Significance
The airway epithelia modulate the inflammatory responses to various pathogens. Pulmonary disease caused by Toxoplasma gondii infection affects human neonates, children, and immunocompromised individuals. However, it is not clear how T. gondii infection impacts airway epithelia. We report the use of a short-circuit current (Isc) technique to determine the Cl− secretion induced by ATP in tracheal epithelia infected by T. gondii. We surprisingly found that the ATP-evoked Cl− secretion in T. gondii-infected mouse tracheal epithelia was significantly suppressed. We also found that the mRNA expression level of the P2Y2 receptor increased significantly in T. gondii-infected mouse trachea, via real-time quantitative PCR. Our study provides previously unidentified insights into the mechanism underlying host impairment caused by T. gondii infection.
Keywords: ATP, Cl− secretion, P2Y2 receptor, pneumonia, cystic fibrosis
Abstract
The airway epithelia initiate and modulate the inflammatory responses to various pathogens. The cystic fibrosis transmembrane conductance regulator-mediated Cl− secretion system plays a key role in mucociliary clearance of inhaled pathogens. We have explored the effects of Toxoplasma gondii, an opportunistic intracellular protozoan parasite, on Cl− secretion of the mouse tracheal epithelia. In this study, ATP-induced Cl− secretion indicated the presence of a biphasic short-circuit current (Isc) response, which was mediated by a Ca2+-activated Cl− channel (CaCC) and the cystic fibrosis transmembrane conductance regulator. However, the ATP-evoked Cl− secretion in T. gondii-infected mouse tracheal epithelia and the elevation of [Ca2+]i in T. gondii-infected human airway epithelial cells were suppressed. Quantitative reverse transcription–PCR revealed that the mRNA expression level of the P2Y2 receptor (P2Y2-R) increased significantly in T. gondii-infected mouse tracheal cells. This revealed the influence that pathological changes in P2Y2-R had on the downstream signal, suggesting that P2Y2-R was involved in the mechanism underlying T. gondii infection in airways. These results link T. gondii infection as well as other pathogen infections to Cl− secretion, via P2Y2-R, which may provide new insights for the treatment of pneumonia caused by pathogens including T. gondii.
The airway epithelium is the initial cell type exposed to both inhaled environmental factors and medications for airway diseases (1). Therefore, epithelial cells are recognized as being very important in host defense. In the lungs of mammals, the first line of defense against pathogen infection is the thin layer of airway surface liquid (ASL) lining the airway surface. Maintenance of the proper height and ion composition of ASL is crucial for proper lung defense. The airway epithelium exhibits several complex regulatory pathways to adjust ASL volume to maintain proper mucociliary clearance (2). These pathways blend ion transport by regulating epithelial sodium channel (ENaC)-mediated Na+ absorption (3) and Ca2+-activated Cl− channel (CaCC) and cystic fibrosis transmembrane conductance regulator (CFTR)-mediated Cl− secretion (4).
It has become increasingly clear that extracellular nucleotides are important regulators of mucus clearance in the airways as a result of their ability to stimulate fluid secretion (5), mucus hydration (6), and cilia beat frequency (7, 8). ATP released by epithelial cells plays a key role as an important autocrine and paracrine signaling molecule (9). In normal airway epithelia, ATP is released in response to local stress in ASL and is sufficient to induce purinoceptor-mediated increases in ASL height and maintain proper mucociliary clearance (10). This unusual ASL level will result in abnormal airway functioning and suggests that ATP has a vital role in airway function. It has been shown that the exogenous application of ATP produces a regulatory effect on airway epithelia ion transport, including the inhibition of Na+ absorption (11, 12) and activation of Cl− secretion (13, 14).
