Abstract
Antibiotics are commonly prescribed in pediatric outpatient settings; however, efforts to decrease inappropriate use have largely focused on inpatients. We obtained baseline metrics to identify conditions that may benefit from establishment of outpatient antimicrobial stewardship interventions (ASP). We evaluated rates and appropriateness of antibiotic prescribing for children with acute otitis media (AOM), community acquired pneumonia (CAP), and skin and soft tissue infections (SSTI) in ambulatory settings within a large healthcare system in the US Midwest. We retrospectively reviewed 77,821 visits and associated diagnostic codes for children less than 17 years seen in ambulatory settings within our health system from August 1, 2009 to July 31, 2010. We measured rates of antibiotic prescribing by location, provider type, patient age, and diagnosis, and assessed concordance with treatment guidelines for AOM, CAP, and SSTI. AOM, CAP, and SSTI comprised about 1/3 of all infections in the study population. Antibiotics were prescribed in 14,543 (18.7%) visits. Antibiotic prescribing rates were 1.1 to 1.2 times higher among Emergency Room (ER) providers compared to Pediatricians and Family Physicians. Antibiotics prescribed for AOM and SSTI were concordant with guidelines in approximately 97% of cases. In contrast, 47% of antibiotics prescribed for treatment of CAP in children < 5 years old were macrolides, which are not recommended first line therapy for CAP in this age group. Antibiotic prescribing for pediatric outpatients within our health system is not guideline-concordant for treatment of CAP.
Keywords: Pediatrics, Antimicrobial stewardship, Otitis Media, Pneumonia, Skin and soft tissue infections
Introduction
Antibiotics are the most commonly prescribed medications to children across all age groups. One fifth of all pediatric ambulatory visits result in an antibiotic prescription [1]. Despite an overall decline in antibiotic prescribing for US children [2,3] inappropriate use of expensive broad-spectrum antibiotics is rising [4], which substantially increases health costs, promotes bacterial resistance, and contributes to increasing rates of antibiotic-associated adverse effects. Outpatient antibiotic overuse has been correlated with higher rates of antibiotic-resistant pathogens [5,6].
In an effort to combat the emergence of drug-resistance, the Infectious Diseases Society of America (IDSA) published guidelines for developing antimicrobial stewardship programs (ASPs). ASPs are coordinated interventions designed to measure and improve the appropriate use of antibiotics, with goals of improving clinical outcomes, minimizing adverse events, limiting emergence of drug-resistance and reducing costs [7]. Development and implementation of clinical pathways for management of common infections is an integral part of ASPs. Accordingly, clinical practice guidelines have been developed for management of common infections including AOM [8], CAP [9], and a common cause of skin and soft tissue infections, methicillin-resistant Staphylococcus aureus infections (MRSA)[10]. Notably, both AOM and pediatric CAP guidelines emphasize the use of narrow-spectrum antibiotics as first line therapy for these conditions [8,9,11,12].
Despite increasing data about inpatient pediatric ASPs, relatively few studies describe the effectiveness of ASPs in pediatric ambulatory settings [13,14]. Further research is needed to describe antibiotic use in community settings and design and implement targeted interventions that optimize antibiotic use among outpatients. The objective of this study was to evaluate the rates and appropriateness of outpatient antibiotic prescribing for children with three common infectious diseases, AOM, CAP and SSTI, in order to identify areas for outpatient stewardship interventions.
Materials and Methods
Study Design and Setting
We performed a retrospective analysis of ambulatory pediatric visits within Franciscan Healthcare-Mayo Clinic Health System (FH-MCHS), which includes locations in western Wisconsin, eastern Minnesota and northeastern Iowa. FHS-MCHS employs 92 providers: 7 pediatricians, 56 family medicine physicians, 15 family medicine physician assistants and nurse practitioners, 13 ER and urgent care physicians, and 1 urgent care physician assistant. This study was approved by the FH-MCHS and Mayo Clinic institutional review boards.
