Abstract
Purpose
The role of consolidative radiotherapy (RT) for stage III and IV DLBCL in the era of rituximab is not well defined. There is evidence that some patients with bulky disease may benefit, but patient selection criteria are not well established. We sought to identify a subset of patients who experienced a high local failure rate after receiving rituximab-based chemotherapy alone and hence may benefit from the addition of consolidative RT.
Methods and Materials
211 stage III and IV DLBCL patients treated between August 1999 and January 2012 were reviewed. Of these, 89 had a complete response to systemic therapy including rituximab and received no initial RT. Kaplan-Meier analysis and Cox proportional hazards regression were performed with local recurrence (LR) as the primary outcome.
Results
Median follow-up was 43.9 months. 50% experienced LR at 5 years. In multivariate analysis tumor ≥ 5 cm and stage III disease were associated with increased risk of LR. Five year LR free survival for patients with ≥ 5 cm lesions were 47.4% versus 74.7% for patients with < 5cm lesions (p = 0.01). In patients with < 5 cm tumors, SUVmax was ≥15 in all patients with LR. Five year LR free survival was 100% in SUV < 15 versus 68.8% in SUV≥15 (p=0.10).
Conclusions
Advanced stage DLBCL patients who are stage III or with disease ≥ 5 cm appear to be at an increased risk for LR. Patients with < 5 cm disease and SUVmax≥15 may be at higher risk for LR. These patients may benefit from consolidative RT following chemoimmunotherapy.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a heterogeneous disease that represents the most common subtype of non-Hodgkin lymphoma in the United States. About two thirds of these patients present with advanced disease. The addition of rituximab to standard chemotherapy has improved outcomes in this disease[1, 2]. However, outcomes remain poor in stage III and IV disease with progression free survival (PFS) and overall survival (OS) at ten years of 37% and 44%, respectively[3].
Relapse after initial treatment typically requires high-dose therapy with autologous stem cell transplant. Prolonged disease free survival rates are poor following relapse[4]. Radiation therapy (RT) is often recommended in the consolidative setting following complete response after chemotherapy, particularly for stage I and II DLBCL patients. Randomized evidence has shown that RT improves the rate of recurrence and possibly survival in stage I and II DLBCL[5, 6]. Other studies conclude that there is no benefit from radiation in this setting[7, 8], but these were all conducted in the pre-rituximab era.
With the dramatic improvement in outcomes in the rituximab era, the question is raised whether radiation therapy is still needed. The role of RT is especially unclear in the setting of advanced stage disease in which systemic relapse is thought to drive outcomes and improved local control with the addition of radiotherapy may be less of an issue. However, there is evidence that consolidative RT can improve outcomes in advanced stage disease. Retrospective series have shown improvement in both PFS and OS with the addition of RT following complete response to chemotherapy[9–11]. Additionally, a recently published prospective nonrandomized trial, including stage III and IV patients, demonstrated improvements in both PFS and OS for ≥70 year old patients with ≥7.5 cm bulky disease with the addition of consolidative RT following R-CHOP[12].
While there may be a benefit to the addition of RT in some patients, advanced stage DLBCL represents a heterogeneous disease. Given the preceding results, we sought to identify a subgroup of patients with advanced stage DLBCL who could benefit from consolidative RT in the modern treatment era. We attempted to clarify this by the identification of adverse risk factors predicting for increased local recurrence (LR) in patients who received a complete response (CR) with rituximab-based chemotherapy. These adverse risk factors would then enable radiation oncologists in better identifying future stage III/IV patients who would likely benefit the most with the addition of consolidative radiotherapy.
Methods and Materials
After obtaining XXXX Institutional Review Board approval, we reviewed the records of 211 histologically confirmed stage III-IV DLBCL patients treated with rituximab in addition to CHOP or CHOP-like chemotherapy at XXXX University between 8/1999 and 1/2012. Patients who received radiation therapy, did not achieve CR, or had involvement of the central nervous system were excluded from our analysis. Imaging response assessment was based on consensus recommendations from the International Harmonization Project in Lymphoma[13].
