Abstract
The discovery of incretin-based medications represents a major therapeutic advance in the pharmacologic management of Type 2 diabetes (T2DM), as these agents avoid hypoglycemia, weight gain and simplify the management of T2DM. Dipeptidyl peptidase-4 (CD26, DPP4) inhibitors (DPP4i) are the most widely used incretin-based therapy for the treatment of T2DM globally. DPP4i are modestly effective in reducing HbA1c (≈0.5%) and while these agents were synthesized with the understanding of the role that DPP4 plays in prolonging the half-life of incretins such as glucagon like peptide-1 (GLP-1) and gastric inhibitory peptide (GIP), it is now recognized that incretins are only one of many targets of DPP4. The widespread expression of DPP4 on blood vessels, myocardium and myeloid cells and the non-enzymatic function of CD26 as a signaling and binding protein, across a wide range of species, suggest a teleological role in cardiovascular regulation and inflammation. Indeed, DPP4 is up regulated in pro-inflammatory states including obesity, T2DM and atherosclerosis. Consistent with this maladaptive role, the effects of DPP4 inhibition appear to exert a protective role in cardiovascular disease at least in pre-clinical animal models. Although 2 large clinical trials suggest a neutral effect on cardiovascular end-points, current limitations of performing trials in T2DM over a limited time horizon on top of maximal medical therapy, must be acknowledged before rendering judgment on the cardiovascular efficacy of these agents. This review will critically review the science of DPP4 and the effects of DPP4i on the cardiovascular system.
Keywords: Cardiovascular disease, DPP4, incretin, Diabetes mellitus, GLP-1
INTRODUCTION
The growing burden of Type 2 Diabetes Mellitus (T2DM) worldwide represents one of the most important challenges for global health. Over 50% of the risk of mortality from T2DM is attributable to cardiovascular (CV) causes, with T2DM contributing significantly to death and disability adjusted life years (DALY)1. While diet and lifestyle approaches are fundamental to the treatment of risk in T2DM, these treatments often fail in many patients necessitating pharmacologic approaches. Dipeptidyl peptidase-4 (DPP4, also known as CD26) is a widely expressed glycoprotein that has gained attention owing to its role in the catalytic degradation of incretins such as glucagon-like peptide-1 (GLP-1) and as a receptor for the Middle Eastern Respiratory Syndrome (MERS) virus. The development of DPP4 inhibitors (DPP4i) as a class of anti-diabetic medications was predicated on the simple notion that these drugs would raise GLP-1/GIP levels resulting in enhancement of insulinotropic effects of glucose. This rather simple construct is now replaced with a much more nuanced understanding of this protein. In this review, we will summarize the structure and function of DPP4 and its known role in physiology. We will review its importance in the pathophysiology of cardiometabolic disorders and provide the evidence to date, on the effects of DPP4 inhibition, both from the context of experimental models, mechanistic human studies and recent clinical trial evidence on CV outcomes.
OVERVIEW OF DPP4 BIOLOGY AND REGULATION
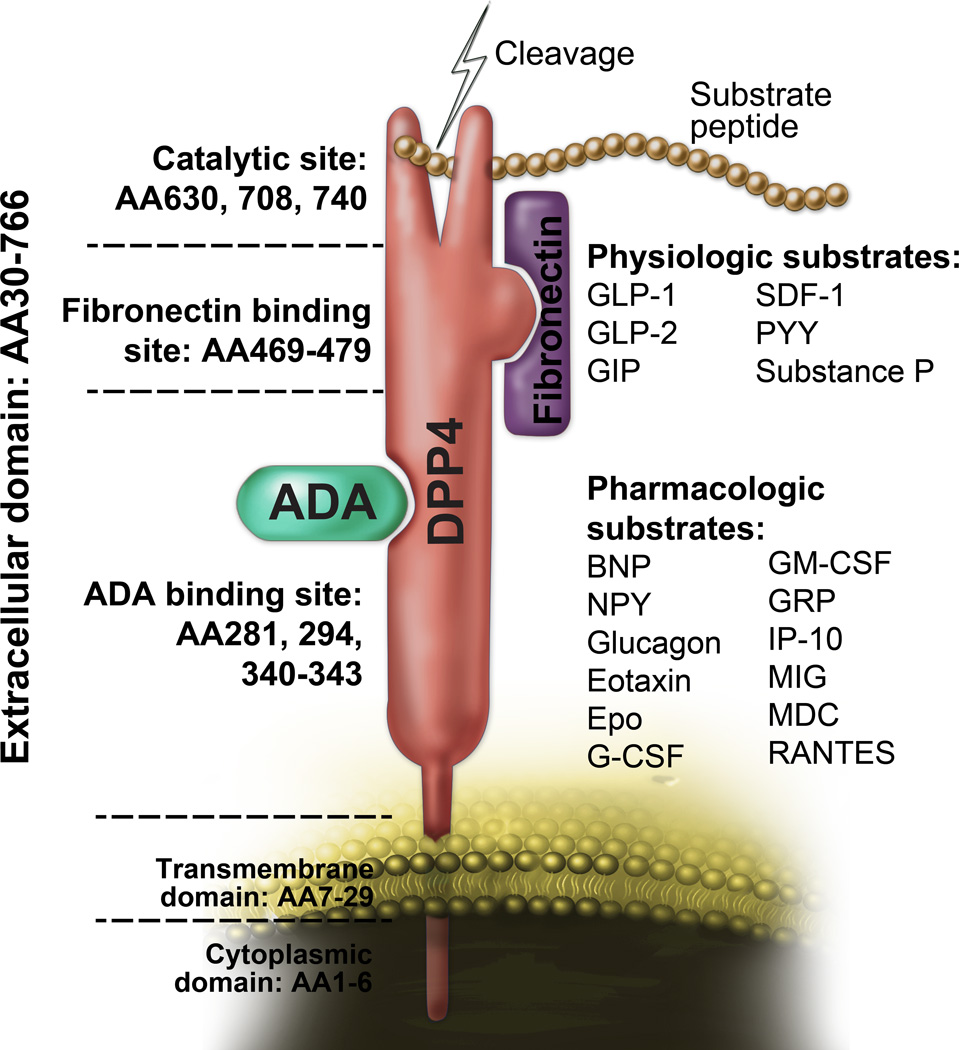
Human DPP4 is a 766 amino acid single-pass type II integral transmembrane glycoprotein that belongs to S9b dipeptidyl peptidase family which also include quiescent cell proline dipeptidase (QPP, also called DPP2), fibroblast activation protein (FAP), DPP8, and DPP9. Monomeric DPP4 has a short N-terminal cytoplasmic portion (6 residues, AA 1–6), a 22-residue transmembrane domain (AA7–29) and an extra-cellular domain, comprised of an eight-blade β-propeller (Arg54-Asn497) and a large α/β-hydrolase domain (Gln508-Pro766) responsible for binding to adenosine deaminase (ADA) and matrix proteins such as fibronectin and collagen2,3. Residues 630, 708 and 740 in the α/β-hydrolase domain are critical for catalytic function of DPP4. While the C terminal α/β-hydrolase domain is relatively conserved, the N-terminal eight-blade β-propeller domain demonstrates sequence variability. The catalytic pocket responsible for cleavage of a number of protein substrates is about 8 Å and contained within a 15Å wide opening at the interface between its hydrolase and propeller domains4. Residue 294 and residues 340–343 within the cysteine-rich segment of DPP4 is essential for ADA binding9. DPP4 exists as a monomer, homodimer, or homotetramer on the cell surface, with the homodimer representing the predominant catalytically active form7. DPP4 is subject to post-translational modification via glycosylation and N-terminal sialytion both of which have been suggested to regulate catalytic activity7,8.
Current understanding of DPP4 molecular regulation is incomplete. The promoter of DPP4 contains a consensus GAS (interferon gamma-activated sequence) sequence at −35 to −27, which has a binding motif for STAT1α. Administration of both interferons and retinoic acid results in the tyrosine phosphorylation of STAT1α, and subsequent nuclear translocation and binding to the GAS motif resulting in DPP4 transcription9. In addition to IL-12 and TNFα, IL-1α has also been shown to regulate the expression of DPP410. The promoter for DPP4 also contains consensus sites for nuclear factor-κB, SP-1, EGFR, AP-1 factor and hepatocyte nuclear factor-1. IL-12 enhances the translation but not transcription of DPP4 in activated lymphocytes, while TNFα decreases cell surface expression of DPP4, suggesting a regulatory role of IL-12 and TNFα in the translation and translocation of DDP411.
DPP4 also circulates as a soluble form in the plasma. Soluble DPP4 (sDPP4) lacks the cytoplasmic and transmembrane domain with preserved catalytic activity. Whether sDPP4 is cleaved from the membrane or is secreted is unclear. For instance, studies investigating viral liver infection suggest that sDPP4 is cleaved from membrane bound DPP412. sDPP4 is also detected in the lumen of secretory granules in pancreatic A cells and in the exocytic secretory lysosomes of natural killer cells suggesting that it may also be processed intracellularly13,14.
PHYSIOLOGICAL ROLE OF CATALYTIC FUNCTION OF DPP4
DPP4 exerts its peptidase function by removing N-terminal dipeptides displaying proline, alanine or serine as the penultimate (P1) amino acid residue. Inactivation of gastric inhibitory polypeptide (GIP) and GLP-1 is responsible for the anti-hyperglycemic effect of DPP4 inhibition. Mice lacking the gene encoding DPP4 are refractory to the development of obesity and hyperinsulinemia and demonstrate improved post-prandial glucose control14,15. GLP-1 and GIP suppress glucagon release, decreases gastric empting, promotes β-cell proliferation, and suppresses β-cell death16–18. Pharmacological inhibition of DPP4 enzymatic activity improves glucose tolerance in wild-type, but not in Dpp4−/− mice. Interestingly DPP4 inhibition improves glucose tolerance in Glp1r−/− mice, indicating that DPP4 contributes to blood glucose regulation by additional substrates such as GIP or through GLP-1R-independent mechanisms15. In addition to gut derived peptides GLP-1 and GIP, the other substrates include a variety of neuropeptides and chemokines. A recent study suggests in addition to chemokines other cytokines such as GM-CSF, G-CSF, IL-3, and Erythropoietin (Epo) could also be cleaved by DPP416. The catalytic activity of DPP4 and its substrates (Figure 1) have been extensively reviewed elsewhere7,17 and we will not go into detail here.
Figure 1. DPP4 functions and structure.
DPP4 consists of a 6-amino-acid cytoplasmic tail, a 22-amino-acid transmembrane domain and a large extracellular domain. The extracellular domain is responsible for the dipeptidase activity and binding to its ligands such as adenosine deaminase (ADA) and fibronectin. AA, amino acid; ADA, adenosine deaminase. Some concepts of this figure were adapted from Zhong J et al. Atherosclerosis. 2013;226:305–314 with modifications.
PHYSIOLOGY OF NON-CATALYTIC FUNCTION OF DPP4
In addition to its well-known peptidase activity, DPP4 also possesses non-catalytic functions through its interaction with a ligands including ADA, caveolin-1, fibronectin, and CXCR4.
Interaction with ADA and Mediation of T cell Co-stimulation
The best known non-catalytic function of DPP4 is to provide co-stimulation for T cells by interacting with ADA18. Activation of DPP4 induced tyrosine phosphorylation of molecules downstream of TCR/CD3, such as p561ck, p59fyn, ZAP-70, MAP kinase, c-Cbl, and phospholipase Cγ19. Interestingly, murine and rat DPP4 do not bind ADA20. By using site-directed mutagenesis, Leu-294 and Val-341 were identified as two ADA-binding sites in Human DPP46. Leu-294 and Val-341 are positioned at the outer strand of the tetramerization blade IV and blade V, respectively20. Therefore, ADA binding has been thought to interfere with tetramerization. Similarly, the glycosylation of Asn281 is likely to influence ADA binding3,20. Thus tetramerization and glycosylation may serve as control mechanisms for ADA binding and may explain why murine DPP4, which lacks glycosylation sites and may not bind ADA.
