Abstract
Antipsychotic drugs (APDs) are provided in the clinic to manage traumatic brain injury (TBI)-induced agitation and aggression. Experimental TBI studies consistently show that daily administration of the APDs, haloperidol (HAL) and risperidone (RISP), hinder recovery. However, it is unknown how long the adverse effects remain after cessation of treatment. To elucidate this clinically relevant issue, anesthetized male rats were randomly assigned to four TBI (controlled cortical impact) and four sham groups administered HAL (0.5 mg/kg), RISP (0.45 mg/kg), bromocriptine (BRO; 5.0 mg/kg, included as a control for D2 receptor action), or vehicle (VEH; 1 mL/kg) 24 h after surgery and once-daily for 19 days. Motor and cognitive recovery was assessed on days 1–5 and 14–19, respectively, and again at 1 and 3 months after drug withdrawal. No overall group differences were observed for motor function among the TBI groups, although the HAL group showed a greater beam-walk deficit on day 5 versus the VEH and BRO groups. Cognitive recovery was significantly impaired in the HAL and RISP groups during the treatment phase versus VEH and BRO. Further, BRO was superior to VEH (p=0.0042). At 1 month, both groups that received APDs continued to exhibit significant cognitive impairment versus VEH and BRO; at 3 months, only the HAL group was impaired. Moreover, the HAL, RISP, and VEH groups continued to be cognitively deficient versus BRO, which also reduced cortical damage. These data replicate previous reports that HAL and RISP impede cognitive recovery after TBI and expand the literature by revealing that the deleterious effects persist for 3 months after drug discontinuation. BRO conferred cognitive benefits when administered concomitantly with behavioral testing, thus replicating previous findings, and also after cessation demonstrating enduring efficacy.
Key words: : antipsychotics, beam-walking, behavior, bromocriptine, controlled cortical impact, functional recovery, learning and memory, Morris water maze, traumatic brain injury
Introduction
Agitation, psychoses, mania, and aggression negatively impact the course of treatment, subsequent recovery, and rehabilitation for traumatic brain injury (TBI) survivors.1 Many TBI patients receive typical and atypical antipsychotic drugs (APDs) for treatment of these maladaptive behaviors, albeit little evidence supports their long-term use. Specifically, the effects of APDs, during and after cessation of treatment, on the fragile homeostasis of the recovering human brain after TBI are still largely uncertain.2–6 Typical APDs, such as haloperidol (HAL), have been used since the early 1950s to reduce positive symptoms of schizophrenia, but are known to induce extrapyramidal symptoms, such as tremor, rigidity, and bradykinesia.7 Moreover, such debilitating side effects limit long-term use, lead to premature termination of treatment, and increase core negative symptoms and cognitive deficits.8 Conversely, although atypical APDs, such as risperidone (RISP), are currently preferred owing to reduced extrapyramidal side effects and superior therapeutic value through affinity at multiple receptors, including serotonergic, dopaminergic, adrenergic, and muscarinic, their long-term effects on cognitive recovery in the TBI population remain unclear.1,4,5
The scientific literature comprising pre-clinical studies focusing on neurobehavioral and mechanistic effects of APDs after experimental TBI is still relatively limited, but describes primarily deleterious effects of these agents on behavioral recovery, especially when the drugs are administered chronically. HAL delayed motor recovery after brain injury when given as an acute 9,10 or chronic treatment paradigm.11 Similarly, a single administration of various doses of HAL (0.1, 1.0, or 10.0 mg/kg) significantly impeded locomotor recovery when given to rats a day after sensorimotor cortex injury.12 Aside from deleterious effects on motor performance, daily HAL treatment (15 days) after fluid percussion injury significantly delayed the acquisition of spatial learning in the Morris water maze (MWM), compared to injury alone.13 In controlled cortical impact (CCI) injury studies, both HAL and RISP delayed motor and cognitive recovery when administered chronically and concomitantly with behavioral testing.14–16 Moreover, both HAL and RISP reinstated motor and cognitive deficits in rats that appeared to be fully recovered, and the cognitive impairments lasted for at least 3 days after discontinuation of treatment.14
In the current study, we sought to assess the persistence of the deleterious effects observed after chronic administration of HAL and RISP on motor recovery, acquisition of spatial learning, and memory retention after CCI injury. Specifically, in order to mimic a clinically relevant course of treatment and neurobehavioral testing, functional outcome was assessed both during drug treatment, as well as at 1 and 3 months after discontinuation. This approach is important, considering that APD-induced long-lasting neurochemical changes may represent an additional hindrance to behavioral recovery when superimposed with underlying pathophysiological mechanisms after TBI. In addition, we included bromocriptine (BRO) as a positive control for D2 receptor action.
Methods
Animals
Sixty adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed in standard steel-wire mesh cages and maintained in a controlled environment with ambient temperature set at 21±1°C and lights set on a 12/12-h light/dark cycle (lights on at 7:00 am). Standard rat chow and water were available ad libitum. Rats were allowed to acclimate to the colony for 1 week before commencing experimental procedures, which were carried out during the light portion of the cycle and were approved by the institutional animal care and use committee at the University of Pittsburgh (Pittsburgh, PA). Every attempt was made to limit the number of animals used and minimize discomfort.
Surgery
Rats weighing 300–325 g on the day of surgery underwent surgical anesthesia, which was induced and maintained with inspired concentrations of 4% and 2% isoflurane, respectively, in a 2:1 ratio of N2O/O2. After endotracheal intubation, rats were secured in a stereotaxic frame, ventilated mechanically, and temperature was maintained at 37±0.5°C with a heating blanket. Employing aseptic procedures, a mid-line scalp incision was made, the skin and fascia were reflected to expose the skull, and a craniectomy (6-mm in diameter) was made in the right hemisphere (encompassing bregma and lambda, and between the sagittal suture and the coronal ridge) with a handheld trephine. The bone flap was removed and the craniectomy was enlarged further with rongeurs. Subsequently, the impacting rod was extended and the impact tip (6 mm, flat) was centered and lowered until it touched the dura mater, then the rod was retracted and the impact tip was advanced 2.8 mm further to produce a brain injury of moderate severity (2.8-mm tissue deformation at 4 m/sec). Immediately after the CCI, anesthesia was discontinued, the incision was sutured, and then rats were extubated and assessed for acute neurological outcome. Sham rats underwent similar surgical procedures, but were not subjected to the impact.
