Abstract
Objective
The objective of this preliminary study was to determine if high-velocity, low-amplitude spinal manipulation (HVLA-SM) thrust duration alters mechanical trunk activation thresholds of nociceptive-specific (NS) lateral thalamic neurons.
Methods
Extracellular recordings were obtained from 18 NS neurons located in 2 lateral thalamic nuclei (ventrolateral [n = 12] and posterior [n = 6]) in normal anesthetized Wistar rats. Response thresholds to electronic von Frey anesthesiometer (rigid tip) mechanical trunk stimuli applied in 3 lumbar directions (dorsal-ventral, 45° caudal, and 45° cranial) were determined before and immediately after the delivery of 3 HVLA-SM thrust durations (time control 0, 100, and 400 milliseconds). Mean changes in mechanical trunk activation thresholds were compared using a mixed model analysis of variance.
Results
High-velocity, low-amplitude spinal manipulation duration did not significantly alter NS lateral thalamic neurons’ mechanical trunk responses to any of the 3 directions tested with the anesthesiometer.
Conclusions
This study is the first to examine the effect of HVLA-SM thrust duration on NS lateral thalamic mechanical response thresholds. High-velocity, low-amplitude spinal manipulation thrust duration did not affect mechanical trunk thresholds.
Key Indexing Terms: Manipulation, Spinal, Thalamus, Nociceptive Neuron, Lumbar Vertebrae, Chiropractic
Spinal manipulation has been shown to be effective in treating neck and low back pain.1–3 Not only are the neurophysiological mechanisms by which this occurs unknown, but a lack of knowledge also exists regarding the effects of clinician-controlled mechanical application parameters of spinal manipulation on neural response. Clinician-controlled mechanical parameters that could potentially impact clinical outcomes of spinal manipulation include the following: magnitude and/or duration of preload, thrust magnitude, thrust duration and the consequent thrust rate, contact point, and/or loading direction relative to patient position.4,5 Basic and clinical studies aimed at determining the relationship between these various mechanical delivery parameters of spinal manipulation and their effects on neurophysiological, biomechanical, and/or patient outcome measures are just beginning to be reported in the literature.4–14 Once delineated, these findings could markedly improve and eventually optimize the utilization and effectiveness of spinal manipulation.
Numerous studies show that high-velocity, low-amplitude spinal manipulation (HVLA-SM) immediately increases mechanical pressure pain thresholds (ie, decreases sensitivity) in both symptomatic and asymptomatic individuals.15–24 These hypoalgesic effects occur both unilaterally and bilaterally as well as proximally and distally to the manipulation site. Evidence from animal models25–29 demonstrates that manual therapy applied to rats affects mechanical withdrawal thresholds in areas of the body distant to those being treated, which is consistent with reported clinical findings. Collectively, this clinical and experimental evidence suggests that HVLA-SM alters central processing of sensory input.
Gillette30 speculated that as many as 40 types of mechanoceptive endings (innocuous as well as nociceptive) in cutaneous and deep paraspinal tissues could be stimulated by spinal manipulation due to their mechanical activation thresholds being below the magnitude of mechanical force applied during a spinal manipulation. Although convergence and modulation of sensory input are known to occur at the level of the spinal cord,31 neuroimaging studies in humans and animals have also demonstrated decreased activation in supraspinal structures involved in pain processing after manual therapy intervention.32–34
The thalamus is a subcortical structure of research interest to manual therapy due to the fact that most ascending somatosensory input converges in it and output from it has the capacity to influence descending projections to nociceptive pathways in the spinal cord dorsal horn.35–37 It receives axonal projections from the spinal cord that relay innocuous (dorsal column pathway) and nociceptive (spinothalamic pathway) input from peripheral receptors.35 These receptors are presumably stimulated by spinal manipulation and theoretically are thought to impact central mechanisms including thalamic neurons.30,38–40 Moreover, there is anatomical evidence of direct projections between the thalamus and subcortical descending pain modulating structures such as the periaqueductal gray (PAG).41–44 Just recently, reciprocal short latency (~5 milliseconds) interactions between the thalamus and PAG have been shown in humans to be associated with pain relief.44 Increasing electrophysiological and neuroreceptor activation evidence implicates thalamic participation in an endogenous analgesic system, which is considered responsible (at least in part) for acupuncture-induced analgesia.37,45–47 Because acupuncture and spinal manipulative interventions both mechanically stimulate receptors in superficial and deep peripheral tissues, they may share a common mechanism of action via the thalamus.
Experimental efforts to better understand the neural effects of spinal manipulation at different levels of central ascending and descending nociceptive processing are needed to help elucidate the mechanisms responsible for its near immediate hypoalgesic effects. Furthermore, identifying which (if any) of the clinician-controlled mechanical parameters of a spinal manipulation can affect convergent ascending central processing will provide critical insight into the central mechanisms and clinical variables responsible for the positive clinical outcomes of spinal manipulation. Toward addressing these areas, a preliminary study was conducted to determine whether HVLA-SM thrust duration alters mechanical trunk activation thresholds of adult rat nociceptive-specific (NS) neurons in lateral thalamic nuclei.
Methods
All methods were approved by the Palmer Institutional Animal Care and Use Committee. Animals were housed individually and exposed to a 12-hour light/dark cycle with food and water ad libitum. For electrophysiological recordings, 9 adult male Wistar rats (320–460 g; Harlan, Indianapolis, IN) were anesthetized with an intraperitoneal injection of 50% urethane (1.2 g/kg) and maintained with supplement doses (5% urethane) administered intravenously as needed.48,49 Anesthetic state III-3 was maintained by monitoring pinch withdrawal, corneal reflex, respiration rate, and vibrissae movements.50 The jugular vein was catheterized for intravenous infusion. The trachea was intubated for PCO2 monitoring. Oxygen concentration, heart rate, and respiration were monitored by a MouseOx system (Starr Life Sciences Corp, Oakmont, PA). Body temperature was monitored with a rectal thermistor and maintained at 37°C with a circulating-water heating pad. The rat’s head was secured in a stereotaxic device (Kopf Instruments, Tujunga, CA) with its dorsal surface positioned horizontally. A small hole was made in the skull and expanded with bone rongeurs. The exposed dura was opened, and the extracellular recording electrode was advanced into the thalamus.
