Abstract
Human skeletal muscle tissue displays specific cellular architecture easily damaged during individual existence, requiring multiple resources for regeneration. Congruent with local prerequisites, heterogeneous muscle stem cells (MuSCs) are present in the muscle interstitium. In this study, we aimed to characterize the properties of human muscle interstitial cells that had the characteristic morphology of telocytes (TCs). Immunocytochemistry and immunofluorescence showed that cells with TC morphology stained positive for c-kit/CD117 and VEGF. C-kit positive TCs were separated with magnetic-activated cell sorting, cultured in vitro and expanded for study. These cells exhibited high proliferation capacity (60% expressed endoglin/CD105 and 80% expressed nuclear Ki67). They also exhibited pluripotent capacity limited to Oct4 nuclear staining. In addition, 90% of c-kit positive TCs expressed VEGF. C-kit negative cells in the MuSCs population exhibited fibroblast-like morphology, low trilineage differential potential and negative VEGF staining. These results suggested that c-kit/CD117 positive TCs represented a unique cell type within the MuSC niche.
Keywords: skeletal muscle, telocytes, c-kit/CD117, VEGF, endoglin/CD105
We pursued the identification of interstitial cells corresponding to the description for TCs within the MuSC niche: presence of two to five cell body prolongations that are very thin, extremely long, with a moniliform aspect [1, 2].
We obtained normal, human skeletal muscle biopsies from patients that were undergoing surgery. All protocols were performed in accordance with international ethical guidelines. Muscle samples were cut into small 5 mm3 pieces and placed in gelatin coated culture flasks to isolate heterogeneous MuSCs. After approximately 10 days, cells reached 70–80% confluence and the tissue was removed. The cells were washed and used for further experiments. The results represent the averages of five separate experiments. The cell population obtained by this cells culture technique was very heterogeneous and we were able to identify typical TCs, apart from fibroblast-like cells and MuSCs. Morphological features of TCs were identified by phase contrast microscopy: oval shape body and long, moniliform prolongations (telopodes), congruent with previous description of TCs (Fig. 1) [3–5].
Fig 1.
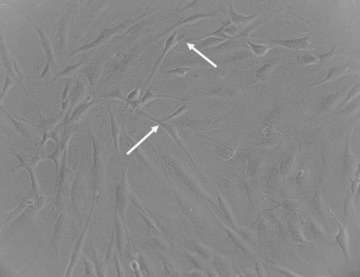
Morphological aspect of skeletal muscle-derived primary cell culture obtained using explant method. TCs are identified based on their characteristics (arrows) within the heterogeneous cell population. Contrast-phase microscopy; magnification 200×.
A number of previous investigators showed that TCs could be identified, apart from their morphological appearance, by staining with antibodies against c-kit/CD117, vimentin, VEGF and CD34 [6, 7]. Most interstitial [8–10] and tissue stem cells lack expression of c-kit/CD117 [11]; thus, we consider this to be an appropriate marker for TCs identification.
We used an anti-c-kit microbeads kit (MACS; Miltenyi Biotec, CA, USA) and the magnetic isolation technique to isolate c-kit positive cells. These cells were expanded in plastic flasks in α-minimum essential medium (αMEM; Gibco BRL, Invitrogen, Carlsbad, CA, USA), supplemented with 10% foetal calf serum (FCS; PromoCell, Heidelberg, Germany) and 2% penicillin/streptomycin mixture (Pen/Strep, 10,000 IU/ml; PromoCell). Immunocytochemistry was performed with the labelled streptavidin-biotin 2-horseradish peroxidase (LSAB2-HRP) visualization system (Dako, Glostrup, Denmark) for identification of vimentin and c-kit positive cells.
Immunofluorescence was performed only on c-kit positive cells. Cells were probed for proliferation/pluripotency markers with primary antibodies to endoglin/CD105, Ki67, Oct4 and VEGF (DakoCytomation; Dako); for immunofluorescence we used AlexaFluor488 and AlexaFluor546 conjugated secondary antibodies (Molecular Probes, Invitrogen) and nuclear counterstaining with DAPI (Sigma-Aldrich, St. Louis, MO, USA).