We abstracted patient age, encounter provider and location, and antibiotics prescribed from the electronic medical records (EMR). We excluded topical, antiviral and anti-parasitic antimicrobials. Diagnosis codes were extracted from the ambulatory billing claim system that uses the International Classification of Diseases, Ninth Revision (ICD-9) codes. These data were integrated with the EMR encounter before final analysis using Statistical Analysis System software (SAS). We excluded inpatient hospital visits, subspecialty and nurse visits, and diagnostic codes for procedures and immunizations. Infection-related diagnostic codes were classified into four categories: AOM, CAP, SSTI, and other infections. Appropriate antibiotics for each indication were defined as those listed as first or second line therapy in published treatment guidelines [8–10]. For AOM treatment, penicillin, amoxicillin, and amoxicillin-clavulanate were considered first line agents, while macrolides, cephalosporins, tetracyclines, and clindamycin were considered second line agents. For CAP, penicillin or amoxicillin were considered first line treatments, as were macrolides in children >5 years, while amoxicillin-clavulanate and second or third generation cephalosporins (in case of penicillin allergy) were considered second line or alternative treatments. For SSTI, we were not able to differentiate purulent lesions from non-purulent lesions; therefore, in accordance with published guidelines [15] we considered agents with activity against either staphylococci or streptococci as first-line therapy: trimethoprim-sulfamethoxazole, clindamycin, doxycycline, minocycline, penicillin, amoxicillin and first generation cephalosporins. Amoxicillin-clavulanate was considered a second line agent for SSTI.
Study population
We included all children ≤17 years, seen at the Franciscan Healthcare - Mayo Clinic Health System (FH-MCHS) from August 1, 2009 until July 31, 2010.
Statistical Analysis
Visit rates for infections were calculated for each of the 4 categories (AOM, CAP, SSTI, and other) and expressed as the number of visits with the given diagnosis per 1,000 patient visits. Visit rates are presented using point estimates and 95% confidence intervals and compared across provider types using the Chi-Square test. Of patients diagnosed with an infection, the percentage prescribed antibiotics was calculated overall and also according to patient age and provider type. Prescription percentages are compared across groups using the Chi-Square test. P-values less than 0.05 were considered significant.
Results and Discussion
Results
Approximately 22,576 children were evaluated during 88,887 visits. We excluded 11,066 (12.4%) visits and 3,350 (18%) medication orders, because of mismatching identifiers or visit dates. Among the 77,821 visits included in the study, antibacterials were prescribed in 14,543 (18.7 %). Patient volume varied widely among the various clinic locations, ranging from 1,967 (2%) to 21,953 (28%). Most patients were seen by providers in Family Medicine (63%), followed by Pediatrics (27%), and Emergency/Urgent care (10%). Across all sites, 27,298 visits (35%) resulted in infection-related diagnoses. The proportion of all infection-related diagnoses was 27.3% for AOM, 3.7% for SSTI, and 3.4% for CAP.
Visit rates and antibiotic prescription rates for AOM, SSTI, and CAP differed significantly across the three provider types (Table 1). For all three diagnoses, visit rates and antibiotic prescription rates were greater in the ER compared to Family Medicine and Pediatric clinics (Table 1).
Table 1.
Visit and antibiotic prescription rates by provider type for pediatric outpatients diagnosed with AOM, CAP, and SSTI at FH-MCHS, August 2009–July 2010
| Visit rate (No. patients with diagnosis/1000 patient visits) (95% CI)a | Antibiotic prescription percentage (95% CI)b | |||||
|---|---|---|---|---|---|---|
| Family Medicine clinics | Pediatric clinics | Emergency Dept. | Family Medicine clinics | Pediatric clinics | Emergency Dept. | |
| AOM | 77.8 (75.4 to 80.3) | 110.5 (106.2 to 114.7) | 145.4 (138.6 to 152.1) | 78.6 (77.3 to 80.0) | 74.1 (72.3 to 75.8) | 89.7 (88.2 to 91.3) |
| CAP | 11.0 (10.0 to 11.9) | 12.1 (10.6 to 13.6) | 14.7 (12.4 to 17.0) | 58.7 (54.4 to 63.0) | 59.9 (53.9 to 65.9) | 72.3 (65.2 to 79.3) |
| SSTI | 11.8 (10.8 to 12.8) | 11.9 (10.5 to 13.4) | 19.3 (16.7 to 21.9) | 69.3 (65.4 to 73.2) | 62.9 (56.9 to 68.8) | 77.3 (71.6 to 83.1) |
| Other infections | 220.2 (216.4 to 224.0) | 179.9 (174.8 to 185.1) | 367.9 (358.7 to 377.1) | 31.2 (30.3 to 32.1) | 23.7 (22.4 to 25.1) | 29.1 (27.7 to 91.3) |
The visit rate represents the number of patients with the given diagnosis per 1,000 patient visits at the given location.
the antibiotic prescription percentage represents the percentage of patient visits at the given location with the given diagnosis that were prescribed an antibiotic.
