Significance
Non-native plants dominate global lists of invasive (harmful) species, yet plants introduced to Britain are both less widespread than native species and not increasing any more than native plants, and changes to native and non-native plant diversity are positively associated. The hypothesis that competitive exclusion will eventually enable introduced plants to drive native species extinct receives no support, according to analysis of extensive British data. A more parsimonious explanation is that both native and introduced plants are responding predominantly to other drivers of environmental change. The negative effects of non-native plants on British biodiversity have been exaggerated, and may also have been exaggerated in other parts of the world.
Keywords: invasive species, biodiversity, Anthropocene, ecology, botany
Abstract
Plants are commonly listed as invasive species, presuming that they cause harm at both global and regional scales. Approximately 40% of all species listed as invasive within Britain are plants. However, invasive plants are rarely linked to the national or global extinction of native plant species. The possible explanation is that competitive exclusion takes place slowly and that invasive plants will eventually eliminate native species (the “time-to-exclusion hypothesis”). Using the extensive British Countryside Survey Data, we find that changes to plant occurrence and cover between 1990 and 2007 at 479 British sites do not differ between native and non-native plant species. More than 80% of the plant species that are widespread enough to be sampled are native species; hence, total cover changes have been dominated by native species (total cover increases by native species are more than nine times greater than those by non-native species). This implies that factors other than plant “invasions” are the key drivers of vegetation change. We also find that the diversity of native species is increasing in locations where the diversity of non-native species is increasing, suggesting that high diversities of native and non-native plant species are compatible with one another. We reject the time-to-exclusion hypothesis as the reason why extinctions have not been observed and suggest that non-native plant species are not a threat to floral diversity in Britain. Further research is needed in island-like environments, but we question whether it is appropriate that more than three-quarters of taxa listed globally as invasive species are plants.
The Global Invasive Species Database (1) lists 3,163 plant (Plantae) and 820 animal (Animalia) species as invasive because they “threaten native biodiversity and natural ecosystems” in the regions to which they have been introduced. Given the relative numbers of animal and plant species that have been described (2–4), this implies that the per species likelihood of being listed as invasive is ∼25 times higher for plants than for animals. For the United Kingdom, 49 of 125 species (39%) categorized as invasive in the same database are plants (1), and a more detailed analysis included 102 plants in a list of 244 non-native species (∼42%, depending on taxonomic designations) that have negative ecological or human effects in Great Britain (5, 6). These numbers imply that non-native plants must be key threats to biodiversity both globally and in Britain. It is surprising, therefore, that examples of regional-scale or species-level extinctions associated with invasive plants are apparently rare (7–12).
Most extinctions associated with introduced species have been caused by invasive predators and diseases encountering “naïve” prey and host species in distant and isolated parts of the world (13–19). Putative examples of competitive exclusion in the invasive species literature have usually turned out to be examples of apparent competition, whereby the invading species is more resistant than native species to a shared pathogen (17–19), rather than traditional interference or exploitative competition. The difference between the effects of invasive plants and those of invasive predators and diseases could, however, simply be a function of time; if non-native plants spread slowly but inexorably, relatively short-term increases could drive regional or global extinctions on centennial or millennial timescales. Introduced plants have certainly contributed to vegetation change in many isolated environments, such as the Hawaiian Islands and the ecologically distinct fynbos vegetation in South Africa (10, 20–22). They can also become abundant in some continental regions, and hence they have the potential to alter ecosystems and exclude native species over long periods of time (23–26). We refer to the proposition that ongoing increases in the distributions and abundances of non-native plants will cause long-term competitive exclusion of native plant species as the “time-to-exclusion hypothesis”.
However, short-term and local gains by non-native species do not automatically result in long-term and large-scale extinctions of native species. Competition may be insufficient as a mechanism to drive many or any native plant species extinct, other than at a local scale (27, 28). A failure of competition to exclude native species at regional or global scales could arise because introduced plants deplete the resources they initially thrive on and accumulate herbivores and diseases, which together apply density-dependent control to introduced species before they can exclude the native plants. In addition, native plants may have the capacity to out-compete or coexist with the invaders, at least in some local environments (29–33).
The time-to-exclusion hypothesis is difficult to test because regional-scale and global exclusions are predicted to take place far into the future. However, it is possible to evaluate two conditions that need to be met if past introductions are likely to cause future extinctions. First, non-native plant species that were established in the past should be continuing to increase more than native species. In contrast, if cover changes of native species are larger than those of the non-natives, it implies that other environmental drivers feature more strongly than biological invasions in altering the composition of communities. Second, although individual non-native species may fail to cause exclusion, this may be achieved through an increasing diversity of aliens, leading to the prediction that changes in native diversity will be negatively correlated with changes in the number of non-native species. Britain provides an excellent testbed for these predictions, partly because plant species have been introduced for several thousand years, providing opportunities for non-native species to spread and increase in numbers, and partly because an extensive stratified random sample of plant species in Britain (the British Countryside Survey) provides robust data to address these two key issues.
Results
Plant Distribution Sizes.
Native plant species dominate Countryside Survey samples of the British flora: native species constituted 83% of the 636 plant species that were recorded in at least one of the 479 study sites in 1990 (native = 529 species; archaeophytes introduced up to 1500 = 60 species; neophytes introduced after 1500 = 47) and 82% of the 677 species recorded in 2007 (native = 553, archaeophyte = 68, neophyte = 56). The apparent differences in species totals between years mainly reflect rare species only recorded in one site in one of the years (Dataset S1). Native species formed 85% of the 531 species that were recorded in at least one site in both years (native = 450, archaeophyte = 51, neophyte = 30) and 89% of the 217 species recorded in at least 10 sites in both years (native = 193, archaeophyte = 16, neophyte = 8).