Acute neurological evaluation
After termination of anesthesia, hindlimb reflexive ability was assessed by gently squeezing the rats' paw every 5 sec and recording the time to elicit a withdrawal response. Return of the righting reflex was also determined as the time required for a rat to turn from the supine to prone position. These natural reflexive responses are sensitive indicators of injury severity and anesthetic effects.17–20
Drug administration
After surgery, rats were randomly distributed among four TBI (n=10 per group) and four sham (n=5 per group) groups and provided HAL (0.5 mg/kg), RISP (0.45 mg/kg), BRO (5.0 mg/kg), or vehicle (VEH; 1.0 mL/kg). HAL (Sigma-Aldrich, St. Louis, MO), RISP (Research Diagnostics, Flanders, NJ), and BRO (Tocris Cookson, Inc., Ballwin, MO) were prepared daily by dissolving in 1:1 dimethyl sulfoxide/saline, which also served as the VEH. The doses of HAL and RISP were chosen because they were reported to be comparable to those used clinically to control psychosis21 and have been used in several TBI studies.14–16 The dose of BRO was chosen based on previous studies revealing significant benefits.22,23 Treatments began 24 h after CCI or sham surgery and were provided intraperitoneally (i.p.) once-daily for 19 days. HAL and RISP were provided after the daily behavioral assessments to circumvent sedative effects that may confound the results. BRO was provided 15 min before daily behavioral assessments because this paradigm has been shown to confer cognitive and neural protection after TBI.22,23
Motor performance: beam-balance and beam-walk
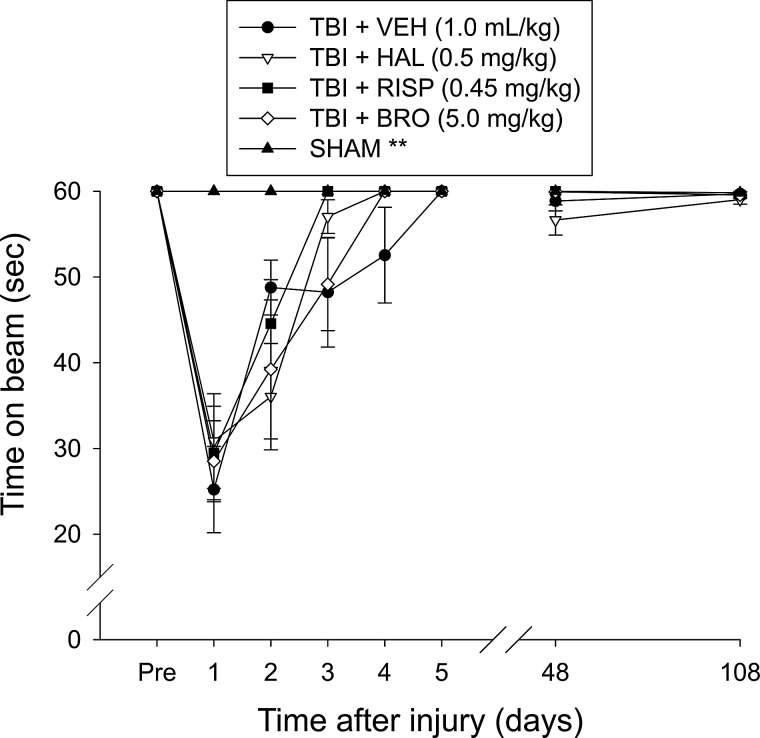
Motor function was evaluated using the well-validated beam-balance (BB) and beam-walk (BW) tasks.22–25 The BB task consists of placing the rat on an elevated (90-cm height from floor) narrow wooden beam (1.5 cm wide) and recording the duration it remains on it for a maximum of 60 sec. The BW task, a modified version of that originally devised by Feeney and colleagues9 and used extensively in our laboratory22–25 as well as those of others,26,27 consists of recording the time to traverse an elevated (90 cm) narrow wooden beam (2.5 wide, 100 cm long) and enter a darkened goal box at the opposite end to escape bright light and high-decibel white noise. All rats were trained to perform the tasks without errors (i.e., maintain their balance for 60 sec and traverse the beam in under 5 sec) preceding TBI or sham injury. A baseline performance assessment was taken on the day of surgery. Testing consisted of providing three trials (60 sec of allotted time with a 30-sec intertrial interval) per day on each task and took place on postoperative days 1–5 while treatments were ongoing and again at postoperative days 48 and 108 (i.e., 1 and 3 months after the withdrawal of treatments). The average daily scores for each subject were used in the statistical analyses.
Cognitive function (acquisition of spatial learning and memory)
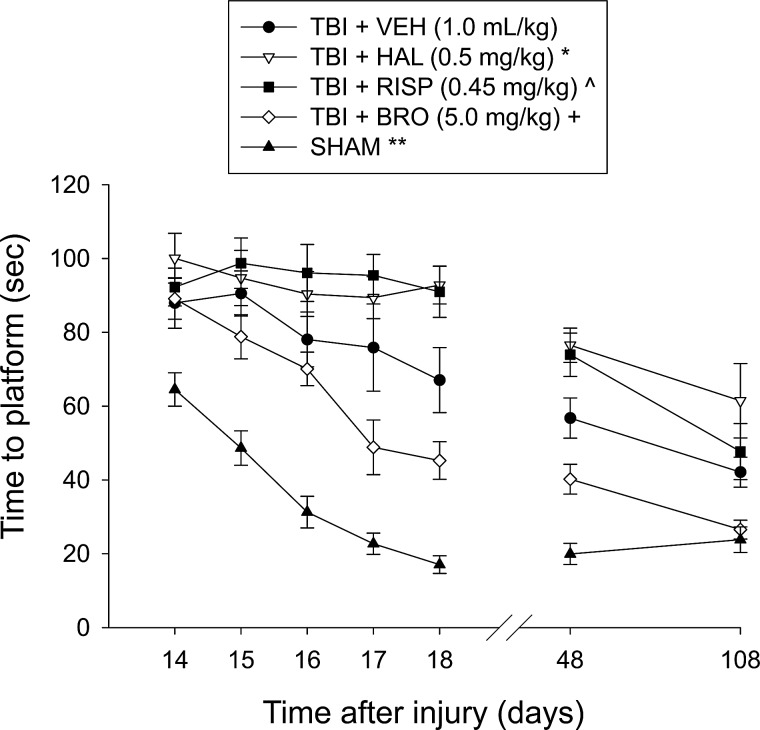
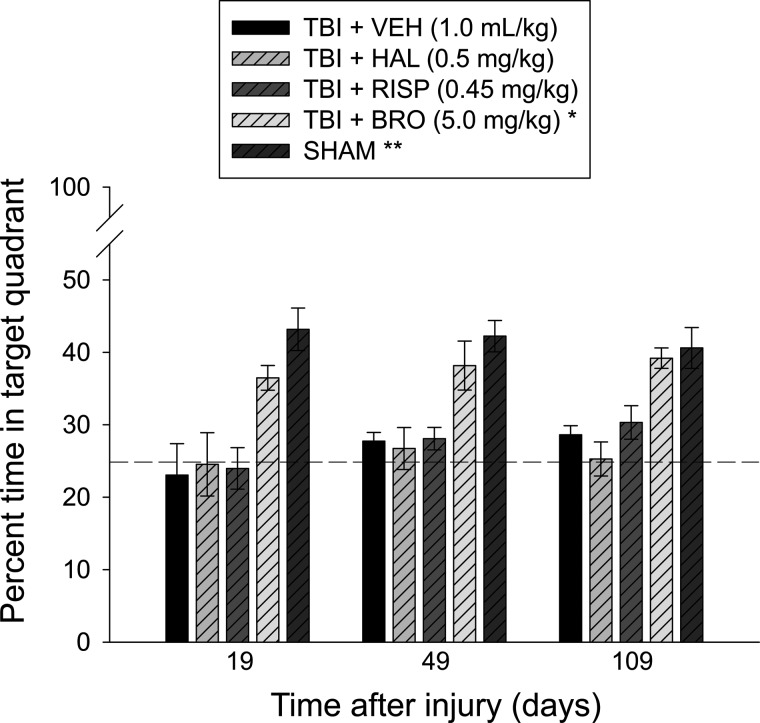
A MWM task28 that is sensitive to cognitive function after TBI22–25,29,30 was used to compare acquisition of spatial learning among the treatment groups. The maze consisted of a plastic pool (180 cm diameter; 60 cm height) filled with water (26±1°C) to a depth of 28 cm and was situated in a room with salient visual cues that remained constant throughout the study. The platform was a clear Plexiglas stand (10 cm diameter, 26 cm high) and was positioned 26 cm from the maze wall in the southwest quadrant of the maze and held constant throughout the study. Acquisition of spatial