Differentiation capacity towards the mesodermal lineages (adipogenic, chondrogenic and osteogenic) was assessed for the entire cell population and also separately for MuSC by employing the appropriate cell culture media. Non-haematopoietic stem cell medium was used for generating osteoblasts/ chondrocytes/adipocytes (Miltenyi Biotec, Bergisch Gladbach, Germany).
Vimentin expression was detected in the cytoplasm of both MuSCs and TCs (Fig. 2). From the entire initial cell population, only 10% were c-kit positive. We present here the morphological appearance of the c-kit positive TCs after separation and following several passages, showing that almost 100% of the cells express c-kit localized at membrane level. On account of the isolated cells characteristic morphology, they will be further referred to as TCs (Fig. 3).
Fig 2.
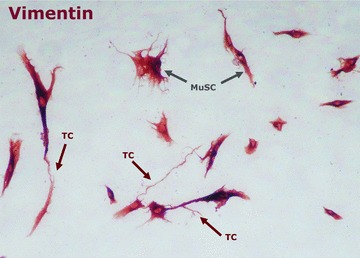
The cytoskeleton protein, vimentin, was ubicuitary expressed in stromal cells. Telocytes were recognized by their distinct morphology of an oval body shape with telopodes. Arrows point to MuSC and telocytes, which confirm the heterogeneity of primary cell cultures obtained from skeletal muscle tissue; magnification 200×.
Fig 3.
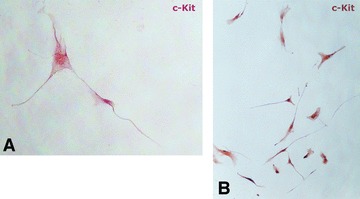
Immunohistochemical analysis of CD117 (c-kit) expressing TCs. Telocytes were isolated by magnetic separation with CD117-specific beads. (A) Single cells show long moniliform prolongations (telopodes); magnification 600×. (B) Overview of in vitro expanded CD117+ population of TCs; magnification 100×.
Cytochemical analysis documented the presence of nuclear Ki67 (proliferation marker) and endoglin/CD105 (vascular proliferation marker; Fig. 4A and B), Oct4 (pluripotency marker; Fig. 4C) and VEGF (vascular proliferation marker; Fig. 4D). Endoglin/ CD105 was previously shown to characterize proliferating endothelial cells [12], while its association with VEGF (vascular endothelial growth factor) presence could be an indicator of TCs abilities to support the well-developed vascular structure required by skeletal muscle tissue. However, the nuclear presence of the Oct4 transcription factor in the absence of Sox2 and Nanog co-regulators in the isolated TCs does not confer stem cells status to the TCs, while it might contribute to their susceptibility to signals from the muscle microenvironment. Further differentiation studies should be conducted before Oct4 expression can be correlated to TCs in vitro behaviour.
Fig 4.
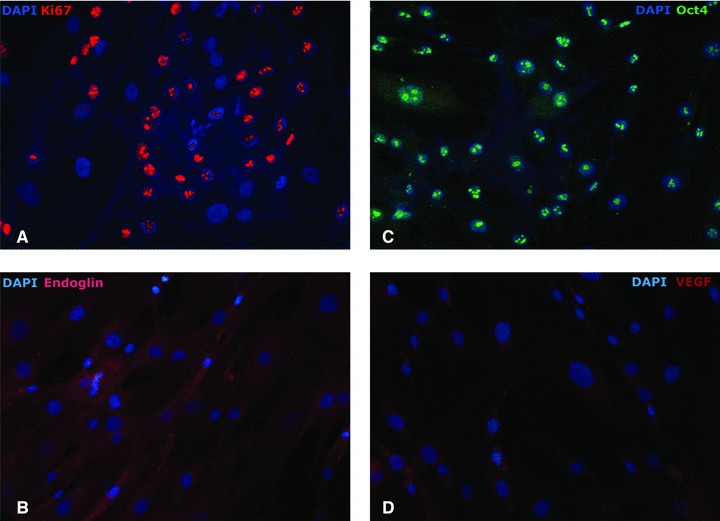
Immunofluorescence of CD117+ TCs, expanded during in vitro culture. A large fraction of cells (60%) showed proliferation. Proliferation was indicated by (A) the presence of Ki67 in the cell nucleus; and (B) endoglin/CD105 expression, at various intensities. (C) Pluripotency-associated transcription factor Oct4 stained positive in the considered cell population. (D) c-kit expression in TCs associated with an intermediate level of VEGF presence in the cytoplasm; magnification 400×.