Electrophysiology
Activity in lateral thalamic neurons was recorded extracellularly with 1,1′-dioctabecyl-3,3,3′,3′-tetramethy-l-indocarbocyanine perchlorate (DiI; Invitrogen, Carlsbad, CA)–coated tungsten microelectrodes (FHC, Bowdoin, ME) having 6 to 8 MΩ impedance as previously described.49,51,52 Thalamic electrode tracks were made in parallel rows, 500 μm apart, and were located between −2.04 and −3.30 mm caudal to bregma and 1.2 and 3.8 mm lateral to midline.49,53 Recording began at 4 mm below the surface of the cortex and ended at 7.5 mm. Lateral thalamic nuclei through which the DiI-labeled electrode passed in each rat included ventrolateral (VL), ventroposterior lateral (VPL), ventroposterior medial (VPM), posterior (Po), and laterodorsal ventrolateral (LDVL), and laterodorsal dorsomedial (LDDM) (Fig 1). The electrode was slowly advanced at a rate of 1 to 5 μm per step using a motorized micromanipulator (Neurostar, Tubingen, Germany) until spontaneous single unit activity was isolated. Neurons with cutaneous receptive fields on the dorsolateral trunk were tested with graded mechanical stimuli (gentle stroking with a nylon brush and noxious pinch with a serrated forceps). Neurons failing to respond to innocuous stroking but responding to trunk pinch were classified as NS neurons. Activity in single thalamic NS neurons was passed through a high impedance probe (HIP511; Grass, West Warwick, RI), amplified (P511K; Grass), recorded, and evaluated off-line using a PC-based data acquisition system (Spike 2; Cambridge Electronic Design, Cambridge, England).
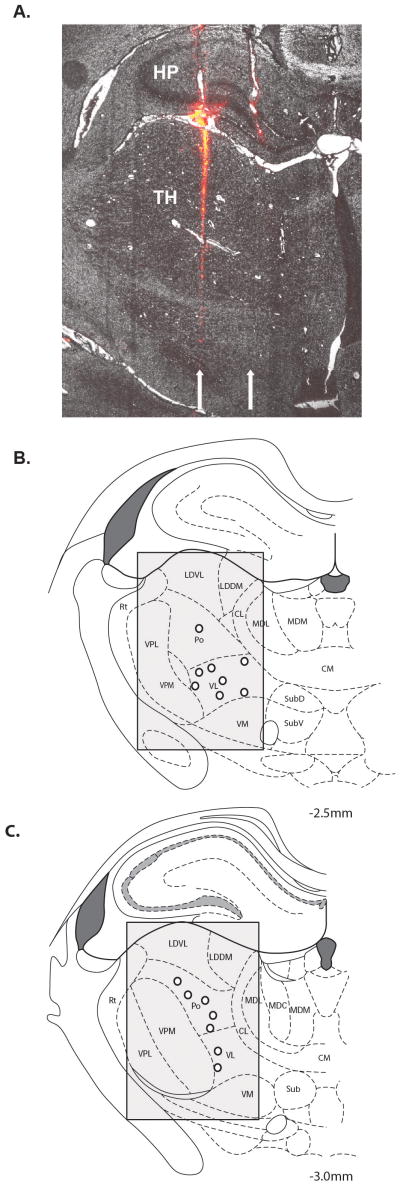
Fig 1.
A, Example of 2 tracks (arrows) from DiI-coated electrodes through the lateral thalamus (TH) and hippocampus (HP) in a coronal brain section at magnification ×40. The medial track was seen in greater detail in adjacent tissue sections. Summary showing the location (open circles) of 15 of 18 lateral thalamic neurons at −2.5 mm (B) and −3.0 mm (C) caudal to bregma that responded to mechanical pressure applied to the trunk. The 3 neurons not shown were located at more rostral thalamic levels. Shading indicates the potential search area, which included the LDDM, LDVL, VL, Po, VPM, and VPL nuclei.
Mechanical Activation Threshold
Once a thalamic neuron responsive to noxious trunk stimulation was located, an electronic von Frey anesthesiometer (with a rigid tip adapter for deep pressure; 0.79 mm2 contact area) (IITC Model 2390; www.iitcinc.com) was used to apply mechanical stimuli (measured in grams) in each of 3 directions on the dorsum of the trunk: dorsal-ventral, 45° caudalward, and 45° cranialward. It was thought that the direction in which a trunk stimulus was applied might differentially affect force transmission to the peripheral mechanoreceptors and thereby impact thalamic response thresholds. Electronic von Frey trunk stimuli were applied within 2 cm of the spine. Mechanical stimulation was applied to the more medial portion of the neuron’s receptive field but not necessarily at its center to take advantage of the more taut skin closer to the spine and to avoid the loose skin of the lateral trunk. Spontaneous activity was present in all neurons and recorded for a minimum of 10 seconds before delivery of any stimulus. The electronic von Frey stimulus was applied during quiescent or minimal tonic spontaneous discharge periods (Fig 2). The presentation order of mechanical stimuli to the trunk (dorsal-ventral, 45° caudalward, and 45° cranialward) and 3 spinal manipulation thrust durations (time control 0, 100, and 400 milliseconds) were randomized to minimize potential ordering effects. Mechanical trunk stimuli were applied serially in all 3 directions immediately before and after delivery of each HVLA-SM duration (time control 0, 100, and 400 milliseconds) until a response was elicited or until 400 g had been delivered. One to four lateral thalamic NS neurons were recorded in each rat.