Novel Roles for DPP4
In 2013, DPP4 was identified as a functional receptor for the spike protein of a novel betacoronavirus species, the Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV) in human and bat cells21. The engagement of the MERS-CoV spike protein S with CD26 mediates viral attachment to host cells initiating infection. The viral receptor-binding domain recognizes blades IV and V of the DPP4 β-propeller22. The residues identified in the virus-DPP4 interface are also involved in ADA binding22. As the ADA-DPP4 interaction has been shown to induce co-stimulatory signals in T cells, this may indicate a possible manipulation of the host immune system by MERS-CoV through competition for the ADA-recognition site. DPP4 receptor binding domain may thus represent a potential treatment strategy for MERS-CoV infection23.
DPP4 on T cells may also interact with caveolin-1 on APCs, resulting in its phosphorylation and leads to activation of downstream NFκB24,25. Activated NFκB in turn upregulates CD8626,27. This process has been suggested to be involved in the pathogenesis of inflammatory disorders28. DPP4 has been reported to bind multiple components of extracellular matrix such as collagen, fibronectin, and the HIV-1 Tat protein2,17. Interactions with matrix components may play a role in sequestration of DPP4 and allow additional functions such as matrix remodeling, metastasis and chemotaxis.
DPP4 IN DIABETES
DPP4 Expression and Diabetes
DPP4 is an important regulator of post-prandial glucose via degradation of GLP-1 and GIP17. Both GLP-1 and GIP are rapidly inactivated by DPP4, resulting in a short half-life (less than 2 min for GLP-1, and less than 2 min in rodents or 7 min in human for GIP)29–31. In contrast to early reports suggesting that GLP-1 levels are decreased in T2DM, recent studies suggest that, in general, T2DM patients do not exhibit reduced GLP-1 secretion in response to an OGTT or meal test. However deteriorating glycemic control may be associated with reduced GLP-1 secretion32. DPP4 enzymatic activity correlates with the degree of glucose homeostasis in T2DM33. DPP4 expression in both visceral and subcutaneous adipose tissue positively correlates with BMI34,35, with visceral adipose tissue (VAT) consistently displaying higher expression36. DPP4 expression positively correlates with the amount of VAT, adipocyte size, inflammation and HbA1c and negatively correlates with glucose infusion rates during euglycemic-hyperinsulinemic clamp36. Within adipose, adipocytes appear to secrete abundant DPP4 with levels exceeding that from macrophages35. Ex vivo release of DPP4 from adipose tissue explants were higher in VAT than in subcutaneous adipose tissue with obese patients displaying higher DPP4 release than lean controls. Secreted DPP4 may in turn exert paracrine effects on insulin signaling in adipocytes and skeletal muscle cells35. Amongst inflammatory cells, T cell expression exceeds that of macrophages and dendritic cells37. Expression of DPP4 increases in peripheral T cells and in the liver of patients with non-alcoholic fatty liver disease with levels correlating with insulin resistance38,39. Interestingly, several widely-used glucose-lowering medications such as metformin and thiazolidinedione have been reported to reduce circulating DPP440–42 and DPP4 on T cells38. DPP4i also appear to exert effects on brown adipose tissue metabolism by increasing UCP-1 and PGC-1a levels, through increase in GLP-143.
Catalytic Inhibition of DPP4 Function in Diabetes
There are currently four DPP4i approved by the FDA and one DPP4i approved by EU: sitagliptin (FDA approved in 2006), saxagliptin (FDA approved in 2009), linagliptin (FDA approved in 2011), alogliptin (FDA approved in 2013), and vildagliptin (EU approved in 2007). They can be broadly divided into two classes based on structure: DPP4 dipeptide structure mimetics and non-peptidomimetics. The first class includes sitagliptin (β-amino acid-based), vildagliptin, and saxagliptin (nitrile containing), whereas the non-peptidomimetics include alogliptin (modified pyrimidinedione) and linagliptin (xanthine-based). There are several others in advanced clinical trials including dutogliptin44 and gemigliptin and at least 2 once weekly DPP4i are in advanced clinical trials. DPP4i demonstrate approximately a 0.4–0.8% lowering of HbA1C, with the degree of lowering depending on the baseline HbA1c, concomitant therapy and patient population45,46. In general the glycemia lowering efficacy with DPP4i is lower than that with sulfonylureas, insulin and thiazolidinediones, but they are significantly better tolerated and devoid of weight gain47–49. The most frequently reported side effects of DPP4i include nasopharyngitis, upper respiratory tract infection, urinary tract infection and headache. In contrast to other members of the class, linagliptin has a primarily non-renal route of excretion and does not need dose adjustment in renal impairment50. Several studies corroborate improvement in β cell function indices including homeostasis model assessment beta cell function index (HOMA-b), fasting proinsulin:insulin ratio, and insulin secretion rates in response to mixed meal challenge51–54.
Non-catalytic Interactions of DPP4 and Inflammation
The mechanism by which DPP4 may potentiate inflammation in T2DM likely involves both its catalytic and non-catalytic function. There is good evidence suggesting a role for DPP4 non-catalytic function in the regulation of T cell activation5,18,25,27. DPP4 expression increases with functional maturation of dendritic cells (DCs) and macrophages from monocytes with a role for upregulation of CD86 expression and activation of the nuclear factor-κB pathway24,26,37. The interaction between DPP4 and ADA may also facilitate T cell activation through regulation of pericellular adenosine levels. Jurkat cell (a T cell line) with a DPP4 mutant devoid of ADA binding activity is sensitive to adenosine-mediated inhibition of T cell proliferation5, indicating that DPP4 on immune cells facilitates adenosine clearance by binding to ADA (Figure 2). In assays using T-cell/DC co-cultures, adenosine dose-dependently suppresses T-cell proliferation, while pre-incubation of DCs with ADA relieved the inhibitory effect of adenosine in a dose-dependent manner. siRNA knockdown of DPP4 on DC suppresses proliferation of both CD4+ and CD8+ T cells while sitagliptin did not37. Both T-cell receptor signaling and Signal transducer and activator of transcription 3 (STAT3), an important signaling molecule that regulates T-cell proliferation and activation, are regulated by DPP4-expressing DC’s37. STAT3 phosphorylation increases on T-cell activation, an effect suppressed by adenosine37. Lymphocyte-specific protein tyrosine kinase (Lck), a Src family tyrosine kinase that is activated with TCR activation and allows binding and phosphorylation of ZAP-70 to mediate downstream signaling events, is prevented from being activated in T cells co-cultured with DPP4 siRNA-transfected DC [as suggested by an increase in phospho-Lck (Tyr505), the inactive form of Lck]37. These results suggest that DPP4 on antigen presenting and T cells may promote adipose inflammation and insulin resistance through its non-catalytic function. However, the precise extent to which non-catalytic function regulates inflammation independent of the catalytic function in T2DM will require experiments in animals expressing human DPP4 that additionally have mutations in enzymatic function as murine DPP4 lacks the ADA binding site20,55.
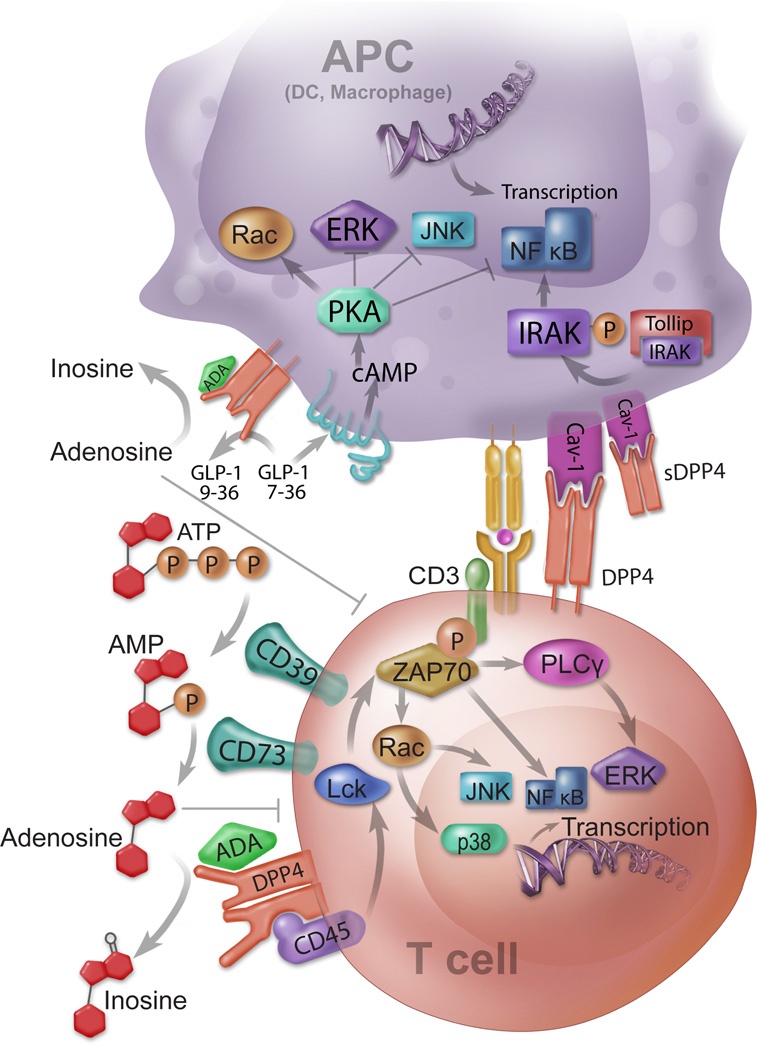
Figure 2. Mechanisms by which DPP4 modulates immune response of relevance to cardiovascular disease.
DPP4 regulates immune response via multiple mechanisms that involve both adaptive and innate immune function. These include: 1) Control of pericellular adenosine levels: adenosine triphosphate (ATP) or ADP is converted into AMP by membrane-bound CD39. AMP is further catalyzed by CD73 and produce adenosine. Adenosine is degraded by ADA bound to membrane-anchored DPP4. Accumulation of adenosine suppresses T cell activation and proliferation; 2) ADA-DPP4 interaction provides co-stimulatory signaling for T cell activation. By signaling through CD45, DPP4 enhances T cell receptor signals to promote T cell activation; 3) Inactivation of Glucagon-Like Peptide-1 (GLP-1). GLP-1 binds to GLP-1R and increases cAMP resulting in Protein Kinase A (PKA) mediated suppression of ERK, JNK, and NFκB; 4) Activation of APCs by interaction with caveolin-1. DPP4 binds to caveolin-1 and activates IRAK and NFκB, leading to the activation of APCs.
EFECTS OF DPP4 INHIBITION ON CARDIOVASCULAR SYSTEM
Effects of DPP4i on Endothelial Cells, NO and Blood Pressure
DPP4 is expressed on endothelial cells and appears to play a physiologic role in the regulation of vascular tone and angiogenesis56. Studies have suggested DPP4/incretin axis has substantial implication in cardiovascular system (Figure 3). Incubation of human umbilical vein endothelial cells (HUVECs) with DPP4i such as alogliptin and vildagliptin has been shown to result in eNOS and Akt phosphorylation paralleled by a rapid increase in NO57,58. DPP4 inhibition relaxes pre-constricted aortic segments in a dose dependent manner, responses unaffected by the GLP-1R antagonist, exendin 9–3957. In one study, vascular relaxation was reduced by endothelial denudation, L-NG-monomethyl-arginine citrate (L-NMMA) and by the soluble guanylate cyclase inhibitor ODQ, while a combination of L-NMMA, and blockers of calcium activated potassium channels completely obviated relaxation in response to DPP4 inhibition, suggesting that both eNOS and potassium channels mediated effects on vascular function57. Inhibition of Src kinase decreased eNOS and Akt phosphorylation in response to DPP4-inhibition NO57,58. DPP4-inhibition-mediated GLP-1R activation may also represent a mechanism for the blood pressure-lowering effect of DPP4i59. However the locus of GLP-1R activation is controversial. Although endothelial cells have been shown to express GLP-1R at least in some studies, at least in one study the antihypertensive effects of GLP-1R activation, by liraglutide did not directly relax preconstricted aortic rings or increase cyclic GMP (cGMP). However, conditioned medium from liraglutide-treated hearts relaxed aortic rings in an endothelium-independent, GLP-1R-dependent manner. The decrease in BP involved an indirect natriuretic effect dependent on ANP release from the atria in a GLP-1R dependent manner59. Several other studies in animal models support a favorable effect of DPP4 inhibition in improving endothelial function and blood pressure57,60,61. In contrast to the positive effects in animals, the effects of DPP4i on endothelial function in humans has been inconsistent62–64. While some of the inconsistency may relate to differences in end-points, methodology and patient characteristics, it is possible that these conflicting results may reflect differential alteration in the levels of substrates and their metabolites by DPP4i, which may vary depending on the study participants. In a double blind study in T2DM, treatment with vildagliptin for 4 weeks improved forearm blood flow in response to intra-arterially delivered aceytylcholine62. In contrast, 2 studies using flow-mediated dilation (FMD) of the brachial artery have shown opposite results with one study showing that both sitagliptin and alogliptin worsen FMD, while in another study sitagliptin improved FMD in association with an increase in CD34+ cells in another study63,64.
