Abstract
Interhemispheric communication plays a critical role to ensure normal brain functions in cognition and behavior. Since non-human primate (NHP) brains resemble most aspects of the human brain, a thorough knowledge of interhemispheric cortical connectivity changes in NHP brains throughout the developmental and aging periods may provide valuable insights for translational and clinical research. In this study, formalin-fixed rhesus monkey brains aged from 1 to 24 years were utilized to examine transcallosal connectivity changes using diffusion spectrum imaging (DSI). It was found that the transcallosal connectivity for most frontal cortical areas, including dorso- and ventro-lateral prefrontal, premotor, and motor cortices, demonstrated pronounced age-related alterations. However, such a pattern was less obvious in temporal, posterior parietal, and visual cortices. The DSI results reveal the age-related evolution pattern of transcallosal connectivity in various cortical areas of macaque brains from infancy to late adulthood, and may have implications for assessing the functional defects or alterations in the associated cortical areas during brain development and aging in humans.
Key words: : aging, brain maturation, DSI, interhemispheric cortical connectivity, non-human primate, white matter tractography
Introduction
Interhemispheric connectivity plays a crucial role in maintaining normal cognitive functions and behaviors. The interruption of interhemispheric connectivity can result in profound functional disorders in patients, including children and adults (Anderson et al., 2011; Dick et al., 2013; Knochel et al., 2012). A thorough knowledge of the progressive alteration of interhemispheric fiber connectivity during normal brain development and aging can provide valuable insights in understanding cognitive development or decline during the entire lifespan.
Since corpus callosum (CC) serves as a bridge to connect both cerebral hemispheres, most studies of interhemispheric connectivity focus on the CC structure directly (Luders et al., 2010; Nowicka and Tacikowski, 2011; van der Knaap and van der Ham, 2011; Wahl et al., 2007). Previous studies have demonstrated that interhemispheric connectivity is an important indicator of brain dysfunction in different stages of human lifespan (Luders et al., 2010; Ota et al., 2006). Earlier resting-state functional magnetic resonance imaging (rsfMRI) studies have also demonstrated that the interhemispheric functional connectivity changes with age in humans (Tomasi and Volkow, 2012; Vergun et al., 2013). However, most studies were based on a comparison between different age groups, or with the assumption of linear relationship with age in development or degeneration stage of the brain (Makris et al., 2007; Tomasi and Volkow, 2012; Vergun et al., 2013). Due to the limited age range in each study, the actual trend or change can be different from one study to another, depending on its specific age coverage in the entire lifespan. In addition, the white matter maturation and degeneration is differentiated across the entire brain regions. Previous results have illustrated that the frontal lobe is lately maturated compared with other regions and also, the age-related degeneration mostly occurs in the frontal brain (Lebel et al., 2008; Pfefferbaum et al., 2000; Sullivan and Pfefferbaum, 2006; Thompson and Nelson, 2001). Age-related alterations in brain white matter of non-human primates (NHPs) have been reported, but most studies either focused on limited brain areas or failed to offer comprehensive interhemispheric connectivity information for the whole brain (Chen et al., 2013; Peters and Sethares, 2002; Phillips and Sherwood, 2012).
Diffusion tensor imaging (DTI)-based fiber tractography has been applied to determine transcallosal interhemispheric connections between different cortical areas (Hofer and Frahm, 2006). However, DTI is limited to delineate the primary diffusion orientation based on the measurement of anisotropic water diffusion, and it is difficult to detect the crossing or touching fibers in an imaging voxel (Wiegell et al., 2000). For example, DTI-based fiber tracking usually shows the dorsal but not lateral cortical fibers from the anterior CC (Hofer and Frahm, 2006). Since homotopic interconnections exist between the hemispheres and also heterotopic interconnections linking functionally different cortices (Clarke and Zaidel, 1994; de Lacoste et al., 1985; Witelson, 1989), the DTI results might be biased due to the failure to detect crossing or touching fibers. In contrast, diffusion spectrum imaging (DSI) allows sufficient angular resolution and can be used to examine crossing fiber tracts in a single voxel (Wedeen et al., 2005). Therefore, DSI-based fiber tractography can provide a novel and unprecedented opportunity for studying complicated fiber connections in vivo or in postmortem studies (Granziera et al., 2009; Meng and Zhang, 2014; Schmahmann et al., 2007; Wedeen et al., 2005, 2008).