The diagnosis of DLBCL was confirmed by hematopathologists at XXXX University according to World Health Organization classification. Staging was based on the Ann Arbor classification[14]. Staging procedures were not standardized but included computed tomography (CT) of the chest, abdomen, and pelvis, or positron emission tomography (PET), LDH, and bone marrow biopsy. Bulky disease was defined as any presenting mass ≥5 cm in CT maximum diameter, including those presenting with multiple bulky sites. We identified and extracted characteristics previously associated with local control (LC), PFS and OS including International Prognostic Index (IPI) score, lactate dehydrogenase (LDH), tumor size, maximum standardized uptake value (SUVmax) on PET, and bone marrow involvement[10, 15–17]. SUVmax determination was done at time of study acquisition by an attending nuclear medicine physician.
Statistical Analysis
Survival functions for LR and distant recurrence (DR) were estimated by the Kaplan- Meier method. LR was defined as the presence of disease recurrence within the initially presenting sites. DR was presence of disease recurrence at all other sites. Time to event was defined as months from completion of chemotherapy. Patients without a LR or DR were censored at last follow-up date. SUVmax ≥ 13 and bulky lesion ≥5 cm were chosen a priori to dichotomize patients based on prior series describing these as relevant cut points[9, 16–18]. Descriptive statistics were reported for patient and disease characteristics. Cox proportional hazards model was used for multivariate analysis. Backward elimination was used with significance level for removal criteria of p > 0.2. Hazard ratios and their 95% confidence intervals were estimated to assess magnitude of risk. The SAS, version 9.3, statistical package (SAS Institute, Cary, NC) was used for all data managements and analyses.
Results
Clinical Characteristics
Two hundred and eleven patients with DLBCL treated with rituximab-based chemotherapy were identified. Of these, 89 patients had a CR as determined by PET or CT and met our inclusion criteria. The median follow-up time was 43.9 months for all patients. The median age was 59 (range 21–81) years. The Ann Arbor stages represented were III (44.9%) and IV (55.1%). A majority had an Eastern Cooperative Oncology Group (ECOG) performance status of either 1 or 0 (96.7%). 56.3% of patients presented with bulky ≥ 5 cm lesions. The median SUVmax was 17.3 (range 2.3–43).
Patients received a median of six cycles of chemotherapy (range 3–8) with 87.6% receiving R-CHOP chemotherapy. The remainder received alternative rituximab-containing regimens at the discretion of their medical oncologist (CVP-cyclophosphamide, vincristine, prednisone; EPOCH-etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin; Hyper-CVAD-cyclophosphamide, vincristine, doxorubicin, dexamethasone, methotrexate and cytarabine were used.) See Table 1 for patient and treatment characteristics.
Table 1.
Patient, Tumor and Treatment Characteristics
| Characteristic | n=89 (%) |
|---|---|
| Median follow-up time in months | 45.9 |
| Age <60 years ≥60 years |
47 (53%) 42 (47%) |
| Gender Male Female |
48 (54%) 41 (46%) |
| Stage III IV |
40 (45%) 49 (55%) |
| ECOG PS 0 1 2+ |
45 (51%) 41 (46%) 3 (3%) |
| B Symptoms Negative Positive |
61 (69%) 28 (31%) |
| Extranodal Sites 0–1 2–5 |
44 (49%) 45 (51%) |
| Bone Marrow Involvement No Yes |
69 (77%) 20 (23%) |
| Bulky ≥ 5 cm No Yes Missing |
28 (44%) 36 (56%) 25 |
| LDH Normal Elevated |
24 (35%) 44 (65%) |
| SUVmax ≥ 13 No Yes Missing |
15 (26%) 42 (74%) 32 |
| SUVmax ≥ 15 No Yes Missing |
19 (33%) 38 (66%) 32 |
| Chemotherapy Received R-CHOP R+Other |
78 (88%) 11 (12%) |
| Chemotherapy Cycles 3–5 6–8 |
7 (8%) 82 (92%) |
Abbreviations: ECOG PS – Eastern Cooperative Oncology Group Performance Status; LDH – lactate dehydrogenase; SUV – standardized uptake value.
