Significance
Positive-strand RNA viruses are major pathogens of plants, animals, and humans. These viruses subvert intracellular membranes for virus replication, and lipids are critical due to interaction with viral and coopted host proteins. To dissect the roles of various lipids in Tomato bushy stunt virus (TBSV) replication, we have developed artificial vesicle-based replication assay. Vesicles consisting of a major phospholipid, namely phosphatidylethanolamine (PE), can support TBSV replication by assembling viral replicase complexes and performing a complete replication cycle. Monitoring PE distribution reveals that PE is enriched at the sites of TBSV replication in plant and yeast cells. Increasing PE level in cells leads to enhanced replication of TBSV and other viruses, suggesting that abundant PE in subcellular membranes has proviral function.
Keywords: plant virus, virus–host interaction, phospholipid, viral replicase complex, host factor
Abstract
Intracellular membranes are critical for replication of positive-strand RNA viruses. To dissect the roles of various lipids, we have developed an artificial phosphatidylethanolamine (PE) vesicle-based Tomato bushy stunt virus (TBSV) replication assay. We demonstrate that the in vitro assembled viral replicase complexes (VRCs) in artificial PE vesicles can support a complete cycle of replication and asymmetrical RNA synthesis, which is a hallmark of (+)-strand RNA viruses. Vesicles containing ∼85% PE and ∼15% additional phospholipids are the most efficient, suggesting that TBSV replicates within membrane microdomains enriched for PE. Accordingly, lipidomics analyses show increased PE levels in yeast surrogate host and plant leaves replicating TBSV. In addition, efficient redistribution of PE leads to enrichment of PE at viral replication sites. Expression of the tombusvirus p33 replication protein in the absence of other viral compounds is sufficient to promote intracellular redistribution of PE. Increased PE level due to deletion of PE methyltransferase in yeast enhances replication of TBSV and other viruses, suggesting that abundant PE in subcellular membranes has a proviral function. In summary, various (+)RNA viruses might subvert PE to build membrane-bound VRCs for robust replication in PE-enriched membrane microdomains.
Many steps in the infection cycles of positive-strand RNA viruses, including entry into the cell, replication, virion assembly, and egress, are associated with subcellular membranes (1–4). Therefore, viruses have to interact with different lipids, such as phospholipids and sterols, which affect the biophysical features of membranes, including the fluidity and curvature (5, 6). The subverted cellular membranes could protect the viral RNA from recognition by the host nucleic acid sensors or from destruction by the cellular innate immune system. In addition, membranes facilitate the sequestration of viral and coopted host proteins to increase their local concentrations and promote macromolecular assembly, including formation of the viral replicase complex (VRC) or virion assembly. To optimize viral processes, RNA viruses frequently manipulate lipid composition of various intracellular membranes (6–13). Overall, the interaction between cellular lipids and viral components is emerging as one of the possible targets for antiviral methods against a great number of viruses. Understanding the roles of various lipids in RNA virus infections is important to ultimately control harmful RNA viruses.
Among the various lipids, the highly abundant phospholipids are especially targeted by RNA viruses (2). In general, phospholipids likely affect the replication of most RNA viruses, which takes place within membranous structures (1, 3, 4). Accordingly, lipidomics analyses of cells infected with Dengue virus and hepatitis C virus (HCV) (8, 9) revealed enhanced virus-induced lipid biosynthesis, resulting in changes in the global lipid profile of host cells. Also, the less abundant regulatory phosphatidylinositol-4-phosphate (PI4P) was shown to be enriched at sites of enterovirus and HCV replication due to recruitment of cellular lipid kinases (7, 14), suggesting that a microenvironment enriched for PI4P facilitates (+)RNA virus replication. However, our knowledge on the roles of various phospholipids in RNA virus replication is currently incomplete. By using tombusviruses, small model RNA viruses of plants that can replicate in a yeast surrogate host (15), which has the advantage of tolerating large changes in different phospholipid composition, a major role for global phospholipid and sterol biosynthesis, have been revealed (16–18). In this paper, a viral replicase reconstitution assay based on artificial phospholipid vesicles identified the essential role of phosphatidylethanolamine (PE) in replication of Tomato bushy stunt virus (TBSV). It has also been shown that TBSV could recruit and enrich PE to the sites of viral replication in yeast and plant cells. Moreover, genetic changes that either increase or decrease PE levels in yeast greatly stimulated or inhibited TBSV replication, confirming the key role of PE in the formation of TBSV replicase.
Results
Efficient Replication of TBSV RNA in Artificial PE Vesicles.
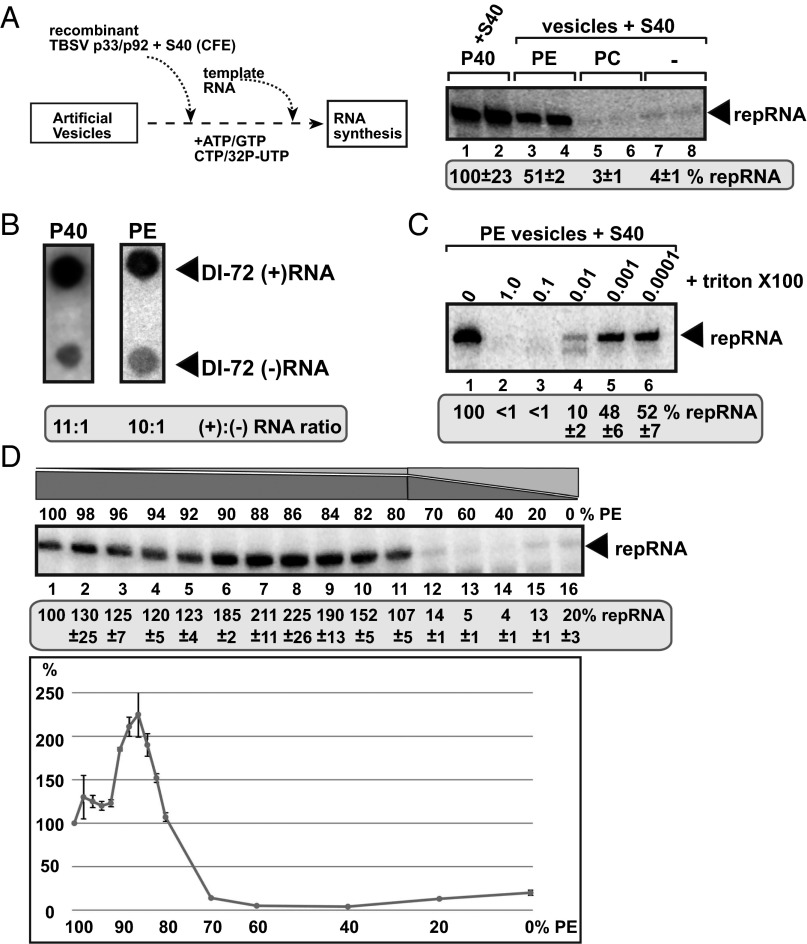
To test what type of phospholipids are required for tombusvirus replication, we developed an artificial vesicle (liposome)-based replication assay involving purified recombinant tombusvirus p33 and p92pol replication proteins, TBSV (+) replicon RNA (repRNA), and cellular cytosolic proteins present in yeast cell-free extract (CFE; Fig. 1A). Interestingly, artificial vesicles prepared from PE supported TBSV repRNA replication, reaching about half of the level that takes place in the standard total membrane fraction of CFE obtained from WT yeast (Fig. 1A, lanes 3 and 4 versus 1 and 2) (19). On the contrary, vesicles consisting of only phosphatidylcholine (PC; Fig. 1A, lanes 5 and 6) or lysophosphatidylethanolamine (lysoPE) showed 5% viral RNA replication activity compared with PE vesicles (Fig. S1A, lanes 3 and 4 and 13 and 14), whereas phosphatidylglycerol (PG), phosphatidylserine (PS), phosphatidylinositol (PI), cardiolipin (CA), and lysophosphatidylcholine (lysoPC) vesicles did not support TBSV RNA replication at detectable levels (Fig. S1A). These data support a model that PE is the only phospholipid required for TBSV replication in vitro, whereas the other phospholipids are not sufficient by themselves to support robust TBSV replication.