NHPs are highly similar to humans in anatomical structures, functional connections, and brain organizations (Hofer et al., 2008; Nakahara et al., 2007; Thiebaut de Schotten et al., 2012). Multiple similarities in fiber association connections have been reported in both human and macaque monkeys (Thiebaut de Schotten et al., 2012). Information of interhemispheric connectivity alteration with age in NHP brains may provide important implications for translational research in brain development and aging. In this study, the cortically specific changes of transcallosal connectivity were evaluated with formalin-fixed macaque brains from infancy to late adulthood using DSI tractography.
Materials and Methods
Animals
All procedures were approved and in compliance with the Institutional Animal Care and Use Committees (IACUC) of Emory University and the NIH guides for the care and use of laboratory animals. Twelve normal rhesus monkeys (Macaca mulatta) of both sexes (six female and six male animals) from 1 to 24 years old (Table 1) with no history of neurological disease or brain injury were utilized. The animals were euthanized by pentobarbital overdose and immediately intracardially perfused with saline followed by 10% buffered formalin. The whole brains were immersed in formalin and kept in a refrigerator for at least 1 month. These brain samples were collected from earlier projects in which the animals in the control groups were euthanized for research purpose or from the cohort of unassigned monkeys sacrificed due to the IACUC end point (such as illness or injury, etc). Each subject's usage and health history was evaluated by the veterinary staff in our facility to ensure the brain was healthy and intact before being recruited into this study.
Table 1.
The Demographic Information of the Macaque Brains
| No. | Sex | Age (years) |
|---|---|---|
| 1 | Female | 1.4 |
| 2 | Female | 2.0 |
| 3 | Male | 3.1 |
| 4 | Female | 5.9 |
| 5 | Male | 6.4 |
| 6 | Male | 9.9 |
| 7 | Female | 10.3 |
| 8 | Male | 13.8 |
| 9 | Female | 14.1 |
| 10 | Female | 20.2 |
| 11 | Male | 22.3 |
| 12 | Male | 22.8 |
MRI experiments
The experiments were carried out on a Bruker Biospec 7.0T spectrometer (Bruker Biospin Corporation) that was equipped with a 12 cm-ID actively shielded gradient insert (400 mT/m). A 7 cm-ID quadrature volume coil was used for signal excitation and reception. The DSI pulse sequence was custom developed and implemented under the software Paravision 5.1. To reduce eddy current effect due to the demand of high b-values (Koch and Norris, 2000), double spin echo acquisition was applied on a 3D EPI sequence with parameters TE/TR=55 msec/500 ms, FOV=70×50×80 cm3, for 1 mm isotropic spatial resolution. Diffusion was encoded with 514 diffusion directions covering a full q-space 3D grid with a five radical grid size and b-value=40,000 sec/mm2, and one B0 image (b-value=0 sec/mm2). The total scan time of each brain sample was 45 h with two averages, yielding a signal to noise ratio (SNR) of around 45 in the B0 image of the whole brain.
Data processing
Dorsolateral prefrontal cortex (dlPFC), ventrolateral prefrontal cortex (vlPFC), dorsal premotor cortex (dPMC), ventral premotor cortex (vPMC), primary motor cortex (PMC), somatosensory cortex (SSC), temporal cortex (TC), posterior parietal cortex (PPC), visual cortex (VC), and CC were selected based on a T1-weighted macaque brain template by referring a rhesus monkey brain atlas (Saleem and Logothetis, 2006). The template was built with isotropic 0.5 mm spatial resolution from coregistered and averaged T1-weighed images of adult macaque monkeys of both sexes in the Yerkes research center.
The cortical areas defined from the template were registered (with 12 DOF linear affine transformation) to the DSI images without diffusion gradients (B0 images) using the FSL software (FMRIB; Oxford) (Jenkinson et al., 2012) (see Fig. 1 and the template in Fig. 2a). In order to examine the registration errors across ages from infancy to late adulthood, the images of monkey brain template were registered to the DSI B0 images of each monkey brain, and the residual errors between two brain images were compared within three age stages (i.e., ≤5years, >5 years but <15 years, and ≥15 years) with one-way ANOVA using the SPSS 22 software (SPSS, Inc.).
FIG. 1.
Coregistration operation evaluation of macaque brains at different ages. (a) Images registered from the T1-weighted template to B0 images (T2-weighted); (b) The differences between B0 images and the corresponding registered images from the template (as shown in a). The residual signal intensity is mostly due to the contrast difference between the two images. The geometry of the difference images looks as similar as the images in (a), indicating good coregistration operation; (c–h) CC and various cortical areas (the masks) determined from the template were registered to individual monkey brains (green color), with the registration information provided by registering the template to the B0 images. CC, corpus callosum; dlPFC, dorsolateral prefrontal cortex; vlPFC, ventrolateral prefrontal cortex; dPMC, dorsal premotor cortex; vPMC, ventral premotor cortex; PMC, primary motor cortex; SSC, somatosensory cortex; PPC, posterior parietal cortex; TC, temporal cortex; VC, visual cortex.