learning began on postoperative day 14 and consisted initially of providing a block of four daily trials (120 sec maximum, 4-min intertrial interval) for 5 consecutive days (days 14–18 postsurgery) to locate the platform when it was submerged 2 cm below the water surface (i.e., invisible to the rat). For each daily block of trials, rats were placed in the pool facing the wall at each of the four possible start locations (north, south, east, and west) in a randomized manner. Each trial lasted until the rat climbed onto the platform or until 120 sec had elapsed, whichever occurred first. All rats were required to remain on the platform for 30 sec before being placed in a heated incubator between trials (4-min intertrial interval). To assess the long-term cognitive effects of a 19-day treatment regimen, a similar daily block of trials was provided at 48 and 108 days postsurgery. Memory retention was assessed on day 19 (i.e., 1 day after the final acquisition training session while treatments were ongoing) and again on days 49 and 109 after cessation of treatments. During these testing phases, the platform was removed from the pool and rats were placed in the maze from the location point most distal to the quadrant where the platform was previously situated (i.e., “target quadrant”) and allowed to freely explore the pool for 30 sec. The percent time spent in the target quadrant was used in the statistical analysis. The behavior data were obtained using a spontaneous motor activity recording and tracking system (San Diego Instruments, San Diego, CA), which, in addition to determining time to the platform, also calculates path length and swim speed.
Histology: Cortical lesion volume
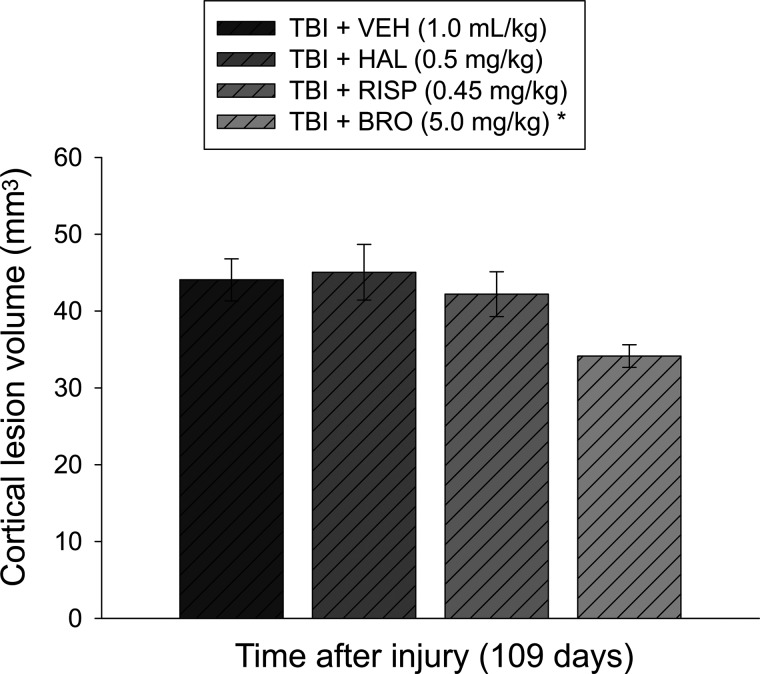
After the last behavioral assessment (i.e., postoperative day 109), rats were anesthetized with pentobarbital (50 mg/kg i.p.) and then perfused transcardially with heparinized 0.1 M phosphate-buffered saline (200 mL; pH 7.4), followed by 10% buffered formalin (300 mL). Brains were extracted, postfixed in 10% buffered formalin for 1 week, dehydrated with alcohols, and embedded in paraffin. Coronal sections (7 μm thick) were cut at 1-mm intervals through the lesion on a rotary microtome and mounted on gelatin-coated glass microscope slides. After drying at room temperature, sections were deparaffinized in xylene, rehydrated, and stained with cresyl violet. Cortical lesion volumes (mm3) were assessed by an observer blinded to experimental conditions using a Nikon Eclipse 90i microscope (Nikon Corporation, Tokyo, Japan). The area of the lesion (mm2) was first calculated by outlining the inferred area of missing cortical tissue for each section (typically 5–7; Nikon NIS-Elements AR 3.22.14 software; Nikon Corp, Tokyo, Japan) and then by summing the lesions obtained, as previously reported.18–20,24
Statistical analyses
Statistical analyses were performed on data collected by observers blinded to treatment conditions using StatView 5.0.1 software (Abacus Concepts, Inc., Berkeley, CA). Motor and cognitive data were analyzed by repeated-measures analysis of variance (ANOVA). Acute neurological data, probe trials, swim speed, and histological findings were analyzed by one-factor ANOVAs. When the ANOVA showed a significant effect, Bonferroni's/Dunn's post-hoc test was utilized to determine specific group differences. Results are expressed as the mean±standard error of the mean (SEM) and were considered significant when p values were ≤0.05 for overall ANOVAs and 0.005 for post-hoc analyses, as dictated by Bonferroni's/Dunn's statistic after correcting for multiple comparisons.
