Significance
Scaffold proteins can serve as critical focal points for association of signaling molecules and downstream pathways that regulate tumor growth and invasion. We demonstrate that low oxygen levels, common in solid tumors, can regulate expression of one member of the AKAP scaffold protein family, AKAP12, in melanoma. Genetic inactivation of AKAP12 leads to decreased migration, invasion, and tumor growth in a mouse model of melanoma. Mechanistically, we discovered a switch in protein kinase A (PKA)-regulated phosphorylations under hypoxia that are dependent on AKAP12 and show that PKA is the critical kinase regulating AKAP12-dependent cellular migration. These results provide novel insight into how the tumor microenvironment modulates signal transduction and biological responses through the regulation of a specific variant of the scaffold protein AKAP12.
Keywords: AKAP12, melanoma, metastasis
Abstract
Scaffold proteins are critical hubs within cells that have the ability to modulate upstream signaling molecules and their downstream effectors to fine-tune biological responses. Although they can serve as focal points for association of signaling molecules and downstream pathways that regulate tumorigenesis, little is known about how the tumor microenvironment affects the expression and activity of scaffold proteins. This study demonstrates that hypoxia, a common element of solid tumors harboring low oxygen levels, regulates expression of a specific variant of the scaffold protein AKAP12 (A-kinase anchor protein 12), AKAP12v2, in metastatic melanoma. In turn, through a kinome-wide phosphoproteomic and MS study, we demonstrate that this scaffolding protein regulates a shift in protein kinase A (PKA)-mediated phosphorylation events under hypoxia, causing alterations in tumor cell invasion and migration in vitro, as well as metastasis in an in vivo orthotopic model of melanoma. Mechanistically, the shift in AKAP12-dependent PKA-mediated phosphorylations under hypoxia is due to changes in AKAP12 localization vs. structural differences between its two variants. Importantly, our work defines a mechanism through which a scaffold protein can be regulated by the tumor microenvironment and further explains how a tumor cell can coordinate many critical signaling pathways that are essential for tumor growth through one individual scaffolding protein.
Scaffold proteins, also known as anchoring proteins, are of crucial importance within cells because they allow dynamic association of signaling molecules, often present in low abundance, by concentrating them at discrete subcellular localizations, forming intricate signal transduction complexes. However, the mechanisms by which scaffold proteins are regulated within the tumor microenvironment and how altering their expression can result in a switch between kinase targets and promote tumor progression remain unknown. Importantly, scaffold proteins have recently been identified as new therapeutic targets, highlighting the necessity of understanding the biology behind these potential targets (1). In this paper, we focus on the scaffold protein AKAP12, which is a member of the larger “A Kinase Anchoring Protein” family that regulates signal transduction from the cell surface to the cytoskeleton. Previous studies have reported that AKAP12 binds to protein kinase A (PKA), protein kinase C (PKC), Src, and the actin cytoskeleton, all involved in tumorigenesis (2). However, the role of the tumor microenvironment in regulating the scaffolding protein AKAP12 and the outputs of these downstream signaling cascades has not been fully investigated.
Hypoxia, or tumor cell oxygen deficiency, is a component of the tumor microenvironment and is strongly linked to metastasis and the poor clinical outcome of patients, through the activation of specific transcriptional, translational, and signaling programs (3–8). Induction of the hypoxia-inducible transcription factor (HIF) family, consisting of HIF-1, -2, and -3, acts to regulate tumorigenic processes in response to low oxygen levels. Under normal oxygen levels, the prolyl-4-hydroxylases (PHDs) hydroxylate HIF-1α on two conserved proline residues, causing its recognition by the von Hippel-Lindau (VHL) tumor suppressor and its subsequent degradation (9). Under states of acute or chronic hypoxia, HIF-1α becomes stabilized because hydroxylation and degradation of this transcription factor by the PHDs is an oxygen-dependent process (10). This stabilization of HIF-1α allows association with HIF-1β (ARNT) and formation of a heterodimer that binds to core sequences within the DNA, 5′-RCGTG-3′, allowing transcription of target genes that can control cellular processes including angiogenesis, glucose metabolism, cell proliferation, and tissue remodeling (11, 12).
Here we report a previously unrecognized role for hypoxia in the induction of the scaffold protein AKAP12, more specifically of its second variant, AKAP12v2, which regulates melanoma tumor growth and metastasis. We demonstrate that AKAP12v2 is hypoxia inducible and a HIF-1α transcriptional target, leading to elevated expression at both the RNA and protein level in human melanoma cell lines and in patient tissues. Functionally, through a kinome-wide study of the phosphoproteome through MS analysis, we identified AKAP12-dependent and oxygen-regulated phosphorylation changes that demonstrate an important role for AKAP12 in cell survival, migration, and invasion. In particular, our analysis identified a switch in PKA substrate phosphorylation in the presence of hypoxia, with its inhibition abrogating migration in an AKAP12-dependent manner. Importantly, we wanted to determine the mechanism behind this shift in AKAP12-dependent PKA-mediated phosphorylation of proteins, and determined this is due to increased expression of AKAP12v2 and localization to the cytoplasm under hypoxia. These studies were expanded using genetic knockdown of AKAP12, which demonstrated that the most significant functional pathways identified in the kinome-wide phosphorylation study, including cell adhesion/motility, were also severely inhibited when AKAP12 is lost in melanoma, both in vitro and in vivo.
Results
AKAP12 Is Elevated in Human Metastatic Melanoma.
