Significance
Genetic, biochemical, and animal model studies have implicated with-no-lysine kinase 4 (WNK4) in regulation of the balance between renal salt reabsorption and K secretion. It has been proposed that serum/glucocorticoid-induced kinase 1 (SGK1)-induced phosphorylation of WNK4 plays an important role in switching from inhibiting KCNJ1 (ROMK) channels during volume depletion to promoting K secretion during increasing K intake. However, it is not understood why a high SGK1 level fails to stimulate ROMK channels during the volume depletion, whereas it increases ROMK channel activity and K secretion during high K intake. The present study provides insights into the mechanism illustrating how SFK-induced phosphorylation of WNK4 regulates ROMK to distinguish the physiologic response to hyperkalemia and volume depletion.
Keywords: hyperkalemia, hypokalemia, PTP-1D, SGK1, volume depletion
Abstract
With-no-lysine kinase 4 (WNK4) inhibits the activity of the potassium channel KCNJ1 (ROMK) in the distal nephron, thereby contributing to the maintenance of potassium homeostasis. This effect is inhibited via phosphorylation at Ser1196 by serum/glucocorticoid-induced kinase 1 (SGK1), and this inhibition is attenuated by the Src-family protein tyrosine kinase (SFK). Using Western blot and mass spectrometry, we now identify three sites in WNK4 that are phosphorylated by c-Src: Tyr1092, Tyr1094, and Tyr1143, and show that both c-Src and protein tyrosine phosphatase type 1D (PTP-1D) coimmunoprecipitate with WNK4. Mutation of Tyr1092 or Tyr1143 to phenylalanine decreased the association of c-Src or PTP-1D with WNK4, respectively. Moreover, the Tyr1092Phe mutation markedly reduced ROMK inhibition by WNK4; this inhibition was completely absent in the double mutant WNK4Y1092/1094F. Similarly, c-Src prevented SGK1-induced phosphorylation of WNK4 at Ser1196, an effect that was abrogated in the double mutant. WNK4Y1143F inhibited ROMK activity as potently as wild-type (WT) WNK4, but unlike WT, the inhibitory effect of WNK4Y1143F could not be reversed by SGK1. The failure to reverse WNK4Y1143F-induced inhibition of ROMK by SGK1 was possibly due to enhancing endogenous SFK effect on WNK4 by decreasing the WNK4–PTP-1D association because inhibition of SFK enabled SGK1 to reverse WNK4Y1143F-induced inhibition of ROMK. We conclude that WNK4 is a substrate of SFKs and that the association of c-Src and PTP-1D with WNK4 at Tyr1092 and Tyr1143 plays an important role in modulating the inhibitory effect of WNK4 on ROMK.
With-no-lysine kinase 4 (WNK4) is expressed in the connecting tubule (CNT) and cortical collecting duct (CCD) (1, 2) and plays an important role in modulating the balance between renal K secretion and Na reabsorption (3–8). The effect of WNK4 on renal K secretion is partially mediated through inhibition of KCNJ1 (ROMK) channels in the CNT and in the CCD. ROMK inhibition is achieved by a stimulation of clathrin-mediated endocytosis (1), an effect that is dependent on intersectin, a scaffold protein containing two Eps15 homology domains (9).
Serum/glucocorticoid-induced kinase 1 (SGK1), a downstream mediator of aldosterone signaling, suppresses the inhibitory effect of WNK4 on ROMK channels through phosphorylation of WNK4 at Ser1169 (2) and Ser1196 (5). Both volume depletion and high K intake increase aldosterone and SGK1 levels (10). However, it is not clear why a high K intake or volume depletion modulates differently the effect of SGK1 on ROMK channels.
Candidate regulators of differential ROMK expression in hyperkalemia and hypovolemia should be regulated in a potassium-dependent manner. One such protein is the protein tyrosine kinase c-Src, whose expression in renal cortex is reduced in states of high potassium intake (11). We have previously demonstrated a key role of c-Src in determining the effect of SGK1 on WNK4 (12). C-Src abolishes SGK1-induced phosphorylation of WNK4 and restores the inhibitory effect of WNK4 on ROMK channels in the presence of SGK1 (13). This effect may play a role in preventing K secretion in the absence of hyperkalemia. High potassium intake, in contrast, will diminish c-Src levels, restore SGK1-induced phosphorylation of WNK4, and lead to increased renal potassium secretion via ROMK.
Whereas protein phosphatase activity has been shown to be involved in c-Src–mediated modulation of the interaction between SGK1 and WNK4 (13), the molecular mechanism of c-Src’s interaction with WNK4 has been elusive. We here identify previously undescribed tyrosine phosphorylation sites in WNK4 that are targets of c-Src. We characterize the effects of tyrosine phosphorylation on the SGK1–WNK4 interaction, as well as WNK4-mediated ROMK inhibition.
Results
c-Src Restores WNK4 Inhibition of ROMK by Reducing SGK1 Phosphorylation.
