Summary
Statin-induced myopathy (SIM) is the most common reason for discontinuation of statin therapy. A polymorphism affecting the gene encoding glycine amidinotransferase (GATM rs9806699 G>A) was previously associated with reduced risk for SIM. Our objective was to replicate the GATM association in a large, multicenter SIM case-control study. Mild and severe SIM cases and age- and gender-matched controls were enrolled. Participants were genotyped, and associations were tested (n=715) using chi-square and logistic regression with consideration for SIM severity and exclusion of subjects with potentially confounding co-medications. The minor allele (A) frequencies of GATM rs9806699 in the controls (n=106), mild SIM (n=324), and severe SIM (n=285) cases were 0.26, 0.28, and 0.29, respectively (p=0.447). The unadjusted odds ratio for the A allele for any SIM (mild or severe) was 1.14 (0.82–1.61; p=0.437), which remained non-significant in all models. Our results do not replicate the association between GATM rs9806699 and SIM.
Introduction
Statins, or 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are highly effective in reducing the risk for major cardiovascular events. The most common reason Americans discontinue their statin therapy is muscle-related side effects (Cohen et al., 2012), or statin-induced myopathy (SIM). SIM can range in severity from mild, tolerable symptoms to life-threatening rhabdomyolysis. Rhabdomyolysis is rare (≤ 0.1% frequency), but SIM has been reported by up to 25% and 60% of current and former statin users, respectively (Cohen et al., 2012). Until the factors for predicting SIM are characterized, patients will continue to experience SIM at unacceptably high rates or experience unnecessary cardiovascular events (as a result of discontinuation of their statin therapy) (McGinnis et al., 2009; Simpson and Mendys, 2010; The West of Scotland Coronary Prevention Study Group, 1997).
Mangravite et al. (2013) recently reported a potential genetic marker for decreased risk of SIM. Using gene expression profiling of lymphoblastoid cell lines (LCLs), they identified an interaction between a cis-eQTL for the glycine amidinotransferase gene (GATM; rs9806699; G>A) and simvastatin exposure (Mangravite et al., 2013). Glycine amidinotransferase is the rate-limiting enzyme required for creatine biosynthesis. Creatine is predominantly synthesized in the liver and kidneys and is subsequently transported to skeletal muscle, providing an important source of cellular energy. The A allele was associated with a greater decrease in GATM RNA expression in simvastatin-exposed LCLs compared to non-exposed control LCLs. Mangravite et al. (2013) subsequently translated these in vitro findings, investigating the effect of the GATM rs9806699 G>A polymorphism in two independent clinical studies (total SIM cases n = 172). The A allele was significantly associated with decreased risk of SIM (meta-analysis odds ratio = 0.60; 95% confidence interval = 0.45–0.81; p = 6 × 10−4). Mangravite et al. (2013) hypothesized that the observed protective effect of the A allele results from the attenuation of the cellular processes necessary for SIM development, and the attenuation results from the diminished myocellular capacity to store energy as phosphocreatine (as a result of decreased creatine availability) associated with the GATM A allele. In a previous issue of Cell Metabolism, Ballard and Thompson (2013) detail and explore the mechanism proposed by Mangravite et al. (2013), concluding that additional investigation is necessary in order to determine the significance of GATM rs9806699 in the development of SIM. A protective effect of GATM rs9806699 was not identified in a prior genome-wide association study (GWAS) (SEARCH Collaborative Group et al., 2008) and, furthermore, GATM deficiency has been identified as a contributing factor of myopathy (Edvardson et al., 2010).
Two replication studies by Carr et al. (2014) (n = 150 SIM cases) and Floyd et al. (2014) (n = 175 SIM cases) did not replicate the association reported by Mangravite et al. (2013). Carr et al. (2014) reported an odds ratio = 0.94 (p = 0.68) and Floyd et al. (2014) reported an odds ratio = 0.84 (95% CI = 0.52–1.36; p = 0.49; excluding fibrate users) for the GATM rs9806699 A allele. Fixed-effect meta-analyses by both Floyd et al. (2014) and Mangravite et al. (2014) both yielded null associations for rs9806699. The following hypotheses have been proposed to explain the failed replications of the GATM rs9806699 association with SIM: 1) low power to detect a modest effect size; 2) the A allele is only protective against mild SIM; 3) the association is masked by confounding co-medications; and 4) patient heterogeneity. An adequately powered study of well-characterized SIM cases is necessary for a more definitive understanding of the clinical validity of GATM rs9806699 in SIM. Herein we address the hypotheses for failed replication of GATM rs9806699 and SIM in our own replication study, using a large, multicenter, independent case-control study that includes both mild and severe SIM.
