Abstract
Activating transcription factor 4 (ATF4) is a critical transcription factor for bone remodeling; however, its role in bone angiogenesis has not been established. Here we show that ablation of the Atf4 gene expression in mice severely impaired skeletal vasculature and reduced microvascular density of the bone associated with dramatically decreased expression of hypoxia inducible factor 1α (HIF-1α) and vascular endothelial growth factor (VEGF) in osteoblasts located on bone surfaces. Results from in vivo studies revealed that hypoxia/reoxygenation induction of HIF-1α and VEGF expression leading to bone angiogenesis, a key adaptive response to hypoxic conditions, was severely compromised in mice lacking the Atf4 gene. Loss of ATF4 completely prevented endothelial sprouting from embryonic metatarsals, which was restored by addition of recombinant human VEGF protein. In vitro studies revealed that ATF4 promotion of HIF-1α and VEGF expression in osteoblasts was highly dependent upon the presence of hypoxia. ATF4 interacted with HIF-1α in hypoxic osteoblasts and loss of ATF4 increased HIF-1α ubiquitination and reduced its protein stability without affecting HIF-1α mRNA stability and protein translation. Furthermore, parathyroid hormone-related protein (PTHrP) and receptor activator of NF-kappaB ligand (RANKL), both well-known activator of osteoclasts, increased release of VEGF from the bone matrix and promoted angiogenesis through the protein kinase C- and ATF4-dependent activation of osteoclast differentiation and bone resorption. Thus, ATF4 is a new key regulator of the HIF/VEGF axis in osteoblasts in response to hypoxia and VEGF release from bone matrix, two critical steps for bone angiogenesis.
INTRODUCTION
Bone is a highly vascularized tissue. Accumulated evidence suggests that angiogenesis is essential for bone formation during skeletal development as well as bone regeneration in response to injury (1-7). Endochondral ossification, which forms most of the skeleton in the body, occurs in close spatial and temporal association with capillary invasion (8). Osteoblasts, which are ideally situated on the trabecular and cortical bone surfaces to sense oxygen tension and respond to hypoxia by activating the hypoxia inducible factor α (HIF-α) pathway, play a critical role in coupling angiogenesis and osteogenesis (3). Mice overexpressing HIF-1α in osteoblasts through selective deletion of the von Hippel–Lindau (pVHL) gene expressed high levels of VEGF and developed extremely dense, heavily vascularized long bones (3). By contrast, long bones in mice lacking HIF-1α in osteoblasts were significantly thinner and less vascularized than those of controls. Further, modulation of the HIF-1α-VEGF axis in osteoblasts greatly impacted bone repair in mice (4-6). These results suggest that osteoblast-mediated angiogenesis plays a critical role in bone formation and repair. It has been demonstrated in more recent studies that osteoclasts, the bone-resorbing cells, also play an important role in regulation of bone angiogenesis (9, 10). However, the molecular and cellular mechanisms that control bone angiogenesis are not fully understood.
Hypoxia-inducible factors (HIFs) are key regulators of hypoxic adaptation and angiogenesis. HIFs form heterodimeric complexes that are composed of an inducible α-subunit (HIF-1α, HIF-2α and HIF-3α) and a constitutively-expressed β-subunit (HIF-β), also known as the aryl hydrocarbon receptor nuclear translocator (ARNT). HIF-α proteins are tightly regulated by oxygen tension via controlled degradation. Under normoxic conditions, following prolyl-4-hydroxylation by a family of three prolyl hydroxylases (PHD1, PHD2, and PHD3) of the critical proline residues in an oxygen-dependent degradation domain (ODD) of HIF-α (11-13), the hydroxylated HIF-α is recognized by pVHL tumor suppressor protein, the substrate-specific adaptor of the Elongin B/C-Cullin2-Rbx E3 ubiquitin ligase complex, and targeted for rapid ubiquitination and proteasomal degradation (14, 15). In hypoxia, on the other hand, the PHD activity is decreased and thus HIF-α hydroxylation is blocked, which leads to HIF-α stabilization, subsequent nuclear import, and dimerization with HIF-β, and eventually activation of the transcription of HIF-responsive genes such as Vegf and promotion of angiogenesis (16). The activity of HIF-α is further regulated by Factor Inhibiting HIF-α (FIH)-mediated hydroxylation of an asparagine in the C-terminal TAD domain of HIF-α (17, 18). HIF-1α regulation is complex as multiple proteins can influence its stability and transcriptional activity, thereby allowing the integration of inputs from different signaling pathways into oxygen signaling including tissue/cell-specific responses and regulation (for review, see (19, 20)).
ATF4 plays a critical role in bone homeostasis (21-26). ATF4 promotes osteoblast function and bone formation by stimulating osteoblast-specific gene expression, amino acid import and the synthesis of type I collagen, proliferation and survival of osteoblasts, and mediation of osteoblastic responses to PTH to increase bone formation (21-23, 27-29). ATF4 is also critical for osteoclast differentiation and bone resorption via direct (24) and indirect (i.e., via up-regulation of RANKL in osteoblasts and bone marrow stromal cells (BMSCs)) (25, 26) mechanisms. While ATF4 is implicated in the activation of VEGF expression in cultured cells in response to stimuli such as endoplasmic reticulum stress and oxidative stress (30-34), the potential role of ATF4 in regulation of angiogenesis at the tissue and organ and animal levels, especially under pathological conditions (e.g., hypoxia), and relevant molecular mechanisms are largely unknown
Using biochemical, cellular, and genetic approaches, we found that loss of ATF4 in mice greatly impaired bone angiogenesis during skeletal development as well as under hypoxia. ATF4 stabilized HIF-1α protein and increased VEGF expression in osteoblasts in response to hypoxia and promoted VEGF release from the bone matrix through osteoclast-mediated bone resorption via the PKC-dependent pathway. These results suggest that ATF4 may be a useful therapeutic target to block abnormal angiogenesis in bone or to increase angiogenesis to promote bone healing.
MATERIALS AND METHODS
Reagents
Tissue culture media and fetal bovine serum were obtained from Thermo Scientific HyClone. PMA, GF109203X, U0126, and CoCl2 were purchased from Sigma Aldrich, PTHrP from PeproTech, recombinant human VEGF, RANKL, OPG and FGF2, and anti-mouse VEGF antibody from R&D System. All other chemicals were of analytical grade.
Atf4-deficient mice
Breeding pairs of Atf4 heterozygous mice (Black Swiss) were described previously (23, 29) and used to generate Atf4 wild-type (WT) (Atf4+/+), heterozygous (Atf4+/−) and homozygous mutant (Atf4−/−) mice for this study. All research protocols were approved by the respective Institutional Animal Care and Use Committees (IACUC) of Nankai University, VA Pittsburgh Healthcare System, or Rush University.
Hypoxia experiments
For in vitro hypoxia experiments, cells were placed in a hypoxia chamber (1% O2) or treated by 150 μM CoCl2 (to rapidly induce hypoxia) for indicated times. In vivo hypoxia experiments were performed as previously described (35) using an oxygen control cabinet system (Manufacturer: Guizhou Aviation Industry Co. Ltd., Guizhou, China; Model: FLYDWC50-IB) (Figure S5). Briefly, mice were subjected to systemic normobaric hypoxia by substituting oxygen with nitrogen at a constant gas flow rate in the closed chamber. Mice were provided with food and water ad libitum, allowed to adjust to the hypoxic environment by gradually decreasing the O2 from 21 to 8% during an adaptation time of 1h, and kept at 8% O2 for 2h per day of continuous hypoxia for 10d. After the last hypoxic exposure, the animals were immediately killed and the dissected bone tissues were processed for the described experiments (Figure 7).