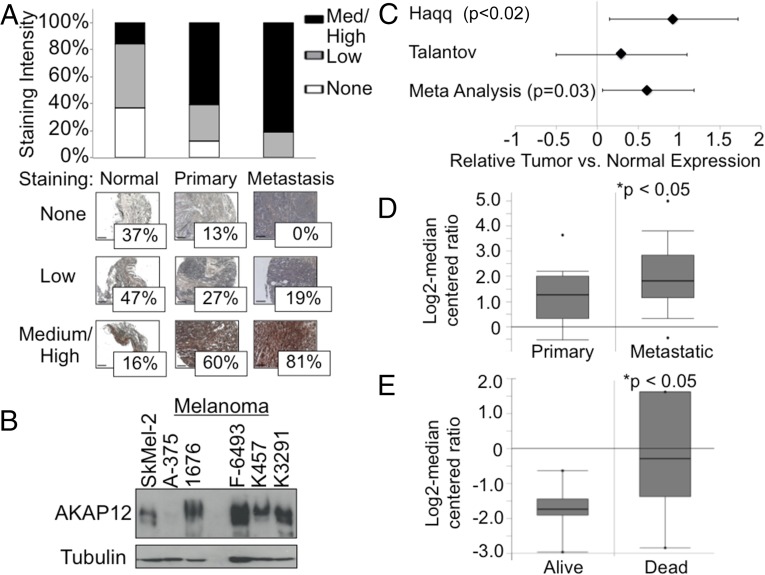
To determine the relevance of our findings in regard to AKAP12 in human melanoma, we examined AKAP12 expression in a melanoma tissue microarray with normal, primary, and metastatic melanoma specimens. We found that metastatic samples had high levels of AKAP12 expression, as do primary tumors, compared with normal skin (Fig. 1A). We also examined a series of malignant melanoma- (A-375) and metastatic melanoma-derived cell lines (Sk-Mel-2, 1676, F-6493, K457, and K3291), in which we found high levels of AKAP12 expression (Fig. 1B). Meta-analysis of two different expression studies from the Oncomine database also showed a statistically significant difference in AKAP12 expression (standardized mean difference) in tumor vs. normal specimens (P = 0.03; Fig. 1C) (13, 14). A separate study by Xu et al. deposited in the Oncomine database revealed that AKAP12 expression is significantly increased in patients with metastatic melanoma vs. primary disease (P < 0.05; Fig. 1D). In addition, data from Bittner et al. in the Oncomine database demonstrate that AKAP12 expression correlates with decreased survival in melanoma patients (P < 0.05; Fig. 1E) (15, 16). These findings highlight an important clinical association between AKAP12 expression in human melanoma and poor survival, supporting a functional role for AKAP12 in human melanoma progression.
Fig. 1.
AKAP12 is elevated in human melanoma and its expression correlates to decreased survival. (A) Core specimens (n = 185) were probed for AKAP12 and scored based on staining intensity. Representative tissues shown. (Scale bar, 100 µm.) (B) Protein lysates probed for AKAP12 expression. (C) Comprehensive meta-analysis of two studies in the Oncomine database show a significant difference in AKAP12 expression (standardized mean difference) in tumor vs. normal specimens. (D) Study by Xu et al. (16) deposited in the Oncomine database showing an increase in AKAP12 expression in patients with metastatic melanoma vs. primary disease. (E) Data from the Oncomine database by Bittner et al. (15) correlating AKAP12 expression and overall survival in melanoma patients.
AKAP12 Is a Hypoxia and HIF Regulated Gene.
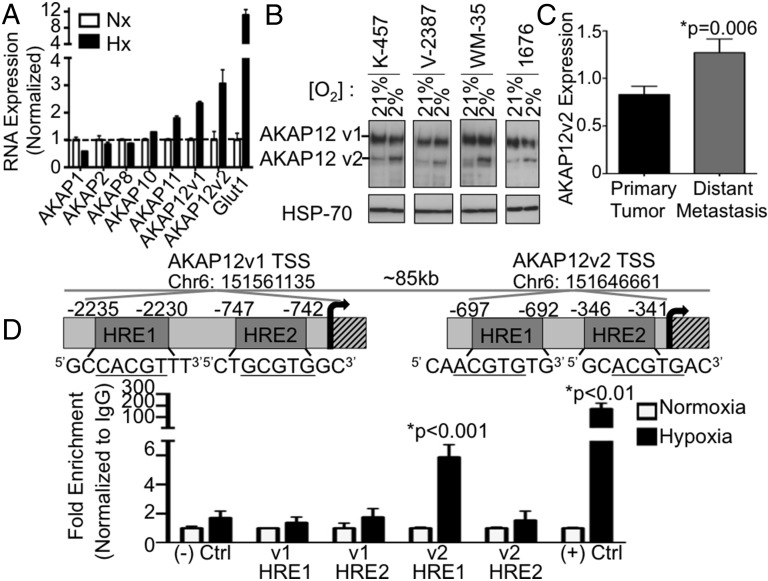
We were interested to follow up on our results in metastatic melanoma, because it is known that both HIF and hypoxia increase the aggressive nature of the disease (17, 18). A panel of AKAP family members in K457 melanoma cells was examined for hypoxic induction, with AKAP12 mRNA induced by hypoxia (Fig. 2A). Next, hypoxic induction of AKAP12 at the protein level was examined, which resulted in specific induction of the smaller variant (AKAP12v2) in melanoma cell lines (Fig. 2B). We then asked whether AKAP12v2 specifically is important for human disease. Using a large dataset from the The Cancer Genome Atlas (TCGA), we determined AKAP12v2 is significantly increased in distant metastasis samples vs. primary tumors, as well as in patients who were deceased by the final follow-up, whereas AKAP12v1 was not significantly increased in either of these situations (Fig. 2C and Fig. S1 A–C). To determine whether the induction of AKAP12 by hypoxia was direct, chromatin immunoprecipitation (ChIP) assays were performed to examine HIF-1α binding to AKAP12 variant 1 (AKAP12v1) and AKAP12 variant 2 (AKAP12v2). It is known that these two variants are derived from separate promoters, resulting in isoforms of 305 and 287 kDa, respectively (19, 20). Therefore, HIF-1α binding to HREs within each promoter was examined, from which we found that HIF-1α binds the AKAP12v2 promoter under hypoxia at a hypoxia response element (HRE) located 697 bp upstream from the transcriptional start site. No HIF binding was detected in the HREs examined for AKAP12v1 (Fig. 2D). This data agrees with our protein data demonstrating hypoxic induction of AKAP12v2 (Fig. 2B) and is the first identification, to our knowledge, of hypoxic regulation of a specific variant of AKAP12.