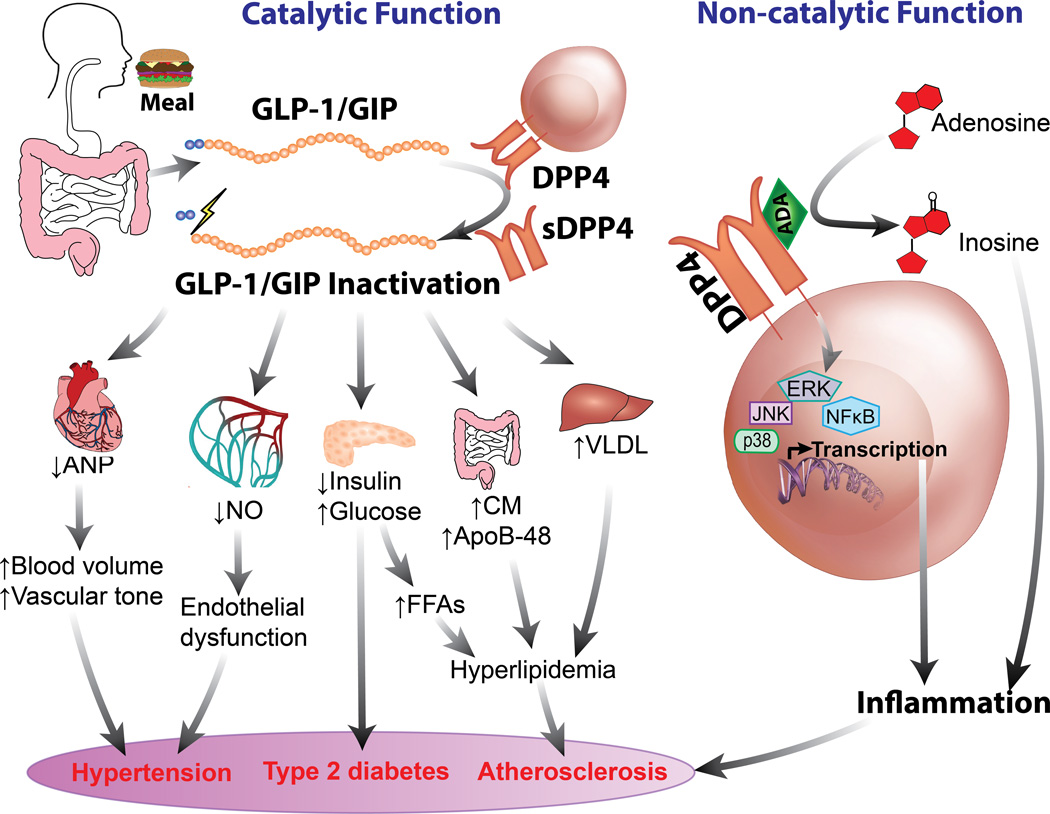
Figure 3. Cardiovascular Effect of DPP4/Incretin Axis.
ADA, adenosine deaminase; ANP, atrial natriuretic peptide; CM, chylomicrons; DPP4, dipeptidyl peptidase 4; FFAs, free fatty acids; NO, nitric oxide; sDPP4, soluble DPP4
While earlier studies in small cohorts appear to suggest a favorable effect of DPP4i in reducing BP these effects have not been consistently observed65,66. An obvious explanation is that the magnitude of effect which is typically small (1–3 mm Hg) may not be easily observed in studies utilizing clinic systolic blood pressure, particularly as a secondary end-point. Indeed a large meta-analysis of published data on DPP4i utilizing clinic BP does not support an effect of on blood pressure67. An alternate hypothesis that has been proffered relates to differential alteration of substrates of DPP4 such as NPY, which shifts the receptor affinity from Y1 to non-Y1 receptors68. DPP4 inhibition has been demonstrated to enhance sodium excretion through interaction with the renal NHE3 exchange protein and does so in both wild-type and Glp1r−/− mice69. The mechanisms may relate to the physical association of the sodium hydrogen exchanger type 3 (NHE3) with DPP4 with inhibition of DPP4 catalytic activity suppressing NHE3-mediated NaHCO3 reabsorption in rat renal proximal tubule, resulting in increased Na+ excretion70. Further the redistribution of the complex of DPP4-NHE3 is believed to represent an adaptive mechanism in chronic hypertension71. It has also been suggested that catalytic inactivation of natriuretic peptides such as brain natriuretic peptides by DPP4 may regulate blood pressure through natriuretic and vasodilatory effects. DPP4 converts BNP to produce BNP(3–32)72, which has reduced vasodilation and natriuretic effects73. In humans however inhibition of DPP4 with sitagliptin acutely does not potentiate BNP levels or its effects on forearm blood flow74. In a post-hoc analysis of SAVOR-TIMI, NT-proBNP levels were not increased with Saxagliptin treatment but were lower when compared to placebo75.
DPP4 Inhibition in Angiogenesis and Stem Cell Homing
Expression of DPP4 on Sca-1+ lin− donor hematopoietic cells negatively regulates homing and engraftment vasculogenesis76. By inhibiting DPP4 catalytic function or deleting DPP4, the transplantation and engraftment efficacy of hematopoietic stem cells was greatly enhanced76. SDF-1 is a substrate for DPP4 and has been implicated in the mobilization and homing of hematopoietic cells in response to G-CSF treatment in experimental ischemia/infarction77,78. DPP4-truncated SDF-1 not only loses its chemotactic activity, but also blocks chemotactic effects of full-length SDF-179. Sitagliptin treatment in patients with T2DM results in a 2-fold increase of circulating EPC with concomitant increase in plasma SDF-180. Short term treatment with DPP4i has also been demonstrated to increase SDF-1 levels and CD34+ cells80,81. The increase in CD34+ cells corresponded to increased homing and deposition to an ischemic hind limb preparation81 and infarcted myocardium82. DPP4 may also regulate HSCs and HPCs by truncating multiple CSFs (other than SDF-1) with consequent loss of their activity. DPP4 knockout or pretreatment of HPCs from human cord blood or mouse bone marrow with DPP4i enhances the proliferative action of GM-CSF, G-CSF, IL-3, and Erythropoietin (Epo)16. DPP4 deficiency or catalytic inhibition promotes hematopoiesis and bone marrow engraftment in mice after radiation or chemotherapy16. Interestingly, DPP4-truncated CSFs blunts the activity of their respective full-length CSF, both in vitro and in vivo with the truncated GM-CSF, demonstrating a higher affinity to GM-CSF receptor16. However, there were also studies showing DPP4 inhibition reduces angiogenesis through inactivation of NPY(1–36). Truncation of NPY by DPP4 leads to a shift of receptor subtype specificity with cleaved NPY(3–36) binding to non-Y1 (Y2, Y3, and Y5) receptors83. Production of NPY(3–36) is required for angiogenic activity as DPP4 inhibition by neutralizing antibody suppresses NPY-mediated endothelial cell migration in an endothelial wound assay84.
Pharmacologic inhibition of DPP4 catalytic function stimulates angiogenesis, with enhances endothelial cell migration, aortic sprouting and angiogenesis in in vivo assays58,85. Src kinase mediated eNOS-Akt activation in response to DPP4 inhibition appears to play an important role in the angiogenic response to DPP4 inhibition86. The effects of DPP4 inhibition in enhancing angiogenesis in-vitro are paralleled by an improvement in hind limb blood flow in a hind-limb ischemia model58. DPP4 inhibition has also been shown to improve healing of chronic foot ulcer in T2DM patients, in parallel with increased HIF-1α and VEGF in the periphery of the ulcer and ulcer capillary density87. Similarly the angiogenic capacity of circulating pro-angiogenic cells in T2DM patients has been shown to be increased in-vivo in response to DPP4 inhibitors88.
Effects on Lipid Metabolism
Administration of DPP4i to hyperlipidemic mice reduces post-prandial lipoprotein levels89. The effects of DPP4i on lipoprotein metabolism been reviewed extensively90,91. A single dose of sitagliptin following a high-fat liquid formula administered under pancreatic clamp conditions, decreased triglyceride (TG)-rich lipoproteins apoB-48 concentration by reducing production rates in healthy subjects92. The magnitude of lipid lowering seen with different DPP4i appears to be comparable and of a similar magnitude to that seen with the GLP-1R agonist exenatide91. In a 4-week single center, randomized double blind study in T2DM, vildagliptin (n=31, 50 mg bid) reduced post-prandial plasma TG, chylomicron apoB-48 following mixed meal challenge, without changes in apoB-100. Vildagliptin also increased intact GLP-1, suppressed inappropriate glucagon secretion93. In a randomized cross over study, alogliptin (25 mg/d, for 7 days) suppressed post-prandial elevation of TG, ApoB-48 and remnant lipoproteins94. In a double-blind cross-over study, sitagliptin over 6 weeks (100 mg/day) significantly decreased post-prandial area plasma apoB-48, TG and VLDL-C95. In a randomized cross over study over 7 days of vildagliptin vs. placebo, with microdialysis catheter based analysis of metabolites, vildagliptin treatment was associated with a reduction in post-prandial lactate and pyruvate production in the skeletal muscle with evidence of enhanced lipolysis. These effects may reflect improvement in hepatic glucose production by the liver due to obviation of insulin resistance by DPP4i and was supported by calorimetry suggesting increased energy expenditure and lipid oxidation rates96.
Effects on Atherosclerosis
A number of DPP4i have been shown to reduce atherosclerosis in experimental models (Table I)97–101. These studies have all consistently noted a reduction in foam cell formation and inflammatory content. In at least 2 of these studies a reduction in migratory capacity of monocytes has been implicated97,98. Pre-treatment with DPP4i for 2 weeks in ApoE−/− mice, markedly reduced the migration of labeled bone marrow derived monocytes to atherosclerotic plaque in response to exogenously administered TNFα and sDPP498. In-vitro Boyden chamber assays using alogliptin and sitagliptin performed in the presence of nanomolar concentration of TNFα confirmed reduced monocyte migration in response to a CCL-2 gradient, which was not inhibited by GLP 9–3998. The effects of DPP4i include reduction in inflammatory gene expression and a reduction in monocytes and T cells98,100,102. Given the effects of DPP4 in co-stimulation and regulation of adenosine, it may suggest a role for DPP4, independent of its effects on substrates such as GLP-197. GLP-1 has been shown to decrease monocyte migration in response to CCL-2 and regulated on activation, normal T cell expressed and secreted (RANTES) in a concentration-dependent manner97. GLP-1 may also exert anti-inflammatory effects via cAMP dependent mechanisms which may reduce NFkB and Erk activation60. Infusion of exendin-4 for 4 weeks reduces neointimal formation103, foam cells104, and atherosclerotic lesion size104,105. In support of a role for GLP-1 in DPP4 mediated effects, administration of antagonists to GLP-1 and GIP [Exendin(9–39) and Pro(3)GIP] attenuated anti-atherosclerotic effects of vildagliptin on atherosclerosis99 (Table I). In humans, one study noted a reduction in carotid intima media thickness progression with sitagliptin compared to placebo over 12 months in newly diagnosed patients with type 2 diabetes with coronary artery disease106.
Table 1.