Fig. 1.
In vitro reconstitution of the TBSV replicase in artificial PE vesicles. (A) Scheme of the replicase assembly assay. Purified recombinant p33 and p92pol replication proteins of TBSV in combination with the TBSV-derived (+)repRNA were added to PE or PC vesicles or the P40 membrane fraction of yeast CFE. The S40 fraction of CFE was also added to each sample to provide soluble host factors required for TBSV VRC assembly. The denaturing PAGE analysis of the 32P-labeled repRNA products obtained is shown. The full-length repRNA is denoted by an arrow. The CFE-based replication assay was chosen as 100% (lanes 1 and 2). (B) Asymmetrical RNA synthesis by TBSV VRCs assembled in PE vesicles. The amounts of TBSV (+)- and (–)-stranded RNA products produced by the reconstituted TBSV VRCs are measured by using the 32P-labeled repRNA probes generated in the in vitro assays. The blot contains the same amount of cold (+)- and (–)-stranded DI-72 RNA. (C) TBSV RNA synthesis by the reconstituted VRCs in PE vesicles requires vesicles/membrane bilayers. The PE vesicles were disrupted by Triton-X100 treatment as shown. The denaturing PAGE analysis of the replicase products is as in panel A. (D) Increased VRC activity in PE vesicles containing a fraction of other phospholipids. The PE vesicles contained the shown percentage of PE plus a mixture of other phospholipids (the ratio in the phospholipid mix was 54.5 of PC, 6.1 of PI, 1.2 of PS, 8.3 of PG, 0.9 of LysoPE, and 0.6 of LysoPC), which was based on their ratios in TBSV-infected N. benthamiana leaves. The denaturing PAGE analysis of the replicase products is as in panel A.
To examine the nature of TBSV replication in PE vesicles, we measured viral (–)- versus (+)-strand RNA synthesis. These experiments revealed that TBSV replication led to the production of ∼10-fold more (+)- than (–)-stranded RNAs, similar to the ratio seen in yeast CFE preparation containing yeast membranes (Fig. 1B) (19). Thus, the in vitro assembled TBSV replicase in artificial PE vesicles can support a complete cycle of replication and asymmetrical RNA synthesis, which is a hallmark of (+)-strand RNA viruses. We also tested if the PE vesicles are required for RNA synthesis by adding various concentrations of Triton X-100, which could disrupt lipid bilayers in the vesicles. Viral RNA synthesis was inhibited up to 90% in the presence of 0.01%, whereas it was completely blocked by the presence of 0.1 or 1.0% Triton X-100 (Fig. 1C), suggesting that the membranous environment is needed for TBSV replication in vitro. To test if TBSV replicase could form a nuclease-resistant compartment, as is the case in cells and in the yeast CFE (19, 20), we performed the in vitro assays in the presence of micrococcal nuclease, which can destroy the unprotected viral RNAs. These studies revealed that TBSV replicase formed in the presence of PE vesicles was much less protective of the viral RNA against the nuclease than the replicase assembled in yeast CFE (Fig. S1B). This finding suggests that not only PE but also additional phospholipids, other types of lipids, or membrane proteins in the yeast CFE might contribute to the assembly of the authentic TBSV replicase.
To identify the optimal PE concentration in the lipid bilayer for supporting the most efficient TBSV replication, we made artificial vesicles containing PE and increasing amounts of a mixture of other phospholipids (including PC, PI, PS, PG, lysoPE, and lysoPC) (Fig. 1D). The highest level of TBSV replication was observed with the vesicles containing 82–90% PE (Fig. 1D). On the other hand, vesicles containing less than 70% PE supported inefficient TBSV replication in vitro. To test the effects of various phospholipids on TBSV replication, we also tested PE and other phospholipids in pair-wise combinations. These in vitro assays revealed that the presence of only 10% of PC or lysoPE in artificial PE vesicles enhanced TBSV replication by more than 50%, whereas these phospholipids were inhibitory when applied in higher than 20% concentrations (Fig. S2 A and B). In contrast, the presence of other phospholipids (PS, PI, CA, and lysoPC) was inhibitory to TBSV replication, except for 10% of PG (Fig. S2B). Thus, various phospholipids (other than PE) have inhibitory effects on TBSV replication when present in higher than 20% amounts. These results indicate that TBSV replication is greatly affected by different kinds of phospholipids.
To test whether phospholipids affect the membrane association of the viral replication proteins, we performed membrane flotation experiments with artificial vesicles and 35S-labeled p33 replication protein. As expected, in the absence of membranes/vesicles, p33 stays at the bottom of the sucrose gradient (Fig. S3B), whereas ∼30% of p33 are present in the top fraction in the presence of either PE or PC vesicles (Fig. S3 C and D). In addition, p33 can strongly associate with PG, PS, and CA vesicles, whereas binding to PI vesicles is poor (Fig. S3). These data suggest that the viral p33 replication protein can bind to different phospholipids. Thus, the inhibitory effects of high PC, PG, PS, and CA concentrations on TBSV replication are not due to the interference of these phospholipids with membrane association of p33 to block TBSV replication in vitro.
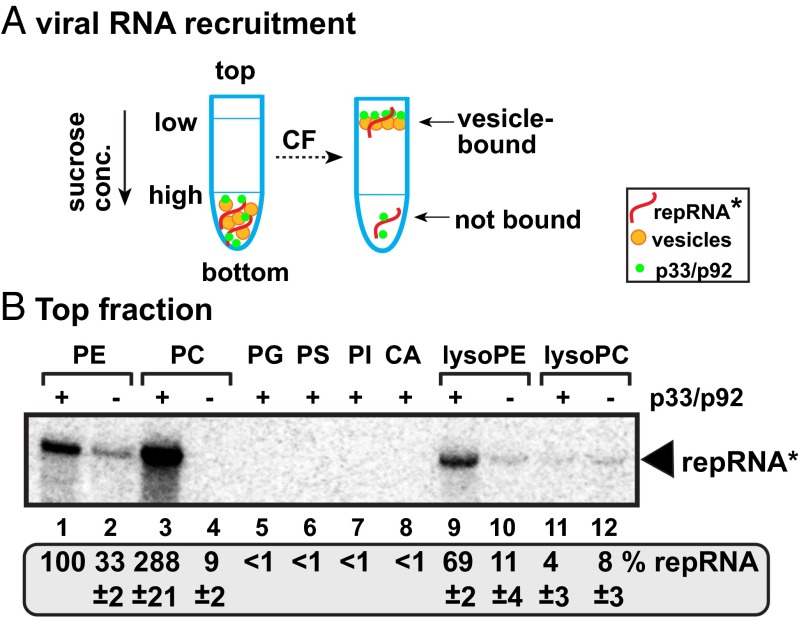
Similar studies with the viral (+)repRNA (Fig. 2A), which has to be recruited with the help of the viral replication proteins to the sites of replication in the membranes (19, 21, 22), revealed that the (+)repRNA bound to PE, PC, and lysoPE vesicles with the highest efficiency when p33/p92pol replication proteins were present (Fig. 2B). The association of the viral (+)repRNA to artificial PG, PS, PI, CA, and lysoPC vesicles was low (Fig. 2B). Therefore, we suggest that binding of p33/p92pol replication proteins to PE, PC, and lysoPE phospholipids facilitates the recruitment of the viral (+)repRNA to membranes, a required step for VRC assembly and RNA replication.
Fig. 2.