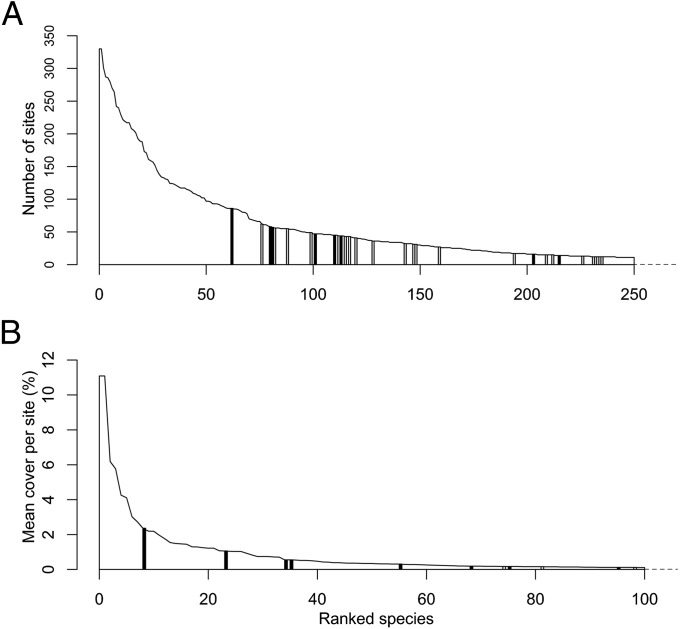
The 50 most widespread plant species, measured by frequency of occurrence in sites in 2007, were all native species; only seven non-native species were in the top 100 (Fig. 1). Of these seven non-natives, three were neophytes (Veronica persica, Acer pseudoplatanus, Brassica napus) and four were archaeophytes (Capsella bursa-pastoris Alopecurus myosuroides, Geranium dissectum, and Viola arvensis). The most widespread native species Holcus lanatus (present in 330 sites in 2007) was much more widespread than either the most widespread neophyte V. persica (86 sites in 2007) or archaeophyte C. bursa-pastoris (62 sites in 2007) (Dataset S1). Native species and archaeophytes were more widespread than neophytes, although native species and archaeophytes did not differ significantly (Fig. 2A and Table 1).
Fig. 1.
The number of sites (A) and mean percentage cover per site (B) of the most widespread (A) and most abundant (B) native species (white polygon with black outline), archaeophytes (gray bars), and neophytes (black bars) recorded during the Countryside Survey in 2007. In A, 250 species (native = 221, archaeophytes = 21, neophytes = 8) are shown. In B, 100 species (native = 92, archaeophytes = 2, neophytes = 6) are shown. Note that the x axes have been truncated. In A, a further 427 species (native = 332, archaeophytes = 47, neophytes = 48) were recorded in Countryside Survey sites in 2007. These species were all recorded in ≤11 sites. In B, a further 171 species (native = 101, archaeophytes = 14, neophytes = 2) recorded in at least 10 sites had mean cover of more than 0% in Countryside Survey sites in 2007. The mean cover of each of these species, per site, was ≤0.088%.
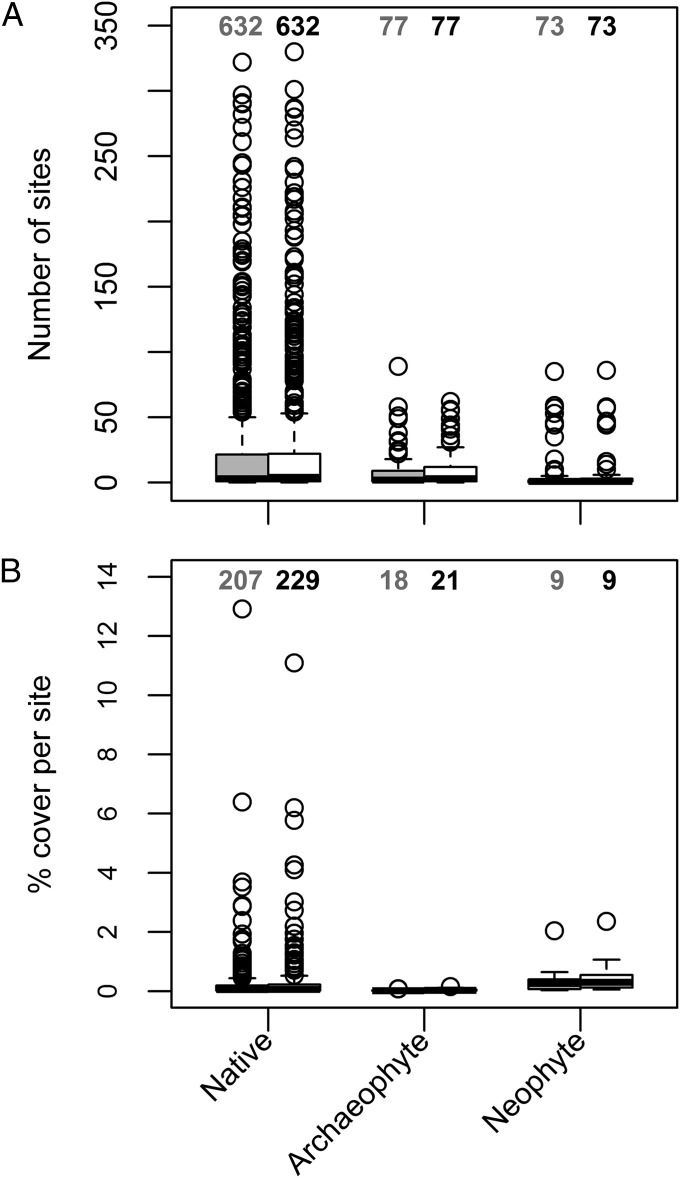
Fig. 2.