Results
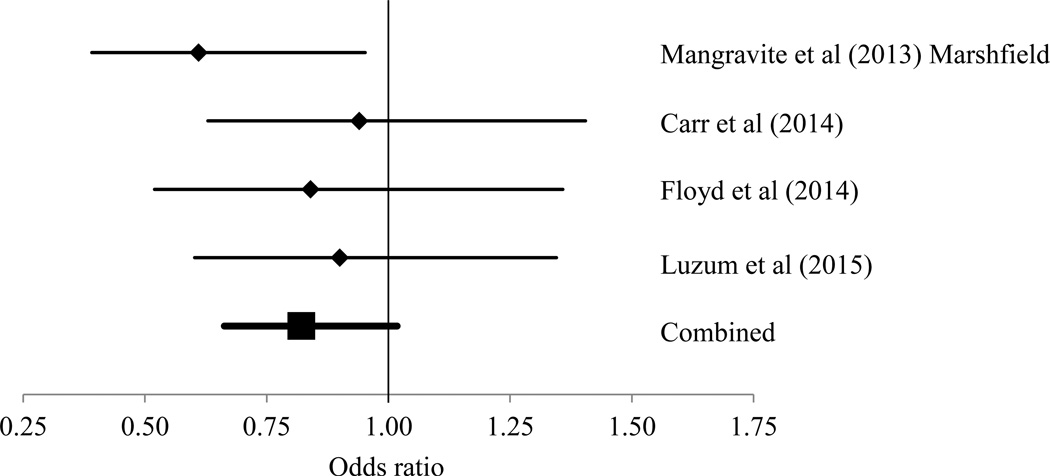
A total of 836 participants were enrolled in this multicenter, case-control study of SIM. Controls were treated with statin therapy for at least 12 months without myopathic symptoms. To enter the study, the participants could have been physician-referred (53%) or self-referred (47%), but the SIM phenotype was evaluated via the same questionnaire in all participants, regardless of the mode of study entry. Caucasian participants with known statin treatment and GATM rs9806699 genotype were included in the analysis (n = 715). Analysis of other racial/ethnic groups was underpowered due to small sample sizes (n < 50 in each non-Caucasian racial/ethnic group). The GATM rs9806699 polymorphism was in Hardy-Weinberg equilibrium in the Caucasian subjects (p = 0.270). Participant characteristics stratified by GATM rs9806699 genotypes and SIM status are displayed in Table 1. The differences in the characteristics and sample sizes between the cases and controls were eliminated in a propensity-matched dataset (see Supplemental Information Table S1). The GATM rs9806699 minor allele and genotype frequencies in the controls, mild SIM, and severe SIM cases are displayed and compared in Table 2. No significant differences in GATM rs9806699 allele or genotype frequencies were found in the complete analytical dataset (n = 715), in the sub-group of subjects without potentially confounding co-medications (n = 386), or in the propensity-matched dataset (n = 188). The GATM rs9806699 polymorphism was not significantly associated with SIM (mild and severe combined), mild SIM alone, or severe SIM alone in the complete analytical dataset (n = 715), the limited dataset of subjects without potentially confounding co-medications (n = 386), or the propensity-matched dataset (n = 188) (Table 3). A summary of all studies investigating the association between GATM variants and SIM is displayed in the Supplemental Data Table S2, and the results for a fixed-effects meta-analysis for rs9806699 are presented in Figure 1. The meta-analysis yielded a marginal, but still null, association between GATM rs9806699 and SIM: odds ratio = 0.82 (95% CI = 0.66–1.02; p = 0.072).
Table 1.