TBSV p33/p92-mediated binding of TBSV RNA to artificial vesicles containing different phospholipids. (A) Scheme of the in vitro binding assay and membrane-flotation experiments. The 32P-labeled TBSV (+)repRNA (DI-72) was incubated with artificial vesicles in the presence of purified recombinant TBSV p33 and p92 (plus the S40 fraction of yeast CFE to provide soluble cellular factors, such as heat shock protein 70), followed by centrifugation in 10–70% sucrose density gradient. The top fraction of the sucrose gradient was tested for the presence of 32P-labeled TBSV (+)repRNA. (B) Denaturing RNA gel analysis of the presence of 32P-labeled TBSV (+)repRNA in the top fraction. The amount of 32P-labeled TBSV (+)repRNA with the PE vesicles was chosen as 100%.
PE Is Enriched at the Sites of TBSV Replication.
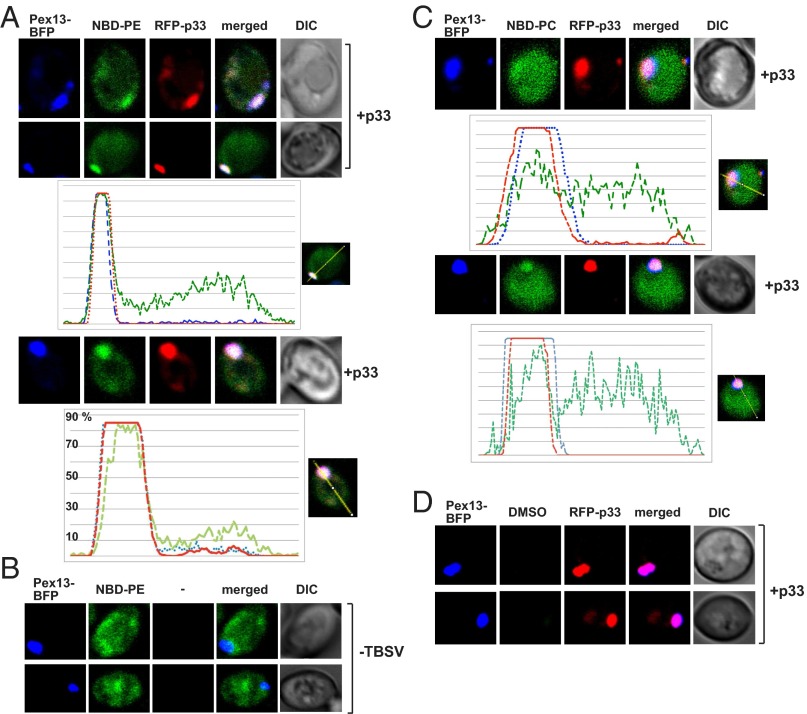
Because TBSV requires membranes with high PE content to assemble the functional VRCs in vitro, we wondered if PE, which is among the most abundant phospholipids in yeast, is enriched at the sites of replication. We used biotinylated duromycin, which specifically binds to PE (23), to monitor the distribution of PE during TBSV replication. Interestingly, PE was highly enriched in the subcellular locations containing GFP-p33 in yeast cells replicating the TBSV repRNA (Fig. 3A). Expression of the p33 replication protein in the absence of other viral compounds was sufficient to promote intracellular redistribution of PE (Fig. 3A). Of all of the p33-positive cells examined, >90% showed redistribution of PE. These PE-enriched sites were colocalized with both the Pex13p-GFP peroxisomal marker and red fluorescent protein (RFP)-p33 (Fig. 3B), indicating that PE is redistributed to the sites of TBSV replication in the peroxisomal membrane. On the contrary, the peroxisomal membrane was not enriched with PE in the absence of TBSV replication proteins and PE was dispersed in many parts of the yeast cell (Fig. 3B, Bottom). We confirmed the above findings using PE molecules with fluorescently labeled fatty-acid chain (named NBD-PE) added to the culture media. Accordingly, NBD-PE was enriched in subcellular locations also containing RFP-p33 and Pex13p-BFP (blue fluorescent protein) (Fig. 4A). On the contrary, NBD-PE was not enriched in the peroxisomal membranes in the absence of TBSV replication proteins (Fig. 4B). Unlike NBD-PE, NBD-PC was not highly enriched at the sites of TBSV replication (Fig. 4C). Based on these data, we propose that PE molecules are efficiently relocalized to and enriched at the sites of viral replication in the peroxisomal membranes with the help of a p33 replication protein.
Fig. 3.
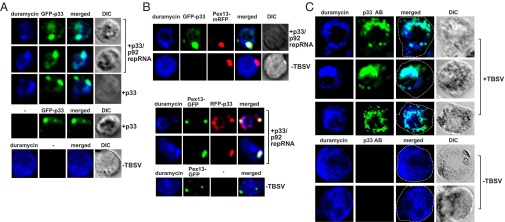
Enrichment of PE at TBSV replication sites in yeast and plant cells. (A) Confocal laser microscopy images show the enrichment of PE and its colocalization with the GFP-tagged TBSV p33 expressed from the GAL1 promoter during TBSV replication (top two images) or when only GFP-p33 was expressed. Differential interference contrast (DIC) images are shown on the right. Localization of PE is detected by using biotinylated duramycin peptide and streptavidin conjugated with Alexa Fluor 405. The bottom image shows the more even cellular distribution of PE in the absence of viral components. Note that the GFP-p33 is functional and fully supports TBSV replication in yeast. (B) Peroxisomal enrichment of PE in the presence of TBSV replication proteins. Peroxisomal membranes are visualized with the help of mRFP-tagged (top images) or GFP-tagged (middle images) yeast Pex13 protein. The bottom image shows the lack of PE enrichment in peroxisomes in the absence of viral components. See further details in A. (C) Enrichment and colocalization of PE with p33/p92 replication proteins in N. benthamiana protoplasts replicating TBSV genomic RNA. The TBSV p33/p92 replication proteins were detected with p33-specific primary antibody and secondary antibody conjugated with Alexa Fluor 488. The images at the bottom show the more even distribution of PE in the absence of viral components. Note that the infectious WT TBSV genomic RNA produced the natural (untagged) p33 and p92 in these experiments. See further details in A.
Fig. 4.
Redistribution and enrichment of exogenous PE in peroxisomes containing the p33 replication protein in yeast cells. (A and B) NBD-PE was added to yeast cultures expressing the TBSV RFP-p33 replication protein or lacking p33. The peroxisomes were visualized by expressing Pex13p-BFP. ImageJ software was used to show the enrichment of PE (green line) in peroxisomes (blue line) containing TBSV p33 (red line). (C) NBD-PC was added to yeast cultures expressing the TBSV RFP-p33 replication protein. See panel A for details. (D) Control panel to show colocalization of Pex13p and p33 in the absence of exogenous PE. DMSO solvent was added to yeast cultures.
To test if similar phenomena also occur in plant cells during TBSV replication, we stained TBSV-infected Nicotiana benthamiana protoplasts (single cells lacking cell walls) with biotinylated duromycin and also treated them with an anti-p33 antibody. Importantly, confocal imaging showed high enrichment of PE in subcellular locations containing the p33/p92 replication proteins (Fig. 3C). On the contrary, the subcellular distribution of PE was dramatically different in uninfected plant cells (Fig. 3C). Based on all these data, the emerging picture is that PE, unlike PC, is efficiently redistributed to and enriched in the peroxisomal membranes in yeast and plant cells to facilitate robust TBSV replication.
Increased PE Level in Yeast and Plant Cells Supporting TBSV Replication.