Fig. 2.
AKAP12v2 is a direct HIF target. (A) qRT-PCR using primers specific to AKAP family members in K457 cells, values normalized to Nx ± SEM. (B) Protein expression in melanoma cell lines following 16 h in Nx or Hx. (C) Average AKAP12v2 expression ± SEM in patient samples from the TCGA. Primary tumor (n = 74) and distant Metastasis (n = 39). (D) HIF-1α ChIP showing induction of both Jumonji domain-containing protein 1A (positive control) and AKAP12 under hypoxia, normalized to IgG. Graph represents results from multiple qRT-PCRs ± SEM.
Although the relationship between HIF-1α and melanocyte cell survival, proliferation, and invasion has been studied extensively, the role of HIF-2α remains less clearly defined. Because both HIF-1α and HIF-2α have been shown to be increased in melanomas, we aimed to test whether HIF-2α is also involved in hypoxic induction of AKAP12v2. To do this, we generated stable K457 cells with shRNA to both HIF-1α and HIF-2α. Using these stable cell lines, we found that knockdown of either HIF-1α or HIF-2α prevented significant induction of AKAP12v2 under hypoxia (Fig. S1D). Taken together, these data suggest that HIF-1α and HIF-2α are involved in hypoxic induction of AKAP12v2.
Functional Role of AKAP12 in Metastatic Melanoma.
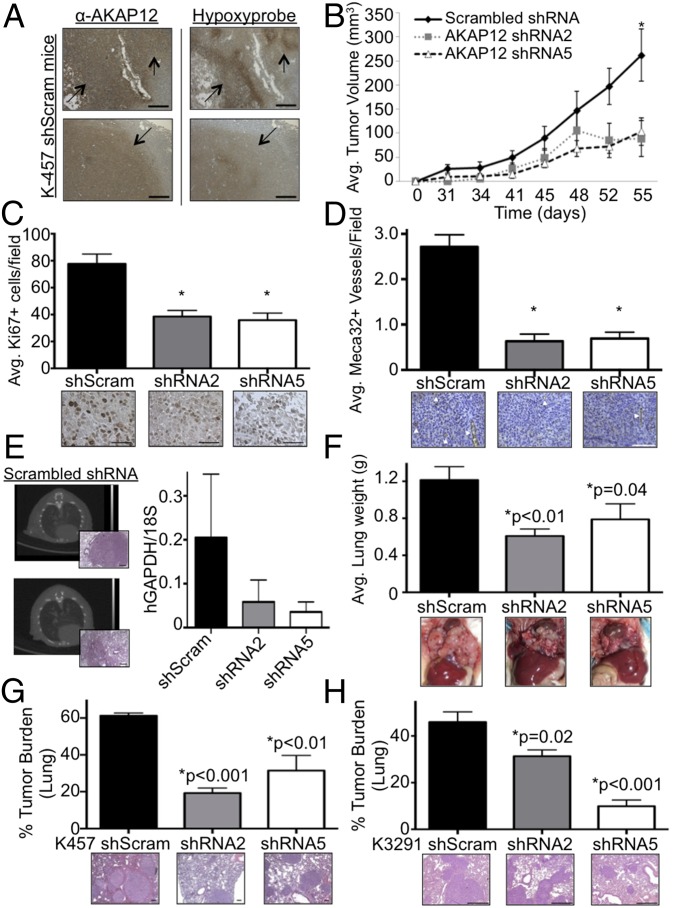
Because we identified AKAP12 as a HIF-regulated gene in melanoma, we next wanted to examine the functional importance of AKAP12 in melanoma tumor growth and metastatic disease in an orthotopic mouse model that mimics the environment in which melanomas normally develop within the dermal layer of skin. To address the relationship of hypoxia and AKAP12 in vivo, mice with AKAP12-expressing K457 cell tumors were injected with pimonidazole before death to mark areas of hypoxia. Indeed, there was overlap between positive AKAP12 staining and hypoxic regions within the tumors, linking AKAP12 expression and hypoxia in vivo (Fig. 3A). Examining primary tumor growth in this model, we found a statistically significant decrease in tumor volume in orthotopic tumors derived from K457 melanoma cell lines stably expressing two different shRNAs against AKAP12 compared with genetically matched K457 shScram cells (Fig. 3B). Inhibition of AKAP12 expression was confirmed at both the RNA and protein level (Fig. S2A). Because tumor growth curves showed differences in primary tumor growth with genetic knockdown of AKAP12, we next assessed proliferation differences by Ki67 staining and found a 50% (shRNA2) and 54% (shRNA5) decrease compared with shScram tumors (Fig. 3C). Examination of apoptosis, through TUNEL staining on primary tumors and flow cytometry in vitro, revealed no differences in cell death between the groups (Fig. S2 C and D). Primary tumors stained with Meca32 showed a significant decrease in vessel density if AKAP12 was inhibited (Fig. 3D). These results demonstrate that AKAP12 increases primary tumor growth through changes in proliferation and angiogenesis but not apoptosis.