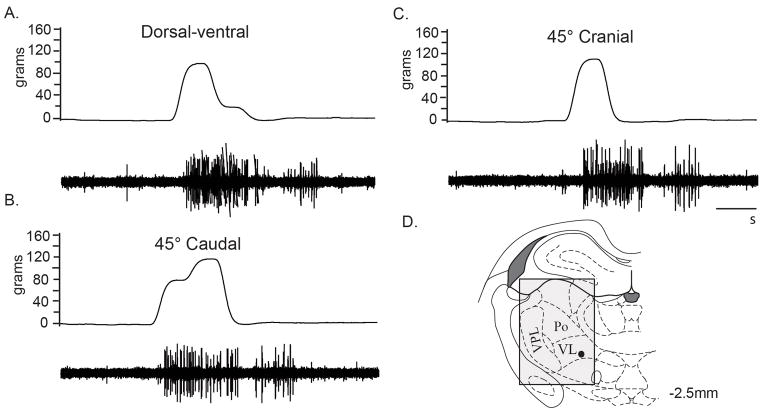
Fig 2.
Trunk mechanical stimuli responses of a single thalamic neuron in the VL nucleus. Raw electrophysiological recordings (lower row) of responses to lumbar trunk electronic von Frey stimuli (upper row) in the dorsal-ventral (96.1 g) (A), 45° caudal (73.9 g) (B), and 45° cranial (106.7 g) (C) directions. D, The location (●) of the trunk responsive VL neuron was at the −2.5 mm caudal to bregma level. The shading indicates the search area. Cal bar, 1 second.
Spinal Manipulation
In clinical settings, the force-time profile of an HVLA-SM can be likened to a triangle wave with a peak load of 31% to 78% body weight (based upon a 70-kg person) being reached in less than 150 milliseconds.54–56 A computer-controlled electronic feedback system (Lever System Model 310; Aurora Scientific, Ontario, Canada) was used in the present study to deliver a linearly increasing dorsal-ventral HVLA-SM thrust force with a peak amplitude of 85% rat body weight over a duration of either 100 or 400 milliseconds. A time control (0-millisecond thrust duration, ie, no thrust force) was included from which potential spontaneous changes in thalamic mechanical responsiveness could be determined. Contact for the HVLA-SM thrust was made on the intact skin overlying the L5 spinous process. High-velocity, low-amplitude spinal manipulations were separated by 5-minute intervals. This intertrial interval appears adequate to mitigate viscoelastic-related tissue changes after an HVLA-SM.14
Histology
After extracellular thalamic recordings, each rat was perfused transcardially with 0.9% saline followed by 4% paraformaldehyde. The brain was removed and stored in a 30% sucrose/10% formalin solution at 4°C until sectioning. Each brain was cut in the coronal plane into 30-μm-thick sections with a cryostat. The sections were mounted on microscope slides, and adjacent sections were stained with cresyl violet.57 1,1′-Dioctabecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate–labeled electrode tracks were located using a Nikon Optiphot microscope equipped with episcopic-fluorescent attachments and illumination system fluorescent attachment and illumination system. Postmortem histologic reconstructions of each DiI-labeled electrode track were made by taking measurements from midline at the appropriate thalamic anterior-posterior distance from bregma combined with carefully maintained records of electrode penetration depths from the cortical surface as previously described.49,52,58
Data Analysis
Changes in directional (dorsal-ventral, 45° caudalward, and 45° cranialward) thalamic mechanical trunk activation thresholds due to HVLA-SM were compared across the 3 thrust durations (time control 0, 100, and 400 milliseconds) using a mixed model analysis of variance. The results were reported as means and 95% confidence intervals (lower, upper 95% CI). P < .05 was considered to be statistically significant.
Results
Electrophysiological activity from 18 NS lateral thalamic neurons was recorded from 9 adult rats. The neurons were classified as NS by their lack of response to innocuous cutaneous stroking of the dorsolateral trunk and their responsiveness to noxious trunk pinch. All 18 NS neurons were found within 2 lateral thalamic nuclei (Po [n = 6] and VL [n = 12]) despite the inclusion of LDVL, LDDM, Po, VL, VPL, and VPM nuclei within our search area (Fig 1). Figure 2 shows an example of a VL NS neuron responding to mechanical trunk stimulation in the dorsal-ventral, 45° cranial, and 45° caudal directions using the electronic von Frey anesthesiometer. In this neuron, pre–HVLA-SM mechanical activation thresholds were similar in magnitude for all 3 directions tested. There was no difference (P = .68) in the mean rate of spontaneous activity between the Po and VL neurons (8.22 imp/s [95% CI, 2.77–13.67] vs 7.23 imp/s [4.35–10.11], respectively). Therefore, results of the neurons from these 2 lateral thalamic nuclei were combined and analyzed together.
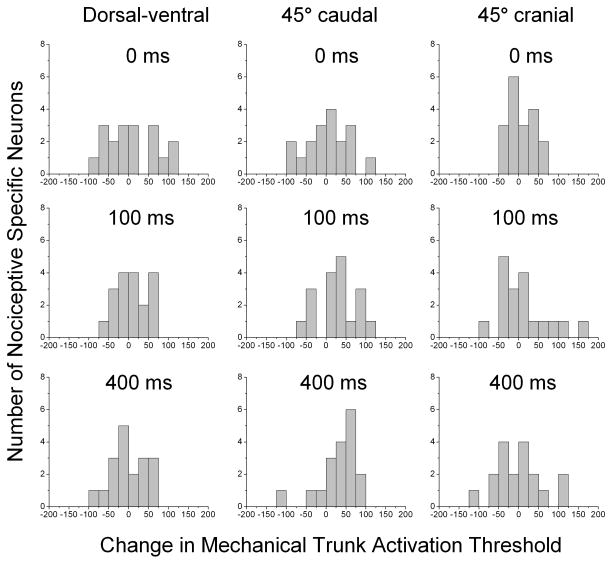
Figure 3 shows the distribution of changes in mechanical trunk activation response thresholds for the 18 NS neurons to each of the 3 mechanical testing directions for each of the 3 HVLA-SM thrust durations. The changes in threshold appear to have a rather similar and symmetrical unimodal distribution across all 3 testing directions for the time control (0-millisecond) duration. When compared with control, the distributions of changes in response threshold for 45° caudal trunk mechanical testing direction following both the 100- and 400-millisecond HVLA-SM thrust durations show distinct shifts to the right unlike the distributions for the dorsal-ventral and 45° cranial trunk mechanical testing directions. A shift to the right indicates an increase in mechanical thresholds (ie, decrease in mechanical sensitivity).