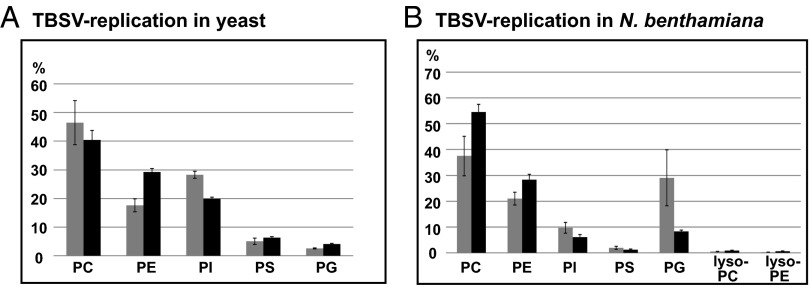
To test if TBSV replication alters phospholipid metabolism in the host cells to facilitate viral replication, we performed lipidomics of yeast cells replicating TBSV repRNA or lacking all TBSV components. These experiments revealed that the relative level of PE increased from 17.6% to 29.3% of total phospholipids in yeast replicating TBSV (Fig. 5A). On the contrary, PC and PI levels, which are two of the most abundant phospholipids, are decreased by ∼6% and 8%, respectively, when yeast supported TBSV replication (Fig. 5A). These data suggest that TBSV replication leads to an increased PE level in yeast. The overall phospholipid content of yeast cells increased by 38%, suggesting that yeast cells are induced by TBSV to produce new phospholipids (Fig. S4A). Overall, the total PE content of yeast cells replicating TBSV increased by ∼2.3-fold. This increased level of PE in yeast cells likely serves the virus’s need to build new membrane-bound replicase complexes. Lipidomics analysis revealed no significant changes in the fatty acid length or saturation status of PE in yeast replicating TBSV versus the control yeast (Fig. S4B).
Fig. 5.
Increased level of PE in yeast and plant cells replicating TBSV. Relative levels of phospholipids in yeast (24 h time point, in A) and plants (from systemic leaves showing symptoms, 6 d after infection, in B) replicating TBSV RNA (black columns) or the TBSV-free control (gray columns) were determined using mass spectrometry analysis.
The relative PE level was also increased in TBSV-infected plant leaves from 21.2% to 28.8% (Fig. 5B). In contrast to yeast, the PC level also increased in plants from 37.8% to 55.4%. The total phospholipid content was also increased in TBSV-infected plant leaves by 20.6%, suggesting active phospholipid synthesis occurring in infected cells. Overall, the lipidomics data support the increased synthesis of PE in TBSV-infected plant cells, similar to its yeast counterpart.
Increased PE Level in cho2Δ Yeast Promotes TBSV Replication.
To examine if PE level can directly affect TBSV replication in cells, we deleted CHO2, which codes for PE methyltransferase (PEMT), in yeast. Cho2p catalyzes the first step in the conversion of PE to PC, and in its absence, PE level is increased up to 40–45%, whereas PC level is reduced down to 15–20% (24, 25). We find that TBSV replication is increased by ∼10-fold in cho2Δ yeast in comparison with WT yeast (Fig. 6A, lanes 5–8 versus 1–4). In addition, the amounts of p33 and p92pol replication proteins were also increased (Fig. 6A). Lipidomics analysis of cho2Δ yeast supporting TBSV replication showed that PE becomes the most abundant phospholipid by reaching up to the ∼42% level (from ∼18% in WT yeast lacking TBSV) and a 2.5-fold higher amount than in WT BY4741 yeast replicating TBSV (Fig. 6B).
Fig. 6.
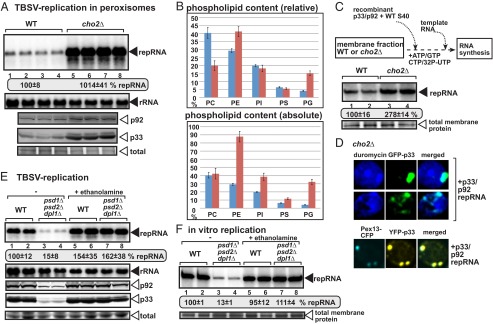
Deletion of the CHO2 PEMT gene enhances TBSV repRNA accumulation in yeast. (A, Top) Replication of the TBSV repRNA in WT and cho2Δ yeast was measured by Northern blotting 24 h after initiation of TBSV replication. Yeast coexpressed the TBSV p33 and p92 replication proteins with the DI-72 (+)repRNA. The accumulation level of repRNA was normalized based on the ribosomal RNA (rRNA). Each sample is obtained from different yeast colonies. (A, Middle and Bottom) The accumulation levels of His6-p92 and His6-p33 were tested by Western blotting. Note that in the absence of Cho2p, which catalyzes the first step in the conversion of PE to PC, the PE level is increased. Each experiment was repeated at least three times. (B) Relative and absolute levels of phospholipids in cho2Δ (red columns) versus WT yeasts (blue columns, measured at 24 h time point) replicating TBSV RNA were determined using mass spectrometry analysis. (C) Enhanced TBSV repRNA replication in CFE prepared from cho2Δ yeast. Shown is the scheme of the CFE-based TBSV replication assay. Purified recombinant TBSV p33 and p92pol replication proteins, DI-72 (+)repRNA in combination with the soluble fraction (S40 fraction from WT yeast), were added to the membranous fraction (P40) of cho2Δ or WT CFEs. Denaturing PAGE analysis of the 32P-labeled repRNA products obtained is shown. The full-length single-stranded repRNA is denoted by an arrow. (D) Confocal laser microscopy images show the enrichment of PE at peroxisomal sites of TBSV p33 accumulation in cho2Δ yeast. See further details in Fig. 3. (E) Depletion of PE in yeast inhibits TBSV replication. PE production was inhibited in a yeast strain (psd1Δ/psd2Δ/dpl1Δ) due to the exclusion of ethanolamine in the culture media (“– ethanolamine” samples). Restoring PE production in yeast (psd1Δ/psd2Δ/dpl1Δ) via the Kennedy pathway due to inclusion of ethanolamine in the culture media led to full recovery of TBSV replication. See further details in panel A. (F) Poor TBSV repRNA replication in CFE prepared from yeast with a depleted PE level. Purified recombinant TBSV p33 and p92pol replication proteins, DI-72 (+)repRNA in combination with the soluble fraction (S40 fraction from WT yeast), were added to the membranous fraction (P40) from a triple mutant (psd1Δ/psd2Δ/dpl1Δ) or WT yeasts. Note that omission of ethanolamine in the culture media (– ethanolamine samples) leads to PE depletion in yeast (psd1Δ/psd2Δ/dpl1Δ), whereas inclusion of ethanolamine in the culture media (“+ ethanolamine” samples) leads to PE production. Denaturing PAGE analysis of the 32P-labeled repRNA products obtained is shown. See further details in panel C.
We also performed in vitro TBSV replicase assembly assay in isolated membrane fractions from CFEs obtained from WT or cho2Δ yeasts (19, 26). The in vitro assembled viral replicase on membranes derived from cho2Δ yeast showed ∼threefold higher activity than comparable viral replicase from WT yeast (Fig. 6C). Because we used the same amounts of the purified recombinant viral proteins and the soluble fraction from WT yeast in the in vitro TBSV replicase assembly assay, the increased TBSV replicase activity in the membrane fraction of cho2Δ CFE suggests that the high accumulation level of TBSV repRNA is due to enhanced replicase activity in cho2Δ yeast (Fig. 6A). The CFE-based assay with comparable amounts of purified TBSV replication proteins suggests that TBSV could build more VRCs when PE is abundant and it excludes that the increased PE level in cho2Δ yeast enhances TBSV replication due solely to the presence of higher amounts of replication proteins (Fig. 6A). Altogether, these findings strongly support the stimulatory function of high PE level on tombusvirus replicase assembly.
Confocal microscopy analysis of cho2Δ yeast showed robust redistribution of PE to the sites of TBSV replication containing the viral replication proteins and peroxisome membranes (Fig. 6D). Thus, similar to the WT yeast, PE becomes highly enriched at the viral replication sites in cho2Δ yeast. To test if the high accumulation of PE in the peroxisome membranes is critical for TBSV replication, we deleted the PEX3 peroxisome biogenesis gene in cho2Δ yeast. In the absence of PEX3, there is no peroxisome or peroxisome membrane remnants in yeast cells (27, 28), and TBSV switches to the ER membranes to perform its replication (29). ER membranes can support as robust a TBSV replication as the peroxisomes in yeast (29–31). We find that TBSV replication is increased by ∼13-fold in cho2Δpex3Δ yeast (Fig. S5), suggesting that TBSV can take advantage of increased PE level in the ER membrane in the absence of peroxisomes. We observed enrichment of PE (Fig. S5B) or NBD-PE (Fig. S5C) at sites containing p33 in the ER membranes. These data highlight the emerging scheme that PE enrichment at the site of replication is critical regardless of peroxisomal or ER localization of the tombusvirus replicase.