Abbreviations: AOM= Acute otitis media, CAP= community acquired pneumonia, SSTI= skin and soft tissue infections, FH-MCHS= Franciscan Healthcare-Mayo Clinic Health System, CI= Confidence Interval.
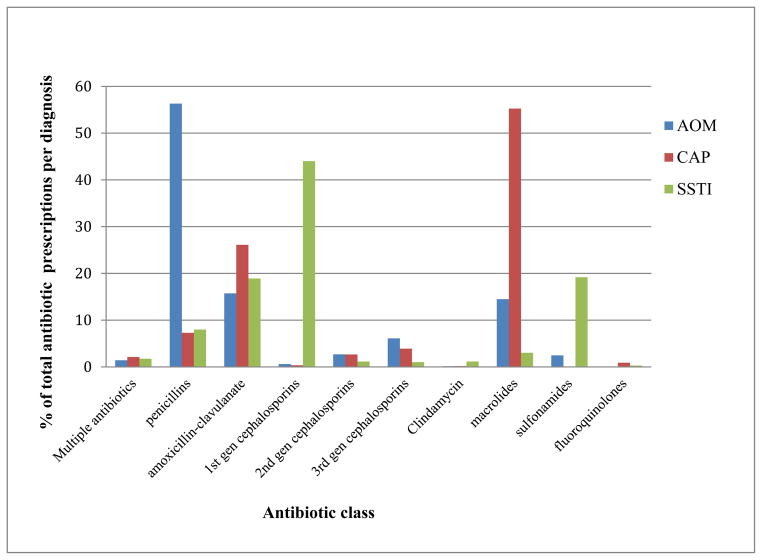
Antimicrobials were prescribed more frequently in patients with AOM compared to CAP or SSTI (79.5%, 61.3% and 69.3% for AOM, CAP and SSTI respectively; p<0.001). Among all antibiotic orders, penicillin and amoxicillin were the most commonly prescribed agents (43.9%), followed by macrolides (20.5%) and beta-lactam/beta-lactamase inhibitors (13.5%). The most commonly prescribed antibiotic classes for AOM, CAP and SSTI were penicillins (56.3%), macrolides (55.2%), and first generation cephalosporins (44%), respectively (Figure 1). Notably, 20% of AOM visits were not associated with an antibiotic prescription. Macrolides were the most commonly prescribed antibiotic for CAP, accounting for 47% of prescriptions in children less than 5 years of age with CAP, and half or more of patients with CAP who received an antibiotic in the emergency room (59%), family medicine clinics (57%), or pediatric clinics (49%) (Figure 1).
Figure 1. Antibiotic prescriptions by infection type in pediatric outpatients at FH-MCHS August 2009–July 2010.
Number of prescriptions per diagnosis: N = 5928 for AOM; N=563 for CAP; N=693 for SSTI. FH-MCHS= Franciscan Healthcare-Mayo Clinic Health System, AOM= Acute otitis media, CAP= community acquired pneumonia, SSTI= skin and soft tissue infections.
Guideline-concordant prescribing varied by diagnosis. Among antibiotics prescribed for AOM, 73.1% were guideline-recommended first line treatments, 24.4% were second line treatments that would only be appropriate in cases of penicillin-allergy, and 2.5% were sulfonamides, which are not recommended for AOM treatment (figure 2) [8]. Prescriptions for CAP were first or second line agents in 77.5 %, and antibiotics that were not recommended in 22.5%. For SSTI, 78.3 % of prescriptions were first line drugs, while 21.7 % were drugs that are not considered first line therapy [10,15].