Fig 3.
Frequency histograms of changes in lumbar trunk electronic von Frey mechanical activation response thresholds for the dorsal-ventral, 45° caudal, and 45° cranial directions of NS lateral thalamic neurons after time control (0-millisecond), 100-, and 400-millisecond HVLA-SM thrust durations. Note the shift to the right in the 45° caudal mechanical testing direction after both the 100-and 400-millisecond thrust durations. HVLA-SM, high velocity low amplitude spinal manipulation; NS, nociceptive specific.
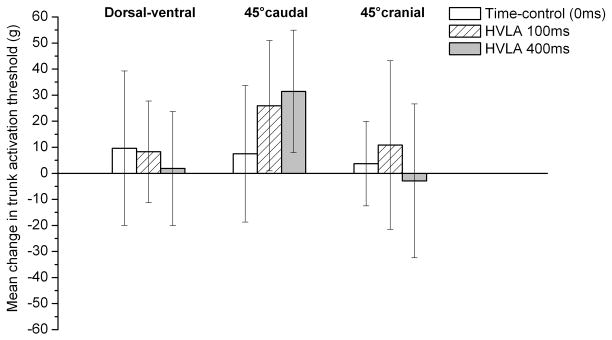
Using a mixed model analysis of variance, mechanical activation thresholds for the NS lateral thalamic neurons were not significantly different between the time control, 100-, or 400-millisecond HVLA-SM thrust duration for any of the 3 mechanical trunk stimuli directions tested (Fig 4). However, the mean changes in mechanical activation threshold for the 100- and 400-millisecond thrust durations in the 45° caudal direction were greater in magnitude than the mean changes in the dorsal-ventral and 45° cranial directions.
Fig 4.
Mean change in lumbar trunk electronic von Frey mechanical activation response thresholds (grams) for the dorsal-ventral, 45° caudal, and 45° cranial directions of NS lateral thalamic neurons after time control 0, 100-, and 400-millisecond HVLA-SM thrust durations. Data are reported as means and 95% CIs (lower, upper). HVLA-SM, high velocity low amplitude spinal manipulation; NS, nociceptive specific.
Discussion
Despite the well-documented clinical hypoalgesic effects associated with HVLA-SM intervention, the central and/or peripheral nervous system mechanisms responsible for this clinical effect remain elusive. Hypotheses for HVLA-SM’s mechanism of action have begun to focus on central neurophysiological interactions due to the nonlocalized nature of HVLA-SM–induced alterations in somatic pain that have been reported.15,38,40 Determining both underlying neurophysiological HVLA-SM mechanisms and how clinician-controlled mechanical parameters of HVLA-SM delivery may ultimately optimize changes in central somatosensory pain modulation has become a priority in both basic4,5,8,14 and clinical6,7,9,10 studies of spinal manipulation. Therefore, we undertook this study as a first step in determining whether there is a relationship between HVLA-SM, thrust duration, and changes in mechanical activation thresholds of thalamic neurons.
The effect of 100- and 400-millisecond HVLA-SM thrust durations on the mechanical trunk activation thresholds of 18 NS lateral thalamic neurons was investigated. Although several lateral thalamic nuclei were included within the search area, the 18 NS neurons in this study were located within either the Po or VL thalamic nucleus. Although the VPL nucleus is typically recognized as being involved in the sensory discriminative component of somatic pain, the lack of NS neurons in the VPL nucleus in the present experiments is likely a reflection of several factors. These factors include (1) the electrode track coordinates not being preferentially selected for any particular lateral thalamic nucleus; (2) the VPL nucleus is smaller than either the VL or Po nucleus; (3) the electrode track penetrations were always performed in the medial to lateral direction, which may have resulted in a more medial nuclei electrode sampling bias; and (4) a large proportion of VPL neurons (up to 80%) are wide dynamic range neurons that respond to both innocuous and noxious mechanical stimuli.59,60 Wide dynamic range neurons were not included in this study.
The data suggest that HVLA-SM duration does not alter mechanical activation thresholds of NS neurons in 2 lateral thalamic nuclei (VL and Po) when the dorsal trunk is mechanically stimulated in the dorsal-ventral, 45° caudal, or 45° cranial direction. Human imaging studies have begun to investigate brain correlates of a counterirritation phenomenon thought to be associated with manual therapy intervention.32,33 For example, a counterirritation stimulus consisting of immersing the left contralateral foot in cold water decreased pain-related activation of several brain areas including the thalamus, primary somatosensory cortices, anterior cingulate cortex, and posterior insular cortex evoked by phasic electrical stimulation of the sural nerve in healthy volunteers.61 Functional magnetic resonance imaging was used in a recent case series study to determine supraspinal activation in response to noxious mechanical stimuli applied before and after spinal manipulation.32 Bilateral activation of the thalami, cerebellum, amygdala, PAG, insular cortex, anterior cingulated cortex, somatosensory cortices, supplementary motor area, and premotor areas occurred with noxious stimulation. Only the insular cortex demonstrated a significant relationship between supraspinal activation and a reduction in subject’s perception of pain postmanipulation. In the future, larger human functional magnetic resonance imaging studies may be used to inform neurophysiology investigations in pursuit of central mechanisms of action underlying spinal manipulation.