The frequency of occurrence (A) and mean percentage cover per site (B) of native species, archaeophytes, and neophytes in 1990 (gray boxes, left-hand box of each species group) and 2007 (white boxes, right-hand box of each species group). Only species recorded in at least 10 sites in each survey year are included in each panel. Sample sizes (numbers of species) are provided at the top of each box. Medians are represented by the horizontal black lines; the top and bottom of each box are the 75th and 25th percentiles, respectively; outliers are represented by hollow dots; and whiskers represent data within 1.5 × interquartile range of the upper and lower quartiles.
Table 1.
Kruskal-Wallis χ2 (d.f.) tests comparing the number of, and changes in, percentage cover (per quadrat per site) and number of sites between native species, neophytes, and archaeophytes
| Response and species groups | Test statistic |
| Number of sites (1990) | |
| All groups | χ2 (2) = 30.27, P < 0.0001 |
| Native vs. neophyte | χ2 (1) = 27.50, P < 0.0001 |
| Native vs. archaeophyte | χ2 (1) = 4.50, P = 0.03 |
| Archaeophyte vs. neophyte | χ2 (1) = 7.43, P = 0.006 |
| Number of sites (2007) | |
| All groups | χ2 (2) = 25.60, P < 0.0001 |
| Native vs. neophyte | χ2 (1) = 24.39, P < 0.0001 |
| Native vs. archaeophyte | χ2 (1) = 2.04, P = 0.15 |
| Archaeophyte vs. neophyte | χ2 (1) = 9.31, P = 0.002 |
| Cover (1990) | |
| All groups | χ2 (2) = 16.79, P < 0.001 |
| Native vs. neophyte | χ2 (1) = 4.45, P = 0.03 |
| Native vs. archaeophyte | χ2 (1) = 11.68, P < 0.001 |
| Archaeophyte vs. neophyte | χ2 (1) = 12.24, P < 0.001 |
| Cover (2007) | |
| All groups | χ2 (2) = 13.85, P < 0.001 |
| Native vs. neophyte | χ2 (1) = 6.52, P = 0.01 |
| Native vs. archaeophyte | χ2 (1) = 6.30, P = 0.01 |
| Archaeophyte vs. neophyte | χ2 (1) = 14.97, P < 0.001 |
| Change in number of sites | |
| All groups | χ2 (2) = 4.29, P = 0.11 |
| Change in cover | |
| All groups | χ2 (2) = 2.44, P = 0.30 |
Significant differences between groups are highlighted in bold; Bonferroni thresholds for P values for three-group comparisons and for pairwise comparisons were 0.025 (repeated tests in 1990 and 2007) and 0.0167 (three pairwise comparisons), respectively.
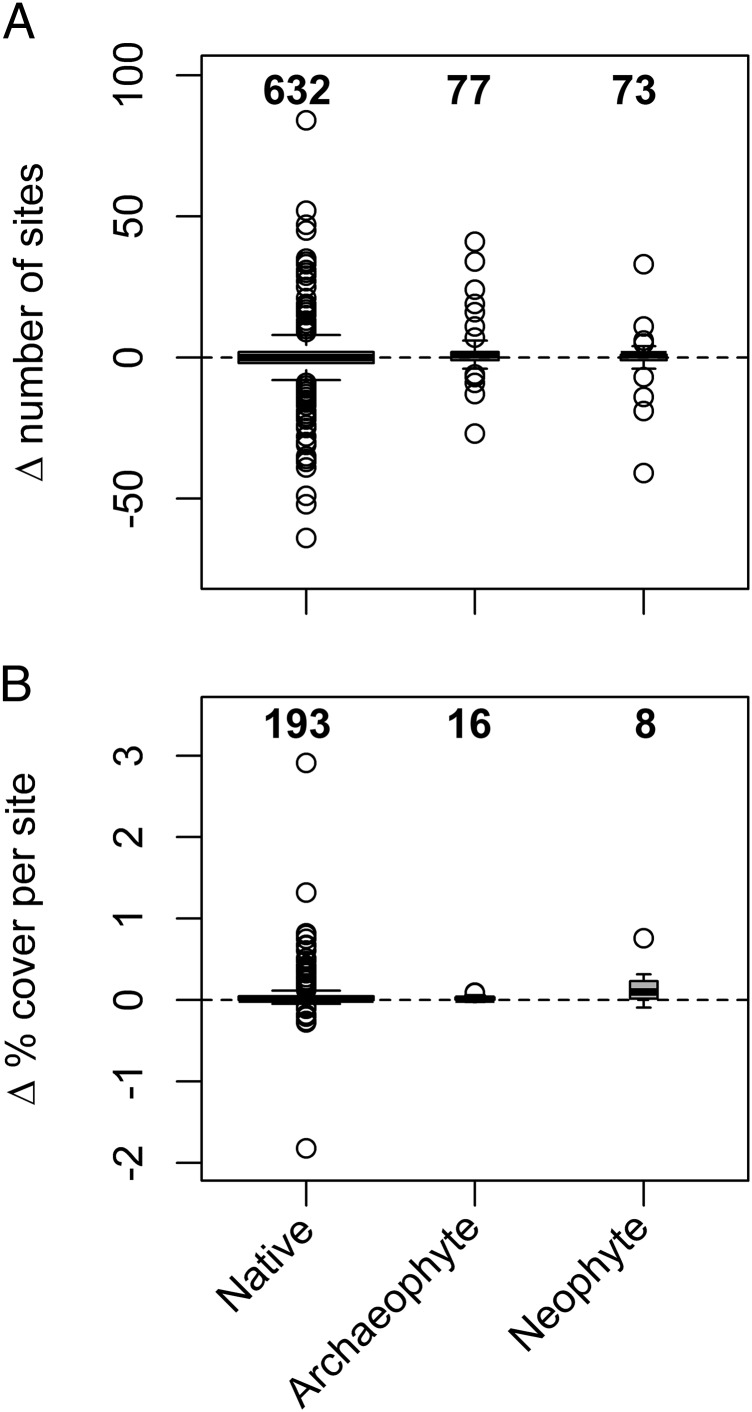
Changes in numbers of occupied sites between 1990 and 2007 were numerically dominated by the native species, and the largest absolute changes were by native species (Fig. 3A), which might have been expected, given that more than 80% of the species considered were native. The frequencies of occurrence of some species increased and others decreased over time, such that there were no significant differences between the three plant categories in the change in number of occupied sites [χ2 (2) = 4.29; P = 0.11; Fig. 3A and Dataset S1].