Results
One rat from the TBI+VEH group was excluded from the study because of an inability to locate the visible platform, which may be indicative of visual acuity deficits. Hence, statistical analyses are based on 59 rats. No significant differences were observed among the sham groups, regardless of treatment in any of the assessments, and thus the data were pooled and analyzed as one group (denoted as “SHAM”).
Acute neurological evaluation
No significant differences were observed among the TBI groups in time to recover the hindlimb withdrawal reflex in response to a brief paw pinch (left range=173.5±7.6 to 182.3±3.9 sec, p>0.005; right range=170.3±4.8 to 178.3±4.5 sec, p>0.005) or for return of righting ability (range, 363.6±16.3 to 396.3±12.8 sec; p>0.005) after cessation of anesthesia. The lack of significant differences with these acute neurological indices suggests that all groups experienced an equivalent level of injury and anesthesia.
Motor performance
Beam-balance
All groups were able to balance on the beam for the allotted 60 sec on each of three trials preceding surgery (Fig. 1). After surgery, all TBI groups were impaired relative to the SHAM group (p<0.0001). On days 1–5 while treatment was ongoing, the overall ANOVA revealed significant group (F4,54=9.963; p<0.0001) and day (F5,270=65.316; p<0.0001) differences, as well as a significant group x day interaction (F20,270=7.047; p<0.0001). The repeated ANOVA on the 5 days of testing did not reveal a significant difference among the TBI groups (p's>0.005). Additionally, no significant differences were detected among any of the groups on either postoperative day 48 or 108 (p's>0.005).
FIG. 1.
Mean (±SEM) time (sec) to maintain balance on an elevated narrow beam before and after TBI or SHAM injury, as well as drug treatment. All TBI groups demonstrated significant deficits relative to the SHAM group on days 1–5, but there no differences among the TBI drug conditions. There were no differences among any of the groups on postoperative days 48 or 108. **p<0.001 versus all TBI groups on days 1–5. TBI, traumatic brain injury; VEH, vehicle; HAL, haloperidol; RISP, risperidone; BRO, bromocriptine.
Beam-walk
There were no differences among the groups in time to traverse the beam preceding surgery owing to the fact that all rats were well trained and reached the goal box in under 4 sec (Fig. 2). After surgery, all TBI groups were impaired relative to the SHAM group (p<0.0001). On days 1–5 while treatment was ongoing, the overall ANOVA revealed significant group (F4,54=79.197; p<0.0001) and day (F5,270=291.304; p<0.0001) differences, as well as a significant group x day interaction (F20,270=27.858; p<0.0001). The repeated-measures ANOVA over the 5 days of testing did not reveal a significant difference among the TBI groups (p's>0.005). However, a single-day analysis on day 5 did reveal a greater beam-walk deficit in the HAL-treated group versus the VEH- and BRO-treated groups (p<0.005). No significant differences were detected among any of the groups on either postoperative day 48 or 108 (p's>0.005).
FIG. 2.
Mean (±SEM) time (sec) to traverse an elevated narrow beam before and after TBI or SHAM injury, as well as drug treatment. All TBI groups exhibited significant impairment versus SHAM. No overall statistical difference was observed among the TBI groups during the first 5 days of testing. However, a single-day analysis on day 5 did reveal a greater beam-walk deficit in the HAL-treated group versus the VEH- and BRO-treated groups (p<0.005). No significant differences were detected among any of the groups on test days 48 or 108 postsurgery. **p<0.0001 versus all TBI groups on days 1–5. TBI, traumatic brain injury; VEH, vehicle; HAL, haloperidol; RISP, risperidone; BRO, bromocriptine.
Cognitive function: Acquisition of spatial learning
Analysis of the water-maze data for postoperative days 14–18, when treatments were ongoing, revealed significant group (F4,54=54.135; p<0.0001) and day (F4,216=19.3; p<0.0001) differences, as well as a significant group x day interaction (F16,216=3.499; p<0.0001). The post-hoc analysis revealed several significant differences among the groups. Specifically, as depicted in Figure 3, all TBI groups were significantly impaired versus SHAM controls (p<0.0001). Further, the TBI+HAL and TBI+RISP groups were significantly impaired versus the TBI+VEH group (p=0.0046 and 0.0020, respectively). Additionally, the TBI+BRO group was able to locate the escape platform significantly quicker over time versus the TBI+HAL (p<0.0001), TBI+RISP (p<0.0001), and TBI+VEH (p=0.0049) groups. There was no difference between the TBI+HAL and TBI+RISP groups (p=0.79). Analyses of the water-maze data on postoperative days 48 and 108 (1 and 3 months after withdrawal of treatments) revealed similar group differences. On day 48, the SHAM control group was significantly better than all TBI groups, regardless of treatment (p≤0.0008). Moreover, the TBI+HAL and TBI+RISP groups continued to be significantly impaired versus the TBI+VEH and TBI+BRO groups (p<0.005), but did not differ from one another (p=0.70). Additionally, the TBI+BRO group was still performing markedly better than the TBI+VEH group (p=0.0046). On day 108, the SHAM group continued to be better than the TBI+HAL, TBI+RISP, and TBI+VEH groups (p≤0.0025), but was no longer different from the TBI+BRO group (p=0.81). The deficit in the TBI+HAL group persisted, given that it differed from the TBI+VEH and TBI+BRO groups (p<0.005). The TBI+RISP group showed some improvement and was no longer different from the TBI+VEH group (p=0.64), but still did not differ from the TBI+HAL group (p=0.12). Finally, the TBI+BRO group remained significantly better than all other TBI groups (p<0.005).
FIG. 3.
Mean (±SEM.) time (sec) to locate a hidden (submerged) platform in a water maze. During postoperative days 14–18, all TBI groups were significantly impaired versus SHAM controls. Moreover, the TBI+HAL and TBI+RISP groups were significantly impaired versus the TBI+VEH group, though they were not different from one another. In contrast, the TBI+BRO group located the escape platform significantly quicker over time, compared to all other TBI groups. These effects persisted at 1 month after drug withdrawal (i.e., day 48). At 3 months after drug withdrawal (i.e., day 108), TBI+HAL rats continued to perform worse than the TBI+VEH group, whereas the TBI+RISP group displayed some improvement and was not significantly different from VEH-treated rats. The TBI+BRO group remained significantly better than all TBI groups. *p<0.005 versus TBI+vehicle and TBI+BRO on days 14–18, 48, and 108; ^p<0.005 versus TBI+vehicle and TBI+BRO on days 14–18 and 48, but only from TBI+BRO on day 108; +p<0.005 versus all TBI groups at each time point; **p<0.0001 versus all TBI groups at each time point, except TBI+BRO on day 108. TBI, traumatic brain injury; VEH, vehicle; HAL, haloperidol; RISP, risperidone; BRO, bromocriptine.