Extracellular ATP and its analogous nucleotides exert their effects through a class of cell surface receptors known as P2 receptors, which are divided into two subfamilies—P2Y and P2X (15). Seven P2Y receptors have been identified in mammals; they contain seven-transmembrane segments, are associated with G-protein activation, and are involved in regulating various biological processes such as secretion, proliferation, differentiation, wound repair, and anti-inflammatory and other processes (16, 17). A knockout study confirmed that the P2Y2 receptor (P2Y2-R) is the dominant P2Y purinoceptor that regulates airway epithelial ion transport, whereas other P2Y receptor subtypes are relatively more important in other nonrespiratory epithelia (18). P2Y2-R is linked to phospholipase C (PLC)-generated inositol (1,4,5) trisphosphate (IP3)-mediated release of [Ca2+]i in human airway epithelia (19).
Toxoplasma gondii is an obligate intracellular parasitic protozoan, and its definitive host is the cat and other feline species. Beside felines, it can also infect many warm-blooded animals including humans, resulting in toxoplasmosis (20). Reactivation of a latent infection in immune-deficient patients such as AIDS and organ transplantation subjects can become life-threatening (21). Besides the mechanical destruction of the host cell, little is known about the mechanisms underlying T. gondii infection resulting in multiorgan injury. Pulmonary disease caused by T. gondii infection is seen in neonates, children, and immunocompromised hosts. Pneumonitis caused by T. gondii in immunocompromised hosts results in dyspnoea, minimal sputum production, and other clinical and radiologic effects. It evokes diffuse disease in the lung, such as interstitial infiltrates, bronchopneumonia, and pulmonary infection (22). Although the key players in host defense against pathogenic microorganisms are airway epithelia, it is not clear how T. gondii infection affects the functions of airway epithelia.
In view of the significant roles of ATP and P2Y2-R in airway epithelia function, we set out to investigate ion transport induction by ATP in tracheal epithelia infected by T. gondii and to elucidate the intracellular signaling pathways involved in the pathogenesis of T. gondii infection. The results uncover a previously unidentified mechanism that enables us to understand the pathogenesis of the infection and could offer potential therapeutic applications for pulmonary toxoplasmosis.
Results
Characteristics of ATP-Induced Short-Circuit Current Response.
Previous studies on extracellular ATP-induced transepithelial Cl− transport in different epithelial cells have shown that the time course for the ATP response is biphasic. Different regulatory pathways are involved in mediating the ATP-induced short-circuit current (Isc), such as the cAMP-dependent and the traditional P2-purinoceptor–linked Ca2+-dependent or P1-like receptor-dependent pathways, which activate different Cl− channels (23–25). In this study, we investigate the Isc response to extracellular ATP at 10 μM in mice trachea epithelia. The normal trachea tissues used in the present study had a transepithelial electrical resistance of 74.93 ± 2.06 Ω/cm2 (n = 4), with a basal Isc (Ib) of 13.38 ± 0.37 μA/cm2 (n = 4) in the unstimulated state when bathed in Krebs–Henseleit (K–H) solution. The transepithelial resistance did not change significantly after ATP stimulation.
ATP added to the apical side induced a biphasic response. This response consisted of a transient upstroke followed by a sustained phase (first peak, 111.85 ± 26.85 μA/cm2; second peak, 69.55 ± 13.80 μA/cm2; Fig. S1A). Removing ambient Cl− (gluconate substitution) made a very highly significant reduction in the whole Isc response (first peak, 32.48 ± 11.42 μA/cm2; second peak, 18.15 ± 5.54 μA/cm2; Fig. S1B) (P < 0.001). A summary of these effects is shown in Fig. S1C. This suggests that the ATP-induced Isc in trachea tissues is primarily due to Cl− currents.