Fig. 3.
AKAP12 knockdown suppresses primary tumor growth and lung metastasis in an orthotopic model of melanoma. (A) Serial sections of intradermal tumors stained for AKAP12 and hypoxia (Hypoxyprobe). Arrows indicate overlap. (B) K457 shScram or shAKAP12 stable cells were injected intradermally into mice and average tumor growth ± SEM is shown (*P ≤ 0.05, two-tailed t test). (C) Average Ki67+ cells per field ± SEM, with three fields per mouse; *P < 0.01, two-tailed t test. Representative images at 40×. (Scale bar, 50 µm.) (D) Average Meca32+ vessels per field ± SEM, with six fields per mouse, *P < 0.001, two-tailed t test. Images at 40×. (Scale bar, 100 µm.) (E) Metastasis was detected in the shScram mice through CT scan and confirmed on H&E sections of the lung (2 of 6; images at 5× in the orthotopic model). (Scale bar, 200 µm.) qRT-PCR on shScram vs. shAKAP12 injected mice to examine RNA expression of hGAPDH/18S within the lungs. (F) K457 shScram or shAKAP12 stable cells were injected into the tail vein of mice, and lung colonization, a measurement of metastatic potential, was assessed (*P = 0.01 and *P = 0.04, one-tailed t test). (G and H) Average tumor burden of tail vein injected mice with K457 (G) and K3291 stable cells (H) is graphed ± SEM (two-tailed t test). Representative H&E shown. [Scale bar, 100 (G) and 400 µm (H).]
We extended our studies to investigate the importance of hypoxia-induced expression of AKAP12 on metastatic potential in our orthotopic mouse model. After primary melanomas were removed surgically, mice were monitored for the development of metastatic lesions, which was detected visually by necropsy and H&E staining, as well as quantified at the RNA level for human specific GAPDH. Three of six shScram mice had visible tumor burden in the thymus or in the lungs, as detected by necropsy or H&E staining. Macroscopic metastases were not observed during necropsy or H&E in lungs from any of the mice injected with AKAP12-deficient cells (0/10; Fig. 3E). These findings were confirmed quantitatively by quantitative real-time PCR (qRT-PCR) analysis of hGAPDH expression in mouse lung tissue. The average expression of hGAPDH was reduced in shAKAP12 lungs compared with shScram lungs (Fig. 3E). These findings demonstrate that in an orthotopic model of human melanoma, AKAP12 regulates both primary tumor growth and metastasis.
To directly assess the role of AKAP12 in late stages of lung metastasis, we performed a study in which equal numbers of K457 cells were injected into the tail vein of mice. This method removes the possibility of different numbers of circulating cells, due to different primary tumor sizes between groups, leading to differences in metastatic disease. To rule out potential differences in survival while in circulation leading to different metastatic burdens, we tested the cells for their ability to survive when detached from a substrate. No differences were observed with or without AKAP12 (Fig. S3A). However, there were differences in overall cell growth and survival when cells were grown in agar or under attachment conditions (Fig. S3 B and C). Lung tumor burden using this tail vein model was determined by measuring total lung weight, which was significantly reduced in shAKAP12 mice compared with shScram mice (Fig. 3F). Analysis of lungs from these mice indicated that K457 shScram-injected mice had 61% tumor burden in their lungs, whereas shAKAP12 mice had statistically less tumor burden: 19% and 31%, respectively (Fig. 3G). Similar results were found using a second cell line (Fig. 3H). These data lead us to conclude that AKAP12 is a critical factor for both early and late stages of melanoma lung metastasis.
Kinome-Wide Phosphoproteomics Identify Phosphorylation Changes Regulated by Hypoxic Induction of AKAP12.
AKAPs were originally discovered as platforms for conducting PKA and PKC phosphorylation and G protein signaling events. Thus, AKAP12 serves in large part as a scaffold for the assembly of protein complexes involved in signal transduction, in particular those mediated by serine/threonine and tyrosine kinases. On this basis, and to gain insight into mechanisms by which AKAP12 could alter oncogenic processes, we used a targeted-phosphoproteomic approach to identify phosphoprotein changes that were AKAP12-dependent under oxic (21% O2) and hypoxic (0.5% O2, 20 h) conditions using the metastatic melanoma cell line K457, stably expressing either shScram or shAKAP12. The complete experimental design is outlined in Fig. S4.