Cognitive function: Swim speed and probe trial
No significant differences in swim speed (range=27.6±1.2 to 31.8±1.0 cm/sec) were observed among the groups. As shown in Figure 4, analysis of the probe data revealed significant memory retention in the SHAM and TBI+BRO groups, as evidenced by a higher percentage of the 30-sec allotted time spent in the target quadrant versus the TBI+HAL, TBI+RISP, and TBI+VEH groups during the period when treatments were ongoing (day 19), as well as nontreatment days 49 and 109 (p<0.005). No statistical differences were revealed between the SHAM and TBI+BRO groups at any time (p>0.005). There were also no differences among the TBI+HAL, TBI+RISP, and TBI+VEH groups at any time point (p>0.005).
FIG. 4.
Mean (±SEM) percentage of time spent in the target quadrant (i.e., where the platform was previously located) after single-probe trials on treatment day 19 and nontreatment days 49 and 109 after controlled cortical impact or sham injury. The TBI+BRO and SHAM groups displayed significant memory retention on all 3 test days, compared to the TBI+HAL, TBI+RISP, and TBI+VEH groups, as evidenced by higher percentages of the 30-sec allotted time spent in the target quadrant. There were no significant differences among the TBI+HAL, TBI+RISP, and TBI+VEH groups at any time point. The dashed line represents performance at the chance level (25%). *p<0.005 versus all TBI groups at each time point; **p<0.0001 versus TBI+VEH, TBI+HAL, and TBI+RISP groups at each time point. No other group comparisons were significant. TBI, traumatic brain injury; VEH, vehicle; HAL, haloperidol; RISP, risperidone; BRO, bromocriptine.
Histology: Cortical lesion volume
The ANOVA for the cortical lesion volume data showed a significant group effect (F3,35=3.269; p=0.03). Subsequently, the post-hoc test revealed that the lesion of 34.1±1.4 mm3 in the TBI+BRO group was significantly smaller than the 44.1±2.7, 45.1±3.6, and 42.2±2.9 mm3 for the TBI+VEH, TBI+HAL, and TBI+RISP groups, respectively (p<0.005; Fig. 5). No other group comparisons were significant (p>0.005).
FIG. 5.
Mean (±SEM) cortical lesion volume (mm3) on postoperative day 109 (i.e., 3 months after discontinuation of drug treatment and after the last behavioral assessment). The TBI+BRO group displayed significantly smaller cortical lesion volumes, compared to the TBI+VEH, TBI+HAL, and TBI+RISP groups. No other group comparisons were significant. *p<0.005 versus all TBI groups. TBI, traumatic brain injury; VEH, vehicle; HAL, haloperidol; RISP, risperidone; BRO, bromocriptine.
Discussion
The aim of the current study was to examine the long-term effects of the typical and atypical APDs, HAL and RISP, respectively, on motor and cognitive recovery after experimental brain trauma produced by a CCI injury that mimics many of the characteristics of human TBI.31 The rationale was that despite previous studies consistently revealing that administering HAL and RISP once-daily for 19 days hinders cognitive recovery after TBI,15,16 these agents are still readily used in the clinic to manage agitation and aggression with little information on their long-term use. Specifically, we were interested in determining how long the APD-induced adverse effects on cognitive processes remain after cessation of treatment. Additionally, we included the D2 receptor agonist BRO as a positive control for D2 receptor mediation as a potential explanation for the observed effects.
Group differences in motor recovery were fairly minimal, with only the HAL group showing a greater beam-walk deficit on day 5 versus the VEH and BRO groups. However, spatial learning recovery was significantly impaired in the HAL and RISP groups during the treatment phase, compared with VEH and BRO. Moreover, both APD groups continued to display significant cognitive impairment versus VEH and BRO at 1 month after cessation of treatment, whereas at 3 months only the HAL group was significantly impaired. Conversely, BRO provided superior cognitive recovery capabilities, compared with VEH, HAL, and RISP, at every time point. Moreover, BRO reduced cortical damage, as indicated by smaller lesion-volume measurements. These findings suggest that the APD-induced recovery delays may be partly mediated by D2 receptors, and that blockade of D2 receptor-signaling pathways play an important role in the detrimental effects of APDs.