Figure 7. Hypoxia/reoxygenation-induced angiogenic response is abolished in Atf4−/− bone.
(A-G) WT and Atf4−/− male mice of 1-month-old age were placed in a hypoxia chamber (8%) for 2h per day for 10d as described in Materials and Methods. Protein extracts and total RNAs were isolated from tibiae (n = 6) for Western blot analysis (A) and qPCR analysis (B-E), respectively. Longitudinal tibial sections from the chondro-osseous junction regions were stained with an anti-CD31 antibody. Representative images are shown (F). Arrows point to microvessels, which were stained brown. Microvessel density of sections from the chondro-osseous junction regions of each group was measured and compared to that of WT sections (normalized to 100%) (G). n = 6. Original magnification, x200, *P < 0.01, versus Normoxia, #P < 0.01, versus WT. (H) A working model for ATF4 promotion of bone angiogenesis.
Imaging of blood vessels
Blood vessels in bone were imaged using μCT. Specimens were prepared in accordance with previously described methods by the Clemens group (3). Briefly, after animals were euthanized, the thoracic cavity was opened, and the inferior vena cava was severed. The vasculature was flushed with 0.9% normal saline containing heparin sodium (100 U/ml) at a pressure of approximately 100 mm Hg via a needle inserted into the left ventricle. The specimens were then pressure fixed with 10% neutral buffered formalin. The vasculature was injected with a radiopaque silicone rubber compound containing lead chromate (Microfil MV-122; Flow Tech). Samples were stored at 4°C overnight for contrast agent polymerization. Mouse calvariae and femurs were dissected from the specimens and soaked for 4 days in 10% neutral buffered formalin to ensure complete tissue fixation. Tissues were subsequently treated for 48h in a formic acid-based solution, Cal-Ex II (Fischer Scientific), to decalcify the bone and facilitate image thresholding of the vasculature from the surrounding tissues. Images were obtained using a high-resolution (16-μm isotropic voxel size) μCT imaging system (μCT40; SCANCO Medical).
Fetal mouse metatarsal angiogenesis assay
This assay was performed as described previously by the Clemens group (3). Briefly, E17.5 embryos were removed from timed-pregnant mice, and metatarsals were dissected. The isolated metatarsals were cultured in 24-well tissue culture plates in 150 μl of α-MEM supplemented with 10% heat-inactivated FBS and 1% of penicillin/streptomycin for 72h. 250 μl of fresh medium were then replaced, and metatarsals were cultured for indicated days, with replacement of medium every 3d. Explants were then fixed in zinc formalin for 15 minutes at RT and subsequently stained for CD31 using a rat polyclonal antiserum against mouse CD31 (BD Biosciences-Pharmingen). Cultures were performed in 6 replicate, and each complete experiment was repeated at least twice. Relative CD31-positive area was measured using an Image Pro Plus 7.0 software (Media Cybernetics, Inc).
TRAP staining of metatarsal sections
Sections of metatarsals from WT and Atf4−/− mice were fixed, decalcified, and stained for TRAP activity as described previously (24). TRAP-positive area was measured using an Image Pro Plus 7.0 software (Media Cybernetics, Inc), which was normalized to total metatarsal area.
ELISA assays
The level of CTX (C-telopeptides), degradation products from type I collagen during osteoclastic bone resorption, in metatarsal culture media was measured using the RatLaps EIA Kit (Immunodiagnostic Systems Limited, Cat# AF-06F1) according to the manufacturer's instruction. The level of VEGF in plasma and metatarsal cultures was measured by using ELISA kits (mouse VEGF ELISA Kit: Cat# MMV00 from R&D Systems Inc, Minneapolis, MN 55413) according to the manufacturer's instructions.
IHC staining
WT and Atf4−/− mice were euthanized and tibiae were fixed in 10% formalin at 4 °C for 24h, decalcified in 10% EDTA (pH 7.4) for 10-14d, and embedded in paraffin. Approximately 6-8 sections were obtained from each area (5-6 samples per group) and analyzed. Five-μm sections of tibiae were immunohistochemically stained with antibodies against HIF-1α (Lifespan Biosciences, LS-B495), pVHL (Santa Cruz Biotechnology, sc-5575), CD31 (BD Biosciences-Pharmingen), and VEGF (Santa Cruz Biotechnology, sc-152) using the EnVision+System-HRP (DAB) kit (Dako North America, Inc) as described previously (22, 24).
Adenoviral infection, qPCR, and Western blot analyses
Cells were infected with adenoviruses expressing ATF4 were described previously (24, 27, 36). The amount of adenovirus was balanced as necessary with a control adenovirus expressing EGFP such that the total amount was constant in each group. RNA isolation and reverse transcription (RT) were previously described (29). qPCR analysis was performed to measure the relative mRNA levels using SYBR Green kit (Bio-Rad Laboratories Inc.). Samples were normalized to Gapdh expression. The DNA sequences of mouse primers used for qPCR were summarized in Table 1. Western blot analysis was performed as previously described (28, 29). Antibodies used are from the following sources: antibodies against pVHL, Runx2, ATF4, VEGF, and anti-rabbit or anti-mouse antibodies conjugated with horse radish peroxidase from Santa Cruz Biotechnology, Inc., mouse monoclonal antibody against HIF-1α from R&D System, and mouse monoclonal antibody against β-actin from Sigma Aldrich.
Table 1.
Real-time PCR primers
| Name | 5′ primer | 3′ primer |
|---|---|---|
| Atf4 | GAGCTTCCTGAACAGCGAAGTG | TGGCCACCTCCAGATAGTCATC |
| Gapdh | CAGTGCCAGCCTCGTCCCGTAGA | CTGCAAATGGCAGCCCTGGTGAC |
| Glut1 | GGGCATGTGCTTCCAGTATGT | ACGAGGAGCACCGTGAAGAT |
| Hif-1α | CAAGATCTCGGCGAAGCAA | GGTGAGCCTCATAACAGAAGCTTT |
| Vegf | GGCTTTACTGCTGTACCTCCACCAT | GCAGTAGCTTCGCTGGTAGACATCC |
| Vegf120 | GCGGATCAAACCTCACCAAA | CTCGGCTTGTCACATTTTTC |
| Vegf165 | ACAGGACAAAGCCAGAAAAACAC | GTTTAACTCAAGCTGCCTCGCCT |
| Vegf188 | GCGGATCAAACCTCACCAAA | GAACAAGGCTCACAGTGAACGC |
| pVhl | TGTGCCATCCCTCAATGTCG | AGGCTCCGCACAACCTGAAG |
In vitro transcription and translation
A cDNA fragment containing a 1710-bp full-length HIF-1α cDNA and a 357-bp 5'-UTR and a 1211-bp 3'-UTR was obtained by RT-PCR from the MDA-MB-231 human breast cancer cell line and subcloned into the pTnT vector. In vitro transcription was conducted to obtain the HIF-1α mRNA template with both 5’ and 3’-UTR using the RiboMAX™ Large Scale RNA Production System-T7 (Promega, P1300) according to the manufacturer's instructions. In vitro translation was performed using the Rabbit Reticulocyte Lysate System (Promega, L4960) and Biotinylated Lysine-tRNA (Promega, L5061) following manufacturer's instructions. Two μl translated protein products were subject to SDS-PAGE, transferred to NC membrance, and detected using the Translation Detection System (Promega L5070).