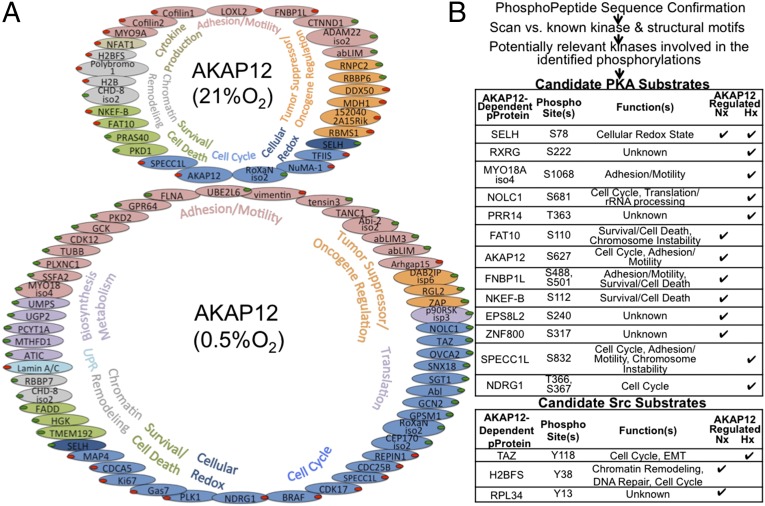
Through MS analysis, we identified specific phosphorylation changes that are dependent on AKAP12 under hypoxia compared with normoxia. Phosphoproteins with known functions that were dependent on AKAP12 under normoxia or hypoxia, and demonstrated changes ±2.5-fold (using two independent shRNAs) are included in the diagram in Fig. 4A. A table of all identified phosphoproteins, including those with unknown function, can be found in Table S1. Importantly, under hypoxia, there was a significant shift in AKAP12-dependent phosphorylation events compared with normoxia.
Fig. 4.
Phosphoproteomic screen identifies kinome-wide AKAP12-dependent phosphorylation changes. (A) AKAP12-dependent phosphorylation changes under 21% and 0.5% O2, consistent in both shRNAs, and colored by function. Red dots indicate repression and green dots indicate induction of phosphorylation (ratio of shAKAP12/shScram). (B) The table contains predicted PKA and Src-specific phosphorylation of the phosphoproteins identified in our screen based on conserved target motif and local amino acid physical characteristics.
To determine whether the phosphoproteins identified were consistent with what we know of scaffolds, we analyzed the dataset using Ingenuity’s IPA software and PhosphoSite Plus, to determine the most significant molecular and cellular functions associated with the phosphoproteins identified in our analysis. The results were consistent with what we know of molecular scaffolds, particularly AKAPs, containing a predominance of signaling proteins (GTPases, kinases and phosphatases, metalloproteases, etc.) and the biological outcome of many signaling cascades (cell cycle, cytoskeletal, motility and adhesion regulators, etc.). More than a third of the AKAP12-dependent phosphoproteins had known roles in invasion and metastasis, with another third having defined roles in other oncogenic processes.
Hypoxia, Through AKAP12, Serves as a Physiological Switch in PKA Signaling.
Next we used conserved target motif and local amino acid physical characteristics to predict kinase-specific phosphorylation of each change identified in our screen. GPS 2.1.2 (group-based prediction system) analysis of the phosphorylations revealed 74 candidate kinases, with almost every site having more than one potential kinase that could phosphorylate it. As expected, several kinases have multiple potential targets, including PKA, Src, and PKC isoforms, all of which are known AKAP12 binding proteins (Fig. 4B and Dataset S1) (2). Interestingly, the number of potential PKA-mediated phosphorylation events was much larger than Src-mediated events under hypoxia (Fig. 4B). Confirmation of one of the specific PKA-induced threonine phosphorylation events from our study, pNDRG1 under hypoxia, is shown in Fig. S4B. These data are consistent with the concept that hypoxia induces the expression of AKAP12v2 that binds PKA and phosphorylates a different set of proteins than found under aerobic conditions. We therefore aimed to further analyze how this is occurring and what effect this might have on tumorigenesis.
Decreased Levels of AKAP12 Reduce Melanoma Migration and Invasion Through PKA-Regulated Phosphorylation Events.
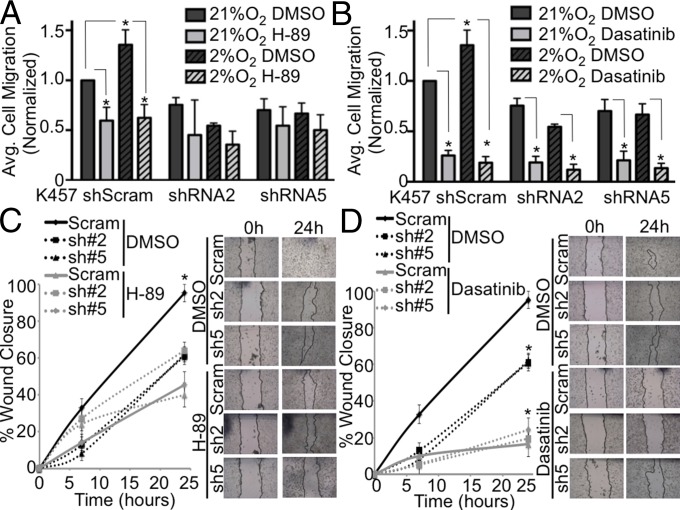
Because AKAP12 is a multifunctional scaffolding protein able to integrate multiple signaling pathways that are hypoxia inducible such as PKA and Src, we hypothesized that hypoxic regulation of AKAP12 could enhance multiple aspects of the metastatic cascade. Specifically, we examined the role of PKA and Src in AKAP12’s involvement in cell migration and invasion. Genetic knockdown of AKAP12 in K457 cells inhibited cell migration through fibronectin-coated transwells under both normoxic and hypoxic conditions compared with control shScram cells (Fig. 5A). Although hypoxia increased cell migration of control shScram cells, hypoxia-induced migration was significantly abrogated in AKAP12-shRNA knockdown cells (Fig. 5A). Because PKA is known to interact with AKAP12, we examined what effect inhibition of PKA activity had on cell migration in the presence and absence of AKAP12 using a well-studied PKA inhibitor, H-89. We found that inhibition of PKA decreased cell migration in AKAP12-expressing cells to similar levels of AKAP12 shRNA knockdown cells (Fig. 5A). Also, H-89 did not significantly alter migration in cells lacking AKAP12. In contrast, using a Src inhibitor, Dasatinib, we saw decreases in cell migration across all samples, indicating that PKA, and not Src, is acting in an AKAP12-dependent manner to regulate cell migration (Fig. 5B). This concept was further examined in wound healing of confluent cell monolayers to determine the role of PKA vs. Src in AKAP12-dependent cell migration. Similar to what we saw with the fibronectin assays, H-89 decreased migration in AKAP12-expressing cells and did not alter migration in cells lacking AKAP12 (Fig. 5C and Fig. S5A). Conversely, inhibition of Src decreased migration independent of AKAP12 expression (Fig. 5D and Fig. S5B).