Patterns of relapse
43.8% of patients developed relapse at initially involved site after rituximab-based chemotherapy alone. The actuarial rate of LC at 5 years was 52.2%. 19 patients (21.4%) developed recurrence exclusively at initially involved sites, and 20 patients (22.5%) developed recurrence both at previously involved sites and distantly. Only 3 (3.4%) patients developed an isolated DR, suggesting that isolated distant recurrences without some form of a local recurrence are uncommon after chemotherapy alone, even in stage III/IV patients with DLBCL.
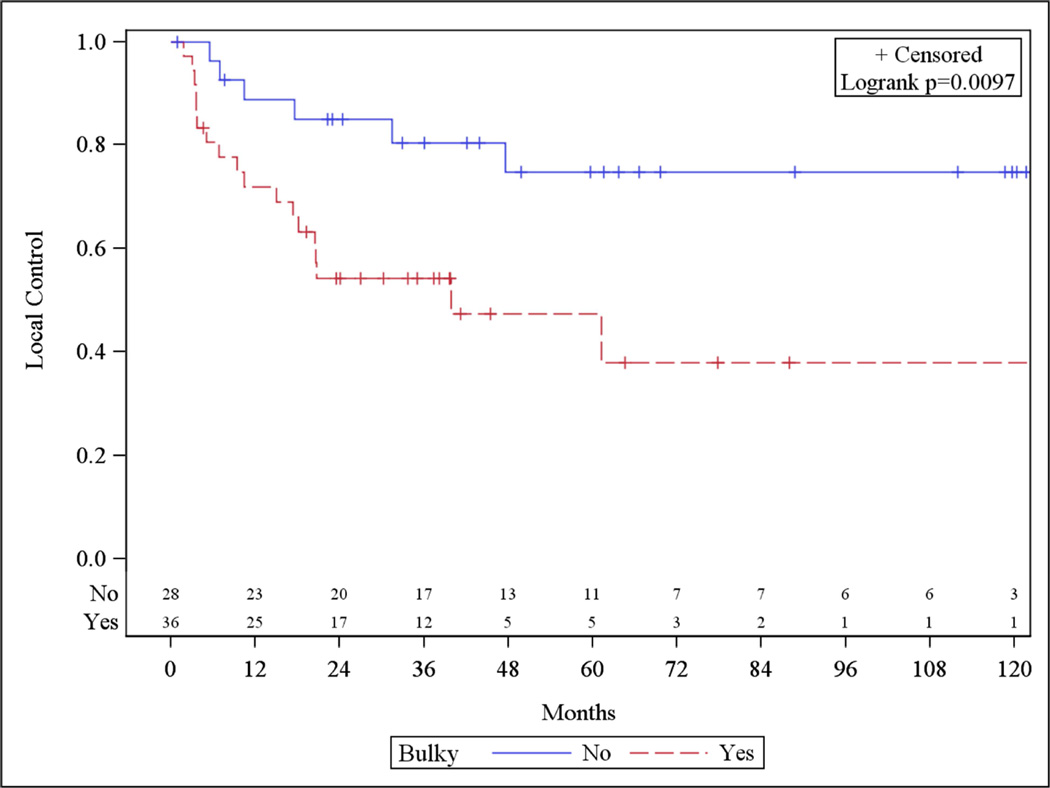
In the univariate analysis, only the presence of bulky disease was associated with LR. Variables including IPI and the presence of extranodal disease were not associated with increased risk of LR (Table 2). There was no association with SUVmax ≥13 (p=0.70) or SUVmax as a continuous variable with increased LR. Actuarial rates of local control at 5 years were 47.4% vs 74.7% (p=0.01) for bulky vs non-bulky disease, respectively (Figure 1). On multivariate analysis, the presence of bulky disease remained associated with increased risk of developing local recurrence (p < 0.01). In addition stage III disease was also associated with increased risk of local recurrence (p=0.03) (Table 3).