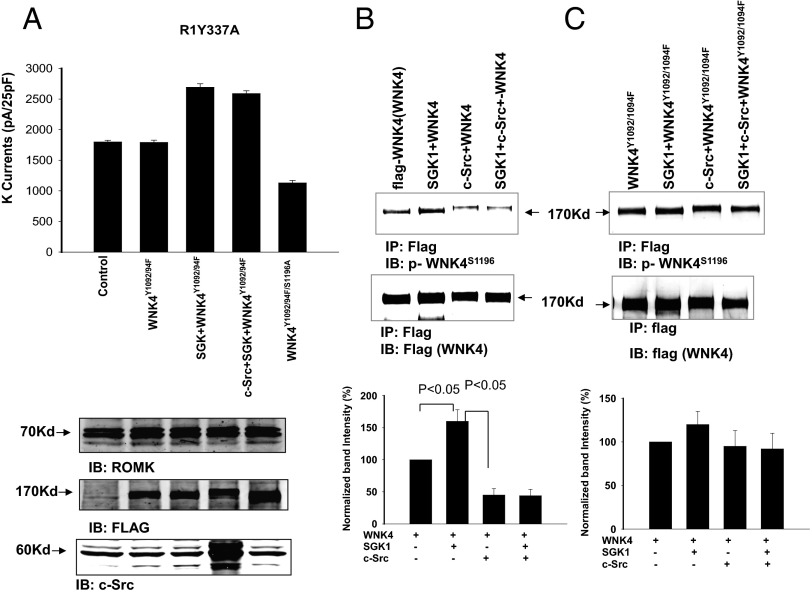
We confirmed the previous finding that WNK4 inhibited ROMK channels (12) and observed that WNK-induced inhibition was reversed by SGK1 and restored by c-Src (Fig. S1). Because c-Src could inhibit ROMK channels by a direct phosphorylation of Tyr337, we repeated the experiments with ROMKY337A mutant (R1Y337A) in which Tyr337 was mutated to phenylalanine to prevent direct tyrosine phosphorylation by c-Src (14). Fig. 1 demonstrates the results of experiments in which K currents at −100 mV were measured with the perforated whole-cell mode in HEK293 cells transfected with R1Y337A and different combinations of SGK1, c-Src, and WNK4.
Fig. 1.
SFK restores the inhibitory effect of WNK4 on ROMK by modulating SGK1-induced WNK4 phosphorylation. The bar graph summarizes experiments in which K currents were measured at −100 mV with the perforated whole-cell recording in HEK cells transfected with ROMK1Y337A and other constructs including WNK4/WNK4S1169/1196D, SGK1, and c-Src. K currents are presented as pA/25 pF. The cells were incubated with a bath solution containing 140 mM KCl, 0.5 mM MgCl2, 1.5 mM CaCl2, and 10 mM Hepes (pH 7.4), and the pipette solution was composed of 140 mM KCl, 2 mM MgCl2, 1 mM EGTA, and 5 mM Hepes (pH 7.4).
As observed with WT ROMK, WNK4 inhibits R1Y337A channel activity; this inhibition is reversed by expression of SGK1 and restored by addition of c-Src (Fig. 1, Left). To test whether the restoration of inhibition was due to a reduction of SGK1-induced WNK4 phosphorylation, we mutated the two known SGK1 phosphorylation sites in WNK4 to aspartate, mimicking constitutive SGK1 phosphorylation (WNK4S1169/1196D) (Fig. 1, Right). WNK4S1169/1196D lost inhibition of ROMK without addition of SGK1 (R1Y337A alone 1,790 ± 50 pA and R1Y337A + WNK4S1169/1196D 2,280 ± 60 pA). Moreover, c-Src did not restore inhibition of ROMK channels in the presence of WNK4S1169/1196D (2,290 ± 50 pA) (n = 6). These results are consistent with a model in which c-Src restores WNK4’s inhibition of ROMK channels by decreasing the phosphorylation at residues 1169 and 1196, which is induced by SGK1 (see below).
c-Src Directly Phosphorylates WNK4 Tyrosine Residues.
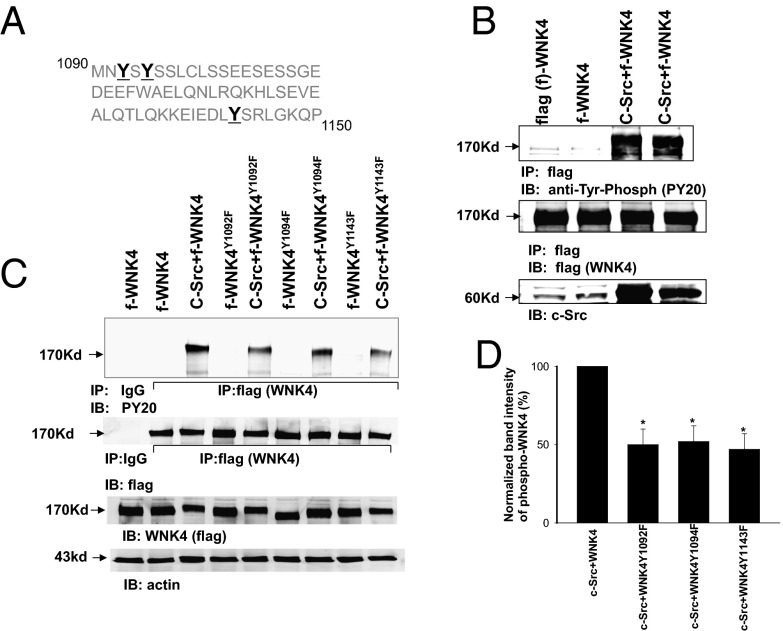
These findings raise the possibility that c-Src might directly phosphorylate WNK4. Motif-based predictions show at least 10 potential c-Src phosphorylation sites on WNK4, and we identified three potential c-Src phosphorylation sites, Tyr1092, Tyr1094, and Tyr1143 in mouse WNK4 (Fig. 2A). These three Tyr residues are conserved among human, mouse, and rat orthologs and are in proximity to the sites of SGK1 phosphorylation. To examine whether c-Src phosphorylates WNK4, we cotransfected HEK293 cells with flag-tagged WNK4 (f-WNK4) and c-Src and harvested WNK4 proteins by immunoprecipitation (IP) of cell lysates with a flag antibody. Tyrosine-phosphorylated WNK4 was detected with an antiphosphotyrosine antibody (PY20). Fig. 2B shows a Western blot demonstrating that coexpression of c-Src dramatically increased tyrosine phosphorylation of WNK4 without affecting WNK4 expression levels. We mutated each of the putative c-Src phosphorylation sites to phenylalanine and examined the effect on overall tyrosine phosphorylation of WNK4 (Fig. 2C). Tyrosine phosphorylation of WNK4Y1092F, WNK4Y1094F, or WNK4Y1143F was significantly lower (50 ± 10%, 52 ± 10% and 47 ± 10% of the control value, respectively) than that of WT WNK4 (n = 5) (Fig. 2D). Moreover, a combined mutation of Tyr1092, 1094, and 1143 to phenylalanine decreased tyrosine phosphorylation level of WNK4 by over 75% (Fig. S2). These results strongly suggest that Tyr1092, Tyr1094, and Tyr1143 are phosphorylated by c-Src and that they are the major sites for the c-Src–induced phosphorylation of WNK4.