Fig. 5.
AKAP12 regulates PKA-mediated cell migration of metastatic melanoma cells. (A and B) Cells exposed to 21% O2 and 2% O2 (or 0.5% O2) that migrated through fibronectin-coated transwells were graphed and normalized to K457 shScram at 21% O2. Graphs are average of two or more independent experiments per condition ± SEM. Cells were treated for 24 h with DMSO, H-89 (15 μM), or Dasatinib (50 nM); *P < 0.05, two-tailed t test. (C and D) Scratch assay in which cells were treated with DMSO, H-89 (15 μM), or Dasatinib (50 nM) for 24 h under 2% O2. Graphs are representative of two or more experiments ± SEM. Images taken at 4×; *P < 0.05, two-tailed t test.
We next examined invasion, using 3D growth assays in a type I collagen matrix. Morphologically, AKAP12-deficient cells exhibited decreased growth and branching, corresponding to decreased invasive potential (Fig. S6A). Inhibitions of PKA with H-89, and Src with Dasatinib, were both able to decrease the invasive potential seen in the AKAP12 expressing cells. Although this is not a quantitative assay, it makes the point that invasive potential is lost when AKAP12 is not present or when PKA and Src are inhibited. We also examined invasion through matrigel-coated transwells both under normoxic and hypoxic conditions. Hypoxia increased invasion in K457 shScram cells, whereas genetic knockdown of AKAP12 suppressed this hypoxia-inducible invasive potential of melanoma cells (Fig. S6B). Similar observations were seen in a second cell line, K3291 (Fig. S6C). Together, these results demonstrate that the presence of AKAP12 and its scaffolding functions are necessary for metastatic melanoma tumor cell migration and invasion in vitro. Mechanistically, this loss of invasive potential appears to be the result of a decrease in AKAP12-mediated PKA signaling, especially under hypoxic conditions.
Signaling Alterations Under Hypoxia Occur due to Changes in AKAP12 Localization.
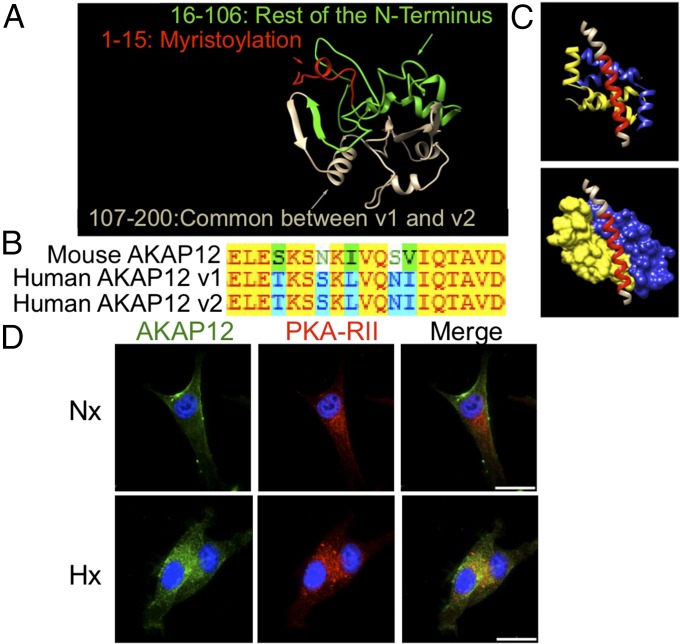
Because we see different PKA-regulated phosphorylation changes that occur under hypoxia vs. normoxia, which are important for melanoma cell migration and invasion, we naturally wanted to follow up these results to gain mechanistic insight into how this was occurring. First, we examined differences between the two variants’ structures to see if this could account for PKA signaling differences. Even though ideal conditions for crystal structure formation of AKAP12 have not yet been identified, we performed ab initio protein folding and protein structure prediction and obtained the predicted structure of AKAP12v1’s N-terminal region (first 200 amino acids), which is illustrated in Fig. 6A. Although the AKAP12 variants share >95% of their coding sequence, AKAP12v1 differs in this N-terminal region and contains a myristoylation site. Because we saw specific differences in AKAP12-dependent PKA substrate phosphorylation from our PhosphoScreen, we next wanted to determine whether there are any structural differences between AKAP12v1 and AKAP12v2 in their PKA binding region. First, examining amino acid sequences, we found that AKAP12v1 and AKAP12v2 have identical amino acid sequences at the PKA binding domain, and the human AKAP12 PKA binding domain is highly conserved compared with mouse (Fig. 6B). We next performed docking studies to predict the structure of AKAP12’s PKA-binding helix and docked it to PKA (Fig. 6C). Importantly, the accuracy of the program we used (I-TASSER) has been validated in the literature. AKAP12v1 and AKAP12v2 demonstrated identical docking, because they have identical sequences within their PKA binding domain.
