In their contribution to PNAS, Beaufort et al. demonstrate that high temperature requirement protein A1 (HtrA1) facilitates TGF-β signaling through processing latent TGF-β binding protein 1 (LTBP-1) (1). Then the authors suggest the down-regulation of TGF-β signaling by lack of HtrA1-mediated LTBP-1 processing as a key mechanism underlying pathogenesis of cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) (1). We applaud the authors for their careful work about HtrA1 but would like to point out that they only studied fibroblasts (FBs), and their data do not support a causative role of this novel mechanism for the pathogenesis of CARASIL.
For CARASIL patients, the main pathology falls on the small penetrating arteries, with lack of vascular smooth muscle cells (VSMCs) in the media and intense adventitial fibrosis (2). Loss of VSMCs is the primary feature of CARASIL, followed by deposition of the granular material in the media and fibrosis of the arterial wall (2, 3). Arterial fibrosis results in the deficiency of contraction of the arteries, leading to ischemic stroke and other clinical symptoms of CARASIL (2, 3). TGF-β promotes fibrosis by stimulating the proliferation and activation of FBs (4). Therefore, attenuation of TGF-β signaling in fibroblasts might not promote CARASIL.
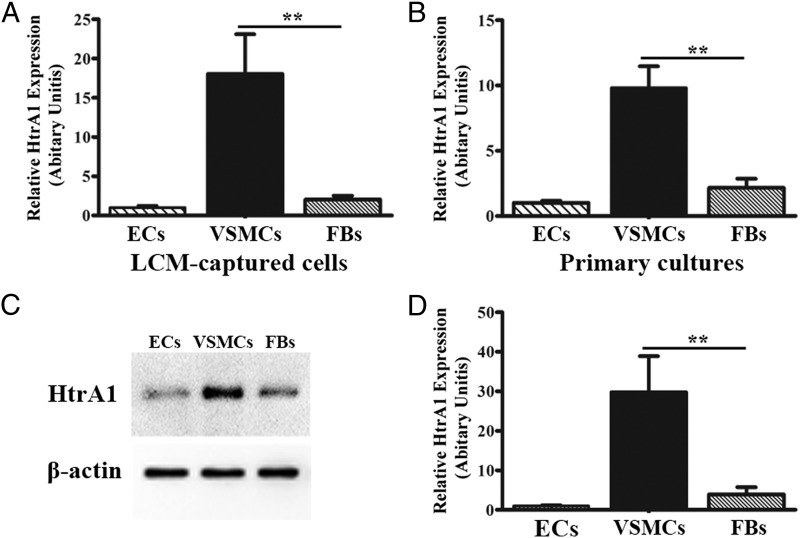
CARASIL is caused by recessive loss-of-function mutations in HtrA1 (5). To provide hints of the expression pattern of HtrA1 in human cerebral small arteries, we examined HtrA1 expression in small coronary arteries of human heart tissues. The endothelial cells (ECs), VSMCs, and peri-vascular FBs were collected from the frozen sections by laser capture microdissection (LCM). The RT-PCR analysis demonstrated that the expression of HtrA1 in VSMCs is remarkably higher than that of FBs (Fig. 1A; 18.04 ± 5.08 vs. 2.03 ± 0.50 AU, P < 0.01). In addition, we prepared primary cells of ECs, VSMCs, and peri-vascular FBs, which are isolated directly from human tissues. Similar to LCM-captured cells, the mRNA expression of HtrA1 is exceedingly high in VSMCs, whereas it is low in ECs and FBs from primary cultures (Fig. 1B). The Western blotting of the whole cell extracts, which measures the protein inside the cells, showed a higher level of HtrA1 in VSMCs than FBs or ECs (Fig. 1C). The ELISA of the conditioned media, which measures secreted HtrA1 protein, also indicated a significantly higher HtrA1 secretion in VSMCs (Fig. 1D). Thus, in small arteries, the majority of HtrA1 exists inside or in the microenvironment of VSMCs.
Fig. 1.
Expression pattern of HtrA1 in small arteries. RT-PCR analysis of HtrA1 expression in LCM-captured (A) and primary-cultured (B) ECs, VSMCs, and FBs from human coronary small arteries. n = 3, **P < 0.01. (C) Immunoblots of HtrA1 and β-actin protein in primary-cultured cells. (D) Quantification of HtrA1 secretion from primary-cultured cells by ELISA. n = 3, **P < 0.01.
VSMCs might be the primary cell type affected by the HtrA1 activities. It is still unclear how the loss of HtrA1 might affect TGF-β signaling in VSMCs. Up-regulation of TGF-β1 induces an increase of VSMC proliferation and migration (4). On the other hand, increased TGF-β1 was observed in VSMCs of the tunica media but not in FBs of the adventitia in CARASIL patients (5). Taken together, the decrease of TGF-β signaling in fibroblasts might not truly contribute to the pathogenesis of CARASIL, and future studies will need to examine the role of HtrA1-mediated LTBP-1 processing in VSMCs.
Footnotes
The authors declare no conflict of interest.
References
- 1.Beaufort N, et al. Cerebral small vessel disease-related protease HtrA1 processes latent TGF-β binding protein 1 and facilitates TGF-β signaling. Proc Natl Acad Sci USA. 2014;111(46):16496–16501. doi: 10.1073/pnas.1418087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arima K, Yanagawa S, Ito N, Ikeda S. Cerebral arterial pathology of CADASIL and CARASIL (Maeda syndrome) Neuropathology. 2003;23(4):327–334. doi: 10.1046/j.1440-1789.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 3.Tikka S, et al. CADASIL and CARASIL. Brain Pathol. 2014;24(5):525–544. doi: 10.1111/bpa.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 5.Hara K, et al. Association of HTRA1 mutations and familial ischemic cerebral small-vessel disease. N Engl J Med. 2009;360(17):1729–1739. doi: 10.1056/NEJMoa0801560. [DOI] [PubMed] [Google Scholar]