Figure 2. Proportion of guideline-concordant antibiotics prescribed for children by type of infection at FH-MCHS August 2009–July 2010.
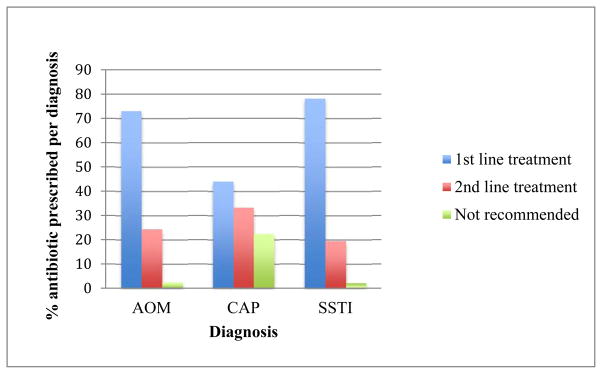
AOM: first line treatment, Amoxicillin or amoxicillin-clavulanate; second line treatment, cefdinir, ceftriaxone and clindamycin; not recommended, sulfonamides. CAP: first line treatment penicillin or amoxicillin, macrolides (for >5 to 17 years); second line/alternative treatment, amoxicillin-clavulanate, second or third generation cephalosporins; not recommended, macrolides (for children < 5 years). SSTI: first line treatment, penicillin, amoxicillin, cephalexin, trimethoprim-sulfamethoxazole, clindamycin, doxycycline and minocycline; second line treatment, amoxicillin-clavulanate; not recommended, second/third generation cephalosporins. Number of prescriptions per diagnosis: N=5844 for AOM, N= 551 for CAP, N= 681 for SSTI. Patients receiving multiple concurrent antibiotics were excluded (N=84 for AOM; N=12 for CAP, and N=12 for SSTI).
Discussion
Overall and diagnosis-specific antibiotic prescribing rates were higher in the ER compared to other outpatient clinics. For management of CAP, antibiotic selection was frequently discordant with published treatment guidelines, with nearly half of children <5 years receiving macrolides rather than first line antibiotics (Figure 2). Our findings demonstrate that quantification and evaluation of antibiotic use, a cornerstone of antimicrobial stewardship, is feasible in outpatient pediatric settings, and identifies CAP treatment as a target for outpatient stewardship interventions.
Although ER visits comprised only 10% of all visits, visits rates for AOM, CAP, and SSTI were consistently higher in the ER compared to outpatient clinics. This undoubtedly reflects the high acuity of care in ER visits compared to clinic visits, many of which were well-child evaluations. Increased acuity among ER visits likely also explains the higher rate of antibiotic prescribing in the ER. Our finding is similar to other studies that noted higher antibiotic prescribing among ER providers and family practitioners than pediatricians [16].
In our health system, 18.7% of the visits resulted in antibiotic prescribing, comparable to the national rate of 21% [1]. Also similar to other studies, within our health system penicillins remained the most commonly prescribed class of antibiotics [1,17] and the macrolide prescription rate was fairly high at 20.5% [1]. Particularly worrisome is the high rate of macrolide use for treatment of CAP in children less than 5 years that we observed. This is in contrast to the recent CAP treatment guidelines, which recommend macrolides for treatment of atypical respiratory pathogens, primarily in school age children and adolescents, and emphasize use of narrow- spectrum penicillins (or cephalosporins for penicillin-allergic patients) as first-line therapy for the most common causative bacteria, Streptococcus pneumoniae [9], which is increasingly resistant to macrolides. In fact, our local pediatric antibiogram for 2010 indicated that Streptococcus pneumoniae was only 50% susceptible to macrolides. Reliance on macrolides for treatment of pediatric CAP has been previously described; national estimates before the publication of CAP guidelines showed that macrolides were the most commonly prescribed antibiotics in pediatric CAP [9]. The higher percentage of macrolide use by family medicine and ER providers compared to pediatricians may reflect the recommended practice for treatment of CAP in adults with macrolides. Our study preceded publication of the pediatric CAP guidelines, likely contributing to some of the guideline-discrepant antibiotic prescribing for CAP within our health system. Conversely, familiarity with AOM and MRSA treatment guidelines, which were published several years prior to our study, likely explains the higher rate of guideline-concordant antibiotic prescribing for these diagnoses.