Fig. 2.
SFK phosphorylates WNK4. (A) Amino acid sequence of mouse WNK4 between residues 1090 and 1150 showing three putative tyrosine phosphorylation sites—Tyr1092, Tyr1094 and Tyr1143 (bold font). (B) Western blot shows that expression of c-Src increases tyrosine phosphorylation of WNK4 in HEK293T cells transfected with flag-tagged WNK4 (f-WNK4) and c-Src. WNK4 was harvested by immunoprecipitation of the cell lysates with a flag antibody and tyrosine phosphorylated WNK4 was detected with PY20 (Top band). The Middle and Low bands demonstrate the expression of WNK4 and c-Src, respectively. (C) Western blot shows tyrosine phosphorylation of WNK4 and WNK4 mutants (WNK4Y1092F, WNK4Y1094F, and WNK4Y1143F) in the absence or presence of c-Src. (D) Bar graph shows the results of densitometric analysis regarding tyrosine phosphorylation of WNK4/mutants in the presence of c-Src. * indicates the significant difference from the control.
We next used established methods with LC-MS analysis to identify the phosphorylation sites in WNK (15). We expressed WNK4 alone in HEK cells in media containing 12C-lysine and 12C14N-arginine or coexpressed WNK4 and c-Src in cells in media containing 13C-lysine and 13C15N-arginine. We isolated WNK4 via IP from the cells cotransfected with c-Src and HA-tagged WNK4 (Fig. S3A). WNK4 proteins were resolved on SDS/PAGE, cut from the gel, and digested with trypsin. Phosphopeptide fractions were obtained via TiO2 enrichment and subjected to LC-MS analysis. The phosphopeptide EIEDLYPSR (m/z = 552.73+2) was unambiguously identified from the phosphopeptide-containing fractions and confirmed phosphorylation at Tyr1143 in WNK4 (Fig. S3B). We performed a separate experiment in which phosphotyrosine peptides were enriched via immobilized antiphosphotyrosine antibodies and subjected to LC-MS analysis. We again observed the phosphopeptide EIEDLYPSR and thus provided additional confirmation of tyrosine phosphorylation. We next sought to show a quantitative increase in WNK4 phosphorylation in the presence of c-Src. We expressed WNK4 alone in HEK cells cultured in media containing 12C-lysine and 12C14N-arginine or coexpressed WNK4 and c-Src in cells cultured in media containing 13C-lysine and 13C15N-arginine (15). This stable isotope labeling by amino acids in cell culture (SILAC) approach allowed us to isolate WNK4 with or without c-Src, mix the proteins 1:1, and directly compare phosphorylation at Tyr1143 (Fig. S3C). Consistent with kinase activity, we saw a marked increase in WNK4 phosphorylation at Tyr1143 in the presence of c-Src and simultaneously observed a stoichiometric decrease in the nonphosphopeptide EIEDLYSR. However, we were not able to identify two additional phosphotyrosine sites (Tyr1092 and Tyr1094) with our phosphoproteomic approaches. Possibly, these tyrosine residues were localized in a region of WNK4 predicted to be replete in tryptic peptides and thus not accessible with phosphoproteomic approaches. This notion was supported by the fact that we did not observe any peptides in a region of WNK4 spanning residues 1072–1122 in our phosphoproteomic analysis. However, Western blots with phosphotyrosine antibody showed strong evidence of tyrosine phosphorylation of WNK4 at positions 1092, 1094, and 1143. Thus, we then aimed to identify potential functional effects of tyrosine phosphorylation at the residues identified.
c-Src Binds to WNK4 Tyr1092.