Limitations
This preliminary study investigated changes in 18 NS neurons located within only 2 lateral thalamic nuclei, VL and Po. No significant differences were found between the time control 0, 100-, and 400-millisecond HVLA-SM thrust durations for any of the 3 mechanical trunk stimuli directions tested. These results indicate that either HVLA-SM does not alter lateral thalamic activation thresholds or the study was underpowered to detect a statistically significant difference due to (1) the small sample size and/or (2) the large variability in mechanical activation thresholds caused by the use of a single application of the electronic von Frey device and/or slippage of the device’s tip on the skin that often occurred at greater loading magnitudes. To better understand whether the variability in our response measure might have prevented us from detecting significant differences in our sample size of 18 neurons, we reviewed thalamic neuron studies with sample sizes of 8 to 42 neurons.62–67 In 1 study that investigated lateral thalamic VPL neurons, changes in extracellular activity after a noxiously evoked mechanical pinch stimulation using an arterial clip were compared in rats with and without unilateral sciatic chronic constriction injury.67 With a sample size of 11 VPL neurons, their mean difference of 36.8 imp/s (SD, 35.4) was statistically significant. Using their mean difference, SD, an α level of .05, and a 2-tailed test, we calculated that a sample size of 18 neurons would have yielded a test with 98% power. This suggests that our sample size of 18 neurons should have been sufficient to find significant differences had our mean variability (SD, 50.3) not been 1.4× greater than in their study.
Our use of a single application of the electronic von Frey mechanical stimulus and the selection of a maximum trunk stimulus of 400 g likely contributed to the variability we observed in thalamic mechanical activation thresholds (Fig 4). The decision to use a single electronic von Frey application to the trunk in each direction before and after HVLA-SM was made to limit potential sensitization and/or tissue damage, which can occur after repeated mechanical stimulation. Testing thalamic neuron responses in 3 directions (dorsal-ventral, 45° caudalward, and 45° cranialward) at 3 HVLA-SM thrust durations (time control 0, 100, and 400 milliseconds) equated to 9 mechanical stimulations per neuron. Had we taken the mean of 3 electronic von Frey applications before and after a single HVLA-SM duration to decrease potential variability, this would have equated to 27 mechanical stimulations per neuron. The maximum mechanical stimulus of 400 g was chosen because the trunk stimulus may not have always been applied to the center of the receptive field. Future basic studies should consider reducing the number of experimental variables tested (mechanical trunk stimulus directions and thrust durations/parameters) while (1) using replicate electronic von Frey mechanical stimulations, (2) reducing the maximum trunk mechanical stimulus delivered, and (3) using an alternate mechanical stimulus such as an arterial clip or other standardized mechanical devices.
Conclusion
To our knowledge, this study is the first to investigate the effects of HVLA-SM thrust duration on neural response properties of supraspinal neurons. The results suggest that HVLA-SM thrust duration does not significantly affect directional trunk mechanical responsiveness of NS neurons in 2 lateral thalamic nuclei (VL and Po). However, before definitive conclusions can be drawn regarding effects of HVLA-SM thrust duration on thalamic neuron responsiveness, additional studies with measures taken to reduce variability should be performed in normal, acute, and chronic animal models. These studies should encompass both lateral and medial thalamic nuclei, brainstem, and cortical structures known to be involved in nociceptive somatosensory processing.
Practical Applications.
This study found that HVLA-SM thrust duration did not alter NS lateral thalamic mechanical trunk responses in any of the 3 directions tested.
This study is a first step in investigating the effects of spinal manipulation thrust duration on supraspinal sensory processing.
Larger studies with measures taken to decrease variability should be performed in normal, acute, and chronic animal models looking at the effects of clinician-controlled biomechanical parameters of HVLA-SM on NS as well as wide dynamic range neurons in the thalamus and other central structures known to be involved in nociceptive somatosensory processing.
Acknowledgments
The authors thank Darlene Burke (University of Louisville-KSCIRC Neuroscience Core supported by grant 8P30GM103507–National Institute of General Medical Sciences, NIH) for statistical analyses and Amanda Hussman for microscopy assistance with histologic reconstructions.
Funding Sources
This work was supported by grants from the Australian Spinal Research Foundation (LG2010-11) and NIH National Center for Complementary and Alternative Medicine (K01AT005935) to WRR. This work was conducted in a facility constructed with support from Research Facilities Improvement Grant number C06 RR15433 from the National Center for Research Resources.
Footnotes
Potential Conflicts of Interest
No conflicts of interest were reported for this study
Contributorship Information
Concept development (provided idea for the research): W.R.R.
Design (planned the methods to generate the results): W.R.R., J.G.P., R.S.S.
Supervision (provided oversight, responsible for organization and implementation, writing of the manuscript): W.R.R.
Data collection/processing (responsible for experiments, patient management, organization, or reporting data): W.R.R., R.S.S.
Analysis/interpretation (responsible for statistical analysis, evaluation, and presentation of the results): W.R.R., J.G.P., S.M.O., R.S.S.
Literature search (performed the literature search): W.R.R., S.M.O., R.S.S.
Writing (responsible for writing a substantive part of the manuscript): W.R.R., S.M.O.