Statistical analysis
Data were analyzed with a GraphPad Prism software (4.0). A one-way ANOVA analysis was used, followed by the Tukey test. Results were expressed as means ± standard deviation (SD).Differences with a P < 0.05 was considered as statistically significant.
RESULTS
Atf4 deficiency impairs bone angiogenesis in mice
At necropsy, we found that Atf4−/− calvariae (Figure 1A) and long bones (not shown) were less richly perfused with blood compared with those from their sex- and age-matched wild-type (WT) control littermates, suggesting that deletion of the Atf4 gene may affect bone angiogenesis. To determine if this is the case, we performed contrast-enhanced μCT imaging in Microfil-perfused calvariae and femurs. The vasculature was strikingly impaired in Atf4−/− calvariae and femurs compared to that in WT groups (Figure 1, B and C). ATF4 deficiency significantly reduced vessel volume and vessel number in femurs (Figure 1, D-F). Results from immunohistochemical (IHC) staining of tibial sections using a specific antibody against CD31 revealed that both the size and density of vessels within the metaphyseal periosteum and diaphyseal endosteum from Atf4−/− tibiae were markedly decreased compared to those of WT tibiae (Figure 1, G-I).
Figure 1. Lack of ATF4 severely impairs bone vasculature during development in mice.
(A) Representative photograph of calvariae from two-month-old male WT and Atf4−/− mice. (B-D) Representative μCT images of vasculature in Microfil-perfused calvariae (B) and femur (C and D) from two-month-old male WT and Atf4−/− mice. (E and F) μCT analysis of vessel volume and number within femurs from Microfil-perfused two-month-old male WT and Atf4−/− mice. n = 3, *P < 0.01 (versus WT). (G-I) IHC staining. Longitudinal tibial sections from 1-month-old male WT and Atf4−/− mice (n = 5-6) were stained with an anti-CD31 antibody or control IgG. Representative images from the metarphyseal periosteum (G, top) and diaphyseal endosteum (G, bottom) regions are shown. Original magnification, x100, arrows point to microvessels, which were stained brown.
Expression of VEGF is dramatically reduced in Atf4−/− osteoblasts located on bone surfaces
We next examined whether ATF4 regulates vascular endothelial growth factor A (VEGFA; hereafter referred to as VEGF) expression in bone. Quantitative real-time RT-PCR (qPCR) analysis revealed that the mRNA levels of Vegf and its 3 isoforms (Vegf120, Vegf165, and Vegf188) were all significantly decreased in Atf4−/− versus WT tibiae (Figure 2A and Figure S1). As expected, Atf4 mRNA was minimal in Atf4−/− tibiae (Figure 2B). Results from IHC staining revealed that VEGF was strongly detected in osteoblasts of WT tibiae, which was drastically decreased in osteoblasts of Atf4−/− mice (Figure 2C). It should be noted that VEGF was detected in bone matrix, which was also decreased by the lack of ATF4. Surprisingly, the level of serum VEGF was not different in mice of the two genotypes (Figure 2D). Collectively, these results indicate that loss of ATF4 results in a down-regulation of VEGF in the bone environment.
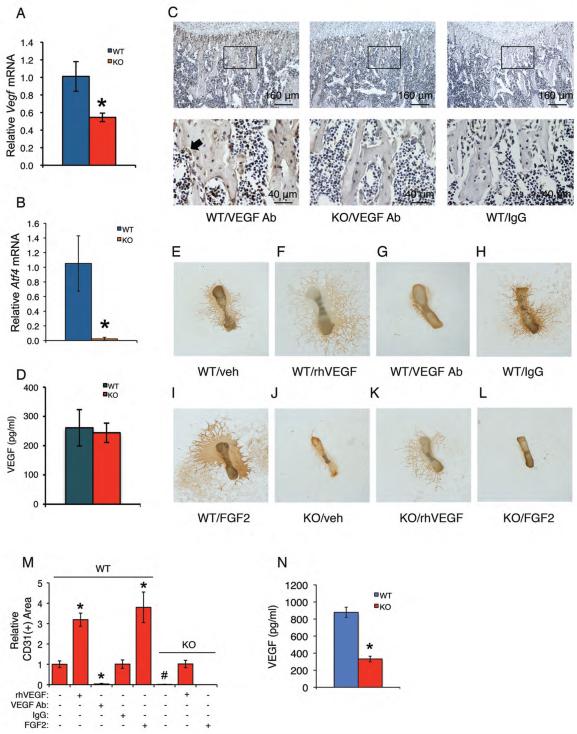
Figure 2. Loss of ATF4 reduces VEGF expression in osteoblasts located on trabecular bone surfaces and abolishes endothelial sprouting from Atf4−/− metatarsals, which is restored by exogenously supplied recombinant human VEGF.
(A and B) qPCR. Total RNAs were isolated from WT and Atf4−/− tibiae (n = 5-6) and analyzed by qPCR using specific primers for Vegf and Atf4 mRNAs, which were normalized to GapdhmRNA. *P < 0.01, versus WT. (C) IHC. Sections of WT and Atf4−/− tibiae (n = 5-6) were stained using specific antibodies against VEGF or normal IgG. VEGF signal was stained brown. Original magnification, x100 (top), x400 (bottom). (D) ELISA. Level of VEGF protein from WT and Atf4−/− serum was measured using an ELISA kit according to the manufacturer's instructions. n = 5-6. (E-M) Metatarsal angiogenesis assays. Metatarsals were dissected from WT and Atf4−/− E17.5 fetuses and cultured in α-MEM for 12 days, followed by IHC staining using anti-CD31 antibody as described in Materials and Methods. Representative images are shown. Microscope: Olympus SZ61. Original magnification, ×20. Endothelial sprouting from WT metatarsals (E) was significantly increased by the treatment of recombinant VEGF (10 ng/ml) (F) and blocked by an anti-VEGF neutralizing antibody (1 μg/ml) (G), but not by control IgG (1 μg/ml) (H). Endothelial sprouting from WT metatarsals was increased by FGF2 (100 ng/ml) (I). No detectable endothelial sprouting from Atf4−/− metatarsals (J). Significant endothelial sprouting from Atf4−/− metatarsals treated with VEGF (K), but not FGF2 (L). Quantitative data of each group (M). n = 6-8, *P < 0.01, versus veh, #P < 0.01, versus WT. (N)ELISA. Level of VEGF protein from WT and Atf4−/− metatarsal cultures was measured using an ELISA kit according to the manufacturer's instructions. n = 6-8, *P < 0.01, versus WT.
Endothelial sprouting is completely lost from Atf4−/− metatarsals, which is restored by exogenously supplied recombinant human VEGF
We performed angiogenesis assays using explants of E17.5 mouse metatarsals. Results showed obvious endothelial sprouting from WT metatarsals (Figure 2E), which was increased by exogenously supplied recombinant human VEGF (Figure 2, F and M) and inhibited by anti-VEGF-neutralizing antibody (Figure 2, G and M), but not by the control IgG (Figure 2, H and M). Strikingly, endothelial sprouting was completely lost in Atf4−/− metatarsals (Figure 2, J and M), and this was restored by exogenous addition of recombinant human VEGF (Figure 2, K and M). While FGF2, a well-known potent angiogenesis inducer, strongly increased endothelial sprouting from WT metatarsals (Figure 2, I and M), it failed to rescue the defect in endothelial sprouting from Atf4−/− metatarsals (Figure 2, L and M). In contrast to results from sera, the level of VEGF was significantly reduced in Atf4−/− metatarsal cultures compared to that in WT metatarsal cultures (P= 0.004, WT versus KO) (Figure 2N). These results suggest that ATF4 deficiency impairs bone angiogenesis probably mainly through down-regulation of VEGF. These results also demonstrate that ATF4 mutant endothelial cells still retain their capacity to a VEGF stimulus.