Table 2.
Univariate Association of Local Recurrence
| Comparison | HR (95% CI) | p Value | |
|---|---|---|---|
| Age | <60 vs ≥60 | 0.98 (0.52–1.84) | 0.94 |
| Gender | Male vs Female | 1.13 (0.60–2.11) | 0.71 |
| ECOG PS | 0 vs 1+ | 0.84 (0.45–1.58) | 0.59 |
| B Symptoms | Absent vs Present | 0.85 (0.43–1.68) | 0.64 |
| Number Extranodal Sites | 0–1 vs 2–5 | 0.86 (0.45–1.63) | 0.64 |
| Bone Marrow Involvement | No vs Yes | 1.23 (0.54–2.79) | 0.62 |
| Stage | III vs IV | 1.21 (0.65–2.28) | 0.54 |
| Bulky ≥5 cm | No vs Yes | 0.31 (0.12–0.79) | 0.01 |
| SUVmax ≥15 | No vs Yes | 0.67 (0.27–1.68) | 0.40 |
| LDH | Normal vs Elevated | 0.64 (0.26–1.54) | 0.32 |
| IPI | ≤2 vs >2 | 1.20 (0.63–2.27) | 0.58 |
| Chemotherapy | R-CHOP vs R+Other | 0.61 (0.19–2.00) | 0.42 |
| Chemotherapy Cycles | <6 vs ≥6 | 1.29 (0.54–3.10) | 0.57 |
Abbreviations: ECOG PS – Eastern Cooperative Oncology Group Performance Status; LDH – lactate dehydrogenase; SUV – standardized uptake value.
Figure 1.
Bulky Disease and Local Control
Table 3.
Multivariate Association of Local Recurrence
| Comparison | HR (95% CI) | p Value | |
|---|---|---|---|
| ECOG PS | 0 vs 1+ | 0.32 (0.09–1.15) | 0.08 |
| Stage | III vs IV | 4.45 (1.13–17.49( | 0.03 |
| Bulky ≥5 cm | No vs Yes | 0.13 (0.03–0.51) | <0.01 |
| SUVmax ≥15 | No vs Yes | 2.47 (0.72–8.53) | 0.15 |
| Chemotherapy Cycles | <6 vs ≥6 | 5.50 (0.60–50.24) | 0.13 |
Abbreviations: ECOG PS – Eastern Cooperative Oncology Group Performance Status; SUV – standardized uptake value.
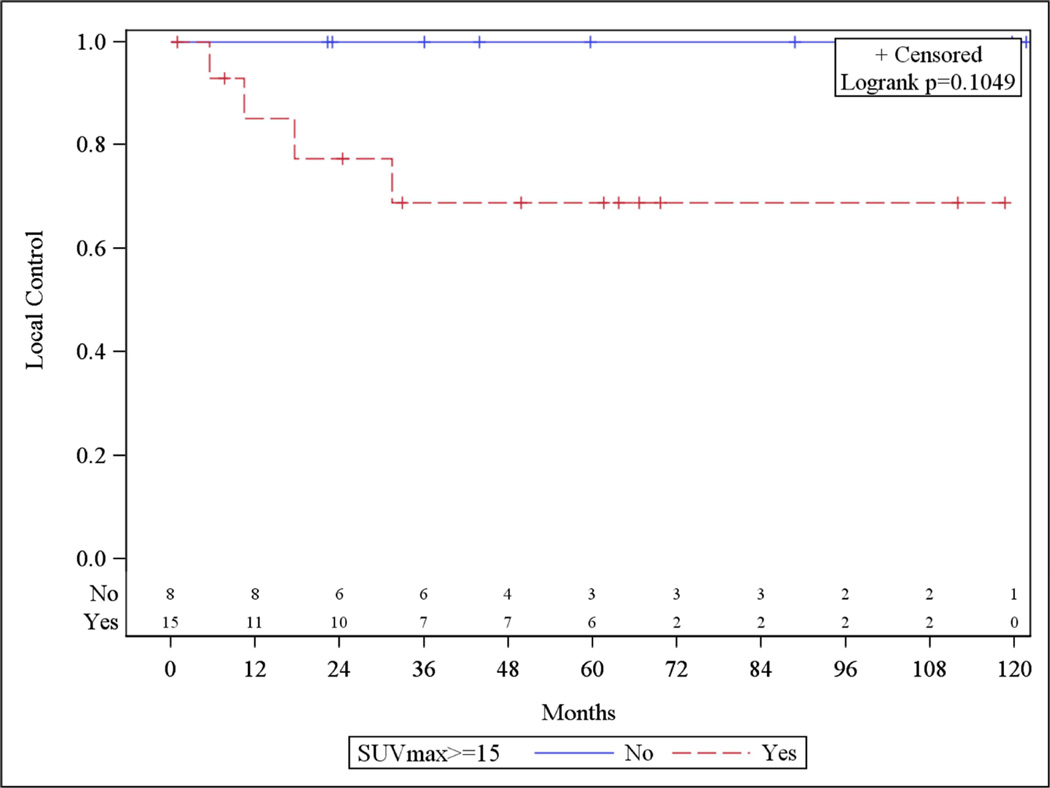
In an exploratory univariate analysis, the subgroup of patients with non-bulky disease (n=28) was evaluated separately. Although, no factor was identified to be significantly associated with LR, all events of LR occurred when SUVmax was ≥15 (Figure 2). This relationship did not exist in patients with bulky lesions.
Figure 2.
SUVmax in Non-bulky Disease and Local Control
23 patients with ≥5 cm or SUVmax ≥15 lesions had evaluable pre and post-treatment imaging. Of these patients 18 (78%) had recurrences within or directly adjacent to previously involved sites.
Discussion