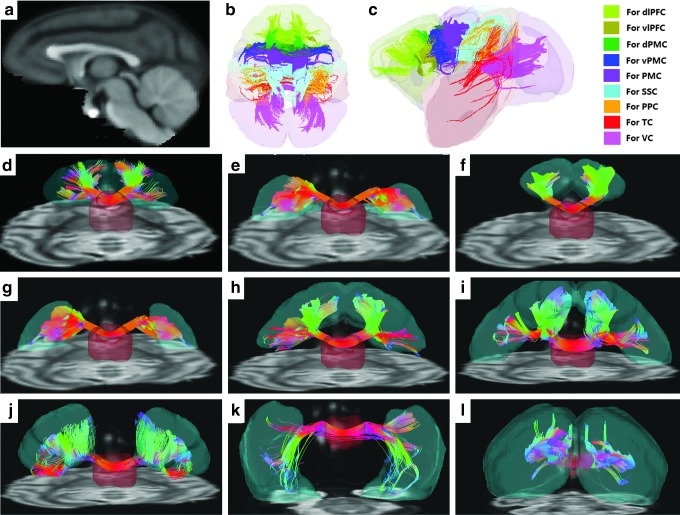
FIG. 2.
Demonstration of the transcallosal fiber tracts for various cortical areas in an adult monkey brain. (a) The sagittal view of a macaque T1-weighted template used to define CC and cortical areas; Axial (b) and sagittal (c) view of the delineated transcallosal fibers, coded with different colors; (d–l) Illustration of the delineated transcallosal fibers crossing CC (light red color in the center of the picture d–l) and the associated cortical areas (light to blue color; from d to l: dlPFC, vlPFC, dPMC, vPMC, PMC, SSC, PPC, TC, and VC). The fiber tracking results are from a fixed adult macaque brain (∼10 years old), overlaid on the DSI B0 images. DSI, diffusion spectrum imaging.
The white matter tracts were constructed using deterministic fiber tracking (Wedeen et al., 2008; Yeh et al., 2013) with the DSI-Studio software (http://dsi-studio.labsolver.org). Cortically transcallosal tractography for the interested cortical areas in both hemispheres was performed in the following two steps. First, the cortical area in the left hemisphere was selected as a seed, and then the CC was used as the waypoint where the fibers are crossing and the fibers terminate in the corresponding cortical area in the right hemisphere, by using 10,000 seeds or iterative fiber tracking numbers. Second, the same procedure was repeated but started with the cortical area in the right hemisphere as a seed. The proportion of the total fibers from the two fiber tracking steps (20,000 tracking numbers in total), or the normalized cortical transcallosal fibers, was used for assessing the transcallosal connection strength for each cortical area. During the data processing, the cortical transcallosal connection strength was defined by calculating the relative values of the fiber numbers connecting different cortical regions through CC. The increased or reduced fiber numbers were associated to the changes of transcallosal connections between the hemispheric cortical areas in each brain.
The relationship between the connection strength and ages of the animals was examined by data fitting with Poisson and quadratic regression models, respectively, using custom-written Matlab scripts (MathWorks). Quadratic model provides a simple correlation with age (Allen et al., 2005; Lebel and Beaulieu, 2011), while Poisson model considers different change patterns during the entire lifespan (Lebel et al., 2010). The following equation was used for the Poisson model:
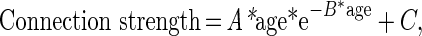 |
in which A, B, and C are the fitting parameters.
To test whether the fitting significance is, indeed, unlikely to occur by chance, permutation test was used in performing multiple comparisons. In the permutation test, each animal's age was randomly assigned to one of the connectivity measurements of all animals and, thus, one permutated data set was obtained; then, the corresponding correlation coefficient value (r) was calculated based on the permutated dataset fitted with Poisson or quadratic models. For 5000 permutations, 5000 correlation coefficient values were obtained (Manly, 1997). Then, the significance of the correlation of the original experimental data was determined based on the distribution of the 5000 correlation coefficient values calculated with the permutated datasets, with a significant level of p<0.05.