Fig. 6.
PKA-mediated phosphorylation changes do not occur through structural differences between AKAP12v1 and AKAP12v2 but through changes in localization. (A) Protein structure and folding prediction of the first 200 amino acids of AKAP12v1, which differs from AKAP12v2, showing the myristoylation motif (red) and N-terminal region (green). (B) Sequence alignment of the PKA-binding region of mouse AKAP12 (1,501–1,519 aa) with human AKAP12v1 (1,541–1,559 aa) and AKAP12v2 (1,443–1,461 aa). Letters = amino acids. Red text on yellow: identical. Green text on white: weakly similar. Black text on green: block of similar. Blue text on blue: conservative. (C) Predicted protein–protein docking of AKAP12 (red) with the PKA regulatory subunit dimer (yellow and blue). (D) K457 Scram cells in normoxia or hypoxia were stained for AKAP12 (green) or PKA-RII (red). Overlap in staining (yellow) can be seen in the hypoxic samples. Representative images at 100×. (Scale bar, 25 μm.)
Because structural modeling and protein–protein docking determined there was not a structural difference between AKAP12v1 and AKAP12v2 causing the AKAP12-dependent shift in PKA substrate phosphorylation, we hypothesized it was likely due to changes in localization, because AKAP12v1’s unique N-terminal region contains a myristoylation site. To address this, we performed immunofluorescence experiments in which we stained for AKAP12 expression along with PKA-RII, a regulatory subunit of PKA that AKAP12 binds with nanomolar affinity. Interestingly, under normoxia, in which we have shown mainly expression of AKAP12v1, we see staining around the cell periphery and no major overlap with PKA-RII, which is known to be intracellular (21). Under hypoxia, a condition in which we see elevated AKAP12v2 protein expression, AKAP12 is mostly intracellular and overlaps with PKA-RII (Fig. 6D and Fig. S7A). We determined the average Pearson’s correlation for overlap between AKAP12 and PKA-RII in cells and found a significant difference between normoxia (0.15 ± 0.04) and hypoxia samples (0.32 ± 0.03, P < 0.01). In addition, in our aforementioned studies using the Src inhibitor, we did not see AKAP12-mediated changes in migration, invasion, or many AKAP12-dependent Src-mediated phosphorylation changes in our phosphoproteome study. This data is supported by the fact that Src is known to be commonly localized at the cell membrane, and our immunofluorescence studies indicate that, under hypoxia, where we see the largest AKAP12-dependent changes in cell migration, invasion, and phosphoproteome changes, AKAP12 is mostly intracellular, where it can interact with PKA but not Src. These studies indicate that the changes we see in AKAP12-dependent PKA-mediated phosphorylation events under hypoxia are due to changes in localization of the protein vs. differences in structural binding between the two variants.
Discussion
Our study brings to light functions of the scaffold protein AKAP12 in promoting melanoma tumor growth and metastasis and emphasizes the critical importance of scaffold proteins in regulating multiple signaling pathways (22). Here we demonstrate that hypoxia, or more specifically HIF-1’s ability to differentially bind to AKAP12v2 vs. AKAP12v1, is a transcriptional regulator of AKAP12v2. Previous work examining exogenous expression of the AKAP12 isoforms and deletion constructs in COS7 cells showed that the coding sequence difference between variants could account for the different subcellular localizations, including: nuclear/cytoplasmic and perinuclear/cell periphery (20, 23). Our modeling studies indicate that the main difference between AKAP12v1 and AKAP12v2 is the myristoylation site that exists at the N-terminal region of AKAP12v1 (Fig. 6A). Under hypoxia, we detect a similar staining pattern (cytoplasmic and concentrated around the nucleus) as previously seen with ectopic forced expression of AKAP12v2 in COS7 cells (20). This result supports the idea that hypoxia is regulating expression of AKAP12v2 and its localization to the cytoplasm, where it can facilitate colocalization of signaling molecules (such as PKA-RII), leading to a change in AKAP12-regulated physiological functions. Although we do see what could be some ER staining in a small fraction of our normoxia samples, as others have shown with AKAP12v1, we believe that the myristoylation site present in this isoform leads to the presence of AKAP12v1 predominately at the cell membrane. Although our work supports other studies that have examined AKAP12 isoform localization patterns, it demonstrates that a component of the microenvironment, hypoxia, can cause a physiological shift in localization of AKAP12, resulting in a shift in PKA target phosphorylation. This shift in PKA signaling is essential for invasion and metastasis. In contrast, the effect Src has on migration is independent of AKAP12.
Collectively, our work expands what is known about the tumor microenvironment and scaffold proteins in tumor progression and also supports the idea of studying scaffold proteins as new therapeutic targets in the future (1). Because we uncovered PKA as an important regulator of AKAP12-dependent migration and invasion of metastatic melanoma cells, the use of a peptide to specifically block PKA binding to AKAP12 would be interesting to examine and a critical first step leading to therapeutic potential down the road. Recent structural data have identified the specific region where the PKA RII subunit binds AKAPs with nanomolar affinity, as well as the PKA RI subunit with micromolar affinity. The development and use of cell soluble scaffold-kinase inhibitors, to specifically block the AKAP12–PKA interaction, would allow selective manipulation of PKA-responsive processes that are regulated through interaction with AKAP12 while limiting nonspecific effects that might be seen with broad spectrum PKA inhibition. Although outside of the scope of this paper, our studies indicate that future efforts to examine therapeutic targeting of the PKA–AKAP12 interaction could be a selective strategy to inhibit migration and invasion of metastatic melanoma cells.