Several in vitro MuSCs differentiation assays were carried in the presence and in the absence of TCs, and demonstrated an enhanced capacity of MuSCs for trilineage differentiation (adipocytes, osteoblasts and chondrocytes) when co-cultured with TCs (in the appropriate differentiation media), compared to that observed with MuSCs alone (Fig. 5). Whether this process can be accounted by direct cell-to-cell contact or mediated by signalling molecules released by TCs is still to be determined.
Fig 5.
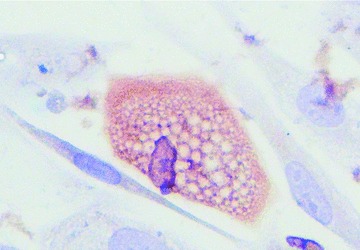
Eighty percent of primary cell cultures readily differentiated into adipocytes (FABP4 positive staining), when they maintained contact with supporting interstitial TCs; magnification 600×.
This paper demonstrated that human skeletal muscle harbours cells with phenotypic characteristics of TCs. This confirmed our hypothesis that human MuSC niche would contain a unique subpopulation of interstitial cells (TCs) that were endowed with proliferative and supportive capabilities.
Acknowledgments
This work was supported by CNCSIS-UEFISCSU, project number PNII-IDEI 1748/2008, and by the Sectorial Operational Programme for Human Resources Development, financed by the European Social Fund, FSE POSDRU/89/1.5/S/60746. Skeletal muscle samples were provided by Prof. Mihai Ionac.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Popescu LM, Faussone-Pellegrini MS. TELOCYTES—a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to Telocytes. J Cell Mol Med. 2010;14:729–40. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popescu LM, Manole E, Serboiu CS, et al. Identification of telocytes in skeletal interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15:1379–92. doi: 10.1111/j.1582-4934.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kostin S, Popescu LM. A distinct type of cell in myocardium: interstitial Cajal-like cells (ICLCs) J Cell Mol Med. 2009;13:295–308. doi: 10.1111/j.1582-4934.2008.00668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gherghiceanu M, Popescu LM. Cardiomyocytes precursors and telocytes in epicardial stem cell niche: electron microscope images. J Cell Mol Med. 2010;14:871–7. doi: 10.1111/j.1582-4934.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Zhang Y, Wen X, et al. Telocytes accompanying cardiomyocyte in primary culture: two- and three-dimensional culture environment. J Cell Mol Med. 2010;14:2641–5. doi: 10.1111/j.1582-4934.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Popescu LM, Manole CG, Gherghiceanu M, et al. Telocytes in human epicardium. J Cell Mol Med. 2010;14:2085–93. doi: 10.1111/j.1582-4934.2010.01129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suciu L, Popescu LM, Gherghiceanu M, et al. Telocytes in human term placenta: morphology and phenotype. Cells Tissue Organs. 2010;192:325–39. doi: 10.1159/000319467. [DOI] [PubMed] [Google Scholar]
- 8.Stokman G, Stroo I, Claessen N, et al. Stem cell factor expression after renal ischemia promotes tubular epithelial survival. PLoS One. 2010;5:e14386. doi: 10.1371/journal.pone.0014386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Huang C, Yang J, et al. Differentiation induction of cardiac c-kit positive cells from rat heart into sinus node-like cells by 5-azacytidine. Tissue Cell. 2011;43:63–74. doi: 10.1016/j.tice.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Ronnstrand L. Signal transduction via the stem cell factor receptor/c-kit. Cell Mol Life Sci. 2004;61:2535–48. doi: 10.1007/s00018-004-4189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gambini E, Pompilio G, Biondi A, et al. C-kit+ cardiac progenitors exhibit mesenchymal markers and preferential cardiovascular commitment. Cardiovasc Res. 2011;89:362–73. doi: 10.1093/cvr/cvq292. [DOI] [PubMed] [Google Scholar]
- 12.Fonsatti E, Maio M. Highlights on endoglin (CD105): from basic findings towards clinical applications in human cancer. J Transl Med. 2004;2:18–24. doi: 10.1186/1479-5876-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]