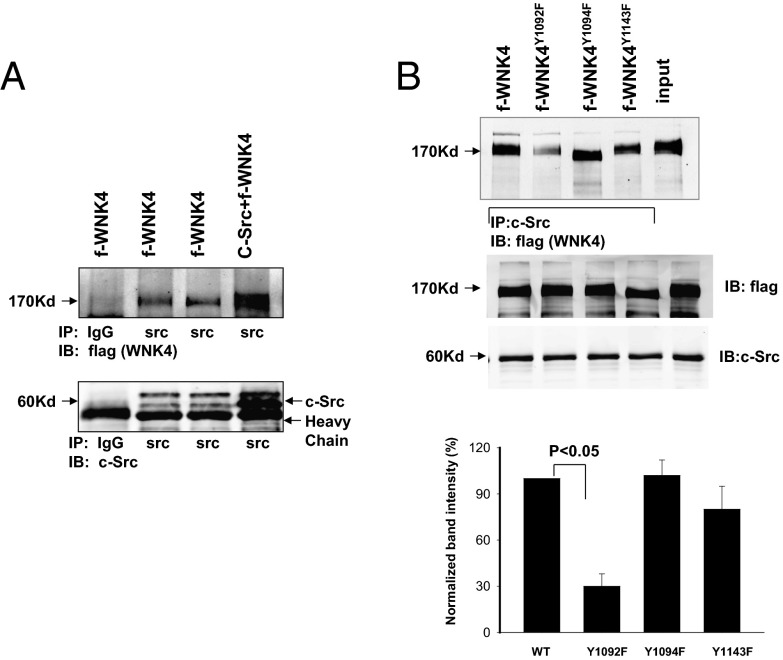
The first phosphorylated tyrosine residue identified (position 1092) is expected to provide a binding site for c-Src through its SH2 domain because amino acid at +2 position of Tyr1092 is also a tyrosine residue that has been shown to enhance c-Src binding by twofold (16, 17). We expressed f-WNK4 and c-Src, immunoprecipitated cell lysates with an anti–c-Src antibody, and subjected resulting proteins to Western blotting for f-WNK4 with an antiflag antibody. Whereas WT WNK4 was consistently coimmunoprecipitated with c-Src (Fig. 3A), WNK4Y1092F was much less efficiently immunoprecipitated with c-Src (70 ± 10% reduction; n = 4; Fig. 3B). In contrast, the association between c-Src and WNK4Y1094F or WNK4Y1143F was similar to that of WT WNK4, suggesting c-Src binding to WNK4 involves Tyr1092.
Fig. 3.
c-Src is associated with WNK4. (A) Western blot shows that WNK4 was immunoprecipitated with c-Src antibody in HEK cells transfected with flag-tagged mouse WNK4 (f-WNK4) and c-Src or (B) in cells transfected with WNK4 mutants (WNK4Y1092F, WNK4Y1094F, and WNK4Y1143F). Bar graph in the bottom of Fig. 4B summarizes the normalized band intensity in comparison with the control value (100%). The normalized value was calculated by comparing the value of total WNK4 and immunoprecipitated WNK4 in the control group with corresponding data in the experimental group.
Phosphorylation of Tyr1092 by c-Src Modulates the Inhibitory Effect of WNK4 on ROMK.
We next tested whether the association between c-Src and WNK4 played a role in mediating WNK4’s inhibition of ROMK channels. We measured Ba2+-sensitive K currents at −100 mV with the perforated whole-cell recording technique in cells transfected with R1Y337A and WT or mutant WNK4 (WNK4Y1092F, WNK4Y1094F, or WNK4Y1092/1094F; Fig. 4A). Consistent with previous results (12), expression of WT WNK4 decreased K currents from 1,750 ± 50 pA to 820 ± 30 pA (n = 10). However, expression of WNK4Y1092F largely abolished the inhibitory effect of WNK4 on ROMK (K current 1,500 ± 30 pA; n = 6). In contrast, expression of WNK4Y1094F still inhibited ROMK channels and decreased K currents to 950 ± 20 pA (n = 6). Mutation of both Tyr1092 and Tyr1094 to phenylalanine completely abolished WNK4’s inhibition of ROMK channels (1,740 ± 40 pA). This observation suggests that phosphorylation of Tyr1092 by c-Src plays a major role in modulating the inhibitory effect of WNK4 on ROMK, whereas the phosphorylation of Tyr1094 is less important, but works synergistically. Moreover, Western blot showed that the tyrosine phosphorylation level of WNKY1092/1094F was 60 ± 10% lower than that of WT WNK4 (n = 4) (Fig. 4B). This suggests that Tyr1092 and Tyr1094 are two important sites for c-Src–mediated tyrosine phosphorylation of WNK4 and play a key role in modulating WNK4’s inhibition of ROMK channels.
Fig. 4.
Mutation of Tyr1092 and Tyr1094 abolishes the effect of WNK4 on ROMK. (A) Bar graph summarizes experiments in which K currents were measured at −100 mV with the perforated whole-cell recording in HEK cells transfected with ROMK1Y337A and WNK4/WNK4 mutants (WNK4Y1094F, WNK4Y1092F, and WNK4Y1092/1094F). K currents are presented as pA/25 pF. The cells were incubated with a bath solution containing 140 mM KCl, 0.5 mM MgCl2, 1.5 mM CaCl2, and 10 mM Hepes (pH 7.4) and the pipette solution contained 140 mM KCl, 2 mM MgCl2, 1 mM EGTA, and 5 mM Hepes (pH 7.4). (B) Western blot demonstrates tyrosine phosphorylation of WNK4 and WNKY1092/1094F in the absence or presence of c-Src. The WNK proteins were enriched by immunoprecipitation with Flag antibody, and tyrosine-phosphorylated WNK4 was detected with PY20.
Phosphorylation at Tyr1092 and Tyr1094 Is Required for c-Src–Induced Restoration of WNK4’s ROMK Inhibition.