Compared with the control data, the ATP induced Cl− secretion was not changed by 100 μM amiloride (an ENaC blocker) (first peak, 107.64 ± 23.55 μA/cm2; second peak, 56.05 ± 8.95 μA/cm2; Fig. S2A), was partially blocked by 100 μM disulfonic acid stilbene (DIDS, a CaCC blocker) (first peak, 37.26 ± 3.18 μA/cm2; second peak, 68.47 ± 7.01 μA/cm2; Fig. S2B), and was significantly blocked by 1 mM diphenylamine-2-carboxylate (DPC, a nonselective Cl− channel blocker) (first peak, 41.72 ± 8.85 μA/cm2; second peak, 15.61 ± 2.18 μA/cm2; Fig. S2C). These results show that DIDS can inhibit a large proportion of the initial Isc response but had no effect on the second phase. A summary of the above data are shown in Fig. S2D. This suggests that the ATP-induced initial Isc response (Cl− secretion) involves activation of the CaCC, whereas the sustained phase may involve other Cl− channels (such as the CFTR-like channel). To further confirm that the ATP-induced Isc-sustained phase is indeed due to the CFTR opening, we used the most specific commercial CFTR blocker (CFTRinh-172) to test the ATP-induced Isc. When the ATP-induced Isc response reached a plateau, 10 μM CFTRinh-172 was applied to the apical side of the trachea tissue. The results showed that 10 μM CFTRinh-172 can inhibit the ATP-induced sustained phase from 69.55 ± 13.80 μA/cm2 to 37.90 ± 9.15 μA/cm2 (Fig. S2 B and E). These results indicate that CaCC and CFTR channels located on the trachea epithelium mediate the ATP-induced Cl− secretion.
ATP can activate PLC through the P2Y receptor, to elevate the intracellular Ca2+ in different cells (26). To determine whether the pathway mentioned above was used in ATP-induced Cl− secretion in mice trachea epithelia, the effects of the P2-R agonist and antagonist were examined. The administration of ATP (10 μM, apical), a P2-R agonist, led to an increase in Isc (Fig. S3A). However, preincubation of the tracheal epithelia with the P2-R antagonist, suramin (100 μM, apical), greatly suppressed the Isc response to ATP (Fig. S3B), and the change in Isc was reduced by 50% (Fig. S3F).
Further experiments were conducted to investigate the role of PLC and intracellular Ca2+ in ATP-induced Cl− secretion response. The Isc response to ATP was eliminated by pretreatment with U73122 (100 μM, apical), which inhibits PLC activation; with BAPTA-AM (100 μM, apical), which chelates intracellular Ca2+; and with 2-aminoethoxydiphenyl borate (2-APB) (100 μM, apical), which blocks IP3 receptors (Fig. S3 C–F). These data demonstrate that PLC activation and intracellular Ca2+ mobilization play significant roles in the ATP-induced Isc response.
The evidence has shown that P2Y2-R is linked to PLC-generated IP3-mediated release of [Ca2+]i in human airway epithelia (19). Together with the above study, it is thus likely that ATP can induce Cl− secretion response via the P2Y2-R.
Inhibitory Effect of T. gondii on ATP-Induced Cl− Secretion.
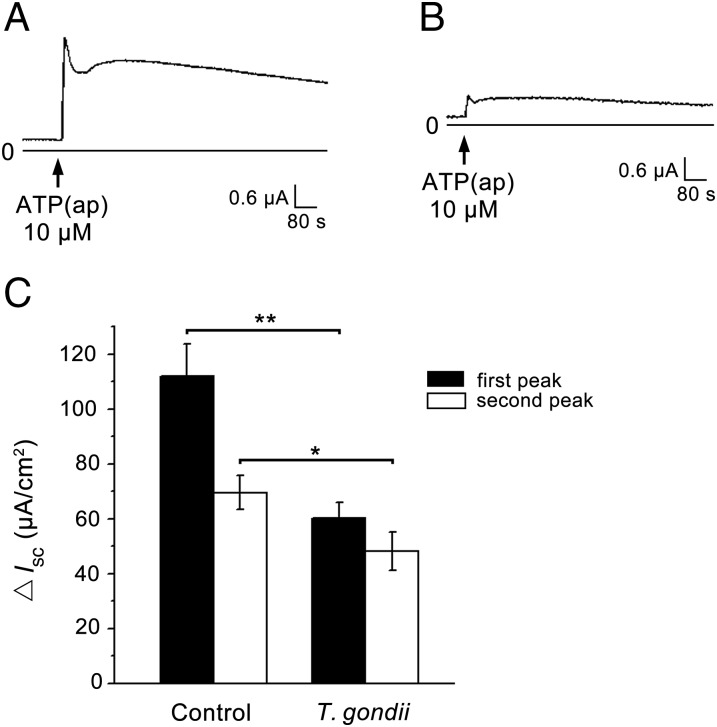
Compared with the normal control group data, the dual peaks of ATP-induced Cl− secretion were reduced significantly by T. gondii infection for 3 d [first peak, 60.19 ± 11.46 μA/cm2 (P < 0.01); second peak, 48.09 ± 14.16 μA/cm2 (P < 0.05); Fig. 1 A and B]. A summary of these effects is shown in Fig. 1C. This suggests that T. gondii can depress ATP-induced Cl− secretion.