Results
As demonstrated in Figure 1, a macaque brain template (T1-weighted) was well coregistered to the DSI B0 images (T2-weighted) of three animal subjects at very different ages (Fig. 1). The residual signal intensity in the difference images is mostly due to the contrast difference between the two images (Fig. 1b). The geometry of the difference images looks as similar as the registered images from the template, indicating good coregistration operation. The registration results of different brain areas in each subject were examined visually and confirmed (Fig. 1c–h). In addition, further quantitative tests indicated that there was no significant difference (p=0.41, with a significance level of p<0.05) in the residual registration errors for the brains at three age stages (i.e., ≤5 years, >5 years but <15 years, and ≥15 years).
The determined transcallosal fiber tracts for various cortical areas in an adult monkey brain (10 years old) were illustrated in Figure 2. As seen in Figure 2d–l, transcallosal fibers for different cortical areas across the whole brain in both dorsal and ventral orientations were successfully determined by DSI tractography.
The changing patterns of transcallosal fibers in different cortical areas of three representative monkey brains were demonstrated in Figure 3. The results indicate that the transcallosal connectivity keeps increasing before the middle age (i.e., ∼13.8 years old), and then declines in the aging period (Figs. 3 and 4). Such change was more pronounced in the frontal lobes and also in SSC (Fig. 3a–f) but less obvious in PPC, temporal, and occipital lobes (Fig. 3g–i).
FIG. 3.
Changes of transcallosal fibers in various cortical areas across the whole brains with three different ages from infancy to late adulthood. Cortically transcallosal fibers for dlPFC (a), vlPFC (b), dPMC (c), vPMC (d), PMC (e), SSC, (f), PPC (g), TC (h), and VC (i) overlaid on the DSI B0 images.
FIG. 4.
Illustrations of Poisson and quadratic curve fittings of the transcallosal connectivity changes in different cortices of the monkey brain from infancy to late adulthood. The interested regions include dlPFC (a), vlPFC (b), dPMC (c), vPMC (d), PMC (e), SSC (f), PPC (g), TC (h), and VC (i). The star (*) indicates that the fitting is statistically significant (p<0.05, after multiple-comparison correction) for the model (see details in Table 2).
Statistics showed different results while fitting the progressive transcallosal connectivity changes with two different fitting models. As shown in Figure 4, the ages at which the transcallosal connectivity reached its maximum were earlier with Poisson model than those with quadratic model (Fig. 4a–f, h, i). In addition, compared with Poisson model, quadratic model failed to delineate the early connectivity changes in PPC (Fig. 4g). As shown in Table 2, the p- and R-values fitted with Poisson model were, respectively, smaller and larger than those fitted with quadratic model, indicating that Poisson model was better than quadratic model for fitting the transcallosal connectivity changes during the lifespan. After multiple comparisons with permutation test, the results showed that the fitting significance took place in dlPFC, vlPFC, dPMC, vPMC, PMC, and TC with the Poisson model, while it was seen only in dlPFC and dPMC with the quadratic model. In addition, as shown in Figure 4, the transcallosal cortical connectivity in the frontal lobe of older adults decreased by about 50% of its maximum value, while such a reduction in temporal and visual cortices was only around 20%.
Table 2.
Fitting Results for the Transcallosal Connectivity of Various Cortical Areas with Age Using Two Regression Models
| Poisson model | Quadratic model | |||||
|---|---|---|---|---|---|---|
| Cortical regions | Age (years) at peak | R | p | Age (years) at peak | R | p |
| dlPFC | 8.1 | 0.89 | 0.0009* | 11.5 | 0.81 | 0.01* |
| vlPFC | 7.3 | 0.76 | 0.02* | 10.6 | 0.75 | 0.03 |
| dPMC | 9.8 | 0.82 | 0.006* | 12.6 | 0.81 | 0.01* |
| vPMC | 10.2 | 0.82 | 0.007* | 12.9 | 0.70 | 0.05 |
| PMC | 8.3 | 0.79 | 0.01* | 11.6 | 0.72 | 0.04 |
| SSC | 7.7 | 0.62 | 0.11 | 10.8 | 0.61 | 0.12 |
| PPC | 4.6 | 0.67 | 0.07 | 8.6 | 0.56 | 0.19 |
| TC | 5.9 | 0.74 | 0.03* | −3.9 | 0.58 | 0.16 |
| VC | 6.3 | 0.66 | 0.07 | 9.4 | 0.46 | 0.34 |
Fitting significance with the models was determined after multiple-comparison corrections with permutation test with a significant level of p<0.05.
dlPFC, dorsolateral prefrontal cortex; dPMC, dorsal premotor cortex; PMC, primary motor cortex; PPC, posterior parietal cortex; SSC, somatosensory cortex; TC, temporal cortex; VC, visual cortex; vlPFC, ventrolateral prefrontal cortex; vPMC, ventral premotor cortex.