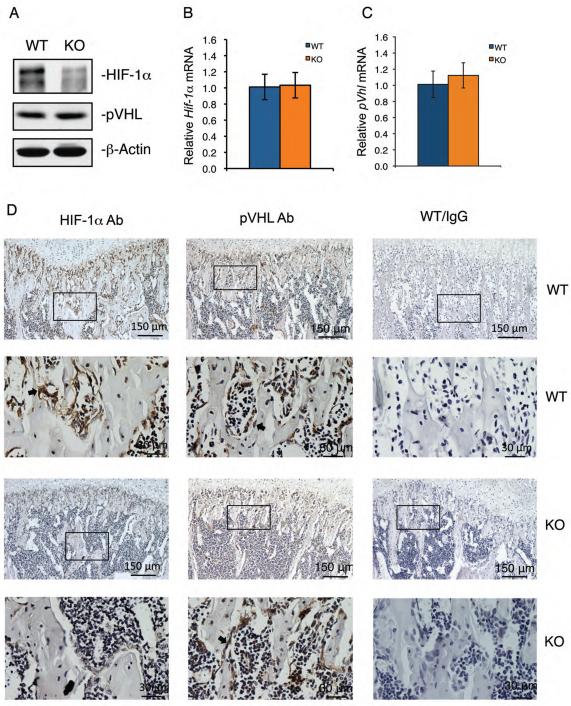
Lack of ATF4 reduces HIF-1α protein levels without altering the expression of pVHL in osteoblasts located on trabecular bone surfaces
We investigated whether ablation of ATF4 decreases the level of HIF-1α, a master regulator of VEGF. Western blot analysis using tibial protein extracts showed that the level of HIF-1α was dramatically decreased in Atf4−/− versus WT tibiae (Figure 3A). HIF-2α was not detectable in bone by Western blotting and was not further investigated (data not shown). In contrast to HIF-1α protein levels, Hif-1α mRNA was expressed at equivalent levels in WT and Atf4−/− tibiae (Figure 3B), suggesting a mechanism involving a post-transcriptional regulation. Likewise, the levels of pVHL mRNA and protein, the HIF-1α recognition subunit of the ubiquitin ligase that promotes HIF-1α ubiquitination and proteasomal degradation, were not altered by the absence of ATF4 (Figure 3, A and C). We examined whether Atf4 deficiency decreased HIF-1α expression in osteoblasts by performing IHC staining of tibial sections using a specific antibody for HIF-1α or control IgG. Results showed that HIF-1α was strongly expressed in osteoblasts of WT tibiae, but was dramatically reduced in osteoblasts of Atf4−/− tibiae (Figure 3D). Similar to the results from Western blot analysis (Figure 3A), pVHL signal detected by IHC was not different in osteoblasts of the two genotypes. Taken together, these results demonstrate that: i) ATF4 deficiency reduces HIF-1α protein levels in osteoblasts in vivo; and ii) this reduction is not due to increased expression of pVHL.
Figure 3. Inactivation of ATF4 greatly decreases the level of HIF-1α protein, but not its mRNA, in osteoblasts located on bone surfaces.
(A) Western blot analysis. Protein extracts were isolated from WT and Atf4−/− tibiae (n = 5-6) and analyzed for HIF-1α and pVHL. β-Actin was used for loading control. (B and C) qPCR. Total RNAs were isolated from WT and Atf4−/− tibiae (n = 5-6) and analyzed by qPCR using specific primers for Hif-1α and pVhl mRNAs, which were normalized to Gapdh mRNA. (D)IHC. Sections of WT and Atf4−/− tibiae (n = 5-6) were stained using specific antibodies against HIF-1α, pVHL or normal IgG. Original magnification, x100 (top), x400 (bottom).
ATF4 increases HIF-1α and VEGF expression in osteoblasts in hypoxia-dependent manner in vitro
To investigate if ATF4 plays a role in hypoxic induction of HIF-1α expression in osteoblasts, primary calvarial osteoblasts isolated from WT and Atf4−/− mice were incubated under hypoxia (1% O2) for the indicated time period. As expected, the level of HIF-1α protein was rapidly increased during hypoxia in primary WT osteoblasts. However, this increase was greatly reduced in primary Atf4−/− osteoblasts (Figure 4A). In contrast, the level of Runx2, a key transcription factor for osteoblast differentiation (37-41), was not affected by hypoxia or a loss of ATF4. Further, adenoviral overexpression of ATF4 dose-dependently increased the levels of endogenous HIF-1α but not Runx2 protein in MC-4 cells, a well-established mouse preosteoblastic cell line (42, 43), in a hypoxia-dependent manner (Figure 4B). Enforced expression of ATF4 did not increase the level of Hif-1α mRNA in hypoxic MC-4 cells (Figure 4C). ATF4 overexpression dramatically increased the levels of Vegf and its 3 isoforms (Vegf120, Vegf165, and Vegf188) and Glut1 (glucose transporter 1) mRNAs, all well-known downstream targets of HIF-1α, in hypoxic MC-4 cells (Figure 4, D, E, and S2. It should be noted that it took a longer time (24h) for hypoxia to induce Vegf and Glut1 mRNA expression in MC-4 cells (Figure 4, D, E, and S2). Thus, ATF4 plays a critical role in up-regulation of HIF-1α and VEGF expression in osteoblasts under hypoxic conditions.
Figure 4. ATF4 increases HIF-1α and VEGF expression in osteoblasts in response to hypoxia and ATF4 interacts with HIF-1α and loss of ATF4 increases HIF-1α ubiquitination and decreases HIF-1α protein stability without affecting HIF-1α mRNA stability and translation.
(A) Hypoxia-induced up-regulation of HIF-1α was reduced by the loss of ATF4. WT and Atf4−/− calvarial osteoblasts were treated with hypoxia (1% O2) for indicated times, followed by Western blot analysis. (B-E) ATF4 up-regulated HIF-1α and Vegf expression in a hypoxia-dependent manner. MC-4 cells were transfected with and without adenoviral vector for ATF4. Twenty-four hours later, cells were cultured in the presence and absence of hypoxia for 6h (B and C), followed by Western blot (B) and qPCR (C) analysis, or 24h (D and E), followed by qPCR analysis for Vegf (D) and Glut1 (E) mRNAs. *P < 0.01, versus AdEGFP, #P < 0.01, versus Normoxia. (F) Hif-1α mRNA stability. WT and Atf4−/− calvarial osteoblasts were treated with transcription inhibitor actinomycin D (Act D) (5 μg/ml) in the presence of 150 μM CoCl2 (i.e., to rapidly induce hypoxia (52)) for indicated times, followed by qPCR for Hif-1α mRNAs, which were normalized to Gapdh mRNAs of the 0h samples of each group. (G) In vitro translation. Translated HIF-1α proteins were subject to Western blotting for HIF-1α. Lane 1: no Hif-1α mRNA template was added, lanes 2-8: equal amounts of Hif-1α mRNA template were added in each reaction. Lane 2: nuclear extracts (NE) from primary WT calvarial osteoblasts, lane 3: NE from primary ATF4 KO calvarial osteoblasts, lane 4: NE from COS-7 transfected with ATF4 expression vector, lane 5: NE from COS-7 transfected with β-galactosidase expression vector (control vector), lane 6: purified His-ATF4 protein, lane 7: elution buffer for purifying the His-ATF4, lane 8: H2O. (H and I) HIF-1α protein stability. Primary WT and Atf4−/− calvarial osteoblasts were exposed to 150 μM cobalt chloride following addition of 10 μg/ml cycloheximide. Lysates of cells harvested at the indicated time intervals were subject to Western blot analysis of HIF-1α expression. Quantitative analysis of HIF-1α, which was normalized to β-actin, from 3 independent experiments (I). *P < 0.01 (versus KO). (J and K) co-IP assays. Whole cell extracts from hypoxic osteoblasts were immunoprecipitated by antibodies against ATF4 (J) or HIF-1α (K), followed by Western blotting for HIF-1α and ATF4. (L and M) HIF-1α ubiquitination. Whole cell lysates from hypoxic osteoblasts from WT and Atf4−/− mice were immunoprecipitated with an anti-HIF-1α antibody, followed by Western blot analysis for ubiquitin (Ub) (J). Quantitative analysis of (Ub)n-HIF-1α from 5 independent experiments (M). *P < 0.01 (versus WT).