The role of RT in the setting of advanced stage DLBCL following complete response to chemotherapy remains controversial. Previous series, including one published from our own institution show excellent rates of LC (90–100%) following RT in advanced stage DLBCL[10, 19]. It is unclear what benefit LC provides for outcomes in this disease, but multiple retrospective studies and a recent prospective trial support the usage of RT in this setting[9–12]. As seen in other cancer sites, improved control of microscopic disease distally due to improved systemic therapy such as rituximab may actually enhance the importance of control at initially involved sites. This is especially true as patients are less likely to die of systemic disease due to improved systemic therapy. Further clarity is needed in identifying the patients who would benefit the most from the addition of RT.
Our results also show that patients with stage III disease more frequently develop relapse at previously involved sites, despite rituximab based chemotherapy. This may reflect that patients with stage IV disease present with more extensive systemic disease burden thus may less likely to have relapse at only initially involved sites. All three patients who had an isolated DR in our study had presented initially with stage IV disease. Additionally, our findings indicate that lesions > 5 cm in size are associated with worse control of disease at initially involved sites with rituximab based chemotherapy alone. 26 of the 28 patients in our series had evaluable pretreatment imaging. Only 4 of these patients would have required two separate radiation treatment fields to encompass areas of bulky disease, the rest would have had their initial bulky disease encompassed in one field. Thus RT to these initially bulky sites is a feasible intervention since a majority of these tend to have one site of bulky involvement and very few appear to have multiple sites of bulk.
Bulky disease is generally accepted as an independent adverse prognostic factor in patients with DLBCL. Bulky lesions may impair drug delivery by lack of vascular flow leading to harboring of resistant clones after chemotherapy[20, 21]. While we have identified 5 cm as a cut point, the exact definition of bulky disease remains uncertain, especially, with tumor sizes ranging between 5–10 cm. There is some thought that rituximab may abrogate the importance of bulky disease as a factor. However, the majority of recently published evidence is consistent with our findings that it remains an important variable[12, 16].