Discussion
In this study, DSI tractography was used to examine the progressive changes of the transcallosal connectivity of macaque brains from infancy to late adulthood. It was found that the transcallosal connectivity for most frontal cortical areas, including dorso- and ventro-lateral prefrontal, premotor, and motor cortices, demonstrated pronounced alteration during brain development and aging. In contrast, such changes were much mitigated in temporal, PPC, and VC. The progressive changes with age follow the Poisson pattern. The DSI results reveal the region-specific changes of the cortical transcallosal connectivity in macaque brains throughout the lifespan.
DSI has numerous advantages over DTI in assessing the transcallosal white matter fibers, although DTI has well demonstrated its robustness and sensitivity in many preclinic and clinic studies (Hofer and Frahm, 2006; Hofer et al., 2008; Meng et al., 2014; Schmahmann et al., 2007). The ability of in vivo DSI tractography for determining the extensive transcallosal fibers across segmented CC has been demonstrated in adult macaque monkeys (Meng and Zhang, 2014). In contrast, ex vivo DSI provides improved SNR and spatial resolution, enabling a better detection of white matter bundles. Therefore, it could be utilized to assess the transcallosal connectivity of the different cortical areas in fixed monkey brains, and furthermore, to evaluate the temporal changes of the transcallosal connectivity in the lifespan.
Using DSI tractography, cortically transcallosal connection strength can be evaluated by calculating the fiber numbers connecting different cortical regions through CC. Such method has been exploited previously in evaluating the hippocampal network connections in rhesus monkeys (Koo et al., 2013). In addition, there are alterative measurements, such as zero-displacement probability and q-space inverse variance (Assaf et al., 2000; Wu et al., 2008), which can be used for evaluating diffusion process in the tissue as well.
The age-related changes were analyzed with two different regression models. The results showed that the Poisson model had advantage over the quadratic regression model (Fig. 4 and Table 2). Poisson model considers varying slops for connectivity changes in both developmental and aging periods. In contrast, the quadratic regression model assumes even slops across both the periods (Fjell et al., 2010), though the quadratic regression model provides a simple way to account for the correlated terms over most of the age range (Allen et al., 2005; Lebel and Beaulieu, 2011). As illustrated in Figure 4, the transcallosal connectivity for different cortical areas changed faster in the developmental period than in the aging period, suggesting that the neural fibers maturate quickly during brain development but degenerate slowly during aging, in good agreement with previous studies (Lebel et al., 2010).
It has been reported that the whole brain white matter percentage increases after birth for approximately 5 years in macaque monkeys (Malkova et al., 2006), and the thickness of CC keeps increasing with age in developing macaque brains (Li et al., 2011). Previous DTI studies in human or NHP brain development have demonstrated that fractional anisotropy (FA) values of white matter fibers increase from infancy to the juvenile stage or young adulthood, showing a progressive maturation procedure in the central nervous system (Alexander et al., 2007; Hermoye et al., 2006; Huppi and Dubois, 2006; Yan et al., 2014b), though the white matter maturation progresses differently in different brain structures (Lebel and Beaulieu, 2011; Lebel et al., 2008). Obviously, the findings of the transcallosal connectivity changes from infancy to young adulthood are consistent with the increases in white matter volume, CC thickness, and FA of most white matter fibers in the developing brain. In addition, previous studies in human and NHP brains have demonstrated that the white matter integrity decreases over time during aging (Chen et al., 2013; Yan et al., 2014a). It was also reported that the percentages of normal sheath profiles in prefrontal cortex and CC decreased dramatically in monkeys that were older than 20 years (Peters and Sethares, 2002). The earlier findings are in good agreement with the decreased connectivity observed in the transcallosal connectivity of elder macaque brains.
As seen in Figure 4 and Table 2, the transcallosal connectivity of the frontal lobe increased from 1 year old, reached a maximum at 7–10 years, and then significantly decreased after 15 years old when fitted with the Poisson model. Such changing patterns remain similar in most cortical areas, including dlPFC, vlPFC, dPMC, vPMC, PMC, and SSC (Fig. 4a–f). Specifically, decreasing transcallosal connectivity in the frontal cortex occurs about 1–3 years later than those in parietal, temporal, and occipital lobes (as fitted with Poisson model; Fig. 4g–i and Table 2). In addition, the transcallosal connectivity of the prefrontal, premotor, motor, and temporal cortices correlated significantly with age when fitted with the Poisson model, though there were less cortical areas whose transcallosal connectivity was significantly correlated with the quadratic model (Fig. 4 and Table 2). The temporal changes of transcallosal connectivity in the frontal lobe of monkey brain agree well with the previous results of late white matter maturation in the prefrontal cortex and the frontal lobe connectivity in humans (Lebel et al., 2008; Thompson and Nelson, 2001). Our results are also in good agreement with the finding that the areas of the anterior CC increased more slowly than those of the posterior CC in humans from 5 to 18 years old (Giedd et al., 1999). In this study, the elongated period of increasing interhemispheric connectivity in the frontal areas of developing brains would be attributed to the prolonging maturation of the cortices themselves and the neuronal pathways involved with the associated areas.