ATF4 interacts with HIF-1α and loss of ATF4 increases HIF-1α ubiquitination and decreases HIF-1α protein stability without affecting Hif-1α mRNA stability and translation
Reduced HIF-1α protein levels in Atf4−/− osteoblasts could result from reduced HIF-1α mRNA stability and/or translation and/or protein stability. To differentiate these possibilities, we first examined if loss of ATF4 affects the stability of Hif-1α mRNA by performing the actinomycin D (a transcription inhibitor) experiments. Results showed that the half-life of Hif-1α mRNAs from WT and Atf4−/− calvarial osteoblasts under hypoxic condition was not significantly different (~7h) (Figure 4F). We next determined if ATF4 impacts HIF-1α protein translation by performing in vitro transcription and translation using the Rabbit Reticulocyte Lysate System (Promega) and Biotinylated Lysine-tRNA (Promega) in the presence and absence of ATF4. Results showed that the levels of in vitro translated HIF-1α protein were not significantly altered with or without the presence of ATF4 (Figure 4G). We therefore examined if loss of ATF4 alters the stability of HIF-1α protein by performing the cycloheximide (a protein synthesis inhibitor) experiments. To do this, HIF-1α expression was analyzed in lysates of cobalt-treated primary calvarial osteoblasts from WT and Atf4−/− mice at serial time intervals following addition of cycloheximide. Results revealed that HIF-1α protein decayed with a half-life of < 5 min in Atf4−/− osteoblasts, compared with ~20 min in WT osteoblasts (Figure 4, H and I). In contrast, Runx2 protein decayed at a similar rate in WT and Atf4−/− osteoblasts (Figure 4H). Collectively, these results establish that loss of ATF4 decreases HIF-1α protein stability in hypoxic osteoblasts.
To investigate the molecular mechanisms whereby ATF4 stabilizes HIF-1α, we performed co-immunoprecipitation (co-IP) assays to determine if ATF4 interacts with HIF-1α. Results showed HIF-1α was present in the anti-ATF4 immunoprecipitates (Figure 4J). Reciprocal co-IP assays revealed that ATF4 was present in the anti-HIF-1α immunoprecipitates (Figure 4K). We next determined if loss of ATF4 impacts HIF-1α ubiquitination. Aliquots of whole-cell extracts from WT and Atf4−/− calvarial osteoblasts exposed to hypoxia were subject to co-IP assays using an anti-HIF-1α antibody, followed by Western blot analysis using an anti-ubiquitin antibody. Compared with WT osteoblasts, Atf4−/− osteoblasts demonstrated a much higher level of polyubiquitinated HIF-1α (Figure 4, L and M).
Atf4 deficiency decreases VEGF release from bone matrix by reducing osteoclast differentiation and bone resorption
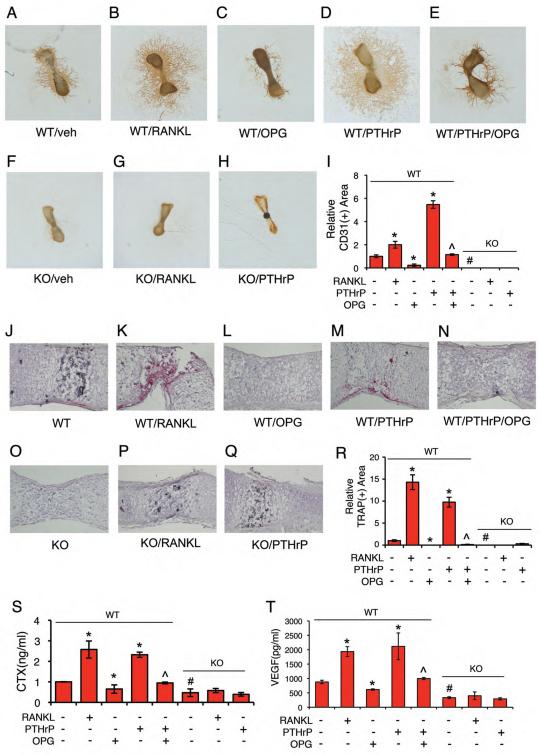
Accumulated evidence suggests that osteoclasts, the bone-resorbing cells, play a role in regulation of bone angiogenesis (9, 10). To determine whether ATF4 regulation of bone angiogenesis involves osteoclast function, WT and Atf4−/− metatarsals (E17.5) were treated with or without activators (RANKL, PTHrP) or an inhibitor (OPG) of osteoclast differentiation, followed by measurements of endothelial sprouting, TRAP (an osteoclast-specific enzyme) activity in metatarsals, and the levels of CTX (an indicator of in vivo osteoclast differentiation and bone resorption) and VEGF in metatarsal cultures. Results showed that RANKL and PTHrP significantly increased endothelial sprouting (Figure 5, B, D and I), TRAP activity (Figure 5, K, M, and R), and the levels of CTX (Figure 5S) and VEGF (Figure 5T) in WT groups. In contrast, OPG, a soluble decoy receptor that blocks RANKL binding to RANK and thereby inhibits osteoclast differentiation (44-46), reduced basal and PTHrP-induced endothelial sprouting (Figure 5, C, E, and I), TRAP activity (Figure 5, L, N, and R), and CTX (Figure 5S) and VEGF (Figure 5T) in WT groups. Basal TRAP activity was undetectable in the Atf4−/− metatarsals (Figure 5O), confirming a critical role of ATF4 in osteoclast differentiation (24). Further, the level of CTX was significantly reduced in Atf4−/− versus WT metatarsal cultures (P<0.01, WT versus KO)(Figure 5S). Strikingly, both RANKL- and PTHrP-stimulated increases in endothelial sprouting (Figure 5, F-I), TRAP activity (Figure 5, O-R), and CTX (Figure 5S) and VEGF (Figure 5T) were abolished by the lack of ATF4. Thus, Atf4 deficiency decreases VEGF expression in osteoblasts as well as VEGF release from bone matrix due to reduced osteoclast activity and bone resorption.
Figure 5. ATF4 regulates VEGF release from bone matrix and bone angiogenesis by activating osteoclast differentiation and bone resorption.