Depletion of PE in Yeast Blocks TBSV Replication.
To study if depletion of PE has a negative effect on TBSV replication, we generated a yeast strain with deletion of three genes involved in PE production—PSD1, PSD2, and DPL1 (32). Briefly, the de novo synthesis of PE from PS was eliminated via deletion of the two phosphatidylserine decarboxylase genes, psd1Δ and psd2Δ, whereas the Kennedy pathway was inhibited through deletion of dihydrosphingosine-1-phosphate, dpl1Δ, and exclusion of ethanolamine from the culture media (33). We found that TBSV repRNA accumulation was inhibited by ∼7.5-fold in yeast (psd1Δ/psd2Δ/dpl1Δ) with depleted PE content (Fig. 6E, lanes 3 and 4 versus 1 and 2). Restoring PE production in yeast (psd1Δ/psd2Δ/dpl1Δ) via the Kennedy pathway due to inclusion of ethanolamine in the culture media led to full recovery of TBSV replication (Fig. 6E, lanes 7 and 8). Thus, PE seems to be required for TBSV replication in yeast, whereas the PSD1, PSD2, and DPL1 genes are not directly involved in the replication process.
To further test the role of PE in TBSV replication, we prepared the membrane fraction from yeast with depleted PE content, followed by reconstituting the functional TBSV replicase using purified recombinant p33 and p92 replication proteins, the viral (+)repRNA, and the soluble fraction from CFE of WT yeast. Interestingly, the CFE membrane fraction with depleted PE supported ∼eightfold less TBSV replication than the CFE membrane fraction from WT yeast (Fig. 6F, lanes 3 and 4 versus 1 and 2). Because only the membrane fractions were different in these CFE-based experiments, it is likely that the depleted PE level in psd1Δ/psd2Δ/dpl1Δ yeast membrane is responsible for the poor TBSV replicase assembly/activity in vitro. As discussed above, the CFE-based assay with comparable amounts of purified TBSV replication proteins suggests that TBSV could build less VRCs when PE is depleted in subcellular membranes. The CFE-based assay also excludes that the low PE level in psd1Δ/psd2Δ/dpl1Δ yeast inhibits TBSV replication due solely to the presence of reduced amounts of replication proteins (Fig. 6E). Altogether, these findings strongly support that the PE level plays a direct role in tombusvirus replicase assembly.
PE Level Also Affects Replication of Other Tombusviruses and the Insect Nodamura Virus in Yeast.
To test if the proviral role of PE also extends to other viruses, we analyzed replication of the closely related Cucumber necrosis tombusvirus (CNV), which also replicates on peroxisomal membranes. Similar to TBSV, CNV replication was increased in cho2Δ yeast (Fig. S6A) and the expression of CNV p33 replication protein alone induced the relocalization of PE in yeast cells (Fig. S6B). In contrast, depletion of the PE level in psd1Δ/psd2Δ/dpl1Δ yeast resulted in less than 10% CNV replication (Fig. S6C, lanes 3 and 4 versus 1 and 2). Thus, the proviral role of PE seems to be similar for TBSV and CNV.
To study if viruses replicating in other subcellular compartments could take advantage of the increased PE level in cho2Δ yeast, we used Carnation Italian ringspot virus (CIRV, a tombusvirus), which replicates in the outer mitochondrial membranes (26, 34). CIRV accumulation is increased by ∼fivefold in cho2Δ yeast (Fig. 7A). Moreover, the p36 replication protein of CIRV induced the efficient enrichment of PE in the same subcellular locations that harbor p36 (Fig. 7B). Depletion of the PE level in psd1Δ/psd2Δ/dpl1Δ yeast reduced CIRV replication to ∼10% of that found in WT yeast (Fig. 7C, lanes 3 and 4 versus 1 and 2), confirming the essential role of PE in CIRV replication. Replication of another mitochondrial RNA virus, the unrelated Nodamura virus (NoV) insect RNA virus (35), also benefitted from the increased PE level in cho2Δ yeast (Fig. 7D). Interestingly, protein A replication protein of NoV is localized at highly PE-enriched subcellular locations (Fig. 7E). Depletion of the PE level reduced NoV replication by ∼sixfold in psd1Δ/psd2Δ/dpl1Δ yeast (Fig. 7F, lanes 3 and 4 versus 1 and 2), further supporting a proviral role of PE in NoV replication. Therefore, we suggest that replication of different tombusviruses and NoV in peroxisomal or mitochondrial subcompartments depends on the PE level in yeast. Similar to TBSV, the replication proteins of these viruses can likely induce the efficient enrichment of PE at the sites of virus replication, suggesting that different RNA viruses use active, albeit yet unraveled, mechanisms to create a PE-enriched microenvironment in infected cells.
Fig. 7.
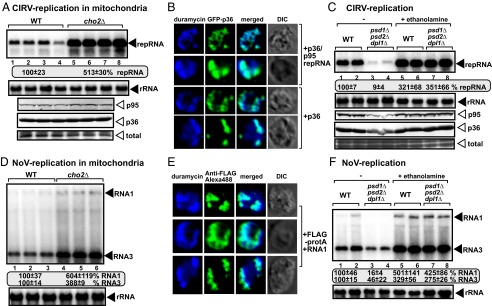
Increased PE level facilitates CIRV and NoV RNA accumulation in cho2Δ yeast. (A, Top) Replication of CIRV repRNA in WT and cho2Δ yeast was measured by Northern blotting 24 h after initiation of CIRV replication. Yeast coexpressed the CIRV p36 and p95 replication proteins with the (+)repRNA. (A, Middle and Bottom) The accumulation levels of His6-p95 and His6-p36 were tested by Western blotting. Each experiment was repeated. (B) Confocal laser microscopy images show the enrichment of PE at mitochondrial sites of CIRV p36 accumulation in WT yeast. See further details in Fig. 3. (C) Depletion of PE in yeast (psd1Δ/psd2Δ/dpl1Δ) inhibits CIRV replication. See further details in Fig. 6E. (D) Replication of NoV RNA1 and RNA3 in WT and cho2Δ yeast was measured by Northern blotting 24 h after initiation of NoV replication. (E) Confocal laser microscopy images show the enrichment of PE at mitochondrial sites of NoV Flag-tagged protA replication protein accumulation in WT yeast. See further details in Fig. 3. (F) Depletion of PE in yeast (psd1Δ/psd2Δ/dpl1Δ) inhibits NoV replication. See further details in Fig. 6E.
Discussion
PE Is an Essential Host Factor Subverted for TBSV Replication.
It is universally accepted that plant and animal positive-strand RNA viruses require cellular membranes for their propagation in infected cells (1–4). These viruses replicate in various subcellular compartments that contain a unique composition of lipids. However, it is currently poorly understood how different lipids could affect the viral replication process. By using the highly tractable tombusviruses, we show that PE is a coopted host factor that plays an essential role in viral replication. The supporting evidence includes (i) the requirement of PE for in vitro viral replicase assembly based on artificial vesicles that facilitate TBSV replication only when PE is present above 70%, (ii) the viral replication protein induces a relative increase in PE level at the expense of other phospholipids in yeast and plant cells replicating TBSV, (iii) highly localized enrichment of PE in the peroxisomal membranes (the sites of tombusvirus replication) to promote tombusvirus replication, (iv) cell-based results showing increased TBSV replication in cho2Δ yeast that contains a high level of PE due to a defect in conversion of PE into PC, (v) a yeast strain with depleted PE level supports low-level viral replication, and (vi) in vitro CFE-based assay to reconstitute TBSV replicase shows enhanced replicase activity in a highly PE-enriched membrane fraction from cho2Δ yeast and poor replicase activity when a PE-depleted yeast membrane fraction is used.