Chronic HAL treatment, which acts as a high-affinity D2 postsynaptic receptor blocker, has been associated with behavioral alterations, but it can also produce a variety of cellular and anatomical changes in the central nervous system (CNS). Studies have reported HAL-related structural synaptic changes in the rat striatum, one of the pivotal subcortical nuclei involved in motor, limbic, and associative behavior. Specifically, morphometric analyses of axodendritic synapses revealed that approximately 12% of axon terminals appeared to have doubled their size and synaptic vesicle number after 16 weeks of HAL administration, with no effects on vesicle density, suggesting enhanced dopamine turnover.32 Administration of HAL for 3 weeks in rats has been shown to produce depleting effects on tyrosine hydroxylase in the substantia nigra, which persisted for 1 month after drug withdrawal.33
APD-induced detrimental effects on cognitive function and/or recovery after an insult may also be partly mediated by the cholinergic transmitter system, which is involved in learning and memory processes.34,35 Recently, Huang and colleagues demonstrated that both short- (7 days) and long-term (45 days) administration of HAL and RISP produced significant brain decreases in choline acetyltransferase expression, the enzyme responsible for acetylcholine synthesis,36 an effect similarly observed in our laboratory as a direct result of brain trauma.18 Drug treatment in the current study ceased at 19 days, but it is appropriate to infer that long-lasting neuropathological effects are likely, considering that even acute HAL injections affect network dynamics in the corticobasal ganglia pathway. Yael and colleagues demonstrated that neuronal subpopulations within the striatum are differentially affected by acute HAL application (0.5 mg, subcutaneously) by performing multi-electrode recordings in freely moving rats. Changes included reduced firing rates for medium spiny neurons and GABAergic fast-spiking interneurons, as well as increased firing rates for the cholinergic tonically active interneurons.37 Extended effects of APDs in the brain after drug withdrawal may be the result of either lasting presence of drug traces in brain tissue or persistent treatment-related signaling cascade alterations. Earlier studies reported signs of central dopaminergic blockade in the rat brain for at least 1 month after acute moderate doses of HAL, when challenged with an acute dose of the nonselective dopaminergic receptor agonist, apomorphine.38 In humans, the brain elimination half-life of HAL is approximately 1 week, with HAL concentrations in postmortem brain tissue measuring 10–30 times higher than optimal serum concentrations used in the treatment of schizophrenia for up to several weeks after withdrawal.39 Moreover, central D2 receptor occupancy has been detected through positron emission tomography for a year after discontinuation of a depot-based HAL delivery preparation.40
APDs are also thought to induce cellular dysfunction through loss of antioxidant enzymes and increased lipid peroxidation,8 as well as oxidative stress, mitochondrial dysfunction, and even cell death.41–43 In particular, increased dopamine turnover could, in turn, result in excessive production of damaging free radicals, such as hydrogen peroxide, superoxide radical, and hydroxyl radical, through oxidation processes and reacting with iron or copper ions.44 In a study by Parikh and colleagues, both HAL and RISP were administered chronically to rats for either 45 or 90 days. Both courses of HAL treatment induced oxidative stress in the brain by significantly reducing the levels of antioxidant defense enzymes and increasing lipid peroxidation, whereas chronic RISP did not produce any alterations.45 These findings align with the reduced long-term deleterious effect of RISP on recovery of spatial learning in the current study, given that cognitive performance was not worse than VEH at 3 months after termination of drug treatment. Reduced negative outcomes after atypical APDs are perhaps to be expected. The hallmark of their mechanism of action is acting on multiple receptor sites in the CNS, such as serotonergic, dopaminergic, adrenergic, and muscarinic receptors, therefore producing less extrapyramidal side effects and detrimental effects on recovery.1,4,5 Nevertheless, although RISP appears to be better tolerated and less detrimental in both humans and rodents, several lines of research support relatively negative behavioral and brain-related outcomes, as previously mentioned. For example, regulation of neuronal survival and mechanisms of neurotoxicity in primary rat cortical neuron cultures were previously differentially affected by HAL and RISP treatment. Treatment with HAL, but not RISP, induced caspase-dependent apoptotic neuronal death induction by reducing cellular survival signaling, which possibly contributes to the differential clinical therapeutic efficacy of these drugs.41 Interestingly, treatment with BRO attenuated haloperidol-induced neuronal toxicity, perhaps by attenuating HAL-induced mitochondrial dysfunction and production of hydroxyl free radicals,41 which may partly explain the markedly enhanced spatial learning in the BRO group in our study, compared to VEH-treated injured rats. Conversely, 28 days of treatment with both HAL and RISP impeded working memory in a cross-maze task,46 whereas extended chronic administration (up to 320 days) of these APDs impaired various performance parameters in the acquisition and performance of a two-radial-arm maze task and a five-choice serial reaction-time task.47
BRO has significant antioxidant properties48,49 that have been associated with improved outcomes after experimental and clinical TBI.22,23,50 The positive effects of BRO include neuroprotective properties against 6-hydoxydopamine-induced decreases in mouse striatal dopamine and its metabolites, as well as dose dependently reducing the number of hydroxyl free radicals in an in vitro Fenton's reagent system.51 In a randomized, double-blind, crossover design counterbalanced for the order of drug administration, McDowell and colleagues provided patients with both 2.5 mg of BRO and a placebo. They reported beneficial effects of BRO on a multitude of cognitive tests thought to engage prefrontal cortical-mediated executive functions, such as the dual task, the trail making test, the Stroop Test, verbal fluency, and the Wisconsin Card Sorting Test.50 However, a 6-week double-blind, placebo-controlled, crossover study with BRO titrated to a dose of 5 mg twice a day did not reveal positive effects on a range of measures of attentional function after TBI. Nevertheless, the investigators recommended the use of lower doses or intermittent administration of BRO to obtain more encouraging outcomes.52 We have reported previously that chronic BRO treatment reduced working memory and spatial acquisition deficits, as well as improved histopathology (i.e., rescuing morphologically intact hippocampal CA3 neurons), compared to the injured VEH-treated group, after CCI.22 Subsequently, we also demonstrated that acute systemic BRO treatment (15 min before CCI or sham injury) provided behavioral and histological protection, seen as improved spatial learning and a greater percentage of surviving hippocampal CA3 neurons, respectively, as well as a reduction in TBI-induced malondialdehyde, the end product of lipid peroxidation, in multiple brain regions.23