Studies that evaluate CV effect of DPP4i
| Intervention | Dose | Duration | Subject | Disease | Major findings | Reference |
|---|---|---|---|---|---|---|
| Angiogenesis | ||||||
| Diprotin A or Val-Pyr |
5mM Diprotin A or Val-Pyr | 15 min in vitro treatment |
Mouse | Bone marrow transplantation |
DPP4 inhibition or deletion improved hematopoietic stem cell homing and engraftment. |
Christopherson KW et al. 200476 |
| Diprotinin-A or P32/98 |
10 µM diprotinin-A or P32/98 | 3–4 days | Mouse, HMVEC |
Angiogenesis | Pharmacological inhibition of CD26/DPP4 enhanced endothelial growth both in vitro and in vivo. |
Takasawa W 201085 |
| Sitagliptin | 10 or 20 mg/kg/d (oral gavage) | 7 weeks | Mouse | Hind limb ischemia | Sitagliptin treatment augmented ischemia-induced increases in SDF-1 and improved blood flow in ischemic limb. In addition, sitagliptin promoted EPC mobilization and homing to ischemic tissue. |
Huang CY et al. 201281 |
| Linagliptin | 10 nM or 0.5 µM | 4 h in vitro treatment |
HUVEC | endothelial cell damage |
Linagliptin inhibition inhibits AGE-induced ROS production in HUVEC. | Ishibashi Y et al. 2013139 |
| Vildagliptin | 1 nM, 5 nM or 10 nM | 6 h | Mouse, HUVEC |
Hind limb ischemia | Vildagliptin stimulated ischemia-induced revascularization through an eNOS signaling. |
Ishii M et al. 201458 |
| Myocardial infarction | ||||||
| Sitagliptin | 250 mg/kg/d (in vivo) or 5 µmol/L (in vitro infusion) | 8 weeks feeding or 20 min in vitro infusion |
Mouse | MI | Sitagliptin improved functional recovery after I/R injury via increasing cardiac pAKT, pGSK3beta, and atrial natriuretic peptide |
Sauvé M et al. 2010107 |
| Diprotin A | 10 mM | 6 h | Mouse, HUVEC |
Thrombosis | Diprotin A enhanced the amount of Tissue Factor encountered and induced the adherence of platelets under flow conditions. |
Krijnen PA et al. 2012140 |
| Sitagliptin | 300 mg/kg/d | 2 weeks | Fischer F344 rat |
MI | Sitagliptin improved passive left ventricular compliance, increased endothelial cell density, reduced myocyte hypertrophy, and decreased the abundance of collagen 1. |
Connelly KA et al. 2013108 |
| Kidney disease | ||||||
| Sitagliptin | 1µmol/L | In vitro treatment | Rat | Renovascular response |
Sitagliptin enhanced angiotensin II-induced increase of perfusion pressure in isolated kidneys from both lean and obese ZSF1 rats |
Tofovic et al. 2010125 |
| Vildagliptin | 1 or 10 mg/kg (i.v.) | One dose | Rat | Renal I/R injury | Vildagliptin reduced tubular necrosis, Bax/Bcl-2 and CXCL10 mRNA expression, and serum creatinine level after renal I/R. |
Glorie LL et al. 2012129 |
| Vildagliptin | 4 or 8 mg/kg/d | 24 weeks | Rat | Kidney injury | Vildagliptin decreased proteinuria, albuminuria, and urinary albumin/creatinine ratio, improved creatinine clearance, and inhibited interstitial expansion, glomerulosclerosis, and the thickening of the glomerular basement membrane in diabetic rats. |
Liu et al. 2012127 |
| Sitagliptin | 200 mg/kg/day | 8 weeks | Rat | Kidney injury | Sitagliptin suppressed nephrectomy-induced activation of PI3K–Akt and FoxO3a. Sitagliptin treatment also reduced apoptosis by decreasing cleaved caspase-3 and −9 and Bax levels. |
Joo et al. 2013128 |
| MK0626 | 33 mg/kg chow (~ 10 mg/kg/d) | 16 weeks | Mouse | Obesity-induced renal injury |
MK0626 prevented high-fructose/high-fat diet-induced glomerular and tubular injury independent of blood pressure/insulin sensitivity. |
Nistala R et al. 2014126 |
| Linagliptin | 83 mg/kg rat chow | 8 weeks | Zucker obese rat |
Obesity-related glomerulopathy |
Linagliptin enhanced filtration barrier remodeling, improved proteinuria, increased active GLP-1 and SDF-1α, and improved oxidant markers. |
Nistala R et al. 2014130 |
| Linagliptin | 5 mg/kg/d in drinking water | 4 weeks | Mouse | kidney fibrosis | Linagliptin ameliorated kidney fibrosis in diabetic mice without altering the blood glucose levels associated with the inhibition of EndMT and the restoration of microRNA 29s. |
Kanasaki et al. 2014131 |
| Blood pressure | ||||||
| Alogliptin | Various doses (in vitro) | In vitro treatment | Mouse aorta, HUVEC |
Vascular function | Alogliptin relaxed pre-constricted aortic segments in a dose dependent manner. Alogliptin induced eNOS and Akt activation in HUVEC cells is independent of GLP-1. |
Shah Z et al. 201157 |
| Sitagliptin | 40 mg/kg twice daily | 8 days | SHR rat | Hypertension | Sitagliptin decreased blood pressure in young SHR rats but not adult SHRs. | Pacheco BP et al. 2011141 |
| Sitagliptin | 10 mg/kg/d | 2 weeks | SHR rat | Hypertension and hypertensive kidney disease |
Sitagliptin treatment improved endothelium-dependent relaxation in renal arteries, restored renal blood flow, and reduced systolic blood pressure in SHR rats. |
Liu L et al. 2012142 |
| Saxagliptin | 10 mg/kg/d | 8 weeks | SHR rat | Hypertension and endothelial dysfunction |
Saxagliptin treatment reduced blood pressure in SHRs, an effect that was accompanied an increase in aortic and glomerular NO release with reductions in peroxynitrite levels. |
Mason RP et al. 2012143 |
| Linagliptin | 83 mg/kg in diet (~ 4 mg/kg/d) |
8 weeks | ZO rat | Hypertension | Linagliptin blunted elevated blood pressure progression in ZO rats without reducing left ventricular hypertrophy, fibrosis, or oxidative stress |
Aroor AR et al. 201361 |
| Atherosclerosis | ||||||
| Alogliptin | 15 mg/kg/d gavage | 24 weeks | Mouse | Diabetic atherosclerosis |
Alogliptin reduced atherosclerotic lesions and TLR4-mediated upregulation of IL-6 and IL-1β in diabetic ApoE−/− mice. |
Ta NN et al. 2011144 |
| Alogliptin | 40 mg/kg/d | 12 weeks | Mouse | Atherosclerosis | Alogliptin reduced atherosclerotic plaque and vascular inflammation in atherosclerosis-prone Ldlr−/− and ApoE−/− mice. |
Shah Z et al. 201198 |
| PKF275-055 (vildagliptin analogue) |
100 µm/[kg d] in drinking water |
4 weeks | Mouse | Atherosclerosis | PKF275-055 reduced foam cell formation, atherosclerotic plaque, and macrophage accumulation in the aortic wall. |
Terasaki et al. 2012 101 |
| Sitagliptin | 1.1% in diet | 12 weeks | Mouse | Atherosclerosis | Sitagliptin reduced plaque macrophage infiltration and plaque MMP-9 levels, increased plaque collagen content but did not change overall lesion size |
Vittone F et al. 2012 97 |
| Anagliptin | 0.3% in diet | 16 weeks | Mouse | Atherosclerosis | DPP4 inhibition reduced accumulation of monocytes and macrophages in the vascular wall and SMC content in plaque areas. |
Ervinna N et al. 2013 102 |
| vildagliptin | 0.003% w/v in drinking water (equal to 3 mg/kg/d) |
4 weeks | Mouse | Diabetic atherosclerosis | Anagliptin confers a substantial anti-atherosclerotic effect in both nondiabetic and diabetic mice, which was abolished by incretin blockers Exendin(9–39) or (Pro(3))GIP. |
Terasaki et al. 2013 99 |
| Sitagliptin | 0.3% in diet | 16 weeks | Mouse | Atherosclerosis | Sitagliptin treatment reduced atherosclerotic plaque size, collagen fiber, MCP-1, and IL-6 in plaques, serum levels of soluble vascular cell adhesion molecule-1 and P-selectin, and increased activation of AMPK and Akt.in aortas. |
Zeng et al. 2014 100 |
| Heart failure | ||||||
| Vildagliptin | 30 mg/kg/d | 4 weeks | Rat | Heart failure | Vildagliptin reversed diabetic diastolic left ventricular dysfunction and pressure- overload-induced left ventricular dysfunction. |
Shigeta et al. 2012 112 |
| Sitagliptin | 30 mg/kg/d | 3 weeks | Pig | Overpacing-induced heart failure |
Sitagliptin increased stroke volume and preserved glomerular filtration rate in pigs with pacing-induced heart failure |
Gomez et al. 2012 113 |
| Saxagliptin | 10 mg/kg/d | Up to 7 weeks | Mouse | Dilated cardiomyopathy |
Saxagliptin treatment improved glucose tolerance but not survival in a transgenic murine model of dilated cardiomyopathy. |
Vyas et al. 2012 114 |
| Vildagliptin | 10 mg/kg/d | 4 weeks | Mouse | Heart failure | Vildagliptin ameliorated TAC-induced left ventricular enlargement and dysfunction, and improved survival rate on day 28. |
Takahashi et al. 2013 115 |
| Vildagliptin | 30 mg/kg/d | 7 days | Rat | Cardiac hypertrophy | Vildagliptin attenuated the β-adrenergic stimulation-induced cardiac hypertrophy as well as cardiomyocyte hypertrophy and perivascular fibrosis. |
Miyoshi et al. 2014 116 |
| MK0626 | 33 mg/kg in diet (~ 10 mg/kg/d) |
16 weeks | Mouse | Diastolic dysfunction |
MK0626 improved western diet-induced insulin resistance and diastolic relaxation, accompanied by reduced myocardial oxidant stress and fibrosis. |
Bostick et al. 2014 117 |
| Linagliptin Sitagliptin |
1.5 mg/kg/d (linagliptin) 20 mg/kg/d (sitagliptin) |
7 weeks | Rat | Heart failure with preserved ejection fraction |
DPP4 inhibition prevented the development of cardiac diastolic dysfunction induced by subtotal nephrectomy, without change in renal function or structure improvement |
Connelly et al. 2014 118 |
CV, Cardiovascular; CVD, cardiovascular disease; I/R, ischemia-reperfusion; MI, myocardial infarction; RLP-C, remnant lipoprotein cholesterol; T2DM, type 2 diabetes; TAC, transverse aortic constriction.
Effect on Myocardial Function and Ischemia-Reperfusion
Genetic disruption of the Dpp4 in mice is not associated with baseline abnormalities in cardiac structure or function. TABLE 1 reviews the effects of DPP4i on models of ischemia re-perfusion107–109. In response to experimental infarction, Dpp4−/− mice exhibit improved survival with a trend towards reduction in infarct size107. Hearts from Dpp4−/− mice contained higher levels of phosphorylated AKT (pAKT), pGSK3 and ANP, well know pro-survival pathways. In a post-infarction model, sitagliptin treatment led to an improvement in passive left ventricular compliance, increased endothelial cell density, reduced myocyte hypertrophy, and reduced collagen 1108. Treatment with sitagliptin resulted in improved post-infarction survival without change in infarct size, an effect associated with upregulation of pro-survival pathways HO-1 and Akt, pathways activated by GLP-1107. Whether the results in response to DPP4 inhibition are due to an increase of DPP4 target proteins such as GLP-1 cannot be definitively inferred from studies to date. Recent studies seem to contest the role of direct effects of GLP-1 on myocardial cells and invoke a role for GLP-1 dependent atrial natriuretic peptide synthesis from atrial cells, since myocytes do not appear to express GLP-1 receptors59.
DPP4 and Heart Failure
DPP4 levels correlate with heart failure with plasma DPP4 activity being significantly higher in patients with more advanced heart failure110,111. Pharmacologic DPP4 inhibition in models of diabetic cardiomyopathy, pressure overload model of heart failure have been consistently shown to improve ventricular remodeling and survival as well as ameliorating severity of heart failure112–118. Similarly, both pharmacological and genetic DPP4 inhibition reversed diabetic diastolic left ventricular dysfunction and pressure-overload-induced left ventricular dysfunction112. Diabetes is well known to impair angiogenic ability through SDF-1α dependent pathways in cardiac tissue119. DPP4 inhibition (both pharmacological and genetic) reversed SDF-1α-dependent microvasculopathy and diabetes-associated diastolic left ventricular dysfunction112. Improved left ventricular function assessed by ejection fraction, mitral annular systolic velocity, and peak systolic velocity has also been observed in humans with DPP4 inhibition (Sitagliptin, single dose or 4 weeks of treatment)120,121. While the effects of DPP4 inhibition on heart failure have been directly attributed to increase in GLP-1, the extent to which these effects are driven by GLP-1 is unclear. This is especially true given recent evidence contesting the presence of GLP-1 receptors in the myocytes and dependence of atrial synthesis of ANP for GLP-1 effects59. Moreover the effects of GLP-1 itself are controversial. GLP-1 agonism has also been shown to increase left ventricular ejection fraction122–124 (all in short-term), however, reports on long-term CV effect of DPP4i and GLP-1 analogs are limited.