Participant characteristics overall and stratified by GATM rs9806699 G>A genotype and SIM status.
| Cases (n = 609; 85%) | Controls (n = 106; 15%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | All (n =715) |
A/A (n = 43; 6%) |
A/G (n = 261; 37%) |
G/G (n = 305; 43%) |
A/A (n = 7; 1%) |
A/G (n = 41; 6%) |
G/G (n = 58; 8%) |
ap-value |
| Male gender | 374 (52%) | 22 (51%) | 134 (51%) | 170 (56%) | 4 (57%) | 18 (44%) | 26 (46%) | 0.615 |
| Age (years) | 58 (11) | 58 (15) | 58 (16) | 58 (15) | 64 (13) | 61 (13) | 60 (17) | 0.686 |
| bAtorvastatin dose equivalents (mg) | 20 (10) | 20 (30) | 10 (30) | 20 (10) | 15 (30) | 10 (20) | 10 (10) | 0.374 |
| Atorvastatin | 392 (55%) | 26 (60%) | 144 (55%) | 148 (49%) | 4 (57%) | 27 (66%) | 43 (74%) | 0.006 |
| Simvastatin | 143 (20%) | 4 (9%) | 53 (20%) | 75 (25%) | 2 (29%) | 6 (15%) | 3 (5%) | |
| Rosuvastatin | 83 (12%) | 10 (23%) | 29 (11%) | 39 (13%) | 0 (0%) | 2 (5%) | 3 (5%) | |
| Pravastatin | 52 (7%) | 1 (2%) | 20 (8%) | 24 (8%) | 0 (0%) | 2 (5%) | 5 (9%) | |
| Lovastatin | 30 (4%) | 0 (0%) | 11 (4%) | 13 (4%) | 0 (0%) | 4 (10%) | 2 (3%) | |
| Other statin | 15 (2%) | 2 (5%) | 4 (2%) | 6 (2%) | 1 (14%) | 0 (0%) | 2 (3%) | |
| Coronary artery disease | 137 (19%) | 8 (19%) | 57 (22%) | 64 (21%) | 1 (14%) | 3 (7%) | 4 (7%) | 0.045 |
| Myocardial infarction | 97 (14%) | 6 (14%) | 39 (15%) | 44 (14%) | 2 (29%) | 5 (12%) | 2 (3%) | 0.219 |
| Diabetes | 109 (15%) | 8 (19%) | 41 (16%) | 48 (16%) | 2 (29%) | 4 (10%) | 6 (10%) | 0.628 |
| Hypertension | 338 (47%) | 24 (56%) | 130 (50%) | 154 (50%) | 2 (29%) | 10 (24%) | 17 (29%) | 0.001 |
| Smoker | 245 (34%) | 19 (44%) | 104 (40%) | 103 (34%) | 2 (29%) | 7 (17%) | 9 (16%) | 0.001 |
| Family history of heart disease | 345 (48%) | 21 (49%) | 138 (53%) | 164 (54%) | 3 (43%) | 9 (22%) | 10 (17%) | < .001 |
| Family history of muscle disease | 42 (6%) | 4 (9%) | 18 (7%) | 16 (5%) | 0 (0%) | 1 (2%) | 3 (5%) | 0.758 |
| Metabolic muscle disease | 19 (3%) | 3 (7%) | 5 (2%) | 11 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.217 |
| Inflammatory muscle disease | 55 (8%) | 3 (7%) | 25 (10%) | 26 (9%) | 0 (0%) | 0 (0%) | 1 (2%) | 0.114 |
| Hypothyroidism | 91 (13%) | 3 (7%) | 41 (16%) | 40 (13%) | 0 (0%) | 3 (7%) | 4 (7%) | 0.200 |
| Heavy alcohol consumption | 17 (2%) | 1 (2%) | 8 (3%) | 8 (3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.768 |
| Obesity | 122 (17%) | 11 (26%) | 52 (20%) | 54 (18%) | 0 (0%) | 3 (7%) | 2 (3%) | 0.008 |
| Liver disease | 17 (2%) | 0 (0%) | 11 (4%) | 6 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.326 |
| Kidney disease | 14 (2%) | 1 (2%) | 7 (3%) | 5 (2%) | 0 (0%) | 0 (0%) | 1 (2%) | 0.881 |
| cParticipants taking ≥ 1 co-medication(s) that could independently cause myopathy | 34 (5%) | 2 (5%) | 13 (5%) | 18 (6%) | 0 (0%) | 1 (2%) | 0 (0%) | 0.480 |
| cParticipants taking ≥ 1 co-medication(s) that could treat SIM | 260 (36%) | 21 (49%) | 114 (44%) | 109 (36%) | 0 (0%) | 7 (17%) | 9 (16%) | <.001 |
| cParticipants taking ≥ 1 co-medication(s) that could increase statin exposure | 77 (11%) | 7 (16%) | 31 (12%) | 29 (10%) | 0 (0%) | 3 (7%) | 7 (12%) | 0.665 |
| cParticipants taking ≥ 1 co-medication(s) that could decrease statin exposure | 7 (1%) | 1 (2%) | 2 (1%) | 2 (1%) | 0 (0%) | 1 (2%) | 1 (2%) | 0.333 |
Continuous variables are presented as median (interquartile range) and compared with the Kruskal-Wallis test. Categorical variables are presented as counts (%) and compared with the chi-square or Fisher’s exact test where necessary. P-values are for the comparison of all groups stratified by GATM rs9806699 genotype and SIM status. Bolded values are for p < 0.05.