We examined the effect of WNK4 on ROMK channels in cells transfected with R1Y337A, SGK1, c-Src, and WNK4Y1092/1094F. We again measured Ba2+-sensitive K currents at −100 mV (Fig. 5A). c-Src failed to restore the inhibitory effect of WNK4 on ROMK channels in cells transfected with WNK4Y1092/1094F (K currents were 2,420 ± 60 pA (n = 6) in cells transfected with c-Src, R1Y337A, SGK1, and WNK4Y1092/1094F, a value similar to the 2,440 ± 60 pA without c-Src). However, the additional mutation of WNK4 Ser1196 to alanine restored WNK4’s inhibitory effect on ROMK channels and decreased K currents to 850 ± 30 pA (n = 6). The results are consistent with the notion that c-Src acts at least in part by reducing phosphorylation at WNK4 Ser1196 (14), and that this effect is lost in the WNK4Y1092/1094F mutant. This notion is confirmed by direct measurement of phosphorylation at this site with an antibody specific for phosphorylation at WNK4 S1196 (Fig. 5 B and C). As reported previously, SGK1 increases phosphorylation at Ser1196 in WT WNK4 (13), and this phosphorylation is inhibited by coexpression of c-Src. Further, basal phosphorylation at Ser1196 is higher in the WNK4Y1092/1094F mutant, and this phosphorylation is not significantly reduced by addition of c-Src.
Fig. 5.
Mutation of SGK1-phosphorylation site (Ser1196) restores the inhibitory effect of WNK4 on ROMK. (A) Bar graph summarizes experiments in which K currents were measured at −100 mV with the perforated whole-cell recording in HEK cells transfected with ROMK1Y337A, WNK4Y1092/94F + R1Y337A, SGK1 + R1Y337A + WNK4Y1092/94F, c-Src + SGK1 + R1Y337A + WNK4Y1092/94F, and R1Y337A + WNK4Y1092/94F/S1196A. The expression of R1Y337A, flag-tagged-WNK4 mutants, and c-Src is shown at the Bottom. Western blot shows the effect of c-Src on SGK1-induced phosphorylation of WNK4 (B) and on WNK4Y1092/94F (C) with p-WNK4S1196 antibody. The flag-tagged WNK4 was harvested by immunoprecipitation of the cell lysates with flag antibody. The bar graph at the Bottom summarizes the normalized band intensity in comparison with the control (without SGK1 and c-Src).
WNK4Y1143F Prevents SGK1-Induced Reversion of WNK4’s ROMK Inhibition.
WNK4Y1143F inhibited ROMK channels as potently as WT WNK4 (Fig. 6; K currents reduced from 1,780 ± 50 pA to 780 ± 30 pA; n = 7). However, whereas SGK1 reverses the inhibitory effect of WT WNK4 on ROMK, SGK1 failed to reverse the inhibitory effect of WNK4Y1143F on ROMK channels (K current 800 ± 30 pA, n = 6). Interestingly, pharmacologic inhibition of endogenous SFK with PP1 (1 μM) can reverse this inhibition (K currents increased to 1,560 ± 30 pA; n = 6) in cells transfected with WNK4Y1143F + SGK1. These findings are consistent with the possibility that SGK1 normally reverses WNK4’s inhibition of ROMK via an effect that requires WNK4Y1143.
Fig. 6.
SGK1 reverses the inhibitory effect of WNK4 but fails to abolish the effect of WNK4Y1143F on ROMK. Bar graph summarizes experiments in which K currents were measured at −100 mV with the perforated whole-cell recording in HEK cells transfected with R1Y337A, SGK1, and WNK4/WNK4Y1143F in the presence of SFK inhibitor (1 μM PP1).
PTP-1D Interacts with WNK4 via Tyr1143 to Reduce the Inhibitory Effect of SFK on ROMK.
We reasoned that protein tyrosine phosphatases (PTPs) might play a role in the c-Src–SGK1–WNK4 interaction. In particular, if Tyr1143 represented a binding site for PTPs, mutation of Tyr1143 might inhibit PTP binding and activity, thereby enhancing SFK effects. This hypothesis was tested by FLAG immunoprecipitation from cells transfected with flag-tagged WNK4. Fig. 7A shows a Western blot demonstrating that PTP-1D was coimmunoprecipitated with WNK4, indicating an interaction of these two proteins. Mutation of Tyr1143 to phenylalanine diminished this interaction, whereas the same substitution at Tyr1092 or Tyr1094 had no effect on PTP-1D binding (Fig. 7B). Moreover, the association between WNK4 and PTP-1D was significantly decreased by 60 ± 10% (n = 4) in c-Src cotransfected cells, suggesting c-Src competed with PTP-1D for WNK4 binding (Fig. 7C).
Fig. 7.
WNK4 is associated with protein tyrosine phosphatase type 1D (PTP-1D). (A) Western blot shows that PTP-1D is pulled down by immunoprecipitation with flag antibody in HEK293 cells transfected with flag-tagged WNK4 (f-flag). (B) Immunoprecipitation of endogenous PTP-1D with WNK4 or WNK4 mutants in HEK293 cells transfected with f-WNK4, WNK4Y1143F, WNK4Y1092F, and WNK4Y1094F. The cell lysates were immunoprecipitated with either IgG or PTP-1D. (C) Western blot demonstrates that coexpression of c-Src diminished the WNK4–PTP-1D association. The endogenous PTP-1D was immunoprecipitated with flag antibody in flag-WNK4 transfected cells. IgG served as a negative control and the expression of PTP-1D (input) in HEK293 cells is shown (Lower).