Effects on Renal Injury and CKD Progression
DPP4 inhibition has been shown to exert renoprotective effects characterized by reduction in oxidative stress, podocyte injury, mesenchymal expansion, fibrosis and proteinuria in experimental models (TABLE 1)125–131. These effects appear to occur without changes in blood pressure or insulin sensitivity126. Treatment with sitagliptin and linagliptin has also been shown to reduce proteinuria132,133. In a 6-month open label trial in 35 patients, albumin creatinine ratio was reduced by at least two-fold in patients with a range of albumin excretion from normoalbunimuria to macroalbuminuria [urinary albumin-to-creatinine ratio (ACR)>300]. A pooled analysis of four completed studies (n=217) of subjects with T2DM and prevalent albuminuria (defined as a [ACR] of 30–3,000 mg/g creatinine) receiving linagliptin on top of stable doses of RAAS inhibitors, demonstrated a modest 28% placebo adjusted reduction in ACR at 6 months independent of blood pressure.
Sitagliptin protects renal function in T2DM patients as evidenced by reduced urinary albumin-to-creatinine ratio Renovascular response to angiotensin II may also be enhanced by DPP4 inhibition. Sitagliptin (1µmol/L) significantly enhanced angiotensin II-induced increase of perfusion pressure in isolated kidneys from both lean (18.2 ± 5.9 vs. 3.4 ± 1.3 mmHg) and obese (17.8 ± 8.2 vs. 5.5 ± 1.3 mmHg) ZSF1 rats125. Improvement in filtration barrier remodeling130 and suppression of fibrosis131 may also serve as alternative mechanisms of DPP4i in protecting renal function in chronic kidney disease.
CARDIOVASCULAR CLINICAL TRIALS WITH DPP4i
Table II lists completed and ongoing randomized controlled clinical trials with DPP4i. SAVOR-TIMI53 was designed as a superiority trial and failed to meet the pre-specified superiority outcome of saxagliptin versus placebo in a high-risk patient population with established vascular disease and/or risk factors. In the EXAMINE trial, designed as a safety trial in a very high risk post-acute coronary syndrome population, the pre-specified end-point of non-inferiority was met and alogliptin was non-inferior to placebo with regards to CV outcomes. In both EXAMINE and SAVOR-TIMI53 trials, the primary outcome composed of CV death, myocardial infarction (MI) and ischemic stroke were non-inferior in DPP4i group compared to placebo group134,135. In both trials the median duration of follow-up was <4 years, the majority of whom were on evidence based therapies. These factors are widely felt to be to have been responsible for lack of benefit with these agents. In SAVOR-TIMI 53, baseline NT-proBNP was measured in 12,301 patients. More patients treated with saxagliptin were hospitalized for heart failure compared to placebo (HR 1.27; 95%CI 1.07–1.51; p=0.007). Differences in heart failure were seen in less than 6 months with rates at 12-months of 1.9% vs.1.3% (HR 1.46, 95%CI 1.15–1.88, p=0.002, with no difference thereafter). Subjects at greatest risk for heart failure had prior heart failure, eGFR ≤60 ml/min and/or elevated baseline levels of NT-proBNP (risk in highest NT-proBNP quartile of 2.1%)75. Whether the association with heart failure applies to other DPP4i will need to be addressed in ongoing CV outcome trials of DPP4i136. In consistence with this finding, a slight increase of heart failure hospitalization in patients with alogliptin compared to those with placebo (3.9% vs. 3.3%, HR = 1.19, 95% CI, 0.90–1.58) in EXAMINE trial, although no statistical significance was observed137,138.
Table 2.
DPP4i CV outcome trials
| Drug (class) | Landmark name |
Study population | Primary outcome | Dosing | Estimated enrollment |
Duration (years) |
End date | Identifier |
|---|---|---|---|---|---|---|---|---|
| Alogliptin | EXAMINE | T2DM with ACS (within >15 to < 90 days) |
MACE | 6.25 mg, 12.5 mg, or 25 mg qd‡ |
5,400 | 4.75 | 2013 | NCT00968708 |
| Saxagliptin |
SAVOR TIMI 53 |
T2DM with multiple CRF‖ |
Composite | 2.5 mg or 5 mg qd |
16,500 | 4 | 2013 | NCT01107886 |
| Sitagliptin | TECOS | T2DM with preexisting CVD |
Composite | 50 mg or 100 mg qd‡ |
14,000 | 5 | 2014 | NCT00790205 |
| Linagliptin | CAROLINA | T2DM with preexisting CVD |
Composite | 5 mg qd | 6,000 | 7.5 | 2018 | NCT01243424 |
| Linagliptin | CARMELINA | T2DM with impaired renal function or CVD |
Composite | 5 mg once daily | 8,300 | 4.5 | 2018 | NCT01897532 |
| Omarigliptin (MK3102) |
MK-3102-015 AM1 |
T2DM with inadequate glycemic control on diet/exercise therapy and oral antihyperglycemic agent monotherapy |
Any adverse event |
25 mg once weekly |
585 | 1.5 | 2014 | NCT01697592 |
| Omarigliptin (MK3102) |
MK-3102-018 | T2DM with preexisting CVD |
Composite | 25 mg once weekly |
4,000 | 5 | 2017 | NCT01703208 |
CVD, cardiovascular disease; T2DM, type 2 diabetes; MACE, Major Adverse Cardiac Events (defined as a composite of CV death, nonfatal MI and nonfatal stroke; these events were adjudicated by an independent cardiovascular endpoints committee); Composite, a composite defined as CV-related death, nonfatal MI, nonfatal stroke, or unstable angina requiring hospitalization
CONCLUSION
The development of DPP4i has evolved from the understanding of its role in regulation of incretin hormones. However the widespread expression of this molecule in a variety of cell types clearly underscores a previously unrecognized role for this molecule in physiological processes. Despite the plethora of enzymatic substrates that serve as targets, the inhibition of enzymatic function in animals and humans appears to be well-tolerated and safe with modest reduction in HbA1c. The pre-ponderance of evidence from inhibiting DPP4 enzymatic function in animal models of disease seems to suggest salutary effects including in heart failure and in totality seem to suggest the possibility of beneficial CV effects in humans. At least 2 large randomized clinical trials focused on CV outcomes in high risk patients with type 2 diabetes on best medical therapy appear to show no differences compared to placebo with regards to the composite end-point of CV death, MI and stroke over a duration less than 4 years of drug treatment. The non-catalytic function of DPP4, include its role as a co-stimulatory molecule and binding to a number of matrix proteins including ADA in humans, points to larger role for DPP4 outside of its catalytic function. Future mechanistic studies will need to focus on the relative contribution of the catalytic versus non-catalytic function in cardiometabolic disease while clinical trials will need to address the heart failure safety signals and demonstrate a beneficial effect of this class in CV complications associated with T2DM.
Acknowledgments
SOURCES OF FUNDING
Dr. Zhong is supported by grants from AHA (13POST17210033), and NSFC (81101553/H1604).
Non-standard Abbreviations and Acronyms
- ADA
adenosine deaminase
- CV
cardiovascular
- DC
dendritic cell
- DPP4
dipeptidyl peptidase-4
- DPP4i
DPP4 inhibitors
- Epo
erythropoietin
- FMD
flow-mediated dilation
- GIP
gastric inhibitory peptide
- GLP-1
glucagon like peptide-1
- HOMA-b
homeostasis model assessment beta cell function index
- HUVECs
human umbilical vein endothelial cells
- MERS
middle eastern respiratory syndrome
- sDPP4
soluble DPP4
- T2DM
type 2 diabetes
- VAT
visceral adipose tissue
Footnotes
DISCLOSURES
Dr. Rajagopalan has received funding from Takeda Pharmaceuticals.
REFERENCES
- 1.Seshasai SRK, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loster K, Zeilinger K, Schuppan D, Reutter W. The cysteine-rich region of dipeptidyl peptidase IV (CD 26) is the collagen-binding site. Biochem Biophys Res Commun. 1995;217:341–348. doi: 10.1006/bbrc.1995.2782. [DOI] [PubMed] [Google Scholar]
- 3.Richard E, Arredondo-Vega FX, Santisteban I, Kelly SJ, Patel DD, Hershfield MS. The binding site of human adenosine deaminase for CD26/Dipeptidyl peptidase IV: the Arg142Gln mutation impairs binding to cd26 but does not cause immune deficiency. J Exp Med. 2000;192:1223–1236. doi: 10.1084/jem.192.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasmussen HB, Branner S, Wiberg FC, Wagtmann N. Crystal structure of human dipeptidyl peptidase IV/CD26 in complex with a substrate analog. Nat Struct Biol. 2003;10:19–25. doi: 10.1038/nsb882. [DOI] [PubMed] [Google Scholar]
- 5.Dong RP, Tachibana K, Hegen M, Munakata Y, Cho D, Schlossman SF, Morimoto C. Determination of adenosine deaminase binding domain on CD26 and its immunoregulatory effect on T cell activation. J Immunol. 1997;159:6070–6076. [PubMed] [Google Scholar]
- 6.Abbott CA, McCaughan GW, Levy MT, Church WB, Gorrell MD. Binding to human dipeptidyl peptidase IV by adenosine deaminase and antibodies that inhibit ligand binding involves overlapping, discontinuous sites on a predicted beta propeller domain. Eur J Biochem. 1999;266:798–810. doi: 10.1046/j.1432-1327.1999.00902.x. [DOI] [PubMed] [Google Scholar]
- 7.Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan H, Meng W, Kilian C, Grams S, Reutter W. Domain-specific N-glycosylation of the membrane glycoprotein dipeptidylpeptidase IV (CD26) influences its subcellular trafficking, biological stability, enzyme activity and protein folding. Eur J Biochem. 1997;246:243–251. doi: 10.1111/j.1432-1033.1997.00243.x. [DOI] [PubMed] [Google Scholar]
- 9.Bauvois B, Djavaheri-Mergny M, Rouillard D, Dumont J, Wietzerbin J. Regulation of CD26/DPPIV gene expression by interferons and retinoic acid in tumor B cells. Oncogene. 2000;19:265–272. doi: 10.1038/sj.onc.1203292. [DOI] [PubMed] [Google Scholar]
- 10.Nemoto E, Sugawara S, Takada H, Shoji S, Horiuch H. Increase of CD26/dipeptidyl peptidase IV expression on human gingival fibroblasts upon stimulation with cytokines and bacterial components. Infect Immun. 1999;67:6225–6233. doi: 10.1128/iai.67.12.6225-6233.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salgado FJ, Vela E, Martin M, Franco R, Nogueira M, Cordero OJ. Mechanisms of CD26/dipeptidyl peptidase IV cytokine-dependent regulation on human activated lymphocytes. Cytokine. 2000;12:1136–1141. doi: 10.1006/cyto.1999.0643. [DOI] [PubMed] [Google Scholar]
- 12.Andrieu T, Thibault V, Malet I, Laporte J, Bauvois B, Agut H, Cahour A. Similar increased serum dipeptidyl peptidase IV activity in chronic hepatitis C and other viral infections. J Clin Virol. 2003;27:59–68. doi: 10.1016/s1386-6532(02)00128-2. [DOI] [PubMed] [Google Scholar]
- 13.Grondin G, Hooper NM, LeBel D. Specific localization of membrane dipeptidase and dipeptidyl peptidase IV in secretion granules of two different pancreatic islet cells. J Histochem Cytochem. 1999;47:489–498. doi: 10.1177/002215549904700407. [DOI] [PubMed] [Google Scholar]