Atorvastatin dose equivalents were calculated as follows: fluvastatin dose/8, lovastatin dose/4, pravastatin dose/4, rosuvastatin dose*4, and simvastatin dose/2 (Stone et al., 2014). Dose equivalents were missing for cerivastatin (n = 6) and pitavastatin (n = 1).
Categories of potentially confounding co-medications are defined in the Supplemental Experimental Procedures section. GATM = gene encoding glycine amidinotransferase; SIM = statin-induced myopathy
Table 2.
GATM rs9806699 minor allele and genotype frequencies in the controls, mild SIM cases, and severe SIM cases.
| All subjects (n = 715) | ||||
| Control (n = 106) |
Mild SIM (n = 324) |
Severe SIM (n = 285) |
p-value | |
| MAF (A) | 0.26 | 0.28 | 0.29 | 0.447 |
| A/A | 0.07 | 0.08 | 0.06 | 0.436 |
| G/A | 0.39 | 0.40 | 0.47 | |
| G/G | 0.55 | 0.52 | 0.48 | |
| Subjects without confounding co-medications (n = 386) | ||||
| Control (n = 80) |
Mild SIM (n = 165) |
Severe SIM (n = 141) |
p-value | |
| MAF (A) | 0.28 | 0.26 | 0.25 | 0.605 |
| A/A | 0.09 | 0.07 | 0.04 | 0.598 |
| G/A | 0.38 | 0.39 | 0.43 | |
| G/G | 0.54 | 0.55 | 0.54 | |
| Propensity-matched dataset (n = 188) | ||||
| Control (n = 94) |
Mild SIM (n = 44) |
Severe SIM (n = 50) |
p-value | |
| MAF (A) | 0.26 | 0.31 | 0.28 | 0.489 |
| A/A | 0.07 | 0.07 | 0.04 | 0.475 |
| G/A | 0.37 | 0.48 | 0.48 | |
| G/G | 0.55 | 0.45 | 0.48 | |
MAF = minor allele frequency; SIM = statin-induced myopathy
Table 3.
Logistic regression modeling results
| All subjects (n = 715) | |||||
| Model | Outcome | Covariates | Odds ratio | 95% CI | p-value |
| 1 | control vs SIM (mild and severe) | none | 1.14 | (0.82–1.61) | 0.437 |
| 2 | control vs SIM (mild and severe) | age + sex | 1.17 | (0.78–1.77) | 0.447 |
| 4 | control vs mild SIM only | none | 1.11 | (0.79–1.58) | 0.548 |
| 5 | control vs mild SIM only | age + sex | 1.15 | (0.75–1.77) | 0.517 |
| 6 | control vs severe SIM only | none | 1.18 | (0.81–1.72) | 0.382 |
| 7 | control vs severe SIM only | age + sex | 1.19 | (0.76–1.85) | 0.453 |
| Subjects without potentially confounding medications (n = 386) | |||||
| Model | Outcome | Covariates | Odds ratio | 95% CI | p-value |
| 8 | control vs SIM (mild and severe) | none | 0.90 | (0.60–1.34) | 0.598 |
| 9 | control vs SIM (mild and severe) | age + sex | 1.00 | (0.61–1.62) | 0.982 |
| 11 | control vs mild SIM only | none | 0.93 | (0.61–1.42) | 0.737 |
| 12 | control vs mild SIM only | age + sex | 1.06 | (0.63–1.77) | 0.833 |
| 13 | control vs severe SIM only | none | 0.86 | (0.55–1.36) | 0.523 |
| 14 | control vs severe SIM only | age + sex | 0.91 | (0.53–1.57) | 0.740 |
| Propensity-matched dataset (n = 188) | |||||
| Model | Outcome | Covariates | Odds ratio | 95% CI | p-value |
| 15 | control vs SIM (mild and severe) | none | 1.19 | (0.74–1.90) | 0.475 |
| 16 | control vs SIM (mild and severe) | age + sex | 1.33 | (0.75–2.36) | 0.338 |
| 17 | control vs mild SIM only | none | 1.23 | (0.70–2.16) | 0.482 |
| 18 | control vs mild SIM only | age + sex | 1.70 | (0.80–3.60) | 0.170 |
| 19 | control vs severe SIM only | none | 1.08 | (0.61–1.88) | 0.800 |
| 20 | control vs severe SIM only | age + sex | 1.17 | (0.60–2.28) | 0.648 |
CI = confidence interval; SIM = statin-induced myopathy
Figure 1.