(A-I) Metatarsal angiogenesis assays. RANKL (50 ng/ml) increased (B) and OPG (300 ng/ml) decreased (C) endothelial sprouting from WT metatarsals. PTHrP (100 nM) increased endothelial sprouting in WT metatarsals (D), which was inhibited by OPG (E). Neither RANKL nor PTHrP increased endothelial sprouting from Atf4−/− metatarsals (F-H). Quantitative data of each group (I). n = 5-6. Microscope: Olympus SZ61. Original magnification, x20. (J-R) TRAP staining. WT and Atf4−/− metatarsal sections treated as indicated were stained for TRAP activity. Quantitative analysis of TRAP-positive area, which was normalized to metatarsal section area (R). n = 5-6. Original magnification, x100. (S and T) ELISA. Metatarsals were dissected from WT and Atf4−/− E17.5 fetuses and cultured in α-MEM for 14d. Culture media were harvested for CTX and VEGF assays as described in Materials and Methods. n = 5-6, *P < 0.01, versus veh, #P < 0.01, versus WT, ^P < 0.01, versus PTHrP alone.
PKC pathway regulates VEGF release from bone matrix and endothelial sprouting from metatarsals via ATF4-dependent osteoclast activation
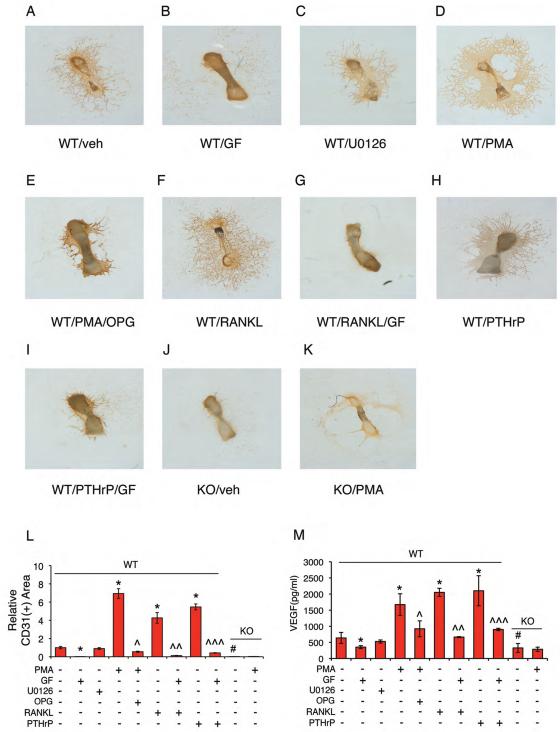
To define signaling pathways that regulate bone angiogenesis, E17.5 metatarsals were treated with or without the indicated inhibitors or activator for various pathways. We observed that endothelial sprouting from WT metatarsal cultures was inhibited by GF109203X (PKC inhibitor), but not by U0126 (Erk1/2 inhibitor) (Figure 6, A-C and L). In contrast, PMA (PKC activator) strikingly induced endothelial sprouting in WT metatarsal cultures in a dose- and time-dependent manner (Figures 6, D, L and S3). The PMA-induced increase in endothelial sprouting was blocked by treatment with OPG (Figure 6, E and L), suggesting the involvement of osteoclast activation in this regulation. Indeed, PMA increased osteoclast differentiation in metatarsals (Figures S4). Further, RANKL- and PTHrP-induced increases in endothelial sprouting were completely suppressed by the PKC inhibition (Figure 6, F-I and L). As shown in Figure 6M, the level of VEGF in metatarsal cultures was decreased by GF109203X but increased by PMA. Further, RANKL- and PTHrP-induced increases in VEGF were blocked by the PKC inhibition. Importantly, PMA failed to induce VEGF and endothelial sprouting in the absence of ATF4 (Figure 6, J-M). Collectively, these results suggest that PKC promotion of VEGF release from the bone matrix and bone angiogenesis is through ATF4-dependent osteoclast activation and bone resorption.
Figure 6. PKC regulates VEGF release from bone matrix and bone angiogenesis through ATF4-dependent osteoclast activation and bone resorption.
(A-L) Metatarsal angiogenesis assays. Endothelial sprouting in WT metatarsals (A) was completely inhibited by GF109203X (2 μM) (PKC inhibitor) (B), but not by U0126 (2 μM) (Erk1/2 inhibitor) (C). PMA (2 μM) (PKC activator) increased endothelial sprouting in WT metatarsals (D), which was suppressed by OPG (E). RANKL- and PTHrP-induced endothelial sprouting was inhibited by GF109203X (F-I). No detectable endothelial sprouting from Atf4−/− metatarsals (J). PMA failed to increase endothelial sprouting from Atf4−/− metatarsals (K). Quantitative data of each group (L). n = 5-6. Microscope: Olympus SZ61. Original magnification, x20. (M) ELISA. Levels of VEGF protein from WT and Atf4−/− metatarsal cultures treated as indicated above were measured. *P < 0.01, versus veh, #P < 0.01, versus WT, ^P < 0.01, versus PMA alone, ^^P < 0.01, versus RANKL alone, ^^^P < 0.01, versus PTHrP alone.
Loss of ATF4 abolishes hypoxia/reoxygenation induction of HIF-1α and VEGF expression and bone angiogenesis in vivo
To evaluate if ATF4 plays a role in regulation of bone angiogenesis under pathological conditions, we investigated whether ATF4 is required for hypoxia/reoxygenation induction of HIF-1α and VEGF expression and angiogenesis in bone. One-month-old WT and Atf4−/− male mice were placed in a hypoxia chamber (Figure S5) (8% O2) 2h per day for 10d. Results showed that in vivo hypoxia significantly increased the expression levels of HIF-1α protein and Vegf and Glut1 mRNAs in WT tibiae, but not in Atf4−/− tibiae (Figure 7, A-C). In contrast, the levels of Hif-1α and pVhl mRNAs were not altered by either hypoxia or ATF4 deficiency (Figure 7, D and E). CD31 staining of sections of the tibial chondro-osseous junction regions revealed that in vivo hypoxia/reoxygenation increased microvessel density by 1.8 fold in WT tibiae (P < 0.01, normoxia versus hypoxia), but this increase was lost in Atf4−/− tibiae (Figure 7, F and G). Taken together, these results demonstrate that ATF4 plays a critical role in regulating expression of HIF-1α and VEGF and angiogenesis in bone under hypoxic conditions.
DISCUSSION
Recognition of the importance of angiogenesis for bone formation and regeneration has raised a fundamental question regarding the molecular mechanisms whereby bone angiogenesis is modulated during skeletal development and under hypoxic conditions. This study establishes that ATF4 is a key regulator of VEGF expression in local bone microenvironment, which contributes to increased bone angiogenesis. Loss of ATF4 dramatically reduced the levels of VEGF in osteoblasts located on bone surfaces, in bone matrix, and in metatarsal culture media without decreasing its serum level. Of particular significance, in vivo hypoxia/reoxygenation induction of VEGF expression and formation of microvessels in bone was completely lost by the loss of ATF4 in vivo. Further, overexpression of ATF4 increased endogenous VEGF expression in osteoblasts, which was highly dependent upon the presence of hypoxia. Results from these loss- and gain-of-function studies and the fact that the Atf4 deficiency-induced defect in endothelial sprouting from the embryonic metatarsals can be completely rescued by exogenous addition of recombinant human VEGF protein strongly suggest that ATF4 controls bone angiogenesis mainly through modulating the level of VEGF in the bone microenvironment. It also indicates that lack of ATF4 does not prevent endothelial cells from responding to VEGF.