Critical review (revised manuscript for intellectual content, this does not relate to spelling and grammar checking): W.R.R., J.G.P., S.M.O., R.S.S.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Bronfort G, Haas M, Evans R, Kawchuk G, Dagenais S. Evidence-informed management of chronic low back pain with spinal manipulation and mobilization. Spine J. 2008;8:213–25. doi: 10.1016/j.spinee.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Goertz CM, Pohlman KA, Vining RD, Brantingham JW, Long CR. Patient-centered outcomes of high velocity, low-amplitude and spinal manipulation for low back pain: a systematic review. J Electromyogr Kinesiol. 2012;22:670–91. doi: 10.1016/j.jelekin.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Bronfort G, Evans R, Anderson AV, Svendsen KH, Bracha Y, Grimm RH. Spinal manipulation, medication, or home exercise with advice for acute and subacute neck pain: a randomized trial. Ann Intern Med. 2012;156:1–10. doi: 10.7326/0003-4819-156-1-201201030-00002. [DOI] [PubMed] [Google Scholar]
- 4.Reed WR, Cao DY, Long CR, Kawchuk GN, Pickar JG. Relationship between biomechanical characteristics of spinal manipulation and neural responses in an animal model: effect of linear control of thrust displacement versus force, thrust amplitude, thrust duration and thrust rate. Evid Based Complement Alternat Med. 2013;2013:492039. doi: 10.1155/2013/492039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reed WR, Long CR, Kawchuk GN, Pickar JG. Neural responses to the mechanical parameters of a high velocity. low amplitude spinal manipulation: effect of preload parameters. J Manipulative Physiol Ther. 2014;37:68–78. doi: 10.1016/j.jmpt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nougarou F, Dugas C, Loranger M, Page I, Descarreaux M. The role of preload forces in spinal manipulation: experimental investigation of kinematic and electromyographic responses in healthy adults. J Manipulative Physiol Ther. 2014;37:287–93. doi: 10.1016/j.jmpt.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Page I, Nougarou F, Dugas C, Descarreaux M. The effect of spinal manipulation impulse duration on spine neuromechanical responses. J Can Chiropr Assoc. 2014;58:141–8. [PMC free article] [PubMed] [Google Scholar]
- 8.Reed WR, Pickar JG, Sozio RS, Long CR. Effect of spinal manipulation thrust magnitude on trunk mechanical activation thresholds of lateral thalamic neurons. J Manipulative Physiol Ther. 2014;37:277–86. doi: 10.1016/j.jmpt.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nougarou F, Dugas C, Deslauriers C, Page I, Descarreaux M. Physiological responses to spinal manipulative therapy: investigation of the relationship between electromyographic responses and peak force. J Manipulative Physiol Ther. 2013;36:557–63. doi: 10.1016/j.jmpt.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Descarreaux M, Nougarou F, Dugas C. Standardization of spinal manipulation therapy in humans: development of a novel device designed to measure dose-response. J Manipulative Physiol Ther. 2013;36:78–83. doi: 10.1016/j.jmpt.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Cao DY, Reed WR, Long CR, Kawchuk GN, Pickar JG. Effects of thrust amplitude and duration of high-velocity, low-amplitude spinal manipulation on lumbar muscle spindle responses to vertebral position and movement. J Manipulative Physiol Ther. 2013;36:68–77. doi: 10.1016/j.jmpt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colloca CJ, Keller TS, Harrison DE, Moore RJ, Gunzburg R, Harrison DD. Spinal manipulation force and duration affect vertebral movement and neuromuscular responses. Clin Biomech. 2006;21:254–62. doi: 10.1016/j.clinbiomech.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Reed WR, Long CR, Pickar JG. Effects of unilateral facet fixation and facetectomy on muscle spindle responsiveness during simulated spinal manipulation in an animal model. J Manipulative Physiol Ther. 2013;36:585–94. doi: 10.1016/j.jmpt.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaillant M, Edgecombe T, Long CR, Pickar JG, Kawchuk GN. The effect of duration and amplitude of spinal manipulative therapy (SMT) on spinal stiffness. Man Ther. 2012;17:577–83. doi: 10.1016/j.math.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coronado RA, Gay CW, Bialosky JE, Carnaby GD, Bishop MD, George SZ. Changes in pain sensitivity following spinal manipulation: a systematic review and meta-analysis. J Electromyogr Kinesiol. 2012;22:752–67. doi: 10.1016/j.jelekin.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Carnero J, Fernandez-de-Las-Penas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epicondylalgia. J Manipulative Physiol Ther. 2008;31:675–81. doi: 10.1016/j.jmpt.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-de-Las-Penas C, Perez-de-Heredia M, Brea-Rivero M, Miangolarra-Page JC. Immediate effects on pressure pain threshold following a single cervical spine manipulation in healthy subjects. J Orthop Sports Phys Ther. 2007;37:325–9. doi: 10.2519/jospt.2007.2542. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez-de-Las-Penas C, Alonso-Blanco C, Cleland JA, Rodriguez-Blanco C, Alburquerque-Sendin F. Changes in pressure pain thresholds over C5-C6 zygapophyseal joint after a cervicothoracic junction manipulation in healthy subjects. J Manipulative Physiol Ther. 2008;31:332–7. doi: 10.1016/j.jmpt.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Maduro deCamargo V, Alburquerque-Sendin F, Berzin F, Stefanelli VC, Rodrigues de Souza DP, Fernandez-de-Las-Penas C. Immediate effects on electromyographic activity and pressure pain thresholds after a cervical manipulation in mechanical neck pain: a randomized controlled trial. J Manipulative Physiol Ther. 2011;34:211–20. doi: 10.1016/j.jmpt.