Discussion
WNK family kinases play an important role in regulating renal K secretion through modulating Na delivery to the distal nephron and regulating apical K-secretory channels (4, 6, 18–20). WNK1, WNK3, and WNK4 have been shown to inhibit ROMK channels (1, 19, 20) and the inhibitory effect of WNK4 is achieved by stimulating clathrin-dependent endocytosis (1, 21). The inhibitory effect of WNK1 is blocked by a kidney-specific splice form of WNK1 (KS-WNK1) (7, 8, 22), in which an alternative 5′ exon replaces the first four exons of WNK1 (23). Because expression of KS-WNK1 is modulated by a dietary K intake, interaction between WNK1 and KS-WNK1 may play a role in regulating ROMK channels and renal K secretion (22). The inhibitory effect of WNK4 is also blocked by SGK1, which phosphorylates WNK4 at Ser1169 and Ser1196 (2, 5), thereby switching WNK4 from inhibition to stimulation of ROMK channels. The role of Ser1169 and Ser1196 in regulating ROMK channels was strongly suggested by the previous finding that mutation of either Ser1169 or Ser1196 to aspartate abolished the effect of WNK4 on ROMK channels (13). Moreover, we observed that the stimulatory effect of SGK1 on ROMK channels was largely abolished in cells transfected with either WNK4S1169A or WNK4S1196A, in which the serine residues that are targets of SGK1 were mutated to alanines (Fig. S4). Thus, the SGK1–WNK4 interaction plays a role in regulating ROMK channel activity.
However, the stimulatory effect of SGK1 on WNK4’s phosphorylation was absent in the presence of high SFK activity (12). A large body of evidence has demonstrated that SFK plays an important role in inhibiting ROMK channels in the CCD (24–29). We have previously demonstrated that SFK phosphorylates the ROMK1 channel at Tyr337 (14) thereby facilitating internalization of ROMK channels (24). A low K intake has been shown to increase tyrosine phosphorylation of ROMK channels (11), leading to inhibition of ROMK channels and decreasing K secretion in the CCD. In addition to direct phosphorylation, SFK regulates ROMK channel activity by suppressing SGK1-induced phosphorylation of WNK4, thereby restoring WNK4-induced inhibition of ROMK channels (12, 13). Such a mechanism could play an important role in suppressing ROMK channel activity during volume depletion, which maintains a high SFK activity (11, 14, 24).
The main finding of the present study is that WNK4 is a previously unknown target of SFK phosphorylation. We discovered three specific WNK4 sites that are phosphorylated by c-Src—Tyr1092, Tyr1094, and Tyr1143. Two of these sites—Tyr1092 and Tyr1094—are required for the modulatory effect of SFK on the SGK1–WNK4 interaction. First, mutation of Tyr1092 and Tyr1094 to phenylalanine abolished the effect of c-Src on Ser1196 phosphorylation in the presence or absence of SGK1. Second, c-Src failed to restore the inhibitory effect of WNK4 on ROMK channels in cells transfected with WNK4Y1092/1094F. Two lines of evidence suggest that c-Src–induced phosphorylation at Tyr1092 may play a major role, whereas phosphorylation at Tyr1094 plays a minor role in mediating the effect of c-Src on WNK4–SGK1 interaction: (i) mutation of Tyr1092 largely abolished the inhibitory effect of WNK4 on ROMK channels; and (ii) mutation of Tyr1092 and Tyr1094 completely abolished the inhibitory effect of WNK4, whereas mutation of Tyr1094 alone did not significantly affect WNK4’s inhibition of ROMK channels. We speculate that dephosphorylation of WNK4 at Tyr1092 and Tyr1094 enhances the stimulatory effect of endogenous SGK1 on WNK4, thereby reversing the inhibitory effect of WNK4 on ROMK channels. When Tyr1092 and Tyr1094 are phosphorylated by SFK, WNK4 should be in inhibitory mode for ROMK channels even in the presence of SGK1. A role of WNK4 phosphorylation at Tyr1092 and Tyr1094 in modulating the function of WNK4’s switch domain was suggested by the finding that WNK4 inhibited ROMK channels in cells transfected with WNK4Y1092/1094F/S1196A, whereas WNK4Y1092/1094F failed to inhibit ROMK channels. This observation indicates that the phosphorylation status of Tyr1092 and Tyr1094 may regulate the SGK1 effect on WNK4 phosphorylation thereby affecting WNK4 inhibition of ROMK.
The second finding was that PTP-1D was associated with WNK4 and that the third site we identified, Tyr1143, was a binding site for PTP-1D to WNK4. This notion was supported by the finding that mutation of Tyr1143 diminished the association of PTP-1D with WNK4, whereas other mutations had no significant effect. The physical association of PTP-1D and WNK4 likely plays an important role in dephosphorylation of tyrosine phosphorylation sites in WNK4, thereby facilitating the effect of SGK1 on WNK4. This notion was supported by the observation that elimination of the putative PTP-1D binding site by mutating Tyr1143 abolished SGK1-induced reversal of the inhibitory effect of WNK4 on ROMK channels. Furthermore, the observation that inhibition of endogenous SFK activity restored the effect of SGK1 and reversed the inhibitory effect of WNK4 on ROMK channels strongly suggests that elimination of the PTP-1D binding site may indirectly amplify SFK activity by inhibition of PTP-1D activity.
Finally, the observation that expression of c-Src increased the phosphorylation of Tyr1143 and diminished the association of PTP-1D with WNK4 suggests that the binding affinity of PTP-1D to WNK4 was also affected by SFK-induced phosphorylation. Therefore, the role of WNK4 phosphorylation at Tyr1143 may be to decrease the association of PTP-1D with WNK4 and to suppress PTP-1D activity, thereby increasing WNK4’s tyrosine phosphorylation at Tyr1092. We speculate that phosphorylation of Tyr1092 may play a role in activation of serine/threonine protein phosphatase, whereas the phosphorylation of Tyr1143 may lead to inhibition of PTP activity.