Essential Role of PE in TBSV VRC Assembly.
Based on in vitro approaches, PE plays a major function during the assembly of the VRCs that synthesize the viral RNAs. We show that active VRCs are only formed and functional in the presence of artificial PE vesicles, whereas other phospholipids are insufficient to support efficient VRC assembly. Moreover, other phospholipids are inhibitory to active VRC assembly when present in 20% or higher concentration in artificial vesicles. In addition, a CFE membrane fraction prepared from cho2Δ yeast, which contains a high PE level, supports greatly enhanced TBSV replication in vitro, suggesting more efficient VRC assembly when PE is abundant in membranes. In contrast, the CFE-based assay using yeast membranes with depleted PE is remarkably inefficient in supporting TBSV replication in vitro.
Interestingly, PE does not seem to be essential at the very early steps of replication (before the VRC assembly), because the TBSV p33 replication protein can associate not only with PE but other phospholipids too. Also, PC is even more efficient than PE for the p33/p92-driven recruitment of the viral (+)repRNA to artificial membranes in vitro (Table 1). After the initial binding to the membranes, p33 will likely induce the local enrichment of PE that is required for VRC assembly. In addition, binding of p33/p92 to PE might stabilize the replication proteins, because we observed elevated levels of p33/p92 in cho2Δ yeast and decreased levels of replication proteins in psd1Δ/psd2Δ/dpl1Δ yeast in comparison with WT yeast. We have shown previously that phospholipids are important for p33/p92 stability in yeast (17).
Table 1.
Role of various phospholipids in TBSV replication
| Vesicles | Replication | p33 membrane association | RNA recruitment |
| PE | + | + | + |
| PC | – | + | + |
| PG | – | + | – |
| PS | – | + | – |
| PI | – | – | – |
| CA | – | + | – |
| lysoPE | – | + | + |
| lysoPC | – | + | – |
In contrast to the preassembly of p33/p92 and the viral (+)RNA in membranes, for which PE might not be essential, we find that PE is absolutely critical for the final assembly of the TBSV VRC. Accordingly, the development of artificial vesicle-based TBSV replication unambiguously demonstrates that TBSV requires PE for active VRC assembly and viral RNA synthesis. TBSV replicase assembled on the PE vesicles could support a complete cycle of RNA replication, including (–)- and (+)-RNA synthesis in an asymmetrical manner, producing ∼10 times more (+)-strands than (–)-strands. Asymmetrical replication of the RNA genome is one of the hallmarks of (+)-strand RNA viruses. However, optimal TBSV replication also requires additional phospholipids, because the highest level of TBSV RNA synthesis was observed with vesicles containing ∼15% additional phospholipids and ∼85% PE.
In comparison with yeast CFE, which supports twofold more efficient TBSV replication than PE vesicles, the artificial PE vesicles cannot fully protect the viral RNA from nucleases during in vitro RNA synthesis, suggesting that PE is not sufficient to allow the formation of complete authentic TBSV VRCs. It is possible that sterols and coopted cellular proteins in combination with additional phospholipids are also needed for the formation of spherule structures (vesicles with narrow neck-like openings), which are the characteristic replication structures for TBSV (36–38) and many other RNA viruses (1–4). We suggest that the subverted PE molecules, due to their conical molecular structures, facilitate the formation of spherules by introducing a negative curvature into lipid bilayers. Indeed, these TBSV-induced negatively deformed membranes are visualized by electron microscopy in yeast cells missing the ESCRT Vps4p AAA+ ATPase protein (37), which is required for spherule formation.
TBSV Builds a PE-Enriched Microenvironment for Replication.
Based on TBSV replication studies with artificial vesicles, the emerging picture is that TBSV requires a high local concentration of PE at the sites of replication (above 70%; Fig. 1). However, PE is below that level in subcellular membranes, such as peroxisomes, in the virus-free stage. Therefore, TBSV likely stimulates the cell to increase the PE level via new PE/phospholipid synthesis, as shown by lipidomics data from yeast and plant cells. Then, the newly synthesized PE molecules are likely subverted for TBSV replication in a currently unknown manner. Another way to increase the local concentration of PE is to redistribute PE from various subcellular membranes to the site of replication. Indeed, confocal microscopy images show the robust accumulation of PE at peroxisomal sites where TBSV p33 replication protein accumulates (to form VRCs). Interestingly, PE molecules (presented as NBD-PE) provided in the yeast culture media found their ways to the p33-containing peroxisomal membrane sites, suggesting that PE is efficiently redistributed to the sites of TBSV replication from the preexisting cellular PE pool. Overall, it seems that TBSV induces cellular PE synthesis as well as subcellular redistribution of PE, resulting in a PE-enriched microenvironment, which serves the virus’s need during VRC assembly.
A Wide-Spread Role of PE in (+)RNA Virus Replication?
Because many (+)RNA viruses build vesicle-like structures for replication that requires membrane deformation and negative membrane curvature (1, 3, 4), it is possible that these viruses also depend on local enrichment of PE at the replication sites. We directly tested the role of PE in replication of several (+)RNA viruses by using cho2Δ yeast, which lacks one of the PEMTs to convert PE to PC (25), with an especially high cellular PE level. Interestingly, the TBSV-related CNV (peroxisomal replication) and CIRV (mitochondrial replication) and the unrelated NoV (mitochondrial replication) all supported enhanced replication when PE is abundant in membranes of cho2Δ yeast. Moreover, PE was shown to become highly enriched at the sites of viral replication protein accumulation, which represent VRCs for these viruses. Also, “forcing” TBSV to switch to ER membranes in the absence of peroxisomes in cho2Δ yeast (due to the pex3Δ background) still resulted in efficient TBSV replication, suggesting that tombusviruses could take advantage of abundant PE in various subcellular membranes. In summary, the emerging picture from our work is that various (+)RNA viruses could subvert cellular PE to build VRCs in the PE-enriched microenvironment, leading to efficient viral replication in infected cells.
In addition to the above presented critical role for PE in tombusvirus and NoV VRC assembly and replication, other phospholipids could also be exploited by plus-strand RNA viruses. For example, PC biosynthesis is increased in poliovirus-infected cells, leading to formation of replication organelles from newly made lipids (39, 40). Inhibition of PC biosynthesis in Drosophila cells reduced the replication of Flock house virus (FHV) (41). However, the absence of direct binding between the FHV replication protein and PC complicates the interpretation of the PC role in the replication process (41). Interestingly, Drosophila cells contain an unusually high level of PE (41), which might naturally facilitate FHV replication. A template-dependent RNA-dependent RNA polymerase extracted from FHV-infected cells was stimulated by phospholipids, including PC and PE (42), suggesting that phospholipids are important for FHV replication in vitro. However, the precise function of PC in replication or VRC assembly in the case of poliovirus or FHV is currently unknown.
Experimental Procedures
In Vitro TBSV Replication Assay Using Artificial Phospholipid Vesicles.
The procedure for in vitro replication assay using phospholipid vesicles was adapted from the previously published procedure using purified yeast organelles (26), except that the 100,000 × g supernatant (S100) was replaced with the S40 fraction of CFE. Briefly, 2 µL of phospholipid vesicles and 1 µL of the S40 fraction were incubated at 25 °C for 1 h in 8 μL buffer containing 30 mM Hepes-KOH (pH 7.4); 150 mM potassium acetate; 5 mM magnesium acetate; 0.6 M sorbitol; 15 mM creatine phosphate; 1 mM ATP, CTP, and GTP; 0.025 mM UTP; 0.1 μL [32P]UTP; 0.1 mg/mL creatine kinase; 0.1 μL RNase inhibitor (Thermo Scientific); 10 mM DTT; 0.5 μg DI-72 RNA transcript; and 0.5 μg MBP-tagged recombinant TBSV p33 and p92 replication proteins. Then, the reaction mix was incubated for 3 h in 16 μL cell-free replication buffer B (30 mM Hepes-KOH pH 7.4, 150 mM potassium acetate, 5 mM magnesium acetate) with 15 mM creatine phosphate; 1 mM ATP, CTP, and GTP; 0.025 mM UTP; 0.2 μL [32P]UTP; 0.1 mg/mL creatine kinase; 0.2 μL RNase inhibitor; 10 mM DTT; and 0.05 mg/mL actinomycin D. After reaction, total RNA was extracted and analyzed in a denaturing gel. Determination of the viral (+)RNA/(–)RNA ratio as well as micrococcal nuclease treatment was described previously (26).