In summary, the current study yielded several relevant outcomes. Specifically, these data replicate previous reports that HAL and RISP impede cognitive recovery after TBI and expand the literature by revealing that the deleterious effects persist for up to 3 months after drug discontinuation. BRO conferred cognitive benefits when administered concomitantly with behavioral testing, thus replicating previous findings.22,23 Moreover, the BRO-mediated benefits persisted after cessation of treatment demonstrating enduring efficacy. The clinical implications of these behavioral findings are that chronic use of HAL and RISP to control TBI-induced agitation and aggression may result in persistent deleterious effects on cognition. In marked contrast, the long-lasting beneficial effects on spatial learning and memory retention with BRO support the use of this agent, and possibly other D2 receptor agonists, as an efficacious therapy for clinical TBI. Indeed, amantadine has recently made a relatively successful translation from the bench53 to bedside.54 Last, the divergent cognitive outcomes detected between HAL and RISP versus BRO suggests that the adverse effect of these APDs may be mediated by D2 receptor antagonism. Further support for this hypothesis stems from work showing that olanzapine, a third-generation APD with less-robust DA activity, did not negatively impact cognitive performance. 13
Acknowledgments
This work was supported, in part, by the National Institutes of Health (grant nos.: NS084967, NS060005, and HD069620; to A.E.K.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chew E., and Zafonte R.D. (2009). Pharmacological management of neurobehavioral disorders following traumatic brain injury—a state-of-the-art review. J. Rehabil. Res. Dev. 46, 851–878 [DOI] [PubMed] [Google Scholar]
- 2.Vogenthaler D.R. (1987). An overview of head injury: its consequences and rehabilitation. Brain Inj. 1, 113–127 [DOI] [PubMed] [Google Scholar]
- 3.Stanislav S.W. (1997). Cognitive effects of antipsychotic agents in persons with traumatic brain injury. Brain Inj. 11, 335–341 [DOI] [PubMed] [Google Scholar]
- 4.Elovic E.P., Lansang R., Li Y., and Ricker J.H. (2003). The use of atypical antipsychotics in traumatic brain injury. J. Head Trauma Rehabil. 18, 177–195 [DOI] [PubMed] [Google Scholar]
- 5.Elovic E.P., Jasey N.N., and Eisenberg M.E. (2008). The use of atypical antipsychotics after traumatic brain injury. J. Head Trauma Rehabil. 23, 132–135 [DOI] [PubMed] [Google Scholar]
- 6.Lombard L.A., and Zafonte R.D. (2005). Agitation after traumatic brain injury: considerations and treatment options. Am. J. Phys. Med. Rehabil. 84, 797–812 [DOI] [PubMed] [Google Scholar]
- 7.Karl T., Duffy L., O'Brien E., Matsumoto I., and Dedova I. (2006). Behavioural effects of chronic haloperidol and risperidone treatment in rats. Behav. Brain Res. 171, 286–294 [DOI] [PubMed] [Google Scholar]
- 8.Pillai A., Parikh V., Terry A.V., Jr., and Mahadik S.P. (2007). Long-term antipsychotic treatments and crossover studies in rats: differential effects of typical and atypical agents on the expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 41, 372–386 [DOI] [PubMed] [Google Scholar]
- 9.Feeney D.M., Gonzalez A., and Law W.A. (1982). Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science 217, 855–857 [DOI] [PubMed] [Google Scholar]
- 10.Feeney D.M., Weisend M.P., and Kline A.E. (1993). Noradrenergic pharmacotherapy, intracerebral infusion and adrenal transplantation promote functional recovery after cortical damage. J. Neural Transplant Plast. 4, 199–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feeney D.M., and Westerberg V.S. (1990). Norepinephrine and brain damage: alpha noradrenergic pharmacology alters functional recovery after cortical trauma. Can. J. Psychol. 44, 233–252 [DOI] [PubMed] [Google Scholar]
- 12.Goldstein L.B., and Bullman S. (2002). Differential effects of haloperidol and clozapine on motor recovery after sensorimotor cortex injury in rats. Neurorehabil. Neural Repair 16, 321–325 [DOI] [PubMed] [Google Scholar]
- 13.Wilson M.S., Gibson C.J., and Hamm R.J. (2003). Haloperidol, but not olanzapine, impairs cognitive performance after traumatic brain injury in rats. Am. J. Phys. Med. Rehabil. 82, 871–879 [DOI] [PubMed] [Google Scholar]
- 14.Kline A.E., Massucci J.L., Zafonte R.D., Dixon C.E., DeFeo J.R., and Rogers E.H. (2007). Differential effects of single versus multiple administrations of haloperidol and risperidone on functional outcome after experimental traumatic brain trauma. Crit. Care Med. 35, 919–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kline A.E., Hoffman A.N., Cheng J.P., Zafonte R.D., and Massucci J.L. (2008). Chronic administration of antipsychotics impede behavioral recovery after experimental traumatic brain injury. Neurosci. Lett. 448, 263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman A.N., Cheng J.P., Zafonte R.D., and Kline A.E. (2008). Administration of haloperidol and risperidone after neurobehavioral testing hinders the recovery of traumatic brain injury-induced deficits. Life Sci. 83, 602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng J.P, Hoffman A.N., Zafonte R.D., and Kline A.E. (2008). A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 194, 79–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kline A.E., McAloon R.L., Henderson K.A., Bansal U.K., Ganti B.M., Ahmed R.H., Gibbs R.B., and Sozda C.A. (2010). Evaluation of a combined therapeutic regimen of 8-OH-DPAT and environmental enrichment after experimental traumatic brain injury. J. Neurotrauma 27, 2021–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monaco C.M., Mattiola V.V., Folweiler K.A., Tay J.K., Yelleswarapu N.K., Curatolo L.M., Matter A.M., Cheng J.P., and Kline A.E. (2013). Environmental enrichment-mediated benefits in female rats after brain trauma further supports this paradigm as a preclinical model of neurorehabilitation. Exp. Neurol. 247, 410–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bondi C.O., Cheng J.P., Tennant H.M., Monaco C.M., and Kline A.E. (2014). Old dog, new tricks: the attentional set-shifting test as a novel cognitive behavioral task after controlled cortical impact injury. J. Neurotrauma 31, 926–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosengarten H., and Quartermain D. (2002). The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three-and eighteen-month-old rats. Prog. Neuropsychopharmacol. Bio. Psychiatry 26, 1047–1054 [DOI] [PubMed] [Google Scholar]
- 22.Kline A.E., Massucci J.L., Marion D.W., and Dixon C.E. (2002). Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. J. Neurotrauma 19, 415–425 [DOI] [PubMed] [Google Scholar]
- 23.Kline A.E., Massucci J.L., Ma X., Zafonte R.D., and Dixon C.E. (2004). Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J. Neurotrauma 21, 1712–1722 [DOI] [PubMed] [Google Scholar]