Forest plot of odds ratios and 95% confidence intervals from individual studies of the association between GATM rs9806699 and SIM and the combined results from the fixed-effect meta-analysis. Data excluding fibrate users (Mangravite et al [2013] Marshfield cohort and Floyd et al [2014]) and any potentially interacting medications (Luzum et al [2015]) was used, except for the data from Carr et al (2014) because concomitant medication information was not available. The 95% confidence intervals from the Carr et al (2014) study were also not available, and thus they were estimated using the standard error from our data (lowest standard error of all studies).
Discussion
The protective effect of GATM rs9806699 G>A reported by Mangravite et al. (2013) was not replicated in our case-control analyses of SIM in 715 Caucasians. Our null findings are consistent with those reported in two other recent studies by Carr et al. (2014) and Floyd et al. (2014). Furthermore, our analysis addressed several of the limitations that were proposed as causative for the previously failed replications. Our study has the largest number of SIM cases (n = 609) among the previously reported association studies (n = 175 was largest number of SIM cases in the study by Floyd et al. [2014]) and sufficient power to detect at least a 25% reduction in the risk of SIM with GATM rs9806699 (i.e., 80% power to detect an odds ratio ≤ 0.75 in our overall sample) (Demidenko, 2007). We tested specifically whether the A allele was protective against mild SIM, severe SIM, or both groups combined. We performed analyses limited to a sub-group of participants without potentially confounding co-medications, and in a propensity-matched dataset in which differences in characteristics and sample sizes between cases and controls were eliminated. In all of these analyses, the association between GATM rs9806699 and SIM reported by Mangravite et al. (2013) was not observed. Notably, a direct comparison of the patient characteristics in our study to the Mangravite et al (2013) study is not possible with the available published data. A few patient characteristics can be found in the original Marshfield (Mareedu et al., 2009) and SEARCH (SEARCH Collaborative Group et al., 2008) publications for the overall patient samples, but the characteristics of the sub-groups used specifically in the Mangravite et al (2013) analysis are unavailable. However some notable differences between our study and the overall Marshfield and SEARCH studies include the statin treatment (our study is the only one to include rosuvastatin) and gender (the SEARCH trial was 83% male).
Adding our data to the previous data by Mangravite et al (2013), Carr et al (2014), and Floyd et al (2014) to a fixed-effect meta-analysis still yielded a null association; although it tended toward significance (p = 0.072). Notably, the Mangravite et al (2013) data from the SEARCH trial could not be included in our meta-analysis because rs9806699 was not genotyped. Other variants in GATM in linkage disequilibrium with rs9806699 (rs1719247 and rs1346268) were statistically significant in the SEARCH trial. Because our meta-analysis tended toward significance, it is possible that the addition of the SEARCH trial data to a meta-analysis (if SEARCH had genotyped rs9806699) could have yielded a significant association. Therefore the A allele may have a small protective effect in a limited population of patients not treated with confounding co-medications, but further studies are needed to determine the clinical relevance of that scenario.
Patient phenotypic heterogeneity among studies of GATM rs9806699 and SIM (and SIM studies in general) is a major issue. SIM symptoms can include muscle pain, tenderness, stiffness, cramping, weakness, and/or fatigue. Patients often present with muscle symptoms but without creatine kinase (CK) elevations or present with CK elevations and no muscle symptoms (Baker and Samjoo, 2008). No widely accepted standard definition of SIM has been established (Rosenson et al., 2014). The subjective (muscle complaints) and objective (biochemical markers) measures delineating SIM vary significantly among studies (Rosenson et al., 2014). Mangravite et al. (2013) defined cases of SIM as patients with muscle symptoms and serum CK concentrations > 3 times the upper limit of normal (xULN) and >10×ULN. In contrast, our study did not use CK in the definition of SIM because only 34% of the combined mild and severe SIM participants in this retrospective study reported measurement of CK. Arguably, the absence of CK data in our definition of SIM may be a limitation; however, Mangravite et al. (2013) did not find an association between GATM rs9806699 and CK concentrations, and CK does not routinely correlate with the presence or severity of muscle symptoms. Furthermore, the current cholesterol treatment guidelines by the American College of Cardiology and the American Heart Association recommend monitoring CK levels only after severe muscle symptoms occur. Monitoring CK levels is not performed for the purpose of establishing a diagnosis of SIM but for identifying the presence of rhabdomyolysis (Stone et al., 2014). We argue that any muscle effect severe enough to trigger a decrease or discontinuation of statin therapy is clinically relevant; muscle symptoms in the absence of CK elevations have been documented (Phillips, et al., 2002).