We have previously demonstrated that low Na intake decreases the expression of PTP-1D, whereas it does not alter the expression of c-Src in comparison with control animals (13). It is conceivable that low expression of PTP-1D induced by low Na intake enhances c-Src–induced phosphorylation of WNK4, thereby decreasing SGK1-induced phosphorylation of WNK4 and switching WNK4 to an inhibitory mode for K secretion. Fig. 8A illustrates the possible mechanism by which SFKs modulate the WNK4–SGK1 interaction during volume depletion. In addition to the inhibition of ROMK by direct tyrosine phosphorylation, SFKs such as c-Src bind to WNK4, thereby stimulating tyrosine phosphorylation of WNK4 at Tyr1092 and Tyr1143. The phosphorylation at Tyr1092 in turn activates serine/threonine protein phosphatase (PP) such as type 1 PP, whereas the phosphorylation at Tyr1143 inhibits PTP-1D activity. The activated serine/threonine protein phosphatase is expected to decrease SGK1-induced phosphorylation of WNK4 thereby locking WNK4 in an inhibitory mode for ROMK channels (13). In contrast, high K intake has been shown to decrease the expression of SFK, whereas it does not alter the expression of PTP-1D (29). Thus, relatively high expression of PTP-1D during high K intake is expected to facilitate PTP-1D binding to WNK4 and increase PTP-induced dephosphorylation of WNK4 (Fig. 8B), thereby enhancing the effect of SGK1 on WNK4’s phosphorylation and switching WNK4 to a stimulatory mode for K secretion. We conclude that WNK4 is a substrate of both SFK and PTP-1D and that tyrosine phosphorylation of WNK4 regulates the effect of the SGK1–WNK4 interaction on ROMK channels and K secretion.
Fig. 8.
The scheme illustrates the mechanism by which SFK modulates the interaction between WNK4 and SGK1 during volume depletion (A) and during high K intake (B). The dotted line and solid line represent an enhanced and a diminished signaling pathway, respectively. Gray font means inhibition. The plus and minus signs represent stimulation and inhibition, respectively. PP1, type 1 serine/threonine protein phosphatase; PTP, protein tyrosine phosphatase; ROMK, renal outer medullary K channel (Kir1.1); SFK, Src-family tyrosine kinase; SGK1, serum/glucocorticoid-induced protein kinase 1.
Materials and Methods
The methods for electrophysiology, purification of flag-WNK4, preparation of protein samples, immunoprecipitation, and Western blot are described in SI Materials and Methods.
Cell Culture and Transient Transfection.
HEK293T cells (American Type Culture Collection) were used for transient expression of the proteins including WNK4, SGK1, c-Src, and ROMK channels. The cells were grown in Dulbecco's modified Eagle medium (DMEM; Invitrogen) supplemented with 10% (vol/vol) FBS (Invitrogen) in 5% CO2 and 95% air at 37 °C. Cells were grown to 50–70% confluence for transfection, and the corresponding cDNAs were simultaneously applied to the cells using TurboFect transfection reagent in according to the manufacturer’s protocol (Fermentas). Briefly, a cDNA mixture (0.4 μg ROMK, 0.4 μg SGK1, 0.4 μg c-Src, and 0.4 μg WNK4) was diluted with 200 μL serum-free DMEM and further mixed with 4 μL TurboFect transfection reagent for the transfection of cells cultured in a 35-mm Petri dish. Cells transfected with vector alone were used as a control, and their background currents were subtracted from the experimental groups. After a 15-min incubation at room temperature, the mixture of the transfection agents was applied to the cells followed by an additional 24-h incubation before use.
TiO2 Enrichment and LC-MS/MS.
Proteins of interest were excised from gels and digested with trypsin. Peptides were extracted with 0.5% trifluoroacetic acid, dried, resuspended, and applied to a TiO2 TopTip microspin column (Glygen). Unbound peptides were washed off and bound peptides were eluted with a 1:33 solution of saturated ammonia. Protein digests and SILAC experiments were analyzed by LC-MS/MS. Identified sites of phosphorylation were confirmed using a second spectrometer. Spectra were searched with Mascot 2.1 with improved phosphopeptide scoring. Phosphopeptide identities were confirmed by manual inspection.
Experimental Materials and Statistics.
Antibodies for c-Src, PTP-1D, and tyrosine phosphorylation (PY20) were purchased from Santa Cruz Biotechnology. Antibodies for SGK1, Flag, actin, IgG, and HA were obtained from Sigma. Anti-WNK4S1196 phospho antibody was generated by J.R. All chemicals including PP1 (4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]-pyrimidine) were purchased from Sigma. The data are presented as mean ± SEM. We used a one-way ANOVA test to determine the statistical significance. P < 0.05 was considered to be significant.
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health Grants DK54983 (to W.-H.W.) and KO1DK089006 (to J.R.) and R.P.L. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503437112/-/DCSupplemental.