Fig. 3.
Changes in the frequency of occurrence (A) and mean percentage cover per site (B) of native species, archaeophytes, and neophytes in 1990 (gray boxes, left-hand box of each species group) and 2007 (white boxes, right-hand box of each species group). Only species recorded in at least 10 sites in both survey years are included in each panel. Sample sizes (numbers of species) are provided at the top of each box. Medians are represented by the horizontal black lines; the top and bottom of each box are the 75th and 25th percentiles, respectively; outliers are represented by hollow dots; and whiskers represent data within 1.5*interquartile range of the upper and lower quartiles.
Plant Cover.
Eleven non-native plant species were in the top 100 by plant cover, of which eight were the more recently introduced neophytes (Fig. 1B and Dataset S1). The most abundant native species Lolium perenne had a higher mean percentage cover per site (mean cover in 2007 = 11.09%) than the most abundant neophyte (Picea sitchensis, 2.36%) or the most abundant archaeophyte (Castanea sativa, 0.17%); C. sativa only ranked 74th (six neophytes ranked ahead of it: P. sitchensis, B. napus, A. pseudoplatanus, Lolium multiflorum, Picea abies, and Pinus contorta; Fig. 1B and Dataset S1). The median cover per neophyte species was significantly greater than that of archaeophytes in both years, and of native species in 2007 (Fig. 2B and Tables S1 and S2). Native species were more abundant than archaeophytes in both surveys (Fig. 2B and Tables S1 and S2). Nonetheless, almost all species of all three categories had very low cover (<<1%; Dataset S1).
The majority of species (60%; n = 130: native = 114, archaeophyte = 10, neophyte = 6) increased in cover between the two periods; 48 species showed no change in cover (22%; native = 43, archaeophyte = 5, neophyte = 0); and the cover of 39 species declined (18%; native = 36, archaeophyte = 1, neophyte = 2). The largest declines and increases were of native grasses: L. perenne (−1.88%), Nardus stricta (−0.28%), Poa trivialis (+1.32%), and H. lanatus (+2.91%) (Dataset S1).
There were no significant differences among native species, archaeophytes, and neophytes in terms of changes in plant cover between 1990 and 2007 [Fig. 3B; χ2 (2) = 2.44; P = 0.30]. Summed across increasing plant species, 9.6 times as much cover change is associated with increased cover of native species compared with non-natives (sum cover change per quadrat per site of natives = 17.47%, archaeophytes = 0.36%, neophytes = 1.46%). Native species continue to form the clear majority of widespread and abundant species (Figs. 1 and 2) and to dominate changes in abundance (Fig. 3B).
Diversity Changes.