Membrane Flotation Assay.
[35S]methionine-labeled TBSV p33 (43) was incubated with different artificial phospholipid vesicles as previously described using purified yeast organelles (26). The membrane flotation assay was performed as previously described (44) with minor modifications. Briefly, the reaction mixture (24 μL) was mixed with 126 μL 85% (wt/vol) sucrose in Hepes buffer in a final concentration of 71.25%, then overlaid with 900 μL 65% (wt/vol) sucrose and 150 μL 10% (wt/vol) sucrose in Hepes buffer (30 mM, pH 7.4). The gradient was centrifuged at 134,000 × g for 16 h at 4 °C in a swinging bucket rotor (Beckman TLS-55).
TBSV RNA Recruitment Assay.
For the viral RNA recruitment assay, artificial phospholipid vesicles were mixed with MBP-tagged recombinant TBSV p33 and p92 replication proteins (0.5 μg each) as in the in vitro replication assay, except that UTP was omitted from the reaction mixture, and 1 μg/μL yeast tRNA was added as a nonspecific competitor and 1 μL radioactive [32P]UTP-labeled DI-72 RNA transcripts was added. After 1 h incubation at 25 °C, the reaction mixtures were subjected to membrane flotation assay in sucrose gradients as described above. Total RNA from the top fraction of each gradient was extracted and analyzed in a denaturing RNA gel (5% polyacrylamide gel containing 8 M urea).
Imaging of PE Distribution and Viral Protein Localization in Yeasts and Plant Protoplasts.
Yeast cultures were grown in glucose-containing media overnight and switched to galactose-containing media with an initial 0.3 OD600. To prepare spheroplasts, overnight cultures were harvested and the yeast cells were fixed with 3.7% formaldehyde for 40 min at room temperature in the dark, washed twice with 0.1 M potassium phosphate (pH 7.5), and then resuspended in SPP (0.1 M potassium phosphate pH 7.5, 1.2 M sorbitol) with zymolase 20T (1 mg/mL). Cells were incubated at 30 °C for 1 h, and then incubated with SPP with 50 mM NH4Cl for 15 min to quench free aldehyde groups. Spheroplasts were collected after washing twice with SPP and then applied to poly-l-lysine–coated slides. Slides were immersed in methanol for 6 min and acetone for 30 s, respectively, at –20 °C. Biotinylated duramycin was added into PBS (pH 7.4) containing 0.05% Nonidet P-40 and 1% BSA (15 μg/mL) and incubated overnight with the fixed cells at –4 °C. Slides were washed and incubated with Streptavidin conjugated with Alexa Fluor 405 (Life Technologies) for 1 h before imaging. In each experiment, ∼20 cells were observed under the confocal laser microscope.
The distribution of PE was also monitored using fatty acid-labeled NBD-PE and NBD-PC internalization. M-C6–NBD-PE [1-myristoyl-2-(6-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]hexanoyl)-sn-glycero-3-phosphoethanolamine] and M-C6–NBD-PC [1-myristoyl-2-(6-[(7-nitro-2–1,3-benzoxadiazol-4-yl)amino]hexanoyl)-sn-glycero-3-phosphocholine] (Avanti Polar lipids, Inc.) were dissolved in DMSO in 8 mM concentration (45) and stored at –20 °C. WT yeast transformed with pESC-mRFP-T33 and pRS315-Pex13p-BFP was pregrown in SC medium, then cultured in SC medium containing 2% galactose with 80 μM NBD-PE or NBD-PC at 0.5 OD600, and incubated at 23 °C for 16 h. Cells were washed with SCNaN3 medium (45) (SC medium with 2% sorbitol and 20 mM sodium azide) and subjected to confocal laser microscope analysis.
N. benthamiana protoplasts were prepared and eletroporated with in vitro-transcribed TBSV full-length genomic RNA as described previously (46). Protoplasts were fixed with 3.7% formaldehyde in protoplast culture medium (46), applied to poly-l-lysine–coated slides, and processed using the above procedure for PE staining. For dual staining of p33 and PE, the anti-p33 primary antibody was diluted (1:400) and incubated with fixed protoplasts in PBS containing 1% BSA/0.05% Nonidet P-40 overnight. After washing three times with PBS/1% BSA/0.05% Nonidet P-40, cells were incubated with anti-mouse secondary antibody conjugated to Alexa Fluor 488 (Life Technologies) for 1 h before imaging.
Confocal images were obtained with an Olympus FV1000 microscope (Olympus America). BFP/Alexa 405, GFP/Alexa 488, and RFP were excited using 405 nm, 488 nm, or 543 nm lasers, respectively. Images were obtained sequentially and merged using Olympus FLUOVIEW 1.5 software. Relative fluorescence intensity was estimated by ImageJ software and analyzed further using Microsoft EXCEL software.
Supplementary Material
Acknowledgments
The authors thank Dr. Herman B. Scholthof (Texas A&M) for the the anti-p33 primary antibody. This work was supported by National Science Foundation Grant MCB-1122039 (to P.D.N.). The lipid analyses described in this work were performed at the Kansas Lipidomics Research Center Analytical Laboratory. The Kansas Lipsidomics Research Center was supported by National Science Foundation Grants EPS 0236913, MCB 0920663, DBI 0521587, and DBI 1228622; the Kansas Technology Enterprise Corporation; K-IDeA Networks of Biomedical Research Excellence of the National Institutes of Health Grant (P20GM103418); and Kansas State University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418971112/-/DCSupplemental.