Materials and Methods
K457 and K3291 cells were used in in vitro assays following standard protocols. Cells were maintained in DMEM supplemented with 10% (vol/vol) FCS, 1% glutamine, and 1% penicillin-streptomycin. Standard in vitro assay protocols were followed and any changes to standard Western blot analysis, cell migration and invasion, ChIP, IHC, IF, qRT-PCR, wound healing, in vivo tumor growth and metastasis, structural analysis, and phosphoproteomic screen, as well as sequences for shRNA stable cells, are described in SI Materials and Methods. Mice were cared for under approval of the Institutional Animal Care and Use Committee at Stanford. Statistical analysis were two-tailed t tests, with P ≤ 0.05 considered statistically significant.
Supplementary Material
Acknowledgments
We thank Brandon Dyer, Chris Betzing, and Thomas Graeber for discussions and Jeffrey Silva, Hongbo Gu, and Kenna Schultz for technical aid. This work was supported by National Institutes of Health Grants CA67166 and CA116685 (to A.J.G.), CA120526 (to M.B.P.), CA140919 and CA129967 (to W.Z.), and T32CA121940 (to E.C.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1418164112/-/DCSupplemental.
References
- 1.Jameson KL, et al. IQGAP1 scaffold-kinase interaction blockade selectively targets RAS-MAP kinase-driven tumors. Nat Med. 2013;19(5):626–630. doi: 10.1038/nm.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malbon CC, Tao J, Shumay E, Wang HY. AKAP (A-kinase anchoring protein) domains: Beads of structure-function on the necklace of G-protein signalling. Biochem Soc Trans. 2004;32(Pt 5):861–864. doi: 10.1042/BST0320861. [DOI] [PubMed] [Google Scholar]
- 3.Lee JW, et al. Protein kinase C-delta regulates the stability of hypoxia-inducible factor-1 alpha under hypoxia. Cancer Sci. 2007;98(9):1476–1481. doi: 10.1111/j.1349-7006.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanna SC, et al. HIF1α and HIF2α independently activate SRC to promote melanoma metastases. J Clin Invest. 2013;123(5):2078–2093. doi: 10.1172/JCI66715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–975. doi: 10.1038/nrc2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poellinger L, Johnson RS. HIF-1 and hypoxic response: The plot thickens. Curr Opin Genet Dev. 2004;14(1):81–85. doi: 10.1016/j.gde.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Villanueva J, Herlyn M. Melanoma and the tumor microenvironment. Curr Oncol Rep. 2008;10(5):439–446. doi: 10.1007/s11912-008-0067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007;2007(407):cm8. doi: 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 10.Jaakkola P, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 11.Mole DR, et al. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem. 2009;284(25):16767–16775. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vasseur S, et al. DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci USA. 2009;106(4):1111–1116. doi: 10.1073/pnas.0812745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haqq C, et al. The gene expression signatures of melanoma progression. Proc Natl Acad Sci USA. 2005;102(17):6092–6097. doi: 10.1073/pnas.0501564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talantov D, et al. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res. 2005;11(20):7234–7242. doi: 10.1158/1078-0432.CCR-05-0683. [DOI] [PubMed] [Google Scholar]
- 15.Bittner M, et al. Molecular classification of cutaneous malignant melanoma by gene expression profiling. Nature. 2000;406(6795):536–540. doi: 10.1038/35020115. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, et al. Gene expression changes in an animal melanoma model correlate with aggressiveness of human melanoma metastases. Mol Cancer Res. 2008;6(5):760–769. doi: 10.1158/1541-7786.MCR-07-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widmer DS, et al. Hypoxia contributes to melanoma heterogeneity by triggering HIF1α-dependent phenotype switching. J Invest Dermatol. 2013;133(10):2436–2443. doi: 10.1038/jid.2013.115. [DOI] [PubMed] [Google Scholar]
- 18.Lee JT, Herlyn M. Microenvironmental influences in melanoma progression. J Cell Biochem. 2007;101(4):862–872. doi: 10.1002/jcb.21204. [DOI] [PubMed] [Google Scholar]
- 19.Choi MC, et al. AKAP12/Gravin is inactivated by epigenetic mechanism in human gastric carcinoma and shows growth suppressor activity. Oncogene. 2004;23(42):7095–7103. doi: 10.1038/sj.onc.1207932. [DOI] [PubMed] [Google Scholar]
- 20.Streb JW, Kitchen CM, Gelman IH, Miano JM. Multiple promoters direct expression of three AKAP12 isoforms with distinct subcellular and tissue distribution profiles. J Biol Chem. 2004;279(53):56014–56023. doi: 10.1074/jbc.M408828200. [DOI] [PubMed] [Google Scholar]
- 21.Diviani D, Scott JD. AKAP signaling complexes at the cytoskeleton. J Cell Sci. 2001;114(Pt 8):1431–1437. doi: 10.1242/jcs.114.8.1431. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Bergami P, Fitchman B, Ronai Z. Understanding signaling cascades in melanoma. Photochem Photobiol. 2008;84(2):289–306. doi: 10.1111/j.1751-1097.2007.00254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Streb JW, Miano JM. Cross-species sequence analysis reveals multiple charged residue-rich domains that regulate nuclear/cytoplasmic partitioning and membrane localization of a kinase anchoring protein 12 (SSeCKS/Gravin) J Biol Chem. 2005;280(30):28007–28014. doi: 10.1074/jbc.M414017200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.