Recently, Gerber et al. demonstrated improvement in antibiotic prescribing in outpatient settings after implementation of an ASP intervention involving provider education and audit and feedback. In this study, broad-spectrum antibiotic prescribing decreased from 26.8% to 14.3% in the intervention group compared to 28.4% to 22.6% in the control group. Furthermore, after the intervention, guideline-discordant prescribing for CAP decreased from 15.7% to 4.2% [14]. Unfortunately, the benefits of this intervention were not durable after discontinuation of audit and feedback [18]. In our institution, the results of the current study led to new antimicrobial stewardship efforts including provider education sessions, and dissemination of treatment guidelines in both electronic and pocket card formats. Future antimicrobial stewardship efforts within our health system should focus on increasing provider awareness of pediatric CAP guidelines and follow-up evaluation of macrolide use. Achieving sustained, appropriate antimicrobial prescribing will likely require multiple interventions like provider education sessions, electronic decision support systems that facilitate guideline-concordant antibiotic ordering, and ongoing audit of antibiotic orders with appropriate feedback to prescribers.
Study strengths are that we examined a large inclusive sample of all pediatric outpatients in our health system, demonstrating the feasibility of obtaining antibiotic utilization data in a large multicenter outpatient practice. The study period spanned over one-year to avoid seasonal variation in illness that may affect antibiotic prescribing.
Study limitations
Electronic data retrieval of antibiotics prescribed outside of our health system or by telephone was not feasible. We did not evaluate appropriate dosing or length of antibiotic treatment. We did not capture medication allergies or microbiology results and thus were unable to distinguish if use of second-line or alternative antibiotics were, in fact, appropriately prescribed to avoid drug allergy or to treat atypical respiratory pathogens causing CAP. We did not collect detailed demographic or clinical data and thus could not adjust rates based on patient-level factors. In addition, we were unable to capture the “watchful waiting” option for otitis media, so the antibiotic prescription rate may be falsely elevated for AOM. Furthermore, clinical outcomes and costs associated with antibiotic prescribing were beyond the scope of the study. Lastly, although the multi-site approach in our study enhances generalizability, we cannot generalize our findings to other practices with different patient populations and antimicrobial resistance rates.
Conclusions
Antibiotic prescribing for pediatric outpatients within our health system is guideline-concordant for treatment of AOM and SSTI but not CAP. Antimicrobial stewardship interventions are needed to optimize outpatient management of CAP in children, especially among ER providers, the group with the highest antibiotic prescription rates. Interventions to increase provider awareness of the pediatric CAP guidelines, and decision support at the time of antibiotic ordering for CAP have potential to improve appropriate antibiotic prescribing. Furthermore, as AOM is the diagnosis for which most antibiotics are prescribed in children, continued attention to judicious prescribing for AOM and routine use of the “watchful waiting” option remains essential.
Acknowledgments
We thank the following for their work: Cullen Finnian, Debra Clanin, Sherry Grass, and Cheryl Reagles. We also acknowledge the support from Dr. Jennifer Brumm and the Pediatric Department staff at FH-MCHS La Crosse. Dr. Saleh is the recipient of a SOAR-grant (System-Oriented and Applied Research) from the Mayo Clinic Health System and received support through the Mayo Clinic Center for Clinical and Translational Science.
Funding was provided by a Mayo Clinic SOAR (System-Oriented and Applied Research) grant. This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH).