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Mansilla-Ferragut P, Fernandez-de-Las PC, Alburquerque-Sendin F, Cleland JA, Bosca-Gandia JJ. Immediate effects of atlanto-occipital joint manipulation on active mouth opening and pressure pain sensitivity in women with mechanical neck pain. J Manipulative Physiol Ther. 2009;32:101–6. doi: 10.1016/j.jmpt.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Srbely JZ, Vernon H, Lee D, Polgar M. Immediate effects of spinal manipulative therapy on regional antinoceptive effects in myofascial tissues in healthy young adults. J Manipulative Physiol Ther. 2013;36:333–41. doi: 10.1016/j.jmpt.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Vernon H. Qualitative review of studies of manipulation-induced hypoalgesia. J Manipulative Physiol Ther. 2000;23:134–8. doi: 10.1016/s0161-4754(00)90084-8. [DOI] [PubMed] [Google Scholar]
- 23.Vicenzino B, Paungmali A, Buratowski S, Wright A. Specific manipulative therapy treatment for chronic lateral epicondylalgia produces uniquely characteristic hypoalgesia. Man Ther. 2001;6:205–12. doi: 10.1054/math.2001.0411. [DOI] [PubMed] [Google Scholar]
- 24.Yu X, Wang X, Zhang J, Wang Y. Changes in pressure pain thresholds and basal electromyographic activity after instrument-assisted spinal manipulative therapy in asymptomatic participants: a randomized, controlled trial. J Manipulative Physiol Ther. 2012;35:437–45. doi: 10.1016/j.jmpt.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Grayson JE, Barton T, Cabot PJ, Souvlis T. Spinal manual therapy produces rapid onset analgesia in a rodent model. Man Ther. 2012;17:292–7. doi: 10.1016/j.math.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Skyba DA, Radhakrishnan R, Rohlwing JJ, Wright A, Sluka KA. Joint manipulation reduces hyperalgesia by activation of monoamine receptors but not opioid or GABA receptors in the spinal cord. Pain. 2003;106:159–68. doi: 10.1016/s0304-3959(03)00320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sluka K, Wright A. Knee joint mobilization reduces secondary mechanical hyperalgesia induced by capsaicin injection into the ankle joint. Eur J Pain. 2001;5:81–7. doi: 10.1053/eujp.2000.0223. [DOI] [PubMed] [Google Scholar]
- 28.Sluka KA, Skyba DA, Radhakrishnan R, Leeper BJ, Wright A. Joint mobilization reduces hyperalgesia associated with chronic muscle and joint inflammation in rats. J Pain. 2006;7:602–7. doi: 10.1016/j.jpain.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Song XJ, Gan Q, Cao JL, Wang ZB, Rupert RL. Spinal manipulation reduces pain and hyperalgesia after lumbar intervertebral foramen inflammation in the rat. J Manipulative Physiol Ther. 2006;29:5–13. doi: 10.1016/j.jmpt.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Gillette RG. A speculative argument for the coactivation of diverse somatic receptor populations by forceful chiropractic adjustments. Man Med. 1987;3:1–14. [Google Scholar]
- 31.Schaible HG, Schmidt R, Willis W. Enhancement of the responses of ascending tract cells in the cat spinal cord by acute inflammation of the knee joint. Exp Brain Res. 1987;66:489–99. doi: 10.1007/BF00270681. [DOI] [PubMed] [Google Scholar]
- 32.Sparks C, Cleland JA, Elliott JM, Zagardo M, Liu W. Using functional magnetic resonance imaging to determine if cerebral hemodynamic responses to pain change following thoracic spine thrust manipulation in healthy individuals. J Orthop Sports Phys Ther. 2013;43:340–8. doi: 10.2519/jospt.2013.4631. [DOI] [PubMed] [Google Scholar]
- 33.Sparks C, Cleland J, Elliott J, Strubhar A. Supraspinal structures may be associated with hypoalgesia following thrust manipulation to the spine: a review of the literature. Phys Ther Rev. 2013;18:112–6. [Google Scholar]
- 34.Malisza KL, Gregorash L, Turner A, Foniok T, Stroman PW. Functional MRI involving painful stimulation of the ankle and the effect of physiotherapy joint mobilization. Magn Reson Imaging. 2003;21:489–96. doi: 10.1016/s0730-725x(03)00074-2. [DOI] [PubMed] [Google Scholar]
- 35.Jones EG. The thalamus. 2. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 36.Ab Aziz CB, Ahmad AH. The role of the thalamus in modulating pain. Malays J Med Sci. 2006;13:11–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Tang JS, Qu CL, Huo FQ. The thalamic nucleus submedius and ventrolateral orbital cortex are involved in nociceptive modulation: a novel pain modulation pathway. Prog Neurobiol. 2009;89:383–9. doi: 10.1016/j.pneurobio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Bialosky JE, Bishop MD, Price DD, Robinson ME, George SZ. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther. 2009;14:531–8. doi: 10.1016/j.math.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henderson CN. The basis for spinal manipulation: chiropractic perspective of indications and theory. J Electromyogr Kinesiol. 2012;22:632–42. doi: 10.1016/j.jelekin.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Boal RW, Gillette RG. Central neuronal plasticity, low back pain and spinal manipulative therapy. J Manipulative Physiol Ther. 2004;27:314–26. doi: 10.1016/j.jmpt.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 41.Krout KE, Loewy AD. Periaqueductal gray matter projections to the midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2000;424:111–41. doi: 10.1002/1096-9861(20000814)424:1<111::aid-cne9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 42.Krout KE, Belzer RE, Loewy AD. Brainstem projections to midline and intralaminar thalamic nuclei of the rat. J Comp Neurol. 2002;448:53–101. doi: 10.1002/cne.10236. [DOI] [PubMed] [Google Scholar]