References
- 1.Kahle KT, et al. WNK4 regulates the balance between renal NaCl reabsorption and K+ secretion. Nat Genet. 2003;35(4):372–376. doi: 10.1038/ng1271. [DOI] [PubMed] [Google Scholar]
- 2.Ring AM, et al. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA. 2007;104(10):4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson FH, et al. Human hypertension caused by mutations in WNK kinases. Science. 2001;293(5532):1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 4.Kahle KT, Ring AM, Lifton RP. Molecular physiology of the WNK kinases. Annu Rev Physiol. 2008;70:329–355. doi: 10.1146/annurev.physiol.70.113006.100651. [DOI] [PubMed] [Google Scholar]
- 5.Rozansky DJ, et al. Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest. 2009;119(9):2601–2612. doi: 10.1172/JCI38323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCormick JA, Yang CL, Ellison DH. WNK kinases and renal sodium transport in health and disease: An integrated view. Hypertension. 2008;51(3):588–596. doi: 10.1161/HYPERTENSIONAHA.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Z, Wang HR, Huang CL. Regulation of ROMK channel and K+ homeostasis by kidney-specific WNK1 kinase. J Biol Chem. 2009;284(18):12198–12206. doi: 10.1074/jbc.M806551200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wade JB, et al. WNK1 kinase isoform switch regulates renal potassium excretion. Proc Natl Acad Sci USA. 2006;103(22):8558–8563. doi: 10.1073/pnas.0603109103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He G, Wang HR, Huang SK, Huang C-L. Intersectin links WNK kinases to endocytosis of ROMK1. J Clin Invest. 2007;117(4):1078–1087. doi: 10.1172/JCI30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallon V, et al. Role of Sgk1 in salt and potassium homeostasis. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R4–R10. doi: 10.1152/ajpregu.00369.2004. [DOI] [PubMed] [Google Scholar]
- 11.Wei Y, Bloom P, Lin D, Gu R, Wang WH. Effect of dietary K intake on apical small-conductance K channel in CCD: Role of protein tyrosine kinase. Am J Physiol Renal Physiol. 2001;281(2):F206–F212. doi: 10.1152/ajprenal.2001.281.2.F206. [DOI] [PubMed] [Google Scholar]
- 12.Yue P, et al. Src family protein tyrosine kinase (PTK) modulates the effect of SGK1 and WNK4 on ROMK channels. Proc Natl Acad Sci USA. 2009;106(35):15061–15066. doi: 10.1073/pnas.0907855106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin DH, et al. Protein phosphatase 1 modulates the inhibitory effect of With-no-Lysine kinase 4 on ROMK channels. Am J Physiol Renal Physiol. 2012;303(1):F110–F119. doi: 10.1152/ajprenal.00676.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin DH, et al. K depletion increases protein tyrosine kinase-mediated phosphorylation of ROMK. Am J Physiol Renal Physiol. 2002;283(4):F671–F677. doi: 10.1152/ajprenal.00160.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rinehart J, et al. Sites of regulated phosphorylation that control K-Cl cotransporter activity. Cell. 2009;138(3):525–536. doi: 10.1016/j.cell.2009.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Songyang Z, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 17.Harrison SC. Variation on an Src-like theme. Cell. 2003;112(6):737–740. doi: 10.1016/s0092-8674(03)00196-x. [DOI] [PubMed] [Google Scholar]
- 18.Ahlstrom R, Yu ASL. Characterization of the kinase activity of a WNK4 protein complex. Am J Physiol Renal Physiol. 2009;297(3):F685–F692. doi: 10.1152/ajprenal.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cope G, et al. WNK1 affects surface expression of the ROMK potassium channel independent of WNK4. J Am Soc Nephrol. 2006;17(7):1867–1874. doi: 10.1681/ASN.2005111224. [DOI] [PubMed] [Google Scholar]
- 20.Leng Q, et al. WNK3, a kinase related to genes mutated in hereditary hypertension with hyperkalaemia, regulates the K+ channel ROMK1 (Kir1.1) J Physiol. 2006;571(Pt 2):275–286. doi: 10.1113/jphysiol.2005.102202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalioti MD, et al. Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet. 2006;38(10):1124–1132. doi: 10.1038/ng1877. [DOI] [PubMed] [Google Scholar]
- 22.Lazrak A, Liu Z, Huang CL. Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA. 2006;103(5):1615–1620. doi: 10.1073/pnas.0510609103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Reilly M, Marshall E, Speirs HJL, Brown RW. WNK1, a gene within a novel blood pressure control pathway, tissue-specifically generates radically different isoforms with and without a kinase domain. J Am Soc Nephrol. 2003;14(10):2447–2456. doi: 10.1097/01.asn.0000089830.97681.3b. [DOI] [PubMed] [Google Scholar]
- 24.Lin DH, et al. Protein tyrosine kinase is expressed and regulates ROMK1 location in the cortical collecting duct. Am J Physiol Renal Physiol. 2004;286(5):F881–F892. doi: 10.1152/ajprenal.00301.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin DH, Sterling H, Wang WH. The protein tyrosine kinase-dependent pathway mediates the effect of K intake on renal K secretion. Physiology (Bethesda) 2005;20:140–146. doi: 10.1152/physiol.00044.2004. [DOI] [PubMed] [Google Scholar]
- 26.Moral Z, et al. Regulation of ROMK1 channels by protein-tyrosine kinase and -tyrosine phosphatase. J Biol Chem. 2001;276(10):7156–7163. doi: 10.1074/jbc.M008671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sterling H, et al. Inhibition of protein-tyrosine phosphatase stimulates the dynamin-dependent endocytosis of ROMK1. J Biol Chem. 2002;277(6):4317–4323. doi: 10.1074/jbc.M109739200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang W, Lerea KM, Chan M, Giebisch G. Protein tyrosine kinase regulates the number of renal secretory K channels. Am J Physiol Renal Physiol. 2000;278(1):F165–F171. doi: 10.1152/ajprenal.2000.278.1.F165. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y, Bloom P, Gu R, Wang W. Protein-tyrosine phosphatase reduces the number of apical small conductance K+ channels in the rat cortical collecting duct. J Biol Chem. 2000;275(27):20502–20507. doi: 10.1074/jbc.M000783200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.