Abbreviations
- ASP
antimicrobial stewardship program
- AOM
Acute otitis media
- CAP
community acquired pneumonia
- SSTI
skin and soft tissue infections
- ER
Emergency room
- MRSA
methicillin-resistant Staphylococcus aureus
- FH-MCHS
Franciscan Healthcare-Mayo Clinic Health System
- EMR
Electronic medical records
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
References
- 1.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–61. doi: 10.1542/peds.2011-1337. [DOI] [PubMed] [Google Scholar]
- 2.Greene SK, Kleinman KP, Lakoma MD, Rifas-Shiman SL, Lee GM, Huang SS, et al. Trends in Antibiotic Use in Massachusetts Children, 2000–2009. Pediatrics. 2012;130(1):15–22. doi: 10.1542/peds.2011-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halasa NB, Griffin MR, Zhu Y, Edwards KM. Decreased number of antibiotic prescriptions in office-based settings from 1993 to 1999 in children less than five years of age. The Pediatr Infect Dis J. 2002;21(11):1023–8. doi: 10.1097/00006454-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Dooling KL, Shapiro DJ, Van Beneden C, Hersh AL, Hicks LA. Overprescribing and inappropriate antibiotic selection for children with pharyngitis in the United States, 1997–2010. JAMA Pediatrics. 2014;168(11):1073–4. doi: 10.1001/jamapediatrics.2014.1582. [DOI] [PubMed] [Google Scholar]
- 5.MacDougall C, Powell JP, Johnson CK, Edmond MB, Polk RE. Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clin Infect Dis. 2005;41(4):435–40. doi: 10.1086/432056. [DOI] [PubMed] [Google Scholar]
- 6.Hicks LA, Chien YW, Taylor TH, Jr, Haber M, Klugman KP. Outpatient antibiotic prescribing and nonsusceptible Streptococcus pneumoniae in the United States, 1996–2003. Clin Infect Dis. 2011;53(7):631–9. doi: 10.1093/cid/cir443. [DOI] [PubMed] [Google Scholar]
- 7.Dellit TH, Owens RC, McGowan JE, Jr, Gerding DN, Weinstein RA, Burke JP, et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America guidelines for developing an institutional program to enhance antimicrobial stewardship. Clin Infect Dis. 2007;44(2):159–77. doi: 10.1086/510393. [DOI] [PubMed] [Google Scholar]
- 8.American Academy of Pediatrics, American Academy of Family physicians, Subcommittee on Diagnosis and management of acute otitis media. Pediatrics. 2004;113(5):1451–65. doi: 10.1542/peds.113.5.1451. [DOI] [PubMed] [Google Scholar]
- 9.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25–76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clini Infect Dis. 2011;52(3):e18–55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 11.Klein JO. The burden of otitis media. Vaccine. 2000;19 (Suppl 1):S2–8. doi: 10.1016/s0264-410x(00)00271-1. [DOI] [PubMed] [Google Scholar]
- 12.Lieberthal AS, Carroll AE, Chonmaitree T, Ganiats TG, Hoberman A, Jackson MA, et al. The diagnosis and management of acute otitis media. Pediatrics. 2013;131(3):e964–99. doi: 10.1542/peds.2012-3488. [DOI] [PubMed] [Google Scholar]
- 13.Finkelstein JA, Davis RL, Dowell SF, Metlay JP, Soumerai SB, Rifas-Shiman SL, et al. Reducing antibiotic use in children: a randomized trial in 12 practices. Pediatrics. 2001;108(1):1–7. doi: 10.1542/peds.108.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Gerber JS, Prasad PA, Fiks AG, Localio AR, Grundmeier RW, Bell LM, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345–52. doi: 10.1001/jama.2013.6287. [DOI] [PubMed] [Google Scholar]
- 15.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJ, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–52. doi: 10.1093/cid/ciu444. [DOI] [PubMed] [Google Scholar]
- 16.Nadeem Ahmed M, Muyot MM, Begum S, Smith P, Little C, Windemuller FJ. Antibiotic prescription pattern for viral respiratory illness in emergency room and ambulatory care settings. Clin Pediatrics. 2010;49(6):542–7. doi: 10.1177/0009922809357786. [DOI] [PubMed] [Google Scholar]
- 17.Vaz LE, Kleinman KP, Raebel MA, Nordin JD, Lakoma MD, Dutta-Linn MM, et al. Recent trends in outpatient antibiotic use in children. Pediatrics. 2014;133(3):375–85. doi: 10.1542/peds.2013-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerber JS, Prasad PA, Fiks AG, Localio AR, Bell LM, Keren R, et al. Durability of Benefits of an Outpatient Antimicrobial Stewardship Intervention After Discontinuation of Audit and Feedback. JAMA. 2014 Oct 10; doi: 10.1001/jama.2014.14042. Epub ahead of print. [DOI] [PubMed] [Google Scholar]