- 24.Sozda C.A., Hoffman A.N., Olsen A.S., Cheng J.P., Zafonte R.D., and Kline A.E. (2010). Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J. Neurotrauma 27, 1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw K.E., Bondi C.O., Light S.H., Massimino L.A., McAloon R.L., Monaco C.M., and Kline A.E. (2013). Donepezil is ineffective in promoting motor and cognitive benefits after controlled cortical impact injury in male rats. J. Neurotrauma 30, 557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dixon C.E., Kraus M.F., Kline A.E., Ma X., Yan H.Q., Griffith R.G., Wolfson B.M., and Marion D.W. (1999). Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 14, 285–294 [PubMed] [Google Scholar]
- 27.Zhang L., Zhang F., Weng Z., Brown B.N., Yan H., Ma X.M., Vosler P.S., Badylak S.F., Dixon C.E., Cui X.T., and Chen J. (2013). Effect of an inductive hydrogel composed of urinary bladder matrix upon functional recovery following traumatic brain injury. Tissue Eng. Part A 19, 1909–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60 [DOI] [PubMed] [Google Scholar]
- 29.Hamm R.J., Dixon C.E., Gbadebo D.M., Singha A.K., Jenkins L.W., Lyeth B.G., and Hayes R.L. (1992). Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma 9, 11–20 [DOI] [PubMed] [Google Scholar]
- 30.Scheff S.W., Baldwin S.A., Brown R.W., and Kraemer P.J. (1997). Morris water maze deficits in rats following traumatic brain injury: lateral controlled cortical impact. J. Neurotrauma 14, 615–627 [DOI] [PubMed] [Google Scholar]
- 31.Bondi C.O., Semple B.D., Noble-Haeusslein L.J., Osier N.D., Carlson S.W., Dixon C.E., Giza C.C., and Kline A.E. (2014). Found in translation: understanding the biology and behavior of traumatic brain injury. Neurosci. Biobehav. Revs. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benes F.M., Paskevich P.A., Davidson J., and Domesick V.B. (1985). The effects of haloperidol on synaptic patterns in the rat striatum. Brain Res. 329, 265–273 [DOI] [PubMed] [Google Scholar]
- 33.Besret L., Caldwell M.A., Torres E.M., and Dunnett S.B. (2000). Antioxidant strategy to counteract the side effects of antipsychotic therapy: an in vivo study in rats. Eur. J. Pharmacol. 408, 35–39 [DOI] [PubMed] [Google Scholar]
- 34.Gold P.E. (2003). Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol. Learn. Mem. 80, 194–210 [DOI] [PubMed] [Google Scholar]
- 35.Hasselmo M.E. (2006). The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 16, 710–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang G.B., Zhao T., Li C.R., Sui Z.Y., Kang N.I., Han E.H., and Chung Y.C. (2012). Choline acetyltransferase expression in rat prefrontal cortex and hippocampus after acute and chronic exposure to amisulpride, haloperidol, and risperidone. Neurosci. Lett. 528, 131–136 [DOI] [PubMed] [Google Scholar]
- 37.Yael D., Zeef D.H., Sand D., Moran A., Katz D.B., Cohen D., Temel Y., and Bar-Gad I. (2013). Haloperidol-induced changes in neuronal activity in the striatum of the freely moving rat. Front. Syst. Neurosci. 7, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Campbell A., Baldessarini R.J., Teicher M.H., and Kula N.S. (1985). Prolonged antidopaminergic actions of single doses of butyrophenones in the rat. Psychopharmacology (Berl.) 87, 161–166 [DOI] [PubMed] [Google Scholar]
- 39.Kornhuber J., Schultz A., Wiltfang J., Meineke I., Gleiter C.H., Zöchling R., Boissl K.W., Leblhuber F., and Riederer P. (1999). Persistence of haloperidol in human brain tissue. Am. J. Psychiatry 156, 885–890 [DOI] [PubMed] [Google Scholar]
- 40.Nyberg S., Farde L., and Halldin C. (1997). Delayed normalization of central D2 dopamine receptor availability after discontinuation of haloperidol decanoate. Preliminary findings. Arch. Gen. Psychiatry 54, 953–958 [DOI] [PubMed] [Google Scholar]
- 41.Ukai W., Ozawa H., Tateno M., Hashimoto E., and Saito T. (2004). Neurotoxic potential of haloperidol in comparison with risperidone: implication of Akt-mediated signal changes by haloperidol. J. Neural Transm. 111, 667–681 [DOI] [PubMed] [Google Scholar]
- 42.Cadet J.L., and Kahler L.A. (1994). Free radical mechanisms in schizophrenia and tardive dyskinesia. Neurosci. Biobehav. Rev. 18, 457–467 [DOI] [PubMed] [Google Scholar]
- 43.Galili R., Mosberg , Gil-Ad I., Weizman A., Melamed E., and Offen D. (2000). Haloperidol-induced neurotoxicity—possible implications for tardive dyskinesia. J. Neural Transm. 107, 479–490 [DOI] [PubMed] [Google Scholar]
- 44.Naidu P.S., Singh A., and Kulkarni S.K. (2002). Carvedilol attenuates neuroleptic-induced orofacial dyskinesia: possible antioxidant mechanisms. Br. J. Pharmacol. 136, 193–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parikh V., Khan M.M., and Mahadik S.P. (2003). Differential effects of antipsychotics on expression of antioxidant enzymes and membrane lipid peroxidation in rat brain. J. Psychiatr. Res. 37, 43–51 [DOI] [PubMed] [Google Scholar]
- 46.Karl T., Duffy L., O'Brien E., Matsumoto I., and Dedova I. (2006). Behavioural effects of chronic haloperidol and risperidone treatment in rats. Behav. Brain Res. 171, 286–294 [DOI] [PubMed] [Google Scholar]
- 47.Hutchings E.J., Waller J.L., and Terry A.V., Jr. (2013). Differential long-term effects of haloperidol and risperidone on the acquisition and performance of tasks of spatial working and short-term memory and sustained attention in rats. J. Pharmacol. Exp. Ther. 347, 547–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshikawa T., Minamiyama Y., Naito Y., and Kondo M. (1994). Antioxidant properties of bromocriptine, a dopamine agonist. J. Neurochem. 62, 1034–1038 [DOI] [PubMed] [Google Scholar]
- 49.Liu X.H., Kato H., Chen T., Kato K., and Itoyama Y. (1995). Bromocriptine protects against delayed neuronal death of hippocampal neurons following cerebral ischemia in the gerbil. J. Neurol. Sci. 129, 9–14 [DOI] [PubMed] [Google Scholar]
- 50.McDowell S., Whyte J., and D'Esposito M. (1998). Differential effect of a dopaminergic agonist on prefrontal function in traumatic brain injury patients. Brain 121, 1155–1164 [DOI] [PubMed] [Google Scholar]
- 51.Ogawa N., Tanaka K., Asanuma M., Kawai M., Masumizu T., Kohno M., and Mori A. (1994). Bromocriptine protects mice against 6-hydroxydopamine and scavenges hydroxyl free radicals in vitro. Brain Res. 657, 207–213 [DOI] [PubMed] [Google Scholar]
- 52.Whyte J., Vaccaro M., Grieb-Neff P., Hart T., Polansky M., and Coslett H.B. (2008). The effects of bromocriptine on attention deficits after traumatic brain injury: a placebo-controlled pilot study. Am. J. Phys. Med. Rehabil. 87, 85–99 [DOI] [PubMed] [Google Scholar]
- 53.Dixon C.E., Kraus M.F., Kline A.E., Ma X., Yan H.Q., Griffith R.G., Wolfson B.M., and Marion D.W. (1999). Amantadine improves water maze performance without affecting motor behavior following traumatic brain injury in rats. Restor. Neurol. Neurosci. 14, 285–294 [PubMed] [Google Scholar]
- 54.Giacino J.T., Whyte J., Bagiella E., Kalmar K., Childs N., Khademi A., Eifert B., Long D., Katz D.I., Cho S., Yablon S.A., Luther M., Hammond F.M., Nordenbo A., Novak P., Mercer W., Maurer-Karattup P., and Sherer M. (2012). Placebo-controlled trial of amantadine for severe traumatic brain injury. N. Engl. J. Med. 366, 819–826 [DOI] [PubMed] [Google Scholar]