In the older adult monkeys, the transcallosal cortical connectivity in the frontal lobe decreased much more than that in the temporal and visual cortices. As shown in Figure 4, compared with most cortical areas in the frontal lobe, the connectivity for SSC, PPC, and VC was not significantly correlated with age by fitting with either the Poisson or quadratic model (Table 2). Although the connectivity for the TC significantly correlated with age when fitted with the Poisson model, the p-value (p=0.03) was much larger than that in other areas whose connectivity significantly correlated with age. The finding that the aging effect is more prominent in the frontal lobe than that in the posterior brain regions, such as cortical areas in the occipital lobe, has been previously revealed by DTI (Pfefferbaum et al., 2000; Sullivan and Pfefferbaum, 2006). The heterogeneity of normal neuroanatomical degeneration was also demonstrated by volumetric measurement in adult human brains which were more than 22 years old, showing that the gray matter in the frontal lobe was the most strongly associated with aging, while that in the occipital lobe was the least associated with it (Allen et al., 2005). Our present results showed that interhemispheric connectivity changes over time were more pronounced in the frontal lobe, which was consistent with what were previously reported in humans (Allen et al., 2005; Pfefferbaum et al., 2000; Sullivan and Pfefferbaum, 2006).
Abnormal alterations in interhemispheric cortical connectivity might be associated with cognitive dysfunctions and abnormal behaviors in the lifespan. A previous rsfMRI study has demonstrated that decreased interhemispheric functional connectivity in sensorimotor cortex, superior temporal gyrus, and superior parietal lobule was relevant to functional defects in autism patients (Anderson et al., 2011). A decline of anterior white matter integrity was also found in elderly people with cognitive impairment (Teipel et al., 2010). Such cognitive disorders could be associated with the abnormal transcallosal connectivity in the relevant cortical regions. Since the life expectancy of macaque monkeys is about 25 years (Primate Info Net, http://pin.primate.wisc.edu), this study consists of macaques from 1 to 24 years old, resembling the human lifespan from infancy to late adulthood. The changing pattern of the connectivity in macaque brain may provide a valuable baseline reference in translational research for the functional defects or alterations in the associated cortical areas during development and aging of the human brain (Paus et al., 2008).
Conclusion
In this work, DSI tractography was used to evaluate the cortically specific changes of transcallosal connectivity across anterior-posterior brain lobes of formalin-fixed macaque brains from infancy to late adulthood. The changes were age-related and varied for different brain lobes. The similarity of the connectivity change patterns in both human and macaque brains suggests that the macaque monkey is a valuable model for translational research of the developmental and aging brain.
Acknowledgments
This project was supported by the National Center for Research Resources P51RR000165 and currently by the Office of Research Infrastructure Programs/OD P51OD011132. The authors are grateful to the pathology department of Yerkes National Primate Research Center for the sample collection and to anonymous reviewers for their valuable suggestions.