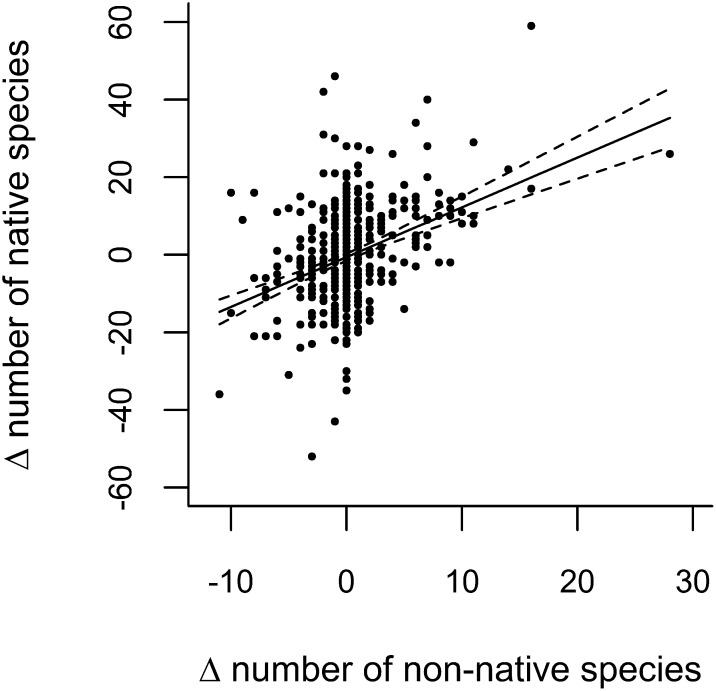
There was a significant positive relationship between changes in the diversity (richness) of native and non-native species in each site between 1990 and 2007 (Fig. 4), suggesting no loss of native diversity with increasing non-native diversity. Non-native species could potentially still contribute to declines in native diversity in the subset of 235 sites that exhibited a net loss of native species, so we repeated some of the above analyses for this subset of sites. Within these sites, 73 species (65 natives, 5 neophytes, and 3 archaeophytes of 155 species that were recorded in 10 or more sites in both survey years) increased in cover between surveys and could potentially contribute to declines in native plant diversity. As in the data set as a whole, the cover changes per species were not significantly different between the three plant categories [χ2 (2) = 5.33; P = 0.07]. The greatest absolute cover increases in these 235 sites were again by native species. The top five native species that increased in cover were H. lanatus (+2.71%), P. trivialis (+1.11%), Molinia caerulea (+0.94%), Trichophorum cespitosum (+0.81%), and Juncus effusus (+0.67%). The three archaeophytes that increased in percentage cover between the two surveys were Avena fatua (+0.13%), Anisantha sterilis (+0.07%), and G. dissectum (+0.02%). The five neophytes that increased were P. sitchensis (+1.14%), B. napus (+0.60%), A. pseudoplatanus (+0.15%), V. persica (+0.05%), and L. multiflorum (+0.04%). For these “increasing” species in these 235 sites, the sum of cover increases for natives it was 12.3% (n = 65 species), for archaeophytes it was 0.22% (n = 3 species), and for neophytes it was 1.98% (n = 5 species), indicating that total increases by native species were 5.6 times greater than total increases by non-native species.
Fig. 4.
Changes in numbers of native plant species as a function of changes in the number of non-native plants species (comprised of neophytes plus archaeophytes) in Countryside Survey plots between 1990 and 2007. Each point represents a site (n = 479 sites). There was a significant positive relationship (line ± 95% confidence interval) between changes in the diversity of native and non-native species (y = −0.58 + 1.28x; R2 = 0.14; P < 0.0001).
Discussion
The time-to-exclusion hypothesis requires species that were introduced a long time ago to continue to expand and become more abundant over time, such that they might eventually drive regional-scale extinctions of native species by competitive exclusion. This was not the case in the present study. Changes in the frequencies of occurrence (distribution) and average plant cover (abundance) in a large, stratified random sample of the British countryside provide no evidence that non-native plant species continue to expand and increase in abundance, relative to native species. Furthermore, native plant species diversity increased in places where non-native plant diversity increased, providing no support for the hypothesis that communities of non-native species will eventually out-compete native plants. This parallels the finding that increased numbers of non-native plant species have established in the United States in locations with high native species richness (34). Non-native species have also increased in locations where humans have created novel environments, particularly in urban environments (35), which were not included in the Countryside Survey. For Britain, at least, the non-native species have supplemented, rather than excluded, the native flora.
Using repeat census field survey data for British plants from 1990 and 2007, we find that the sum total of area changes of native plant species is an order of magnitude greater than the changes to the abundances of non-native species, indicating that native, rather than non-native, plant species dominate vegetation changes. This strong influence of native species arises because there are more native plant species (85% of the 531 plant species recorded in at least one site in both surveys), and they tend to be more widespread (Figs. 1A and 2A), rather than because there were any fundamental differences in the population trajectories of plants that arrived in Britain at different times in the past. These same quadrats only detected 81 (<5%) non-native plant species present in both survey years, of a total of 1,728 non-native plant species in the flora (36), emphasizing that most non-native species remain too localized to have national-scale effects on other species.
The behavior of neophytes and archaeophytes was indistinguishable from that of native species, measured as changes in numbers of sites occupied and in changes in percentage cover (Fig. 3 and Table 1). Some archaeophytes have continued to spread, as required by the time-to-exclusion hypothesis, but others have contracted and declined in abundance (Dataset S1). Nonetheless, there were two differences between the three groups of species. Native species and archaeophytes were more widespread than neophytes, suggesting that increased time may provide opportunities for range expansion (37), despite the fact that recent rates of change do not differ (Fig. 3A and Table 1). Second, the more recently established neophytes were more abundant than archaeophytes and native species, in terms of mean plant cover per species. The difference between neophytes and native species can be attributed to direct management. Five of the six most abundant neophytes are actively planted for wood products (P. sitchensis, P. abies, P. contorta), vegetable oil (B. napus), and grass forage (L. multiflorum), and hence their high abundance is associated with continuing forestry and farming interventions, rather than being cases of biological invasion after their initial introduction.
When these five neophytes were excluded, native species and neophytes no longer differed significantly in their average cover (P = 0.05 for 1990; P = 0.26 for 2007), although the remaining neophytes still had significantly greater cover than archaeophytes in 2007 (P = 0.05 for 1990; P = 0.005 for 2007; the native/archaeophyte analysis was unaffected; Table S1). Excluding these five neophytes, there were still no significant differences between the three groups of plants in their changes in abundance or distribution (Table S2). These results indicate that there are some differences in the histories and management of the three groups of plants (which is clearly true, given their different times of arrival in Britain), but that their recent performances (distribution and abundance changes) have not differed.