In the present study, we demonstrate that ATF4 is a new key modulator of the levels of HIF-1α in osteoblasts in response to hypoxia. Hypoxic induction of HIF-1α protein was reduced in Atf4−/− osteoblasts in vitro and in bone. Further, adenoviral overexpression of ATF4 in hypoxic osteoblasts dose-dependently increased the level of HIF-1α protein without increasing its mRNA. ATF4 failed to increase the level of HIF-1α in osteoblasts under normoxia when the level of HIF-1α protein is extremely low due to the pVHL-mediated degradation following the PHD hydroxylation (47). Under hypoxic condition, PHD activity is decreased thus blocking hydroxylation, resulting in HIF-1α stabilization. Out results reveal that ATF4 forms a protein-protein complex with preexisting HIF-1α in hypoxic osteoblasts and stabilizes HIF-1α protein by decreasing its ubiquitination and degradation. Therefore, this study defines ATF4 as an important new regulator of HIF-1α protein levels in osteoblasts in response to hypoxia.
Further, we demonstrate that ATF4 additionally favors bone angiogenesis by promoting VEGF release from bone matrix via its actions in osteoclasts and bone resorption. Atf4 deficiency impaired RANKL and PTHrP, both well-known activators of osteoclast differentiation, up-regulation of VEGF and endothelial sprouting from metatarsals, which was accompanied by a dramatic reduction of CTX, an indicator of in vivo osteoclast activity and bone resorption. Studies from our and other groups demonstrated that ATF4 promotes osteoclast differentiation directly (24) and via indirect up-regulation of RANKL expression in osteoblasts (25, 26). The PKC pathway seems to be critical in regulation of VEGF release from bone matrix in an ATF4-dependent manner. PKC activation increased and PKC inhibition decreased the level of VEGF in metatarsal cultures and endothelial sprouting from WT metatarsals. PKC seems to exert its angiogenic effect via activation of osteoclasts because RANKL and PTHrP promotion of VEGF release from bone matrix and endothelial sprouting in metatarsal cultures was completely blocked by PKC inhibition or by treatment with OPG. While these experiments suggest that there is a role for PKC in osteoclast-mediated angiogenesis, they do not exclude a role of PKC in osteoblast-mediated angiogenesis (either directly or via production of RANKL, etc). Future studies will determine this possibility.
Based on findings from this and other studies, we have proposed a working model to explain how ATF4 promotes bone angiogenesis (Figure 7H). ATF4 interacts, stabilizes and up-regulates HIF-1α in hypoxic osteoblasts, leading to increased expression of VEGF. VEGF directly binds to its receptors on endothelial cells and induces angiogenesis or is secreted and stored in bone matrix. In the meanwhile, ATF4 activates osteoclast differentiation directly (24) and indirectly (i.e., via up-regulation of RANKL in osteoblasts) (25, 26). Increased osteoclast activity and bone resorption promotes release of VEGF from the bone matrix. Collectively, ATF4 controls the expression and availability of VEGF, leading to increased bone angiogenesis, which in return favors bone formation and repair (4-6). Thus, ATF4, via its actions in osteoblasts and osteoclasts, plays a critical role in coupling angiogenesis and osteogenesis.
Finally, our findings that ATF4 plays a critical role in the regulation of bone angiogenesis under hypoxia may have therapeutic implications beyond bone development. Hypoxia is a feature of disease states such as cancer skeletal metastases and pathological fractures (48-51). For example, the skeleton is a common site for major cancer metastases (e.g., myeloma, breast, lung and prostate cancers). Hypoxia and angiogenesis are often abnormally increased in bone where metastases occur (48), which favors cancer cell proliferation and growth. Further, in human and rabbit fractures, oxygen partial pressure (pO2) in post-fracture haematomas was extremely low (49, 51), which thereby induces local angiogenesis and promotes healing. Therefore, modulating the level of ATF4 in bone cells (i.e., osteoblasts and osteoclasts) could potentially be used as a strategy to inhibit skeletal metastases or to promote the healing of pathological fractures.
Supplementary Material
ACKNOWLEDGEMENTS
Thanks to Kenneth Patrene (University of Pittsburgh) for providing assistance on μCT imaging analysis of blood vessels, Frank Cackowski (University of Pittsburgh) for help on Microfil perfusion and fetal mouse metatarsal angiogenesis assay, and Chenggang Zhang (Beijing Institute of Radiation Medicine) for help on the in vivo hypoxia/reoxygenation experiments. This work was supported by Chinese Ministry of Science and Technology Grant (2009CB918902) and by the US National Institutes of Health (grant AR059647).
Footnotes
Disclosures
All authors state that they have no conflicts of interest
Author's roles: Study design: GX and KZ. Study conduct: GX, KZ, HJ, SL, HC, ZZ, XZ and YL. Data collection: GX, KZ, HJ, HC, SL, ZZ, XZ and YL. Data analysis: GX and KZ. Data interpretation: GX, KZ, DC, HI, DLG and FJ. Drafting manuscript: GX. GX takes the responsibility for the integrity of the data analysis.
REFERENCES
- 1.Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res. 2009;24:1347–1353. doi: 10.1359/jbmr.090602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maes C, Carmeliet P, Moermans K, Stockmans I, Smets N, Collen D, Bouillon R, Carmeliet G. Impaired angiogenesis and endochondral bone formation in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Mech Dev. 2002;111:61–73. doi: 10.1016/s0925-4773(01)00601-3. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan C, Gilbert SR, Wang Y, Cao X, Shen X, Ramaswamy G, Jacobsen KA, Alaql ZS, Eberhardt AW, Gerstenfeld LC, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A. 2008;105:686–691. doi: 10.1073/pnas.0708474105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobsen KA, Al-Aql ZS, Wan C, Fitch JL, Stapleton SN, Mason ZD, Cole RM, Gilbert SR, Clemens TL, Morgan EF, et al. Bone formation during distraction osteogenesis is dependent on both VEGFR1 and VEGFR2 signaling. J Bone Miner Res. 2008;23:596–609. doi: 10.1359/JBMR.080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Street J, Bao M, deGuzman L, Bunting S, Peale FV, Jr., Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99:9656–9661. [Google Scholar]
- 7.Liu Y, Berendsen AD, Jia S, Lotinun S, Baron R, Ferrara N, Olsen BR. Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest. 2012;122:3101–3113. doi: 10.1172/JCI61209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maes C, Kobayashi T, Kronenberg HM. A novel transgenic mouse model to study the osteoblast lineage in vivo. Ann N Y Acad Sci. 2007;1116:149–164. doi: 10.1196/annals.1402.060. [DOI] [PubMed] [Google Scholar]
- 9.Cackowski FC, Anderson JL, Patrene KD, Choksi RJ, Shapiro SD, Windle JJ, Blair HC, Roodman GD. Osteoclasts are important for bone angiogenesis. Blood. 2010;115:140–149. doi: 10.1182/blood-2009-08-237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruni-Cardoso A, Johnson LC, Vessella RL, Peterson TE, Lynch CC. Osteoclast-derived matrix metalloproteinase-9 directly affects angiogenesis in the prostate tumor-bone microenvironment. Mol Cancer Res. 2010;8:459–470. doi: 10.1158/1541-7786.MCR-09-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang LE, Pete EA, Schau M, Milligan J, Gu J. Leu-574 of HIF-1alpha is essential for the von Hippel-Lindau (VHL)-mediated degradation pathway. J Biol Chem. 2002;277:41750–41755. doi: 10.1074/jbc.M207280200. [DOI] [PubMed] [Google Scholar]