Lack of reproducibility in biomedical research is being increasingly recognized as a major problem (Collins and Tabak, 2014). This is troubling for the research community because it can cause stakeholders, such as patients, clinicians, policymakers, and funding agencies, to lose confidence in biomedical research. Further investigation of potential reasons for the repeated failed replication of the findings by Mangravite et al. (2013) is warranted. The study reported by Mangravite et al. (2013) was robust and of the highest quality: a combination of functional in vitro findings translated to two independent clinical studies. Therefore a false positive finding by Mangravite et al. (2013) is highly unlikely. Alternatively, false null findings by our group, Carr et al. (2014), and Floyd et al. (2014) are also highly unlikely because the association failed replication in three separate clinical studies performed by three independent research groups. A key piece of information that may solve this apparent contradiction is the mechanism by which GATM variation impacts SIM; currently the mechanism can only be hypothesized. Experiments establishing the mechanism of GATM involvement with SIM are necessary and could shed light on the specific conditions, if any, in which GATM affects SIM. If a clear mechanism cannot be established, then the findings by Mangravite et al. (2013) could have been another case of the “winner’s curse.” The “winner’s curse” is a common phenomenon in genetic association literature, in which the initial reported genotype–phenotype association is exaggerated relative to the estimated effect in follow-up studies or cannot subsequently be replicated at all (Talameh and McLeod, 2011; Zollner and Pritchard, 2007).
Notably, our study has certain limitations. The proportion of controls (15%) was small compared to SIM cases (85%). However, we balanced the sample sizes and characteristics of the cases and control using propensity-matching and still found a null association. Controls were defined as participants without myopathic symptoms after six months of statin therapy and monitored for any change in status by questionnaire at 12 months. However, it is possible for the onset of SIM to occur even after 12 months of statin therapy. SIM is exacerbated by exercise (Parker and Thompson, 2012), but exercise was not assessed in this study. Two other polymorphisms in GATM (rs1719247 and rs1346268) remained statistically significant in a fixed-effects meta-analysis by Mangravite et al. (2014) for an association with SIM, but only rs9806699 was genotyped in our study. Using 1000 Genomes CEU data, the extent of linkage disequilibrium for both rs1719247 and rs1346268 with rs9806699 is r2 = 0.85 and D’ = 1.0. Notably, Floyd et al (2014) also performed fixed-effects meta-analyses for rs1719247 and rs1346268, but they found null associations.
In conclusion, we provide further evidence against an association between GATM rs9806699 and SIM in a large, multicenter case-control study of both mild and severe SIM in Caucasian participants. Experiments elucidating the potential mechanism between GATM and SIM are necessary to determine the specific conditions, if any, under which GATM may truly affect SIM.
Experimental Procedures
Participants
Cases and controls (n = 836) were derived from six medical centers in six states and provinces in the United States and in Canada representative of no single geographic location. Data for all participants was collected via a standardized questionnaire administered by a research coordinator. The Principal Investigator, Dr. Vladutiu, reviewed every questionnaire and individually telephoned every case that had gaps of information or inconsistencies. (This communication was documented). We have previously reported our definition of mild versus severe SIM (Vladutiu et al., 2011). Briefly, mildly affected cases were defined as participants with muscle aches and pains associated with the initiation of statin therapy that were reversible with cessation of therapy. Severely affected cases experienced symptoms of incapacitating muscle pain with or without weakness or with weakness alone that led to rhabdomyolysis in a number of cases and serum CK > 4 × ULN in 50% of cases in which it was quantified. Approximately 79% of participants continued to have severe symptoms for ≥ 6 months following discontinuation of statin therapy and 24% experienced progressive worsening with time. The time of onset of symptoms may have varied, but the association with statin therapy must have been clear to the referring physician and the participants themselves. Age- and gender-matched controls were recruited at collaborating centers following at least six months of statin therapy without myopathic symptoms. They were also monitored, via questionnaire, for change in status at 12 months. Details on the data collection and co-medication classifications can be found in the Supplemental Experimental Procedures. The study was approved by the institutional review boards at each study site, and all participants provided written informed consent.