Although the changes in frequencies of occurrence and abundances were only recorded over a period of 17 years, this duration was sufficient to detect a significant positive correlation between diversity changes of native and non-native species, the opposite of what might have been expected if non-native species were in general causing declines in native diversity. Of course, some non-native species become common in some locations, and thereby alter the local flora, and there may be local implications for conservation, but we find no evidence that non-native species drive such changes at a national scale or that they do so any more than native species. In fact, we find the reverse; cover increases by native plants were greater than cover increases by non-native plants.
Whether our conclusions will apply to isolated and endemic-rich floras requires further examination. The glacial history of northern Europe may have resulted in incomplete saturation of the present-day flora (38, 39), and hence an increased capacity to assimilate new introduced species without driving native species extinct. However, Britain is not exceptional in this. A considerable portion of the world’s land surface has undergone major vegetation change since the last glacial maximum (40, 41), and the new vegetation of many regions may not have become saturated with species in the Holocene. The tendency for plant introductions to increase regional diversity, even on oceanic islands (which are also unlikely to be saturated) (27), and for biotic exchanges to increase net diversity on geological timescales (42, 43) suggests that other regions may also be able to assimilate large additional floras without (many) losses. We do not dispute that major vegetation changes associated with invasive plants can arise when new plant functional types arrive in regions that lack them (e.g., 44, 45). However, we suggest they are not representative of changes over much of the Earth’s land surface.
If interspecific competition has been contributing to changes to the composition of British plant communities in recent decades, then it is helpful to consider which species might be responsible. The largest absolute changes, in terms of numbers of sites and cover, were in native, rather than non-native, species. Summed across species, more than nine times as great a total cover increase was achieved by all native species compared with the increases by all non-native plants (combining neophytes and archaeophytes). Native species also dominated abundance changes in the subset of sites where native species diversity declined. Thus, any competitive effects must predominantly have been caused by species that are longstanding members of the native flora, rather than by introduced plant species.
The lack of significant differences in abundance and distribution trajectories of introduced and native plants, some increasing and some decreasing, implies that factors other than date of introduction have been more important determinants of the fates of each species during the last few decades. Changes to the abundances and frequencies of occurrence of plants in the countryside, of which there are many, predominantly represent species-specific responses to environmental drivers, such as nitrogen deposition, changed land management, and climate change (46–49), rather than to invasion. We suggest, therefore, that the prominence of non-native plants in lists of invasive species is likely to be out of proportion to the real threat they pose to other species.
Materials and Methods
Data Acquisition and Species Classification.
Countryside Survey data were downloaded from www.countrysidesurvey.org.uk (accessed August 27, 2014). The Countryside Survey comprises field surveys in 1 km2 sites in England, Wales, and Scotland that were selected to provide a representative sample of environmental types in Great Britain (49). Within each site, detailed surveys of vegetation are carried out. We use data collected from the large “main” quadrats (200 m2), which are randomly placed within each site (50); the number of these quadrats per site averaged 4.81 ± 0.61 SD across the two surveys (49). We use Countryside Survey data from sites visited in both 1990 and 2007, which covers a sufficient period and number of repeat-sampled sites (n = 479 sites) that we could calculate changes in vegetation cover and species occurrence.
Species were classified as native (“natural” postglacial invasion), archaeophytes (introduced up to 1500), and neophytes (introduced after 1500) (35–37, 51); 782 species, classified as native (n = 632 species), archaeophyte (n = 77 species), or neophyte (n = 73 species), were included in the analyses, representing the species that were sufficiently widespread and abundant in Britain to be recorded in the random Countryside Survey main quadrats. We only considered higher plant species for which field recording was reliable and consistent between periods. Therefore, we excluded from analysis a further 248 other higher plant “species” because they were taxonomically ambiguous, leading to identification issues for field workers, or if there was ambiguity over the dates of arrival. Excluded species included genus-only aggregates (n = 42); genus-only records (n = 163); sensu latu records (n = 14); “native hybrids” (n = 4); “native aliens,” for which part of their Great Britain range was through introduction (n = 13); and “alien hybrids” (n = 2). We also excluded marine species (n = 2), for which the survey plots were not appropriate; “alien casuals” (n = 8) that are not thought to be naturalized; and two introduced species (Mahonia japonica and Chenopodium quinoa), whose classifications as neophytes or archaeophytes were uncertain.
Data Analysis.
The absolute changes in the frequency of occurrence (number of 1 km2 sites) and in the percentage cover (per quadrat per site, including zeros) of each species between 1990 and 2007 were calculated. When calculating cover, we included only those species that were recorded in at least 10 sites in both survey years (n = 217 species). To calculate mean percentage cover of each species (per quadrat per site) in 1990 and in 2007, we calculated the mean percentage cover per quadrat in each site (i.e., sum of percentage cover in a site divided by the number of quadrats in that site), summed these values, and then divided by the total number of sites surveyed in both years (n = 479 sites). We included the cover of the excluded species (aggregates etc.; see Data Acquisition and Species Classification.) and of bare ground as part of total cover in the denominator. Absolute changes in the percentage cover and in the frequency of occurrence (number of sites) of each species were calculated by taking the values in 1990 from the values in 2007. Differences between native, archaeophyte, and neophyte species in their percentage cover and in their frequency of occurrence were analyzed using Kruskal-Wallis tests, given the nonnormality of the response variables. Absolute change in the number of native species (max = 632) and the number of non-native species (max = 150, comprised of archaeophytes plus neophytes) recorded in each of the 479 sites between 1990 and 2007 was calculated; a generalized linear model was used to investigate the relationship between change in the diversity of native and of non-native species, using a “TF” error distribution in the GAMLSS package in R. All analysis was conducted using R (52).