- 14.Topham NJ, Hewitt EW. Natural killer cell cytotoxicity: how do they pull the trigger? Immunology. 2009;128:7–15. doi: 10.1111/j.1365-2567.2009.03123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A. 2000;97:6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broxmeyer HE, Hoggatt J, O’Leary Ha, et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18:1786–1796. doi: 10.1038/nm.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhong J, Rao X, Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: Potential implications in cardiovascular disease. Atherosclerosis. 2013;226:305–314. doi: 10.1016/j.atherosclerosis.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261:466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 19.Hegen M, Kameoka J, Dong RP, Schlossman SF, Morimoto C. Cross-linking of CD26 by antibody induces tyrosine phosphorylation and activation of mitogen-activated protein kinase. Immunology. 1997;90:257–264. doi: 10.1046/j.1365-2567.1997.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engel M, Hoffmann T, Wagner L, Wermann M, Heiser U, Kiefersauer R, Huber R, Bode W, Demuth H-U, Brandstetter H. The crystal structure of dipeptidyl peptidase IV (CD26) reveals its functional regulation and enzymatic mechanism. Proc Natl Acad Sci U S A. 2003;100:5063–5068. doi: 10.1073/pnas.0230620100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raj VS, Mou H, Smits SL, et al. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu G, Hu Y, Wang Q, Qi J, Gao F, Li Y, Zhang Y, Zhang W, Yuan Y, Bao J, Zhang B, Shi Y, Yan J, Gao GF. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;500:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raj VS, Smits SL, Provacia LB, van den Brand JMa, Wiersma L, Ouwendijk WJD, Bestebroer TM, Spronken MI, van Amerongen G, Rottier PJM, Fouchier RaM, Bosch BJ, Osterhaus ADME, Haagmans BL. Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4-mediated entry of the Middle East respiratory syndrome coronavirus. J Virol. 2014;88:1834–1838. doi: 10.1128/JVI.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohnuma K, Yamochi T, Uchiyama M, Nishibashi K, Iwata S, Hosono O, Kawasaki H, Tanaka H, Dang NH, Morimoto C. CD26 mediates dissociation of Tollip and IRAK-1 from caveolin-1 and induces upregulation of CD86 on antigen-presenting cells. Mol Cell Biol. 2005;25:7743–7757. doi: 10.1128/MCB.25.17.7743-7757.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnuma K, Takahashi N, Yamochi T, Hosono O, Dang NH, Morimoto C. Role of CD26/dipeptidyl peptidase IV in human T cell activation and function. Front Biosci. 2008;13:2299–2310. doi: 10.2741/2844. [DOI] [PubMed] [Google Scholar]
- 26.Ohnuma K, Yamochi T, Uchiyama M, Nishibashi K, Yoshikawa N, Shimizu N, Iwata S, Tanaka H, Dang NH, Morimoto C. CD26 up-regulates expression of CD86 on antigen-presenting cells by means of caveolin-1. Proc Natl Acad Sci U S A. 2004;101:14186–14191. doi: 10.1073/pnas.0405266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohnuma K, Munakata Y, Ishii T, Iwata S, Kobayashi S, Hosono O, Kawasaki H, Dang NH, Morimoto C. Soluble CD26/dipeptidyl peptidase IV induces T cell proliferation through CD86 up-regulation on APCs. J Immunol. 2001;167:6745–6755. doi: 10.4049/jimmunol.167.12.6745. [DOI] [PubMed] [Google Scholar]
- 28.Ohnuma K, Inoue H, Uchiyama M, Yamochi T, Hosono O, Dang NH, Morimoto C. T-cell activation via CD26 and caveolin-1 in rheumatoid synovium. Mod Rheumatol. 2006;16:3–13. doi: 10.1007/s10165-005-0452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136:3585–3596. doi: 10.1210/endo.136.8.7628397. [DOI] [PubMed] [Google Scholar]
- 30.Pridal L, Deacon CF, Kirk O, Christensen JV, Carr RD, Holst JJ. Glucagon-like peptide-1(7–37) has a larger volume of distribution than glucagon-like peptide-1(7–36)amide in dogs and is degraded more quickly in vitro by dog plasma. Eur J Drug Metab Pharmacokinet. 1996;21:51–59. doi: 10.1007/BF03190278. [DOI] [PubMed] [Google Scholar]
- 31.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 32.Calanna S, Christensen M, Holst JJ, Laferrère B, Gluud LL, Vilsbøll T, Knop FK. Secretion of glucagon-like peptide-1 in patients with type 2 diabetes mellitus: systematic review and meta-analyses of clinical studies. Diabetologia. 2013;56:965–972. doi: 10.1007/s00125-013-2841-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mannucci E, Pala L, Ciani S, Bardini G, Pezzatini A, Sposato I, Cremasco F, Ognibene A, Rotella CM. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia. 2005;48:1168–1172. doi: 10.1007/s00125-005-1749-8. [DOI] [PubMed] [Google Scholar]
- 34.Sell H, Blüher M, Klöting N, Schlich R, Willems M, Ruppe F, Knoefel WT, Dietrich A, Fielding BA, Arner P, Frayn KN, Eckel J. Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care. 2013;36:4083–4090. doi: 10.2337/dc13-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamers D, Famulla S, Wronkowitz N, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–1925. doi: 10.2337/db10-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sell H, Blüher M, Klöting N, Schlich R, Willems M, Ruppe F, Knoefel WT, Dietrich A, Fielding BA, Arner P, Frayn KN, Eckel J. Adipose dipeptidyl peptidase-4 and obesity: correlation with insulin resistance and depot-specific release from adipose tissue in vivo and in vitro. Diabetes Care. 2013;36:4083–4090. doi: 10.2337/dc13-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhong J, Rao X, Deiuliis J, Braunstein Z, Narula V, Hazey J, Mikami D, Needleman B, Satoskar AR, Rajagopalan S. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes. 2013;62:149–157. doi: 10.2337/db12-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SA, Kim YR, Yang EJ, Kwon EJ, Kim SH, Kang SH, Park DB, Oh BC, Kim J, Heo ST, Koh G, Lee DH. CD26/DPP4 levels in peripheral blood and T cells in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:2553–2561. doi: 10.1210/jc.2012-4288. [DOI] [PubMed] [Google Scholar]
- 39.Miyazaki M, Kato M, Tanaka K, Tanaka M, Kohjima M, Nakamura K, Enjoji M, Nakamuta M, Kotoh K, Takayanagi R. Increased hepatic expression of dipeptidyl peptidase-4 in non-alcoholic fatty liver disease and its association with insulin resistance and glucose metabolism. Mol Med Rep. 2012;5:729–733. doi: 10.3892/mmr.2011.707. [DOI] [PubMed] [Google Scholar]
- 40.Lenhard JM, Croom DK, Minnick DT. Reduced serum dipeptidyl peptidase-IV after metformin and pioglitazone treatments. Biochem Biophys Res Commun. 2004;324:92–97. doi: 10.1016/j.bbrc.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 41.Lindsay JR, Duffy NA, McKillop AM, Ardill J, O’Harte FP, Flatt PR, Bell PM. Inhibition of dipeptidyl peptidase IV activity by oral metformin in Type 2 diabetes. Diabet Med. 2005;22:654–657. doi: 10.1111/j.1464-5491.2005.01461.x. [DOI] [PubMed] [Google Scholar]
- 42.Green BD, Irwin N, Duffy NA, Gault VA, O’Harte FP, Flatt PR. Inhibition of dipeptidyl peptidase-IV activity by metformin enhances the antidiabetic effects of glucagon-like peptide-1. Eur J Pharmacol. 2006;547:192–199. doi: 10.1016/j.ejphar.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 43.Shimasaki T, Masaki T, Mitsutomi K, Ueno D, Gotoh K, Chiba S, Kakuma T, Yoshimatsu H. The dipeptidyl peptidase-4 inhibitor des-fluoro-sitagliptin regulates brown adipose tissue uncoupling protein levels in mice with diet-induced obesity. PLoS One. 2013;8:e63626. doi: 10.1371/journal.pone.0063626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pattzi HM, Pitale S, Alpizar M, Bennett C, O’Farrell AM, Li J, Cherrington JM, Guler HP. Dutogliptin, a selective DPP4 inhibitor, improves glycaemic control in patients with type 2 diabetes: a 12-week, double-blind, randomized, placebo-controlled, multicentre trial. Diabetes Obes Metab. 2010;12:348–355. doi: 10.1111/j.1463-1326.2010.01195.x. [DOI] [PubMed] [Google Scholar]
- 45.Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369. doi: 10.1136/bmj.e1369. [DOI] [PubMed] [Google Scholar]
- 46.Park H, Park C, Kim Y, Rascati KL. Efficacy and safety of dipeptidyl peptidase-4 inhibitors in type 2 diabetes: meta-analysis. Ann Pharmacother. 2012;46:1453–1469. doi: 10.1345/aph.1R041. [DOI] [PubMed] [Google Scholar]
- 47.Liu S-C, Tu Y-K, Chien M-N, Chien K-L. Effect of antidiabetic agents added to metformin on glycaemic control, hypoglycaemia and weight change in patients with type 2 diabetes: a network meta-analysis. Diabetes Obes Metab. 2012;14:810–820. doi: 10.1111/j.1463-1326.2012.01606.x. [DOI] [PubMed] [Google Scholar]
- 48.Wu D, Li L, Liu C. Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis. Diabetes Obes Metab. 2014;16:30–37. doi: 10.1111/dom.12174. [DOI] [PubMed] [Google Scholar]
- 49.Karagiannis T, Paschos P, Paletas K, Matthews DR, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:e1369. doi: 10.1136/bmj.e1369. [DOI] [PubMed] [Google Scholar]
- 50.Graefe-Mody U, Friedrich C, Port A, Ring A, Retlich S, Heise T, Halabi A, Woerle HJ. Effect of renal impairment on the pharmacokinetics of the dipeptidyl peptidase-4 inhibitor linagliptin. Diabetes, Obes Metab. 2011;13:939–946. doi: 10.1111/j.1463-1326.2011.01458.x. [DOI] [PubMed] [Google Scholar]
- 51.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- 52.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 53.Van Raalte DH, van Genugten RE, Eliasson B, Möller-Goede DL, Mari A, Tura A, Wilson C, Fleck P, Taskinen MR, Smith U, Diamant M. The effect of alogliptin and pioglitazone combination therapy on various aspects of β-cell function in patients with recent-onset type 2 diabetes. Eur J Endocrinol. 2014;170:565–574. doi: 10.1530/EJE-13-0639. [DOI] [PubMed] [Google Scholar]
- 54.Williams-Herman D, Xu L, Teng R, Golm GT, Johnson J, Davies MJ, Kaufman KD, Goldstein BJ. Effect of initial combination therapy with sitagliptin and metformin on β-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2012;14:67–76. doi: 10.1111/j.1463-1326.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 55.Dinjens WNM, Ten Kate J, Wijnen JT, Van der Linden EPM, Beek CJL, Lenders MHJH, Meera Khan P, Bosman FT. Distribution of adenosine deaminase-complexing protein in murine tissues. J Biol Chem. 1989;264:19215–19220. [PubMed] [Google Scholar]
- 56.Pala L, Rotella C. The role of DPP4 activity in cardiovascular districts: in vivo and in vitro evidence. J Diabetes Res. 2013;2013:590456. doi: 10.1155/2013/590456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shah Z, Pineda C, Kampfrath T, Maiseyeu A, Ying Z, Racoma I, Deiuliis J, Xu X, Sun Q, Moffatt-Bruce S, Villamena F, Rajagopalan S. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vasc Pharmacol. 2011;55:2–9. doi: 10.1016/j.vph.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishii M, Shibata R, Kondo K, Kambara T, Shimizu Y, Tanigawa T, Bando YK, Nishimura M, Ouchi N, Murohara T. Vildagliptin stimulates endothelial cell network formation and ischemia-induced revascularization via an endothelial nitric oxide synthase-dependent mechanism. J Biol Chem. 2014;289:27235–27245. doi: 10.1074/jbc.M114.557835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 60.Matsubara J, Sugiyama S, Sugamura K, et al. A dipeptidyl peptidase-4 inhibitor, des-fluoro-sitagliptin, improves endothelial function and reduces atherosclerotic lesion formation in apolipoprotein edeficient mice. J Am Coll Cardiol. 2012;59:265–276. doi: 10.1016/j.jacc.2011.07.053. [DOI] [PubMed] [Google Scholar]