References
- 1.de Castro IF, Volonté L, Risco C. Virus factories: Biogenesis and structural design. Cell Microbiol. 2013;15(1):24–34. doi: 10.1111/cmi.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belov GA, van Kuppeveld FJ. (+)RNA viruses rewire cellular pathways to build replication organelles. Curr Opin Virol. 2012;2(6):740–747. doi: 10.1016/j.coviro.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Boon JA, Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol. 2010;64:241–256. doi: 10.1146/annurev.micro.112408.134012. [DOI] [PubMed] [Google Scholar]
- 4.Nagy PD, Pogany J. The dependence of viral RNA replication on co-opted host factors. Nat Rev Microbiol. 2012;10(2):137–149. doi: 10.1038/nrmicro2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: Where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9(2):112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schoggins JW, Randall G. Lipids in innate antiviral defense. Cell Host Microbe. 2013;14(4):379–385. doi: 10.1016/j.chom.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu NY, et al. Viral reorganization of the secretory pathway generates distinct organelles for RNA replication. Cell. 2010;141(5):799–811. doi: 10.1016/j.cell.2010.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond DL, et al. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog. 2010;6(1):e1000719. doi: 10.1371/journal.ppat.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perera R, et al. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog. 2012;8(3):e1002584. doi: 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Syed GH, Amako Y, Siddiqui A. Hepatitis C virus hijacks host lipid metabolism. Trends Endocrinol Metab. 2010;21(1):33–40. doi: 10.1016/j.tem.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8(5):422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heaton NS, et al. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci USA. 2010;107(40):17345–17350. doi: 10.1073/pnas.1010811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, et al. Intersection of the multivesicular body pathway and lipid homeostasis in RNA replication by a positive-strand RNA virus. J Virol. 2011;85(11):5494–5503. doi: 10.1128/JVI.02031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reiss S, et al. Recruitment and activation of a lipid kinase by hepatitis C virus NS5A is essential for integrity of the membranous replication compartment. Cell Host Microbe. 2011;9(1):32–45. doi: 10.1016/j.chom.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy PD, Pogany J, Lin JY. How yeast can be used as a genetic platform to explore virus-host interactions: From ‘omics’ to functional studies. Trends Microbiol. 2014;22(6):309–316. doi: 10.1016/j.tim.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Chuang C, Barajas D, Qin J, Nagy PD. Inactivation of the host lipin gene accelerates RNA virus replication through viral exploitation of the expanded endoplasmic reticulum membrane. PLoS Pathog. 2014;10(2):e1003944. doi: 10.1371/journal.ppat.1003944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma M, Sasvari Z, Nagy PD. Inhibition of phospholipid biosynthesis decreases the activity of the tombusvirus replicase and alters the subcellular localization of replication proteins. Virology. 2011;415(2):141–152. doi: 10.1016/j.virol.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma M, Sasvari Z, Nagy PD. Inhibition of sterol biosynthesis reduces tombusvirus replication in yeast and plants. J Virol. 2010;84(5):2270–2281. doi: 10.1128/JVI.02003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogany J, Stork J, Li Z, Nagy PD. In vitro assembly of the Tomato bushy stunt virus replicase requires the host Heat shock protein 70. Proc Natl Acad Sci USA. 2008;105(50):19956–19961. doi: 10.1073/pnas.0810851105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pogany J, Nagy PD. Authentic replication and recombination of Tomato bushy stunt virus RNA in a cell-free extract from yeast. J Virol. 2008;82(12):5967–5980. doi: 10.1128/JVI.02737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pogany J, White KA, Nagy PD. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA is essential for replication. J Virol. 2005;79(8):4859–4869. doi: 10.1128/JVI.79.8.4859-4869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monkewich S, et al. The p92 polymerase coding region contains an internal RNA element required at an early step in Tombusvirus genome replication. J Virol. 2005;79(8):4848–4858. doi: 10.1128/JVI.79.8.4848-4858.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao M. Lantibiotics as probes for phosphatidylethanolamine. Amino Acids. 2011;41(5):1071–1079. doi: 10.1007/s00726-009-0386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boumann HA, et al. Depletion of phosphatidylcholine in yeast induces shortening and increased saturation of the lipid acyl chains: Evidence for regulation of intrinsic membrane curvature in a eukaryote. Mol Biol Cell. 2006;17(2):1006–1017. doi: 10.1091/mbc.E05-04-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Summers EF, Letts VA, McGraw P, Henry SA. Saccharomyces cerevisiae cho2 mutants are deficient in phospholipid methylation and cross-pathway regulation of inositol synthesis. Genetics. 1988;120(4):909–922. doi: 10.1093/genetics/120.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu K, Huang TS, Nagy PD. Authentic in vitro replication of two tombusviruses in isolated mitochondrial and endoplasmic reticulum membranes. J Virol. 2012;86(23):12779–12794. doi: 10.1128/JVI.00973-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang Y, Morrell JC, Jones JM, Gould SJ. PEX3 functions as a PEX19 docking factor in the import of class I peroxisomal membrane proteins. J Cell Biol. 2004;164(6):863–875. doi: 10.1083/jcb.200311131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hettema EH, Girzalsky W, van Den Berg M, Erdmann R, Distel B. Saccharomyces cerevisiae pex3p and pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 2000;19(2):223–233. doi: 10.1093/emboj/19.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jonczyk M, Pathak KB, Sharma M, Nagy PD. Exploiting alternative subcellular location for replication: Tombusvirus replication switches to the endoplasmic reticulum in the absence of peroxisomes. Virology. 2007;362(2):320–330. doi: 10.1016/j.virol.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Pathak KB, Sasvari Z, Nagy PD. The host Pex19p plays a role in peroxisomal localization of tombusvirus replication proteins. Virology. 2008;379(2):294–305. doi: 10.1016/j.virol.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Xu K, Nagy PD. Expanding use of multi-origin subcellular membranes by positive-strand RNA viruses during replication. Curr Opin Virol. 2014;9:119–126. doi: 10.1016/j.coviro.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Kannan M, Riekhof WR, Voelker DR. Transport of phosphatidylserine from the endoplasmic reticulum to the site of phosphatidylserine decarboxylase2 in yeast. Traffic. 2015;16(2):123–134. doi: 10.1111/tra.12236. [DOI] [PubMed] [Google Scholar]
- 33.Birner R, Bürgermeister M, Schneiter R, Daum G. Roles of phosphatidylethanolamine and of its several biosynthetic pathways in Saccharomyces cerevisiae. Mol Biol Cell. 2001;12(4):997–1007. doi: 10.1091/mbc.12.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weber-Lotfi F, Dietrich A, Russo M, Rubino L. Mitochondrial targeting and membrane anchoring of a viral replicase in plant and yeast cells. J Virol. 2002;76(20):10485–10496. doi: 10.1128/JVI.76.20.10485-10496.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Venter PA, Schneemann A. Recent insights into the biology and biomedical applications of Flock House virus. Cell Mol Life Sci. 2008;65(17):2675–2687. doi: 10.1007/s00018-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barajas D, Jiang Y, Nagy PD. A unique role for the host ESCRT proteins in replication of Tomato bushy stunt virus. PLoS Pathog. 2009;5(12):e1000705. doi: 10.1371/journal.ppat.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barajas D, Martín IF, Pogany J, Risco C, Nagy PD. Noncanonical role for the host Vps4 AAA+ ATPase ESCRT protein in the formation of Tomato bushy stunt virus replicase. PLoS Pathog. 2014;10(4):e1004087. doi: 10.1371/journal.ppat.1004087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCartney AW, Greenwood JS, Fabian MR, White KA, Mullen RT. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. Plant Cell. 2005;17(12):3513–3531. doi: 10.1105/tpc.105.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nchoutmboube JA, et al. Increased long chain acyl-Coa synthetase activity and fatty acid import is linked to membrane synthesis for development of picornavirus replication organelles. PLoS Pathog. 2013;9(6):e1003401. doi: 10.1371/journal.ppat.1003401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vance DE, Trip EM, Paddon HB. Poliovirus increases phosphatidylcholine biosynthesis in HeLa cells by stimulation of the rate-limiting reaction catalyzed by CTP: Phosphocholine cytidylyltransferase. J Biol Chem. 1980;255(3):1064–1069. [PubMed] [Google Scholar]
- 41.Castorena KM, Stapleford KA, Miller DJ. Complementary transcriptomic, lipidomic, and targeted functional genetic analyses in cultured Drosophila cells highlight the role of glycerophospholipid metabolism in Flock House virus RNA replication. BMC Genomics. 2010;11:183. doi: 10.1186/1471-2164-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu SX, Ahlquist P, Kaesberg P. Active complete in vitro replication of nodavirus RNA requires glycerophospholipid. Proc Natl Acad Sci USA. 1992;89(23):11136–11140. doi: 10.1073/pnas.89.23.11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang RY, Stork J, Nagy PD. A key role for heat shock protein 70 in the localization and insertion of tombusvirus replication proteins to intracellular membranes. J Virol. 2009;83(7):3276–3287. doi: 10.1128/JVI.02313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stapleford KA, Rapaport D, Miller DJ. Mitochondrion-enriched anionic phospholipids facilitate flock house virus RNA polymerase membrane association. J Virol. 2009;83(9):4498–4507. doi: 10.1128/JVI.00040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grant AM, Hanson PK, Malone L, Nichols JW. NBD-labeled phosphatidylcholine and phosphatidylethanolamine are internalized by transbilayer transport across the yeast plasma membrane. Traffic. 2001;2(1):37–50. doi: 10.1034/j.1600-0854.2001.020106.x. [DOI] [PubMed] [Google Scholar]
- 46.Panaviene Z, Baker JM, Nagy PD. The overlapping RNA-binding domains of p33 and p92 replicase proteins are essential for tombusvirus replication. Virology. 2003;308(1):191–205. doi: 10.1016/s0042-6822(02)00132-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.