- 12.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruick RK. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. Genes Dev. 2003;17:2614–2623. doi: 10.1101/gad.1145503. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 15.Kaelin WG., Jr. Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer. 2002;2:673–682. doi: 10.1038/nrc885. [DOI] [PubMed] [Google Scholar]
- 16.Kallio PJ, Okamoto K, O'Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 1998;17:6573–6586. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16:1466–1471. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 20.Powis G, Kirkpatrick L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol Cancer Ther. 2004;3:647–654. [PubMed] [Google Scholar]
- 21.Yang X, Karsenty G. ATF4, the osteoblast accumulation of which is determined post-translationally, can induce osteoblast-specific gene expression in non-osteoblastic cells. J Biol Chem. 2004;279:47109–47114. doi: 10.1074/jbc.M410010200. [DOI] [PubMed] [Google Scholar]
- 22.Yu S, Franceschi RT, Luo M, Fan J, Jiang D, Cao H, Kwon TG, Lai Y, Zhang J, Patrene K, et al. Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone. PLoS One. 2009;4:e7583. doi: 10.1371/journal.pone.0007583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Yu S, Galson DL, Luo M, Fan J, Zhang J, Guan Y, Xiao G. Activating transcription factor 4 is critical for proliferation and survival in primary bone marrow stromal cells and calvarial osteoblasts. J Cell Biochem. 2008;105:885–895. doi: 10.1002/jcb.21888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao H, Yu S, Yao Z, Galson DL, Jiang Y, Zhang X, Fan J, Lu B, Guan Y, Luo M, et al. Activating transcription factor 4 regulates osteoclast differentiation in mice. J Clin Invest. 2010;120:2755–2766. doi: 10.1172/JCI42106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 26.Elefteriou F, Benson MD, Sowa H, Starbuck M, Liu X, Ron D, Parada LF, Karsenty G. ATF4 mediation of NF1 functions in osteoblast reveals a nutritional basis for congenital skeletal dysplasiae. Cell Metab. 2006;4:441–451. doi: 10.1016/j.cmet.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao G, Jiang D, Ge C, Zhao Z, Lai Y, Boules H, Phimphilai M, Yang X, Karsenty G, Franceschi RT. Cooperative Interactions between Activating Transcription Factor 4 and Runx2/Cbfa1 Stimulate Osteoblast-specific Osteocalcin Gene Expression. J Biol Chem. 2005;280:30689–30696. doi: 10.1074/jbc.M500750200. [DOI] [PubMed] [Google Scholar]
- 28.Yu S, Jiang Y, Galson DL, Luo M, Lai Y, Lu Y, Ouyang HJ, Zhang J, Xiao G. General transcription factor IIA-gamma increases osteoblast-specific osteocalcin gene expression via activating transcription factor 4 and runt-related transcription factor 2. J Biol Chem. 2008;283:5542–5553. doi: 10.1074/jbc.M705653200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu S, Franceschi RT, Luo M, Zhang X, Jiang D, Lai Y, Jiang Y, Zhang J, Xiao G. Parathyroid hormone increases activating transcription factor 4 expression and activity in osteoblasts: requirement for osteocalcin gene expression. Endocrinology. 2008;149:1960–1968. doi: 10.1210/en.2007-1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roybal CN, Hunsaker LA, Barbash O, Vander Jagt DL, Abcouwer SF. The oxidative stressor arsenite activates vascular endothelial growth factor mRNA transcription by an ATF4-dependent mechanism. J Biol Chem. 2005;280:20331–20339. doi: 10.1074/jbc.M411275200. [DOI] [PubMed] [Google Scholar]
- 31.Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol. 2010;30:1007–1013. doi: 10.1161/ATVBAHA.110.204354. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh R, Lipson KL, Sargent KE, Mercurio AM, Hunt JS, Ron D, Urano F. Transcriptional regulation of VEGF-A by the unfolded protein response pathway. PLoS One. 2010;5:e9575. doi: 10.1371/journal.pone.0009575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dedieu S, Canron X, Rezvani HR, Bouchecareilh M, Mazurier F, Sinisi R, Zanda M, Moenner M, Bikfalvi A, North S. The cytoprotective drug amifostine modifies both expression and activity of the pro-angiogenic factor VEGF-A. BMC Med. 2010;8:19. doi: 10.1186/1741-7015-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pereira ER, Liao N, Neale GA, Hendershot LM. Transcriptional and post-transcriptional regulation of proangiogenic factors by the unfolded protein response. PLoS One. 2010;5:e12521. doi: 10.1371/journal.pone.0012521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 36.Yang S, Xu H, Yu S, Cao H, Fan J, Ge C, Fransceschi RT, Dong HH, Xiao G. Foxo1 mediates insulin-like growth factor 1 (IGF1)/insulin regulation of osteocalcin expression by antagonizing Runx2 in osteoblasts. J Biol Chem. 2011;286:19149–19158. doi: 10.1074/jbc.M110.197905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Banerjee C, McCabe LR, Choi JY, Hiebert SW, Stein JL, Stein GS, Lian JB. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J Cell Biochem. 1997;66:1–8. doi: 10.1002/(sici)1097-4644(19970701)66:1<1::aid-jcb1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 38.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation [see comments]. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 39.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts [see comments]. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 40.Mundlos S, Otto F, Mundlos C, Mulliken JB, Aylsworth AS, Albright S, Lindhout D, Cole WG, Henn W, Knoll JH, et al. Mutations involving the transcription factor CBFA1 cause cleidocranial dysplasia [see comments]. Cell. 1997;89:773–779. doi: 10.1016/s0092-8674(00)80260-3. [DOI] [PubMed] [Google Scholar]
- 41.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, et al. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development [see comments]. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Christensen K, Chawla K, Xiao G, Krebsbach PH, Franceschi RT. Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. J Bone Miner Res. 1999;14:893–903. doi: 10.1359/jbmr.1999.14.6.893. [DOI] [PubMed] [Google Scholar]
- 43.Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3-E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11:1103–1113. doi: 10.1210/mend.11.8.9955. [DOI] [PubMed] [Google Scholar]
- 44.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, Elliott R, Colombero A, Elliott G, Scully S, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 45.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 47.Moroz E, Carlin S, Dyomina K, Burke S, Thaler HT, Blasberg R, Serganova I. Real-time imaging of HIF-1alpha stabilization and degradation. PLoS One. 2009;4:e5077. doi: 10.1371/journal.pone.0005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dunn LK, Mohammad KS, Fournier PG, McKenna CR, Davis HW, Niewolna M, Peng XH, Chirgwin JM, Guise TA. Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One. 2009;4:e6896. doi: 10.1371/journal.pone.0006896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brighton CT, Krebs AG. Oxygen tension of healing fractures in the rabbit. J Bone Joint Surg Am. 1972;54:323–332. [PubMed] [Google Scholar]
- 50.Maurer P, Meyer L, Eckert AW, Berginski M, Schubert J. Measurement of oxygen partial pressure in the mandibular bone using a polarographic fine needle probe. Int J Oral Maxillofac Surg. 2006;35:231–236. doi: 10.1016/j.ijom.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 51.Kolar P, Gaber T, Perka C, Duda GN, Buttgereit F. Human early fracture hematoma is characterized by inflammation and hypoxia. Clin Orthop Relat Res. 2011;469:3118–3126. doi: 10.1007/s11999-011-1865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu K, Chan W, Heymach J, Wilkinson M, McConkey DJ. Control of HIF-1alpha expression by eIF2 alpha phosphorylation-mediated translational repression. Cancer Res. 2009;69:1836–1843. doi: 10.1158/0008-5472.CAN-08-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.