Retrospective series of patients treated with CHOP chemotherapy from MD Anderson and Milan demonstrated benefits for addition RT for tumors ≥4 cm and ≥6 cm respectively[9, 22]. An exploratory analysis of the relationship between maximum tumor diameter and event free survival was performed on patients treated on the Mabthera International Trial Group Study[16]. This analysis demonstrated a stronger association between bulky disease and event free survival in the setting of CHOP chemotherapy rather than with R-CHOP. However, a significant difference in OS was noted with 6 cm as a cut point with patients receiving R-CHOP. A recent large series form MD Anderson did not show association between bulky disease ≥5 cm and PFS or OS[10]. However, a recently published non-randomized prospective trial showed improved PFS and OS in elderly patients with ≥7.5 cm lesions when analyzed per protocol treatment design[11, 12].
18F-fluorodeoxyglucose (FDG) PET metabolic imaging is a standard method for initial staging and assessment of treatment response in DLBCL. The value of initial SUVmax on disease outcomes has not been widely reported. SUVmax offers a quantitative representation of glucose utilization. This can be a proxy for proliferative rate in DLBCL in vivo23]. Our study is the first to identify SUVmax ≥15 as a potential predictor of LR in patients with non-bulky disease. There was no association between SUVmax and LR in patients with bulky disease. Prior studies have shown association with both SUVmax ≥ 15 and SUVmax ≥ 30 with PFS and OS respectively[24, 25]. While our conclusions regarding this are limited due to small numbers, the association specifically with LR is important because of the potential implication on RT field design. It is unclear from our study how this correlates with molecular proliferative indices or molecular subtypes of DLBCL. These data provide provocative findings that support future investigation in these areas.
Limitations in our study primarily arise from the retrospective nature and the limited numbers in examining subgroups. Additionally, not all patients underwent cytogenetic analysis regarding c-MYC, BCL2 or BCL6 rearrangement. Thus, we were not able to correlate their presence with difference in outcomes. Our study is unique in that it examines a uniform cohort of advanced stage patients, all, who received CR to standard immunochemotherapy, in order to identify factors leading to poor LC. This is a population that has not previously been examined with this question and impacts optimal patient selection for radiation oncologists. Additional risk factors need to be identified that reflect the heterogeneity of this disease to appropriately risk stratify patients needing RT. Well designed randomized trials are needed to provide further clarity to this disease. The ongoing Unfavorable Low-Risk Patients Treated with Densification of R-Chemo Regimens (UNFOLDER) trial (clinical trial registry: NCT00278408) featuring a 2x2 randomization comparing R-CHOP -21 and 14 as well as RT or no RT to ≥7.5 cm bulky disease will eventually help guide decisions on RT. The arms without RT were recently closed on interim analysis suggesting some benefit to RT[26]. Results from this study will further shed light on management of DLBCL patients.
Conclusions
About 50% of patients with advanced stage DLBCL will experience local relapse. Only 3.4% of patients in our study had isolated distant relapse with no component of LR. In advanced stage DLBCL, increased LR is associated with the presence of bulky ≥ 5cm disease and stage III disease. In patients with non-bulky disease, there appears to be an association with SUVmax ≥15 and LR. Identification of these risk factors may aid in the decisions regarding optimal selection of patients for RT after CR to R-CHOP based chemotherapy.
Acknowledgment
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none.