Genotyping
Genomic DNA was prepared from whole blood collected in EDTA tubes with Gentra Puregene DNA isolation kits (Qiagen, Valencia, CA, USA). Genotypes for GATM rs9806699 were obtained using a TaqMan genotyping assay (C__30104701_10; Life Technologies, NY, USA) and analyzed with a MJR Opticon 2 real-time detection system (Bio-Rad, Hercules, CA, USA).
Statistical methods
Continuous variables were summarized as median and interquartile range and compared using the Kruskal Wallis test. Categorical variables were summarized as counts and percentages and compared using chi-squared or Fisher’s exact test where necessary. The Monte Carlo estimate of the exact p-value for Hardy-Weinberg equilibrium (HWE) was calculated in the Caucasian subjects using 10,000 permutations. Genetic associations were evaluated by both allele- and genotype-based tests. The primary analysis was for the association with any SIM (mild or severe), and the secondary analyses assessed the associations with mild SIM alone and severe SIM alone. Univariate and age- and sex- adjusted logistic regression models were used. Sensitivity analyses were performed in a sub-group of participants (n = 386) without potentially confounding co-medications (defined in Supplemental Experimental Procedures) and a propensity-matched dataset. The purpose of propensity-matching was to eliminate the known differences in the characteristics and sample sizes between the cases and controls. The propensity-matched dataset matched control subjects with SIM (mild or severe) subjects 1:1 using a greedy 8→1 matching algorithm (Parsons, 2004; Rosenbaum and Rubin, 1983; D'Agostino, 1998). Cases and controls were matched based on the significantly different characteristics in Table 1 (statin therapy, coronary artery disease, hypertension, smoking status, family history of heart disease, obesity, and co-medications that could treat SIM). A fixed-effect meta-analysis of all studies evaluating the association between rs9806699 and SIM (Table S2) was performed (Senn et al, 2011). Data excluding fibrate users (Mangravite et al [2013] Marshfield cohort and Floyd et al [2014]) and any potentially interacting medications (Luzum et al [2015]) was used, except for the data from Carr et al (2014) because concomitant medication information was not available. The 95% confidence intervals from the Carr et al (2014) study were also not available, and thus they were estimated using the standard error from our data (lowest standard error of all studies). All statistical analyses were performed using SAS version 9.3 (Cary, NC, USA). P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We would like to thank Ms. Shanping Huang at the University at Buffalo for performing the genotyping. We also acknowledge the generous contribution of participants by our collaborators at the following institutions: McMaster University, Hamilton, ON, Canada; Johns Hopkins University, Baltimore, MD; Cedars-Sinai Medical Center, Los Angeles, CA; Medical College of Wisconsin, Milwaukee, WI; and the University of Oklahoma Health Sciences Center, Oklahoma City, OK. We also gratefully acknowledge all participants in the study. The following grants supported the development of the SIM study cohort utilized in this analysis: NIH RO1HL085800 (PI: Vladutiu) and R21AR055704 (PI: Isackson). The following grants supported completion of the analysis: NIH K23GM100372 (PI: Kitzmiller) and American Heart Association 14POST20100054 (PI: Luzum).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
The authors do not have any conflicts of interest to disclose.