- 61.Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, Hayden MR, Johnson MS, Salam M, Whaley-Connell A, Demarco VG. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology. 2013;154:2501–2513. doi: 10.1210/en.2013-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Poppel PCM, Netea MG, Smits P, Tack CJ. Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes Care. 2011;34:2072–2077. doi: 10.2337/dc10-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ayaori M, Iwakami N, Uto-Kondo H, et al. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J Am Heart Assoc. 2013;2:e003277. doi: 10.1161/JAHA.112.003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nakamura K, Oe H, Kihara H, Shimada K, Fukuda S, Watanabe K, Takagi T, Yunoki K, Miyoshi T, Hirata K, Yoshikawa J, Ito H. DPP-4 inhibitor and alpha-glucosidase inhibitor equally improve endothelial function in patients with type 2 diabetes: EDGE study. Cardiovasc Diabetol. 2014;13:110. doi: 10.1186/s12933-014-0110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ogawa S, Ishiki M, Nako K, Okamura M, Senda M, Mori T, Ito S. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J Exp Med. 2011;223:133–135. doi: 10.1620/tjem.223.133. [DOI] [PubMed] [Google Scholar]
- 66.Mistry GC, Maes AL, Lasseter KC, Davies MJ, Gottesdiener KM, Wagner JA, Herman GA. Effect of sitagliptin, a dipeptidyl peptidase-4 inhibitor, on blood pressure in nondiabetic patients with mild to moderate hypertension. J Clin Pharmacol. 2008;48:592–598. doi: 10.1177/0091270008316885. [DOI] [PubMed] [Google Scholar]
- 67.Von Eynatten M, Gong Y, Emser A, Woerle HJ. Efficacy and safety of linagliptin in type 2 diabetes subjects at high risk for renal and cardiovascular disease: A pooled analysis of six phase III clinical trials. Cardiovasc Diabetol. 2013;12:60. doi: 10.1186/1475-2840-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pedrazzini T, Seydoux J, Künstner P, Aubert JF, Grouzmann E, Beermann F, Brunner HR. Cardiovascular response, feeding behavior and locomotor activity in mice lacking the NPY Y1 receptor. Nat Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- 69.Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, Drucker DJ, Vallon V. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol. 2012;303:F963–F971. doi: 10.1152/ajprenal.00259.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Girardi ACC, Fukuda LE, Rossoni LV, Malnic G, Rebouças NA. Dipeptidyl peptidase IV inhibition downregulates Na+ - H+ exchanger NHE3 in rat renal proximal tubule. Am J Physiol Renal Physiol. 2008;294:F414–F422. doi: 10.1152/ajprenal.00174.2007. [DOI] [PubMed] [Google Scholar]
- 71.Crajoinas RO, Lessa LMA, Carraro-Lacroix LR, Davel APC, Pacheco BPM, Rossoni LV, Malnic G, Girardi ACC. Posttranslational mechanisms associated with reduced NHE3 activity in adult vs. young prehypertensive SHR. Am J Physiol Renal Physiol. 2010;299:F872–F881. doi: 10.1152/ajprenal.00654.2009. [DOI] [PubMed] [Google Scholar]
- 72.Brandt I, Lambeir A-M, Ketelslegers J-M, Vanderheyden M, Scharpé S, De Meester I. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem. 2006;52:82–87. doi: 10.1373/clinchem.2005.057638. [DOI] [PubMed] [Google Scholar]
- 73.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC. Des-serine-proline brain natriuretic peptide 3–32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–R901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- 74.Devin JK, Pretorius M, Nian H, Yu C, Billings FT, Brown NJ. Dipeptidyl-peptidase 4 inhibition and the vascular effects of glucagon-like peptide-1 and brain natriuretic peptide in the human forearm. J Am Heart Assoc. 2014;3:e001075. doi: 10.1161/JAHA.114.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scirica BM, Braunwald E, Raz I, et al. Heart Failure, Saxagliptin and Diabetes Mellitus: Observations from the SAVOR - TIMI 53 Randomized Trial. Circulation. 2014;130:1579–1588. doi: 10.1161/CIRCULATIONAHA.114.010389. [DOI] [PubMed] [Google Scholar]
- 76.Christopherson KW, Hangoc G, Mantel CR, Broxmeyer HE. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305:1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 77.Kanki S, Segers VFM, Wu W, Kakkar R, Gannon J, Sys SU, Sandrasagra A, Lee RT. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail. 2011;4:509–518. doi: 10.1161/CIRCHEARTFAILURE.110.960302. [DOI] [PubMed] [Google Scholar]
- 78.Theiss HD, Vallaster M, Rischpler C, et al. Dual stem cell therapy after myocardial infarction acts specifically by enhanced homing via the SDF-1/CXCR4 axis. Stem Cell Res. 2011;7:244–255. doi: 10.1016/j.scr.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 79.Christopherson KW, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–7008. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 80.Fadini GP, Boscaro E, Albiero M, Menegazzo L, Frison V, de Kreutzenberg S, Agostini C, Tiengo A, Avogaro A. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1alpha. Diabetes Care. 2010;33:1607–1609. doi: 10.2337/dc10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang CY, Shih CM, Tsao NW, et al. Dipeptidyl peptidase-4 inhibitor improves neovascularization by increasing circulating endothelial progenitor cells. Br J Pharmacol. 2012;167:1506–1519. doi: 10.1111/j.1476-5381.2012.02102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hocher B, Sharkovska Y, Mark M, Klein T, Pfab T. The novel DPP-4 inhibitors linagliptin and BI 14361 reduce infarct size after myocardial ischemia/reperfusion in rats. Int J Cardiol. 2013;167:87–93. doi: 10.1016/j.ijcard.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 83.Mentlein R, Dahms P, Grandt D, Krüger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- 84.Ghersi G, Chen W, Lee EW, Zukowska Z. Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding. Peptides. 2001;22:453–458. doi: 10.1016/s0196-9781(01)00340-0. [DOI] [PubMed] [Google Scholar]
- 85.Takasawa W, Ohnuma K, Hatano R, Endo Y, Dang NH, Morimoto C. Inhibition of dipeptidyl peptidase 4 regulates microvascular endothelial growth induced by inflammatory cytokines. Biochem Biophys Res Commun. 2010;401:7–12. doi: 10.1016/j.bbrc.2010.08.112. [DOI] [PubMed] [Google Scholar]
- 86.Aronis KN, Chamberland JP, Mantzoros CS. GLP-1 promotes angiogenesis in human endothelial cells in a dose-dependent manner, through the Akt, Src and PKC pathways. Metabolism. 2013;62:1279–1286. doi: 10.1016/j.metabol.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marfella R, Sasso FC, Rizzo MR, et al. Dipeptidyl peptidase 4 inhibition may facilitate healing of chronic foot ulcers in patients with type 2 diabetes. Exp Diabetes Res. 2012;2012:892706. doi: 10.1155/2012/892706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Poncina N, Albiero M, Menegazzo L, Cappellari R, Avogaro A, Fadini GP. The dipeptidyl peptidase-4 inhibitor Saxagliptin improves function of circulating pro-angiogenic cells from type 2 diabetic patients. Cardiovasc Diabetol. 2014;13:92. doi: 10.1186/1475-2840-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flock G, Baggio LL, Longuet C, Drucker DJ. Incretin receptors for glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide are essential for the sustained metabolic actions of vildagliptin in mice. Diabetes. 2007;56:3006–3013. doi: 10.2337/db07-0697. [DOI] [PubMed] [Google Scholar]
- 90.Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114:1788–1803. doi: 10.1161/CIRCRESAHA.114.301958. [DOI] [PubMed] [Google Scholar]
- 91.Zhong J, Maiseyeu A, Rajagopalan S. Lipoprotein Effects of Incretin Analogues and Dipeptidyl Peptidase 4 (DPP-4) Inhibitors. Clin Lipidol. 2015 doi: 10.2217/clp.14.59. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xiao C, Dash S, Morgantini C, Patterson BW, Lewis GF. Sitagliptin, a DPP-4 inhibitor, acutely inhibits intestinal lipoprotein particle secretion in healthy humans. Diabetes. 2014;63:2394–2401. doi: 10.2337/db13-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Matikainen N, Mänttäri S, Schweizer A, Ulvestad A, Mills D, Dunning BE, Foley JE, Taskinen M-R. Vildagliptin therapy reduces postprandial intestinal triglyceride-rich lipoprotein particles in patients with type 2 diabetes. Diabetologia. 2006;49:2049–2057. doi: 10.1007/s00125-006-0340-2. [DOI] [PubMed] [Google Scholar]
- 94.Noda Y, Miyoshi T, Oe H. Alogliptin ameliorates postprandial lipemia and postprandial endothelial dysfunction in non-diabetic subjects: a preliminary report. Cardiovasc. 2013;12:8. doi: 10.1186/1475-2840-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tremblay AJ, Lamarche B, Deacon CF, Weisnagel SJ, Couture P. Effect of sitagliptin therapy on postprandial lipoprotein levels in patients with type 2 diabetes. Diabetes Obes Metab. 2011;13:366–373. doi: 10.1111/j.1463-1326.2011.01362.x. [DOI] [PubMed] [Google Scholar]
- 96.Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, Sweep FCGJ, Luft FC, He Y, Foley JE, Jordan J. Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:846–852. doi: 10.1210/jc.2008-1400. [DOI] [PubMed] [Google Scholar]
- 97.Vittone F, Liberman A, Vasic D, Ostertag R, Esser M, Walcher D, Ludwig A, Marx N, Burgmaier M. Sitagliptin reduces plaque macrophage content and stabilises arteriosclerotic lesions in Apoe−/− mice. Diabetologia. 2012;55:2267–2275. doi: 10.1007/s00125-012-2582-5. [DOI] [PubMed] [Google Scholar]
- 98.Shah Z, Kampfrath T, Deiuliis JA, Zhong J, Pineda C, Ying Z, Xu X, Lu B, Moffatt-Bruce S, Durairaj R, Sun Q, Mihai G, Maiseyeu A, Rajagopalan S. Long-term dipeptidyl-peptidase 4 inhibition reduces atherosclerosis and inflammation via effects on monocyte recruitment and chemotaxis. Circulation. 2011;124:2338–2349. doi: 10.1161/CIRCULATIONAHA.111.041418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terasaki M, Nagashima M, Nohtomi K, Kohashi K, Tomoyasu M, Sinmura K, Nogi Y, Katayama Y, Sato K, Itoh F, Watanabe T, Hirano T. Preventive Effect of Dipeptidyl Peptidase-4 Inhibitor on Atherosclerosis Is Mainly Attributable to Incretin’s Actions in Nondiabetic and Diabetic Apolipoprotein E-Null Mice. PLoS One. 2013;8:e70933. doi: 10.1371/journal.pone.0070933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zeng Y, Li C, Guan M, Zheng Z, Li J, Xu W, Wang L, He F, Xue Y. The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms. Cardiovasc Diabetol. 2014;13:32. doi: 10.1186/1475-2840-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Terasaki M, Nagashima M, Watanabe T, Nohtomi K, Mori Y, Miyazaki A, Hirano T. Effects of PKF275-055, a dipeptidyl peptidase-4 inhibitor, on the development of atherosclerotic lesions in apolipoprotein E-null mice. Metabolism. 2012;61:974–977. doi: 10.1016/j.metabol.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 102.Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S, Sukmawati D, Nomiyama T, Kanazawa A, Kawamori R, Fujitani Y, Watada H. Anagliptin, a DPP-4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo e-deficient mice. Endocrinology. 2013;154:1260–1270. doi: 10.1210/en.2012-1855. [DOI] [PubMed] [Google Scholar]
- 103.Goto H, Nomiyama T, Mita T, Yasunari E, Azuma K, Komiya K, Arakawa M, Jin WL, Kanazawa A, Kawamori R, Fujitani Y, Hirose T, Watada H. Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces intimal thickening after vascular injury. Biochem Biophys Res Commun. 2011;405:79–84. doi: 10.1016/j.bbrc.2010.12.131. [DOI] [PubMed] [Google Scholar]