References
- 1.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. Chop chemotherapy plus rituximab compared with chop alone in elderly patients with diffuse large-b-cell lymphoma. The New England journal of medicine. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, Lopez-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M MabThera International Trial G. Chop-like chemotherapy plus rituximab versus chop-like chemotherapy alone in young patients with good-prognosis diffuse large-b-cell lymphoma: A randomised controlled trial by the mabthera international trial (mint) group. The Lancet Oncology. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 3.Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M, Sebban C, Belhadj K, Bordessoule D, Ferme C, Tilly H. Long-term outcome of patients in the lnh-98.5 trial, the first randomized study comparing rituximab-chop to standard chop chemotherapy in dlbcl patients: A study by the groupe d'etudes des lymphomes de l'adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, Bosly A, Ketterer N, Shpilberg O, Hagberg H, Ma D, Briere J, Moskowitz CH, Schmitz N. Salvage regimens with autologous transplantation for relapsed large b-cell lymphoma in the rituximab era. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller TP, Dahlberg S, Cassady JR, Adelstein DJ, Spier CM, Grogan TM, LeBlanc M, Carlin S, Chase E, Fisher RI. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-hodgkin's lymphoma. The New England journal of medicine. 1998;339:21–26. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 6.Horning SJ, Weller E, Kim K, Earle JD, O'Connell MJ, Habermann TM, Glick JH. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-hodgkin's lymphoma: Eastern cooperative oncology group study 1484. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:3032–3038. doi: 10.1200/JCO.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet C, Fillet G, Mounier N, Ganem G, Molina TJ, Thieblemont C, Ferme C, Quesnel B, Martin C, Gisselbrecht C, Tilly H, Reyes F Groupe d'Etude des Lymphomes de lA. Chop alone compared with chop plus radiotherapy for localized aggressive lymphoma in elderly patients: A study by 12 the groupe d'etude des lymphomes de l'adulte. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:787–792. doi: 10.1200/JCO.2006.07.0722. [DOI] [PubMed] [Google Scholar]
- 8.Reyes F, Lepage E, Ganem G, Molina TJ, Brice P, Coiffier B, Morel P, Ferme C, Bosly A, Lederlin P, Laurent G, Tilly H Groupe d'Etude des Lymphomes de lA. Acvbp versus chop plus radiotherapy for localized aggressive lymphoma. The New England journal of medicine. 2005;352:1197–1205. doi: 10.1056/NEJMoa042040. [DOI] [PubMed] [Google Scholar]
- 9.Ferreri AJ, Dell'Oro S, Reni M, Ceresoli GL, Cozzarini C, Ponzoni M, Villa E. Consolidation radiotherapy to bulky or semibulky lesions in the management of stage iii-iv diffuse large b cell lymphomas. Oncology. 2000;58:219–226. doi: 10.1159/000012104. [DOI] [PubMed] [Google Scholar]
- 10.Phan J, Mazloom A, Medeiros LJ, Zreik TG, Wogan C, Shihadeh F, Rodriguez MA, Fayad L, Fowler N, Reed V, Horace P, Dabaja BS. Benefit of consolidative radiation therapy in patients with diffuse large b-cell lymphoma treated with r-chop chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4170–4176. doi: 10.1200/JCO.2009.27.3441. [DOI] [PubMed] [Google Scholar]
- 11.Dorth JA, Prosnitz LR, Broadwater G, Diehl LF, Beaven AW, Coleman RE, Kelsey CR. Impact of consolidation radiation therapy in stage iii-iv diffuse large b-cell lymphoma with negative postchemotherapy radiologic imaging. International journal of radiation oncology, biology, physics. 2012;84:762–767. doi: 10.1016/j.ijrobp.2011.12.067. [DOI] [PubMed] [Google Scholar]
- 12.Held G, Murawski N, Ziepert M, Fleckenstein J, Poschel V, Zwick C, Bittenbring J, Hanel M, Wilhelm S, Schubert J, Schmitz N, Loffler M, Rube C, Pfreundschuh M. Role of radiotherapy to bulky disease in elderly patients with aggressive b-cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:1112–1118. doi: 10.1200/JCO.2013.51.4505. [DOI] [PubMed] [Google Scholar]
- 13.Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V International Harmonization Project on L. Revised response criteria for malignant lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 14.Carbone PP, Kaplan HS, Musshoff K, Smithers DW, Tubiana M. Report of the committee on hodgkin's disease staging classification. Cancer Res. 1971;31:1860–1861. [PubMed] [Google Scholar]