- 43.Mantyh PW. Connections of midbrain periaqueductal gray in the monkey, I. Ascending efferent projections. J Neurophysiol. 1983;49:567–81. doi: 10.1152/jn.1983.49.3.567. [DOI] [PubMed] [Google Scholar]
- 44.Rinvik E, Wiberg M. Demonstration of a reciprocal connection between the periaqueductal gray matter and the reticular nucleus of the thalamus. Anat Embryol. 1990;181:577–84. doi: 10.1007/BF00174629. [DOI] [PubMed] [Google Scholar]
- 45.Takeshige C, Sato T, Mera T, Hisamitsu T, Fang J. Descending pain inhibitory system involved in acupuncture analgesia. Brain Res Bull. 1992;29:617–34. doi: 10.1016/0361-9230(92)90131-g. [DOI] [PubMed] [Google Scholar]
- 46.Tobaldini G, Aisengard B, Lima MM, Tambeli CH, Fisher L. Ascending nociceptive control contributes to the antinoceptive effect of acupuncture in a rat model of acute pain. J Pain. 2014;15:422–34. doi: 10.1016/j.jpain.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Zhao Z. Neural mechanism underlying acupuncture analgesia. Prog Neurobiol. 2008;85:355–75. doi: 10.1016/j.pneurobio.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Hubscher CH, Johnson RD. Responses of thalamic neurons to input from the male genitalia. J Neurophysiol. 2003;89:2–11. doi: 10.1152/jn.00294.2002. [DOI] [PubMed] [Google Scholar]
- 49.Reed WR, Chadha HK, Hubscher CH. Effects of 17-β-estradiol on responses of viscerosomatic convergent thalamic neurons in the ovariectomized female rat. J Neurophysiol. 2009;102:1062–74. doi: 10.1152/jn.00165.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedberg MH, Lee SM, Ebner FF. Modulation of receptive field properties of thalamic somatosensory neurons by the depth of anaesthesia. J Neurophysiol. 1999;81:2243–52. doi: 10.1152/jn.1999.81.5.2243. [DOI] [PubMed] [Google Scholar]
- 51.Chadha HK, Hubscher CH. Convergence of nociceptive information in the forebrain of female rats: reproductive organ response variations with stage of estrus. Exp Neurol. 2008;210:375–87. doi: 10.1016/j.expneurol.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 52.Massey JM, Hubscher CH, Wagoner MR, et al. Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci. 2006;26:4406–14. doi: 10.1523/JNEUROSCI.5467-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Burlington, MA: Academic Press; 2007. [Google Scholar]
- 54.Herzog W, Conway PJ, Kawchuk GN, Zhang Y, Hasler EM. Forces exerted during spinal manipulative therapy. Spine. 1993;18:1206–12. doi: 10.1097/00007632-199307000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Herzog W. The biomechanics of spinal manipulation. J Bodyw Mov Ther. 2010;14:280–6. doi: 10.1016/j.jbmt.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Triano JJ. Biomechanics of spinal manipulative therapy. Spine J. 2001;1:121–30. doi: 10.1016/s1529-9430(01)00007-9. [DOI] [PubMed] [Google Scholar]
- 57.Hubscher CH, Reed WR, Kaddumi EG, Armstrong JE, Johnson RD. Select spinal lesions reveal multiple ascending pathways in the rat conveying input from the male genitalia. J Physiol. 2010;588:1073–83. doi: 10.1113/jphysiol.2009.186544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hubscher CH. Estradiol-associated variation in responses of rostral medullary neurons to somatovisceral stimulation. Exp Neurol. 2006;200:227–39. doi: 10.1016/j.expneurol.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Palecek J, Willis WD. Responses of neurons in the rat ventral posterior lateral thalamic nucleus to noxious visceral and cutaneous stimuli. Thalamus Relat Syst. 2005;3:25–32. [Google Scholar]
- 60.Poggio GF, Mountcastle VB. The functional properties of ventrobasal thalamic neurons studied in unanesthesized monkeys. J Neurophysiol. 1963:775–806. doi: 10.1152/jn.1963.26.5.775. [DOI] [PubMed] [Google Scholar]
- 61.Piche M, Arsenault M, Rainville P. Cerebral and cerebrospinal processes underlying counterirritation analgesia. J Neurosci. 2009;29:14236–46. doi: 10.1523/JNEUROSCI.2341-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao P, Waxman SG, Hains BC. Sodium channel expression in the ventral posterolateral nucleus of the thalamus after peripheral nerve injury. Mol Pain. 2006:2. doi: 10.1186/1744-8069-2-27. http://dx.doi.org/10.1186/1744-8069-2-27. [DOI] [PMC free article] [PubMed]
- 63.Fischer TZ, Tan AM, Waxman SG. Thalamic neuron hyperexcitability and enlarged receptive fields in the STZ model of diabetic pain. Brain Res. 2009;1268:154–61. doi: 10.1016/j.brainres.2009.02.063. [DOI] [PubMed] [Google Scholar]
- 64.Abdul Aziz AA, Finn DP, Mason R, Chapman V. Comparison of responses of ventral posterolateral and posterior complex thalamic neurons in naive rats and rats with hindpaw inflammation: mu-opioid receptor mediated inhibitions. Neuropharmacology. 2005;48:607–16. doi: 10.1016/j.neuropharm.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Guilbaud G, Benoist JM, Neil A, Kayser V, Gautron M. Neuronal response thresholds to and encoding of thermal stimuli during carrageenin-hyperalgesic-inflammation in the ventro-basal thalamus of the rat. Exp Brain Res. 1987;66:421–31. doi: 10.1007/BF00243316. [DOI] [PubMed] [Google Scholar]
- 66.Guilbaud G, Neil A, Benoist JM, Kayser V, Gautron M. Thresholds and encoding of neuronal responses to mechanical stimuli in the ventro-basal thalamus during carrageenin-induced hyperalgesic inflammation in the rat. Exp Brain Res. 1987;68:311–8. doi: 10.1007/BF00248797. [DOI] [PubMed] [Google Scholar]
- 67.Iwata M, LeBlanc BW, Kadasi LM, et al. High-frequency stimulation in the ventral posterolateral thalamus reverses electrophysiologic changes and hyperalgesia in a rat model of peripheral neuropathic pain. Pain. 2011;152:2505–13. doi: 10.1016/j.pain.2011.07.011. [DOI] [PubMed] [Google Scholar]