- 104.Nagashima M, Watanabe T, Terasaki M, Tomoyasu M, Nohtomi K, Kim-Kaneyama J, Miyazaki A, Hirano T. Native incretins prevent the development of atherosclerotic lesions in apolipoprotein e knockout mice. Diabetologia. 2011;54:2649–2659. doi: 10.1007/s00125-011-2241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, Fujitani Y, Hirose T, Kawamori R, Watada H. Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes. 2010;59:1030–1037. doi: 10.2337/db09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ishikawa S, Shimano M, Watarai M, Koyasu M, Uchikawa T, Ishii H, Inden Y, Takemoto K, Murohara T. Impact of sitagliptin on carotid intima-media thickness in patients with coronary artery disease and impaired glucose tolerance or mild diabetes mellitus. Am J Cardiol. 2014;114:384–388. doi: 10.1016/j.amjcard.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 107.Sauvé M, Ban K, Abdul Momen M, Zhou YQ, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59:1063–1073. doi: 10.2337/db09-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Connelly K, Zhang Y, Advani A, Advani S, Thai K, Yuen D, Gilbert R. DPP-4 Inhibition Attenuates Cardiac Dysfunction and Adverse Remodeling Following Myocardial Infarction in Rats with Experimental Diabetes. Cardiovasc Ther. 2013;31:259–267. doi: 10.1111/1755-5922.12005. [DOI] [PubMed] [Google Scholar]
- 109.Krijnen PAJ, Hahn NE, Kholová I, Baylan U, Sipkens JA, van Alphen FP, Vonk ABA, Simsek S, Meischl C, Schalkwijk CG, van Buul JD, van Hinsbergh VWM, Niessen HWM. Loss of DPP4 activity is related to a prothrombogenic status of endothelial cells: implications for the coronary microvasculature of myocardial infarction patients. Basic Res Cardiol. 2012;107:233. doi: 10.1007/s00395-011-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dos Santos L, Salles TA, Arruda-Junior DF, Campos LCG, Pereira AC, Barreto ALT, Antonio EL, Mansur AJ, Tucci PJF, Krieger JE, Girardi ACC. Circulating dipeptidyl peptidase IV activity correlates with cardiac dysfunction in human and experimental heart failure. Circ Heart Fail. 2013;6:1029–1038. doi: 10.1161/CIRCHEARTFAILURE.112.000057. [DOI] [PubMed] [Google Scholar]
- 111.Gomez N, Matheeussen V, Damoiseaux C, Tamborini A, Merveille AC, Jespers P, Michaux C, Clercx C, De Meester I, McEntee K. Effect of Heart Failure on Dipeptidyl Peptidase IV Activity in Plasma of Dogs. J Vet Intern Med. 2012;26:929–934. doi: 10.1111/j.1939-1676.2012.00942.x. [DOI] [PubMed] [Google Scholar]
- 112.Shigeta T, Aoyama M, Bando YK, Monji A, Mitsui T, Takatsu M, Cheng XW, Okumura T, Hirashiki A, Nagata K, Murohara T. Dipeptidyl peptidase-4 modulates left ventricular dysfunction in chronic heart failure via angiogenesis-dependent and -independent actions. Circulation. 2012;126:1838–1851. doi: 10.1161/CIRCULATIONAHA.112.096479. [DOI] [PubMed] [Google Scholar]
- 113.Gomez N, Touihri K, Matheeussen V, Da Costa AM, Mahmoudabady M, Mathieu M, Baerts L, Peace A, Lybaert P, Scharpé S, De Meester I, Bartunek J, Vanderheyden M, Mc Entee K. Dipeptidyl peptidase IV inhibition improves cardiorenal function in overpacing-induced heart failure. Eur J Heart Fail. 2012;14:14–21. doi: 10.1093/eurjhf/hfr146. [DOI] [PubMed] [Google Scholar]
- 114.Vyas AK, Aerni-Flessner LB, Payne MA, Kovacs A, Jay PY, Hruz PW. Saxagliptin Improves Glucose Tolerance but not Survival in a Murine Model of Dilated Cardiomyopathy. Cardiovasc Endocrinol. 2012;1:74–82. doi: 10.1097/XCE.0b013e32835bfb24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Takahashi A, Asakura M, Ito S, et al. Dipeptidyl-Peptidase IV Inhibition Improves Pathophysiology of Heart Failure and Increases Survival Rate in Pressure-Overloaded Mice. Am J Physiol Heart Circ Physiol. 2013;304:5–7. doi: 10.1152/ajpheart.00454.2012. [DOI] [PubMed] [Google Scholar]
- 116.Miyoshi T, Nakamura K, Yoshida M, Miura D, Oe H, Akagi S, Sugiyama H, Akazawa K, Yonezawa T, Wada J, Ito H. Effect of vildagliptin, a dipeptidyl peptidase 4 inhibitor, on cardiac hypertrophy induced by chronic beta-adrenergic stimulation in rats. Cardiovasc Diabetol. 2014;13:43. doi: 10.1186/1475-2840-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bostick B, Habibi J, Ma L, Aroor A, Rehmer N, Hayden MR, Sowers JR. Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metabolism. 2014;63:1000–1011. doi: 10.1016/j.metabol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Connelly KA, Bowskill BB, Advani SL, Thai K, Chen L-H, Kabir MG, Gilbert RE, Advani A. Dipeptidyl peptidase-4 inhibition improves left ventricular function in chronic kidney disease. Clin Invest Med. 2014;37:E172. doi: 10.25011/cim.v37i3.21384. [DOI] [PubMed] [Google Scholar]
- 119.Gallagher KA, Liu Z-J, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McCormick LM, Kydd AC, Read PA, Ring LS, Bond SJ, Hoole SP, Dutka DP. Chronic dipeptidyl peptidase-4 inhibition with sitagliptin is associated with sustained protection against ischemic left ventricular dysfunction in a pilot study of patients with type 2 diabetes mellitus and coronary artery disease. Circ Cardiovasc Imaging. 2014;7:274–281. doi: 10.1161/CIRCIMAGING.113.000785. [DOI] [PubMed] [Google Scholar]
- 121.Read PA, Khan FZ, Heck PM, Hoole SP, Dutka DP. DPP-4 inhibition by sitagliptin improves the myocardial response to dobutamine stress and mitigates stunning in a pilot study of patients with coronary artery disease. Circ Cardiovasc Imaging. 2010;3:195–201. doi: 10.1161/CIRCIMAGING.109.899377. [DOI] [PubMed] [Google Scholar]
- 122.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-Like Peptide-1 Infusion Improves Left Ventricular Ejection Fraction and Functional Status in Patients With Chronic Heart Failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 123.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of Glucagon-Like Peptide-1 in Patients with Acute Myocardial Infarction and Left Ventricular Dysfunction after Successful Reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 124.Read PA, Khan FZ, Dutka DP. Cardioprotection against ischaemia induced by dobutamine stress using glucagon-like peptide-1 in patients with coronary artery disease. Heart. 2012;98:408–413. doi: 10.1136/hrt.2010.219345. [DOI] [PubMed] [Google Scholar]
- 125.Tofovic DS, Bilan VP, Jackson EK. Sitagliptin augments angiotensin II-induced renal vasoconstriction in kidneys from rats with metabolic syndrome. Clin Exp Pharmacol Physiol. 2010;37:689–691. doi: 10.1111/j.1440-1681.2010.05389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nistala R, Habibi J, Lastra G, Manrique C, Aroor AR, Hayden MR, Garro M, Meuth A, Johnson M, Whaley-Connell A, Sowers JR. Prevention of obesity-induced renal injury in male mice by DPP4 inhibition. Endocrinology. 2014;155:2266–2276. doi: 10.1210/en.2013-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu WJ, Xie SH, Liu YN, Kim W, Jin HY, Park SK, Shao YM, Park TS. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin-induced diabetic rats. J Pharmacol Exp Ther. 2012;340:248–255. doi: 10.1124/jpet.111.186866. [DOI] [PubMed] [Google Scholar]
- 128.Joo KW, Kim S, Ahn S, Chin HJ, Chae D-W, Lee J, Han JS, Na KY. Dipeptidyl peptidase IV inhibitor attenuates kidney injury in rat remnant kidney. BMC Nephrol. 2013;14:98. doi: 10.1186/1471-2369-14-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Glorie LL, Verhulst A, Matheeussen V, Baerts L, Magielse J, Hermans N, D’Haese PC, De Meester I, De Beuf A. DPP4 inhibition improves functional outcome after renal ischemia-reperfusion injury. Am J Physiol Ren Physiol. 2012;303:F681–F688. doi: 10.1152/ajprenal.00075.2012. [DOI] [PubMed] [Google Scholar]
- 130.Nistala R, Habibi J, Aroor A, Sowers JR, Hayden MR, Meuth A, Knight W, Hancock T, Klein T, DeMarco VG, Whaley-Connell A. DPP4 Inhibition attenuates filtration barrier injury and oxidant stress in the zucker obese rat. Obesity (Silver Spring) 2014;22:2172–2179. doi: 10.1002/oby.20833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kanasaki K, Shi S, Kanasaki M, He J, Nagai T, Nakamura Y, Ishigaki Y, Kitada M, Srivastava SP, Koya D. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63:2120–2131. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 132.Hattori S. Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr J. 2011;58:69–73. doi: 10.1507/endocrj.k10e-382. [DOI] [PubMed] [Google Scholar]
- 133.Groop P-H, Cooper ME, Perkovic V, Emser A, Woerle H-J, von Eynatten M. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36:3460–3468. doi: 10.2337/dc13-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 135.Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 136.Standl E, Erbach M, Schnell O. Dipeptidyl-peptidase-4 Inhibitors and Heart Failure: Class Effect, Substance-Specific Effect, or Chance Effect? Curr Treat Options Cardiovasc Med. 2014;16:353. doi: 10.1007/s11936-014-0353-y. [DOI] [PubMed] [Google Scholar]
- 137.F Z, C C, W C, et al. Alogliptin in patients with type 2 diabetes after acute coronary syndromes: Heart failure outcomes and cardiovascular safety in heart failure patients. J. Am. Coll. Cardiol. 2014;63:A117. [Google Scholar]
- 138.Sanon VP, Sanon S, Pham SV, Chilton R. Play of Chance Versus Concerns Regarding Dipeptidyl Peptidase-4 Inhibitors: Heart Failure and Diabetes. Clin Diabetes. 2014;32:121–126. doi: 10.2337/diaclin.32.3.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ishibashi Y, Matsui T, Maeda S, Higashimoto Y, Yamagishi S. Advanced glycation end products evoke endothelial cell damage by stimulating soluble dipeptidyl peptidase-4 production and its interaction with mannose 6-phosphate/insulin-like growth factor II receptor. Cardiovasc Diabetol. 2013;12:125. doi: 10.1186/1475-2840-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Krijnen PAJ, Hahn NE, Kholová I, Baylan U, Sipkens JA, van Alphen FP, Vonk ABA, Simsek S, Meischl C, Schalkwijk CG, van Buul JD, van Hinsbergh VWM, Niessen HWM. Loss of DPP4 activity is related to a prothrombogenic status of endothelial cells: implications for the coronary microvasculature of myocardial infarction patients. Basic Res Cardiol. 2012;107:233. doi: 10.1007/s00395-011-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pacheco BP, Crajoinas RO, Couto GK, Davel APC, Lessa LM, Rossoni LV, Girardi AC. Dipeptidyl peptidase IV inhibition attenuates blood pressure rising in young spontaneously hypertensive rats. J Hypertens. 2011;29:520–528. doi: 10.1097/HJH.0b013e328341939d. [DOI] [PubMed] [Google Scholar]
- 142.Liu L, Liu J, Wong WT, et al. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60:833–841. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]
- 143.Mason RP, Jacob RF, Kubant R, Ciszewski A, Corbalan JJ, Malinski T. Dipeptidyl peptidase-4 inhibition with saxagliptin enhanced nitric oxide release and reduced blood pressure and sICAM-1 levels in hypertensive rats. J Cardiovasc Pharmacol. 2012;60:467–473. doi: 10.1097/FJC.0b013e31826be204. [DOI] [PubMed] [Google Scholar]
- 144.Ta NN, Schuyler CA, Li Y, Lopes-Virella MF, Huang Y. DPP-4 (CD26) Inhibitor Alogliptin Inhibits Atherosclerosis in Diabetic Apolipoprotein E-Deficient Mice. J Cardiovasc Pharmacol. 2011;58:157–166. doi: 10.1097/FJC.0b013e31821e5626. [DOI] [PMC free article] [PubMed] [Google Scholar]