Supplementary Material
Acknowledgments
Thanks to Kevin Walker (Botanical Society of the British Isles), Niall Moore (Non-native Species Secretariat for Great Britain), and two anonymous referees for helpful comments on the manuscript. The Countryside Survey is conducted by the Natural Environment Research Council Centre for Ecology and Hydrology. The Countryside Survey of 2007 was funded by a partnership of nine government-funded bodies led by the Natural Environment Research Council and the Department for Environment, Food and Rural Affairs. G.P. and C.D.T. are supported by Natural Environment Research Council Grant NE/K00381X/1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1423995112/-/DCSupplemental.
References
- 1. Global Invasive Species Database. Welcome to the Global Invasive Species Database. Available at www.issg.org/database/welcome/. Accessed September 11, 2014.
- 2.Mora C, Tittensor DP, Adl S, Simpson AGB, Worm B. How many species are there on Earth and in the ocean? PLoS Biol. 2011;9(8):e1001127. doi: 10.1371/journal.pbio.1001127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z-Q. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa. 2011;3148:1–237. doi: 10.11646/zootaxa.3703.1.1. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z-Q. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness (Addenda 2013) Zootaxa. 2013;3703:1–82. doi: 10.11646/zootaxa.3703.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Roy H, et al. Non-native species in Great Britain: Establishment, detection and reporting to inform effective decision making. Appendix 5. Defra; London: 2012. [Google Scholar]
- 6.Roy HE, et al. GB Non-native Species Information Portal: Documenting the arrival of non-native species in Britain. Biol Invasions. 2014;16(12):2495–2505. [Google Scholar]
- 7.Gurevitch J, Padilla DK. Are invasive species a major cause of extinctions? Trends Ecol Evol. 2004;19(9):470–474. doi: 10.1016/j.tree.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Sax DF, Gaines SD, Brown JH. Species invasions exceed extinctions on islands worldwide: A comparative study of plants and birds. Am Nat. 2002;160(6):766–783. doi: 10.1086/343877. [DOI] [PubMed] [Google Scholar]
- 9.Sax DF, Gaines SD. Species diversity: From global decreases to local increases. Trends Ecol Evol. 2003;18(11):561–566. [Google Scholar]
- 10.Pyšek P, et al. A global assessment of invasive plant impacts on resident species, communities and ecosystems: The interaction of impact measures, invading species' traits and environment. Glob Change Biol. 2012;18(5):1725–1737. [Google Scholar]
- 11.Pearman D, Walker K. Alien plants in Britain, a real or imagined problem? British Wildlife. 2009;21:22–27. [Google Scholar]
- 12.Thomas CD. Local diversity stays about the same, regional diversity increases, and global diversity declines. Proc Natl Acad Sci USA. 2013;110(48):19187–19188. doi: 10.1073/pnas.1319304110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savidge JA. Extinction of an island forest avifauna by an introduced snake. Ecology. 1987;68(3):660–668. [Google Scholar]
- 14.Mack RN, et al. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol Appl. 2000;10(3):689–710. [Google Scholar]
- 15.Blackburn TM, Cassey P, Duncan RP, Evans KL, Gaston KJ. Avian extinction and mammalian introductions on oceanic islands. Science. 2004;305(5692):1955–1958. doi: 10.1126/science.1101617. [DOI] [PubMed] [Google Scholar]
- 16.Duncan RP, Boyer AG, Blackburn TM. Magnitude and variation of prehistoric bird extinctions in the Pacific. Proc Natl Acad Sci USA. 2013;110(16):6436–6441. doi: 10.1073/pnas.1216511110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCallum H. Disease and the dynamics of extinction. Philos Trans R Soc Lond B Biol Sci. 2012;367(1604):2828–2839. doi: 10.1098/rstb.2012.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkinson CT, LaPointe DA. Introduced avian diseases, climate change, and the future of Hawaiian honeycreepers. J Avian Med Surg. 2009;23(1):53–63. doi: 10.1647/2008-059.1. [DOI] [PubMed] [Google Scholar]
- 19.Strauss A, White A, Boots M. Invading with biological weapons: The importance of disease-mediated invasions. Funct Ecol. 2012;26(6):1249–1261. [Google Scholar]
- 20.Vitousek PM, Walker LR. Biological invasion by Myrica faya in Hawai'i: Plant demography, nitrogen fixation, ecosystem effects. Ecol Monogr. 1989;59(3):247–265. [Google Scholar]
- 21.Le Maitre DC, et al. Impacts of invasive Australian acacias: Implications for management and restoration. Divers Distrib. 2011;17(5):1015–1029. [Google Scholar]
- 22.Rundel PW, Dickie IA, Richardson DM. Tree invasions into treeless areas: Mechanisms and ecosystem processes. Biol Invasions. 2014;16(3):663–675. [Google Scholar]
- 23.Ricciardi A, Simberloff D. Assisted colonization is not a viable conservation strategy. Trends Ecol Evol. 2009;24(5):248–253. doi: 10.1016/j.tree.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Essl F, et al. Socioeconomic legacy yields an invasion debt. Proc Natl Acad Sci USA. 2011;108(1):203–207. doi: 10.1073/pnas.1011728108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson DM, Ricciardi A. Misleading criticisms of invasion science: A field guide. Divers Distrib. 2013;19(12):1461–1467. [Google Scholar]
