infection with other gastrointestinal pathogens, pancreatic infection with K. pneumoniae is indeed a cause of substantial morbidity. Identification of patients at particular risk for infection with K. pneumoniae bearing KPC is imperative for the timely initiation of appropriate antibiotic therapy coupled with adequate surgical drainage. In the case of severe acute pancreatitis, we describe successful treatment with drainage, tigecycline, and colistin. The use of both tigecycline and colistin appears to be an important “last line” choice of therapy for SAP and may be considered further in the future.
Acknowledgments
Research reported in this publication was supported by funds and facilities provided by the Cleveland Department of Veterans Affairs, the Veterans Affairs Merit Review Program Award 1I01BX001974 and the Geriatric Research Education and Clinical Center VISN 10, and by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers RO1AI000560, RO1AI63517 (to RAB) and UM1A104681. FP is a Louis Stokes Scholar at Case Western Reserve University and is supported through the Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research.
Author Disclosure Statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Veterans Affairs.
References
- 1.Braykov NP, Eber MR, Klein EY, et al. Trends in resistance to carbapenems and third-generation cephalosporins among clinical isolates of Klebsiella pneumoniae in the United States, 1999–2010. Infect Control Hosp Epidemiol 2013;34:259–268 [DOI] [PubMed] [Google Scholar]
- 2.Munoz-Price LS, Poirel L, Bonomo RA, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13:785–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tzouvelekis LS, Markogiannakis A, Psichogiou M, et al. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: An evolving crisis of global dimensions. Clin Microbiol Rev 2012;25:682–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dionigi R, Rovera F, Dionigi G, et al. Infected pancreatic necrosis. Surg Infect (Larchmt). 2006;7Suppl 2:S49–52 [DOI] [PubMed] [Google Scholar]
- 5.Di Carlo P, Pantuso G, Cusimano A, et al. Two cases of monomicrobial intraabdominal abscesses due to KPC-3 Klebsiella pneumoniae ST258 clone. BMC Gastroenterol 2011;11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet 2008;371:143–152 [DOI] [PubMed] [Google Scholar]
- 7.Haney JC, Pappas TN. Necrotizing pancreatitis: Diagnosis and management. Surg Clin North Am 2007;87:1431–1446, ix [DOI] [PubMed] [Google Scholar]
- 8.Wittau M, Mayer B, Scheele J, et al. Systematic review and meta-analysis of antibiotic prophylaxis in severe acute pancreatitis. Scand J Gastroenterol. 2011;46:261–270 [DOI] [PubMed] [Google Scholar]
- 9.Uhl W, Isenmann R, Buchler MW. Infections complicating pancreatitis: diagnosing, treating, preventing. New Horiz 1998;6:S72–S79 [PubMed] [Google Scholar]
- 10.Bhasin DK, Udawat HP, Rana SS, et al. Intrasplenic pancreatic abscess successfully treated by endoscopic transpapillary drainage through the minor papilla. Gastrointest Endosc 2005;62:192–194 [DOI] [PubMed] [Google Scholar]
- 11.Chong VH. Isolated pyogenic pancreatic abscess mimicking a neoplasm. JOP 2008;9:309–312 [PubMed] [Google Scholar]
- 12.Orzechowska A, Lacey S, Soosay G, Melzer M. Community-acquired Klebsiella pneumoniae meningitis in an alcoholic patient with an infected pancreatic pseudocyst; a case report and review of literature. J Med Case Rep 2007;1:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hughes SJ, Papachristou GI, Federle MP, Lee KK. Necrotizing pancreatitis. Gastroenterol Clin North Am 2007;36:313–323, viii. [DOI] [PubMed] [Google Scholar]
- 14.Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology 2002;223:603–613 [DOI] [PubMed] [Google Scholar]
- 15.Paterson DL. Resistance in gram-negative bacteria: Enterobacteriaceae. Am J Infect Control 2006;34:S20–28 [DOI] [PubMed] [Google Scholar]
- 16.Di Carlo P, Gulotta G, Casuccio A, et al. KPC - 3 Klebsiella pneumoniae ST258 clone infection in postoperative abdominal surgery patients in an intensive care setting: Analysis of a case series of 30 patients. BMC Anesthesiol 2013;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012;56:2108–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: Importance of combination therapy. Clin Infect Dis 2012;55:943–950 [DOI] [PubMed] [Google Scholar]
- 19.Meagher AK, Passarell JA, Cirincione BB, et al. Exposure-response analyses of tigecycline efficacy in patients with complicated skin and skin-structure infections. Antimicrob Agents Chemother 2007;51:1939–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Passarell JA, Meagher AK, Liolios K, et al. Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob Agents Chemother 2008;52:204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacGowan AP. Tigecycline pharmacokinetic/pharmacodynamic update. J Antimicrob Chemother 2008;62Suppl 1:i11–16 [DOI] [PubMed] [Google Scholar]
- 22.Meagher AK, Ambrose PG, Grasela TH, Ellis-Grosse EJ. The pharmacokinetic and pharmacodynamic profile of tigecycline. Clin Infect Dis 2005;41Suppl 5:S333–340 [DOI] [PubMed] [Google Scholar]
- 23.Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012;54:1699–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new Boxed Warning. FDA Drug Safety Communication. www.fda.gov/drugs/drugsafety/ucm369580 Accessed January23, 2014
- 25.Hung WY, Kogelman L, Volpe G, et al. Tigecycline-induced acute pancreatitis: Case report and literature review. Int J Antimicrob Agents 2009;34:486–489 [DOI] [PubMed] [Google Scholar]
- 26.Lim LM, Ly N, Anderson D, et al. Resurgence of colistin: A review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy 2010;30:1279–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]