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander AL, Lee JE, Lazar M, Field AS. 2007. Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. 2005. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiol Aging 26:1245–1260 [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, Abildskov T, Nielsen JA, Cariello AN, Cooperrider JR, Bigler ED, Lainhart JE. 2011. Decreased interhemispheric functional connectivity in autism. Cereb Cortex 21:1134–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assaf Y, Mayk A, Cohen Y. 2000. Displacement imaging of spinal cord using q-space diffusion-weighted MRI. Magn Reson Med 44:713–722 [DOI] [PubMed] [Google Scholar]
- Chen X, Errangi B, Li L, Glasser MF, Westlye LT, Fjell AM, Walhovd KB, Hu X, Herndon JG, Preuss TM, Rilling JK. 2013. Brain aging in humans, chimpanzees (Pan troglodytes), and rhesus macaques (Macaca mulatta): magnetic resonance imaging studies of macro- and microstructural changes. Neurobiol Aging 34:2248–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JM, Zaidel E. 1994. Anatomical-behavioral relationships: corpus callosum morphometry and hemispheric specialization. Behav Brain Res 64:185–202 [DOI] [PubMed] [Google Scholar]
- de Lacoste MC, Kirkpatrick JB, Ross ED. 1985. Topography of the human corpus callosum. J Neuropathol Exp Neurol 44:578–591 [DOI] [PubMed] [Google Scholar]
- Dick AS, Raja Beharelle A, Solodkin A, Small SL. 2013. Interhemispheric functional connectivity following prenatal or perinatal brain injury predicts receptive language outcome. J Neurosci 33:5612–5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Westlye LT, Ostby Y, Tamnes CK, Jernigan TL, Gamst A, Dale AM. 2010. When does brain aging accelerate? Dangers of quadratic fits in cross-sectional studies. Neuroimage 50:1376–1383 [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Rajapakse JC, Vaituzis AC, Liu H, Berry YC, Tobin M, Nelson J, Castellanos FX. 1999. Development of the human corpus callosum during childhood and adolescence: a longitudinal MRI study. Prog Neuropsychopharmacol Biol Psychiatry 23:571–588 [DOI] [PubMed] [Google Scholar]
- Granziera C, Schmahmann JD, Hadjikhani N, Meyer H, Meuli R, Wedeen V, Krueger G. 2009. Diffusion spectrum imaging shows the structural basis of functional cerebellar circuits in the human cerebellum in vivo. PLoS One 4:e5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoye L, Saint-Martin C, Cosnard G, Lee SK, Kim J, Nassogne MC, Menten R, Clapuyt P, Donohue PK, Hua K, Wakana S, Jiang H, van Zijl PC, Mori S. 2006. Pediatric diffusion tensor imaging: normal database and observation of the white matter maturation in early childhood. Neuroimage 29:493–504 [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. 2006. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32:989–994 [DOI] [PubMed] [Google Scholar]
- Hofer S, Merboldt KD, Tammer R, Frahm J. 2008. Rhesus monkey and human share a similar topography of the corpus callosum as revealed by diffusion tensor MRI in vivo. Cereb Cortex 18:1079–1084 [DOI] [PubMed] [Google Scholar]
- Huppi PS, Dubois J. 2006. Diffusion tensor imaging of brain development. Semin Fetal Neonatal Med 11:489–497 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. 2012. FSL. Neuroimage 62:782–790 [DOI] [PubMed] [Google Scholar]
- Knochel C, Oertel-Knochel V, Schonmeyer R, Rotarska-Jagiela A, van de Ven V, Prvulovic D, Haenschel C, Uhlhaas P, Pantel J, Hampel H, Linden DE. 2012. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. Neuroimage 59:926–934 [DOI] [PubMed] [Google Scholar]
- Koch M, Norris DG. 2000. An assessment of eddy current sensitivity and correction in single-shot diffusion-weighted imaging. Phys Med Biol 45:3821–3832 [DOI] [PubMed] [Google Scholar]
- Koo BB, Oblak AL, Zhao Y, Farris CW, Bowley B, Rosene DL, Killiany RJ. 2013. Hippocampal network connections account for differences in memory performance in the middle-aged rhesus monkey. Hippocampus 23:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Beaulieu C. 2011. Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 31:10937–10947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Caverhill-Godkewitsch S, Beaulieu C. 2010. Age-related regional variations of the corpus callosum identified by diffusion tensor tractography. Neuroimage 52:20–31 [DOI] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C. 2008. Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055 [DOI] [PubMed] [Google Scholar]
- Li C, Chan A, Zhang X. 2011. Longitudinal study of the corpus callosum thickness in developing monkeys. Proceedings of the 19th Annual Meeting of ISMRM (International Society of Magnetic Resonance in Medicine): Montreal, p. 2082 [Google Scholar]
- Luders E, Thompson PM, Toga AW. 2010. The development of the corpus callosum in the healthy human brain. J Neurosci 30:10985–10990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Papadimitriou GM, van der Kouwe A, Kennedy DN, Hodge SM, Dale AM, Benner T, Wald LL, Wu O, Tuch DS, Caviness VS, Moore TL, Killiany RJ, Moss MB, Rosene DL. 2007. Frontal connections and cognitive changes in normal aging rhesus monkeys: a DTI study. Neurobiol Aging 28:1556–1567 [DOI] [PubMed] [Google Scholar]