- 26.Simberloff D. Biological invasions: What's worth fighting and what can be won? Ecol Eng. 2014;65:112–121. [Google Scholar]
- 27.Sax DF, Gaines SD. Colloquium paper: Species invasions and extinction: The future of native biodiversity on islands. Proc Natl Acad Sci USA. 2008;105(Suppl 1):11490–11497. doi: 10.1073/pnas.0802290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell KI, Chase JM, Knight TM. A synthesis of plant invasion effects on biodiversity across spatial scales. Am J Bot. 2011;98(3):539–548. doi: 10.3732/ajb.1000402. [DOI] [PubMed] [Google Scholar]
- 29.Strong DR, Lawton JH, Southwood R. Insects on Plants. Blackwell Scientific Publications; Oxford: 1984. [Google Scholar]
- 30.Anderson PK, et al. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 2004;19(10):535–544. doi: 10.1016/j.tree.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 31.Daehler CC. Performance comparisons of co-occurring native and alien invasive plants: Implications for conservation and restoration. Annu Rev Ecol Evol Syst. 2003;34:183–211. [Google Scholar]
- 32.Levine JM, Adler PB, Yelenik SG. A meta-analysis of biotic resistance to exotic plant invasions. Ecol Lett. 2004;7(10):975–989. [Google Scholar]
- 33.Heard MJ, Sax DF. Coexistence between native and exotic species is facilitated by asymmetries in competitive ability and susceptibility to herbivores. Ecol Lett. 2013;16(2):206–213. doi: 10.1111/ele.12030. [DOI] [PubMed] [Google Scholar]
- 34.Stohlgren TJ, Barnett DT, Kartesz JT. The rich get richer: Patterns of plant invasions in the United States. Front Ecol Environ. 2003;1(1):11–14. [Google Scholar]
- 35.Botham MS, et al. Do urban areas act as foci for the spread of alien plant species? An assessment of temporal trends in the UK. Divers Distrib. 2009;15(2):338–345. [Google Scholar]
- 36.Preston CD, Pearman DA, Dines TD. New Atlas of the British & Irish flora. Oxford Univ Press; Oxford: 2002. [Google Scholar]
- 37.de Albuquerque FS, Castro-Díez P, Rueda M, Hawkins BA, Rodríguez MÁ. Relationships of climate, residence time, and biogeographical origin with the range sizes and species richness patterns of exotic plants in Great Britain. Plant Ecol. 2011;212(11):1901–1911. [Google Scholar]
- 38.Svenning JC, Skov F. Limited filling of the potential range in European tree species. Ecol Lett. 2004;7(7):565–573. [Google Scholar]
- 39.Svenning JC, Skov F. Ice age legacies in the geographical distribution of tree species richness in Europe. Glob Ecol Biogeogr. 2007;16(2):234–245. [Google Scholar]
- 40.Harrison SP, Prentice CI. Climate and CO2 controls on global vegetation distribution at the last glacial maximum: Analysis based on palaeovegetation data, biome modelling and palaeoclimate simulations. Glob Change Biol. 2003;9(7):983–1004. [Google Scholar]
- 41.Bartlein PJ, et al. Pollen-based continental climate reconstructions at 6 and 21 ka: A global synthesis. Clim Dyn. 2011;37(3–4):775–802. [Google Scholar]
- 42.Tilman D. Diversification, biotic interchange, and the universal trade-off hypothesis. Am Nat. 2011;178(3):355–371. doi: 10.1086/661245. [DOI] [PubMed] [Google Scholar]
- 43.Pinto-Sánchez NR, Crawford AJ, Wiens JJ. Using historical biogeography to test for community saturation. Ecol Lett. 2014;17(9):1077–1085. doi: 10.1111/ele.12310. [DOI] [PubMed] [Google Scholar]
- 44.Vitousek PM. Biological invasions and ecosystem processes: Towards an integration of population biology and ecosystem studies. Oikos. 1990;57(1):7–13. [Google Scholar]
- 45.Liao C, et al. Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytol. 2008;177(3):706–714. doi: 10.1111/j.1469-8137.2007.02290.x. [DOI] [PubMed] [Google Scholar]
- 46.Maskell LC, Smart SM, Bullock JM, Thompson K, Stevens CJ. Nitrogen deposition causes widespread loss of species richness in British habitats. Glob Change Biol. 2010;16(2):671–679. [Google Scholar]
- 47. Lim J, Crawley MJ, De Vere N, Rich T, Savolainen V. A phylogenetic analysis of the British flora sheds light on the evolutionary and ecological factors driving plant invasions. Ecol Evol 2014;4(22):4258–4269. [DOI] [PMC free article] [PubMed]
- 48.Doxford SW, Freckleton RP. Changes in the large-scale distribution of plants: Extinction, colonisation and the effects of climate. J Ecol. 2012;100(2):519–529. [Google Scholar]
- 49.Carey PD, et al. Countryside Survey: UK Results from 2007. Natural Environment Research Council; Swindon, United Kingdom: 2008. [Google Scholar]
- 50.Maskell LC, et al. Countryside Survey. Vegetation Plots Handbook. Natural Environment Research Council; Swindon, United Kingdom: 2008. [Google Scholar]
- 51.Hulme PE. Relative roles of life-form, land use and climate in recent dynamics of alien plant distributions in the British Isles. Weed Res. 2009;49(1):19–28. [Google Scholar]
- 52.R Core Team 2013 The R Project for Statistical Computing. Available at www.r-project.org/. Accessed February 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.