References
- Baker SK, Samjoo IA. A neuromuscular approach to statin-related myotoxicity. Can. J. Neurol. Sci. 2008;35:8–21. doi: 10.1017/s0317167100007514. [DOI] [PubMed] [Google Scholar]
- Ballard KD, Thompson PD. Does reduced creatine synthesis protect against statin myopathy? Cell. Metab. 2013;18:773–774. doi: 10.1016/j.cmet.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DF, Alfirevic A, Johnson R, Chinoy H, van Staa T, Pirmohamed M. GATM gene variants and statin myopathy risk. Nature. 2014;513:E1. doi: 10.1038/nature13628. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J. Clin. Lipidol. 2012;6:208–215. doi: 10.1016/j.jacl.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Collins FS, Tabak LA. Policy: NIH plans to enhance reproducibility. Nature. 2014;505:612–613. doi: 10.1038/505612a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Demidenko E. Sample size determination for logistic regression revisited. Stat. Med. 2007;26:3385–3397. doi: 10.1002/sim.2771. [DOI] [PubMed] [Google Scholar]
- Edvardson S, Korman SH, Livne A, Shaag A, Saada A, Nalbandian R, Allouche-Arnon H, Gomori JM, Katz-Brull R. l-arginine:glycine amidinotransferase (AGAT) deficiency: clinical presentation and response to treatment in two patients with a novel mutation. Mol. Genet. Metab. 2010;101:228–232. doi: 10.1016/j.ymgme.2010.06.021. [DOI] [PubMed] [Google Scholar]
- Floyd JS, Bis JC, Brody JA, Heckbert SR, Rice K, Psaty BM. GATM locus does not replicate in rhabdomyolysis study. Nature. 2014;513:E1–E3. doi: 10.1038/nature13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangravite LM, Engelhardt BE, Medina MW, Smith JD, Brown CD, Chasman DI, Mecham BH, Howie B, Shim H, Naidoo D, et al. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature. 2013;502:377–380. doi: 10.1038/nature12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangravite LM, Engelhardt BE, Stephens M, Krauss RM. Mangravite et al. reply. Nature. 2014;513:E3. doi: 10.1038/nature13630. [DOI] [PubMed] [Google Scholar]
- Mareedu RK, Modhia FM, Kanin EI, Linneman JG, Kitchner T, McCarty CA, Krauss RM, Wilke RA. Use of an electronic medical record to characterize cases of intermediate statin-induced muscle toxicity. Prev Cardiol. 2009;12:88–94. doi: 10.1111/j.1751-7141.2009.00028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis BD, Olson KL, Delate TM, Stolcpart RS. Statin adherence and mortality in patients enrolled in a secondary prevention program. Am. J. Manag. Care. 2009;15:689–695. [PubMed] [Google Scholar]
- Parker BA, Thompson PD. Effect of statins on skeletal muscle: exercise, myopathy, and muscle outcomes. Exerc. Sport Sci. Rev. 2012;40:188–194. doi: 10.1097/JES.0b013e31826c169e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LS. Performing a 1:N Case-Control Match on Propensity Score. Proceedings of the 29th SAS Users Group International Conference Paper 165-29; 2004. Available at: http://www2.sas.com/proceedings/sugi29/165-29.pdf. [Google Scholar]
- Phillips PS, Haas RH, Bannykh S, Hathaway S, Gray NL, Kimura BJ, Vladutiu GD, England JDF the Scripps Mercy Clinical Research Center. Statin-Associated Myopathy with Normal Creatine Kinase Levels. Ann. Intern. Med. 2002;137:581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA. An assessment by the Statin Muscle Safety Task Force: 2014 update. J. Clin. Lipidol. 2014;8:S58–S71. doi: 10.1016/j.jacl.2014.03.004. [DOI] [PubMed] [Google Scholar]
- SEARCH Collaborative Group. Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy--a genomewide study. N. Engl. J. Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- Senn S, Weir J, Hua TA, Berlin C, Branson M, Glimm E. Creating a suite of macros for meta-analysis in SAS®: A case study in collaboration. Statistics and Probability Letters. 2011;81:842–851. [Google Scholar]
- Simpson RJ, Jr, Mendys P. The effects of adherence and persistence on clinical outcomes in patients treated with statins: a systematic review. J. Clin. Lipidol. 2010;4:462–471. doi: 10.1016/j.jacl.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Talameh JA, McLeod HL. PON1 Q192R and clopidogrel: a case of the winner's curse or inadequate replication? Clin. Pharmacol. Ther. 2011;90:771–774. doi: 10.1038/clpt.2011.226. [DOI] [PubMed] [Google Scholar]
- The West of Scotland Coronary Prevention Study Group. Compliance and adverse event withdrawal: their impact on the West of Scotland Coronary Prevention Study. Eur. Heart. J. 1997;18:1718–1724. doi: 10.1093/oxfordjournals.eurheartj.a015165. [DOI] [PubMed] [Google Scholar]
- Vladutiu GD, Isackson PJ, Kaufman K, Harley JB, Cobb B, Christopher-Stine L, Wortmann RL. Genetic risk for malignant hyperthermia in non-anesthesia-induced myopathies. Mol. Genet. Metab. 2011;104:167–173. doi: 10.1016/j.ymgme.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollner S, Pritchard JK. Overcoming the winner's curse: estimating penetrance parameters from case-control data. Am. J. Hum. Genet. 2007;80:605–615. doi: 10.1086/512821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.