- 15.A predictive model for aggressive non-hodgkin's lymphoma. The international non-hodgkin's lymphoma prognostic factors project. The New England journal of medicine. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 16.Pfreundschuh M, Ho AD, Cavallin-Stahl E, Wolf M, Pettengell R, Vasova I, Belch A, Walewski J, Zinzani PL, Mingrone W, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Corrado C, Scheliga A, Loeffler M, Kuhnt E MabThera International Trial G. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-b-cell lymphoma treated with chop-like chemotherapy with or without rituximab: An exploratory analysis of the mabthera international trial group (mint) study. The Lancet Oncology. 2008;9:435–444. doi: 10.1016/S1470-2045(08)70078-0. [DOI] [PubMed] [Google Scholar]
- 17.Schoder H, Noy A, Gonen M, Weng L, Green D, Erdi YE, Larson SM, Yeung HW. Intensity of 18fluorodeoxyglucose uptake in positron emission tomography distinguishes between indolent and aggressive non-hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:4643–4651. doi: 10.1200/JCO.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 18.Noy A, Schoder H, Gonen M, Weissler M, Ertelt K, Cohler C, Portlock C, Hamlin P, Yeung HW. The majority of transformed lymphomas have high standardized uptake values (suvs) on positron emission tomography (pet) scanning similar to diffuse large b-cell lymphoma (dlbcl) Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2009;20:508–512. doi: 10.1093/annonc/mdn657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Z, Das S, Okwan-Duodu D, Esiashvili N, Flowers C, Chen Z, Wang X, Jiang K, Nastoupil LJ, Khan MK. Patterns of failure in advanced stage diffuse large b-cell lymphoma patients after complete response to r-chop immunochemotherapy and the emerging role of consolidative radiation therapy. International journal of radiation oncology, biology, physics. 2013;86:569–577. doi: 10.1016/j.ijrobp.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Aviles A, Delgado S, Nambo MJ, Alatriste S, Diaz-Maqueo JC. Adjuvant radiotherapy to sites of previous bulky disease in patients stage iv diffuse large cell lymphoma. International journal of radiation oncology, biology, physics. 1994;30:799–803. doi: 10.1016/0360-3016(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 21.Song MK, Chung JS, Sung-Yong O, Lee GW, Kim SG, Seol YM, Shin HJ, Choi YJ, Cho GJ, Shin DH, Yun EY. Clinical impact of bulky mass in the patient with primary extranodal diffuse large b cell lymphoma treated with r-chop therapy. Annals of hematology. 2010;89:985–991. doi: 10.1007/s00277-010-0964-7. [DOI] [PubMed] [Google Scholar]
- 22.Schlembach PJ, Wilder RB, Tucker SL, Ha CS, Rodriguez MA, Hess MA, Cabanillas FF, Cox JD. Impact of involved field radiotherapy after chop-based chemotherapy on stage iii-iv, intermediate grade and large-cell immunoblastic lymphomas. International journal of radiation oncology, biology, physics. 2000;48:1107–1110. doi: 10.1016/s0360-3016(00)00760-4. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Pertovaara H, Korkola P, Vornanen M, Eskola H, Kellokumpu-Lehtinen PL. Glucose metabolism correlated with cellular proliferation in diffuse large b-cell lymphoma. Leukemia & lymphoma. 2012;53:400–405. doi: 10.3109/10428194.2011.622420. [DOI] [PubMed] [Google Scholar]
- 24.Chihara D, Oki Y, Onoda H, Taji H, Yamamoto K, Tamaki T, Morishima Y. High maximum standard uptake value (suvmax) on pet scan is associated with shorter survival in patients with diffuse large b cell lymphoma. International journal of hematology. 2011;93:502–508. doi: 10.1007/s12185-011-0822-y. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki Y, Nawa Y, Miyagawa M, Kohashi S, Nakase K, Yasukawa M, Hara M. Maximum standard uptake value of 18f-fluorodeoxyglucose positron emission tomography is a prognostic factor for progression-free survival of newly diagnosed patients with diffuse large b cell lymphoma. Annals of hematology. 2013;92:239–244. doi: 10.1007/s00277-012-1602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi Z, Esiashvili N, Flowers C, Das S, Khan MK. Renewed interest in the role of consolidative radiotherapy in advanced stage diffuse large b-cell lymphoma. Leukemia & lymphoma. 2013;54:2122–2130. doi: 10.3109/10428194.2013.779687. [DOI] [PubMed] [Google Scholar]