- Malkova L, Heuer E, Saunders RC. 2006. Longitudinal magnetic resonance imaging study of rhesus monkey brain development. Eur J Neurosci 24:3204–3212 [DOI] [PubMed] [Google Scholar]
- Manly B. 1997. Randomization, Bootstrap, and Monte Carlo Methods in Biology, 2nd ed. London: Chapman and Hall [Google Scholar]
- Meng Y, Payne C, Li L, Hu X, Zhang X, Bachevalier J. 2014. Alterations of hippocampal projections in adult macaques with neonatal hippocampal lesions: A Diffusion Tensor Imaging study. NeuroImage 102P2:828–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Zhang X. 2014. In vivo diffusion spectrum imaging of non-human primate brain: initial experience in transcallosal fiber examination. Quant Imaging Med Surg 4:129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara K, Adachi Y, Osada T, Miyashita Y. 2007. Exploring the neural basis of cognition: multi-modal links between human fMRI and macaque neurophysiology. Trends Cogn Sci 11:84–92 [DOI] [PubMed] [Google Scholar]
- Nowicka A, Tacikowski P. 2011. Transcallosal transfer of information and functional asymmetry of the human brain. Laterality 16:35–74 [DOI] [PubMed] [Google Scholar]
- Ota M, Obata T, Akine Y, Ito H, Ikehira H, Asada T, Suhara T. 2006. Age-related degeneration of corpus callosum measured with diffusion tensor imaging. Neuroimage 31:1445–1452 [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. 2008. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Sethares C. 2002. Aging and the myelinated fibers in prefrontal cortex and corpus callosum of the monkey. J Comp Neurol 442:277–291 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. 2000. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med 44:259–268 [DOI] [PubMed] [Google Scholar]
- Phillips KA, Sherwood CC. 2012. Age-related differences in corpus callosum area of capuchin monkeys. Neuroscience 202:202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem KS, Logothetis NK. 2006. A Combined MRI and Histology Atlas of the Rhesus Monkey Brain in Stereotaxic Coordinates. London, UK: Academic Press [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, de Crespigny AJ, Wedeen VJ. 2007. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain 130:630–653 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. 2006. Diffusion tensor imaging and aging. Neurosci Biobehav Rev 30:749–761 [DOI] [PubMed] [Google Scholar]
- Teipel SJ, Meindl T, Wagner M, Stieltjes B, Reuter S, Hauenstein KH, Filippi M, Ernemann U, Reiser MF, Hampel H. 2010. Longitudinal changes in fiber tract integrity in healthy aging and mild cognitive impairment: a DTI follow-up study. J Alzheimers Dis 22:507–522 [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M. 2012. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex 48:82–96 [DOI] [PubMed] [Google Scholar]
- Thompson RA, Nelson CA. 2001. Developmental science and the media. Early brain development. Am Psychol 56:5–15 [DOI] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. 2012. Aging and functional brain networks. Mol Psychiatry 17:471, 549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap LJ, van der Ham IJM. 2011. How does the corpus callosum mediate interhemispheric transfer? A review. Behav Brain Res 223:211–221 [DOI] [PubMed] [Google Scholar]
- Vergun S, Deshpande AS, Meier TB, Song J, Tudorascu DL, Nair VA, Singh V, Biswal BB, Meyerand ME, Birn RM, Prabhakaran V. 2013. Characterizing functional connectivity differences in aging adults using machine learning on resting state fMRI data. Front Comput Neurosci 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. 2007. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J Neurosci 27:12132–12138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. 2005. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 54:1377–1386 [DOI] [PubMed] [Google Scholar]
- Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WY, Dai G, Pandya DN, Hagmann P, D'Arceuil H, de Crespigny AJ. 2008. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage 41:1267–1277 [DOI] [PubMed] [Google Scholar]
- Wiegell MR, Larsson HB, Wedeen VJ. 2000. Fiber crossing in human brain depicted with diffusion tensor MR imaging. Radiology 217:897–903 [DOI] [PubMed] [Google Scholar]
- Witelson SF. 1989. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain 112(Pt 3):799–835 [DOI] [PubMed] [Google Scholar]
- Wu YC, Field AS, Alexander AL. 2008. Computation of diffusion function measures in q-space using magnetic resonance hybrid diffusion imaging. IEEE Trans Med Imaging 27:858–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Li L, Preuss TM, Hu X, Herndon JG, Zhang X. 2014a. In vivo evaluation of optic nerve aging in adult rhesus monkey by diffusion tensor imaging. Quant Imaging Med Surg 4:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y, Nair G, Li L, Patel S, Wilson M, Hu X, Sanchez M, Zhang X. 2014b. In vivo evaluation of optic nerve development in non-human primates by using diffusion tensor imaging. Int J Dev Neurosci 32:64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, Verstynen TD, Wang Y, Fernandez-Miranda JC, Tseng WY. 2013. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 8:e80713. [DOI] [PMC free article] [PubMed] [Google Scholar]