Fig. 1.
Effects of T. gondii infection on ATP-induced Isc currents. Addition of 10 μM ATP to the apical side (ap) of the proximal trachea tissues caused a rise in Isc response (A). This response was found to be partially inhibited by T. gondii pretreatment (B). (C) A summary of the effects of T. gondii on the dual phases of ATP-induced Isc response. Data are presented as mean change in Isc ± SEM (n = 4) and are representative of four independent experiments. *P < 0.05; **P < 0.01.
Inhibitory Effect of T. gondii on ATP-Induced [Ca2+]i Response.
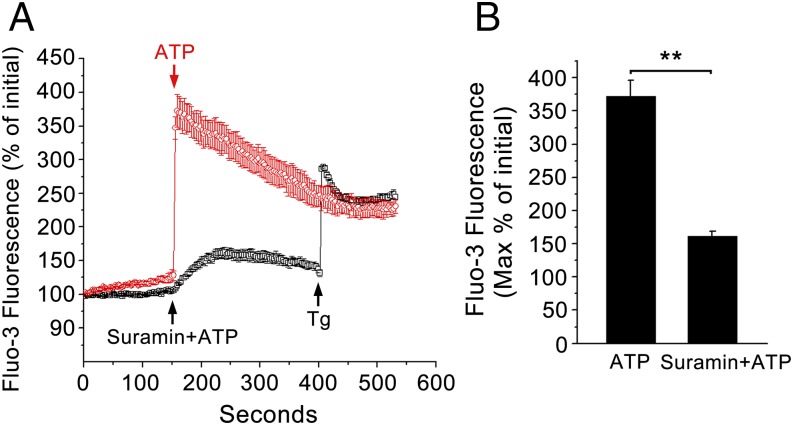
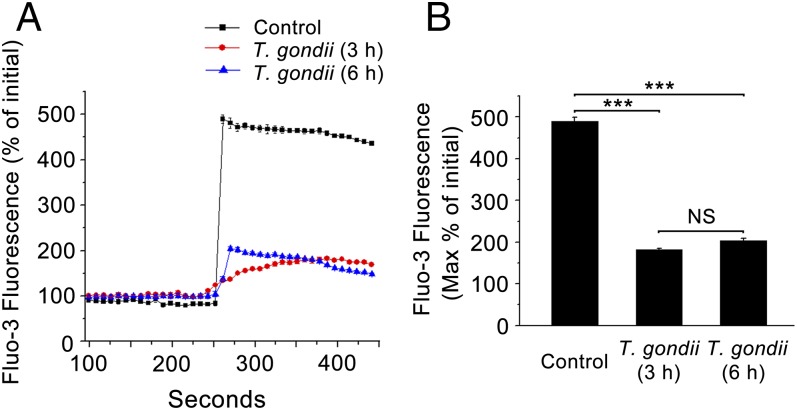
To further investigate intracellular Ca2+ involvement in T. gondii-reduced Cl− secretion in response to ATP, the change of intracellular Ca2+ level was measured by confocal imagining using Fluo3 dye in the presence of extracellular Ca2+. Fig. 2 shows the effect of ATP (10 μM) on the [Ca2+]i in single 16 human bronchial epithelial (16HBE) cells. It can be seen that 10 μM ATP stimulation gave rise to a Ca2+ peak and that in the presence of the P2-R antagonist, suramin, ATP did not lead to a corresponding [Ca2+]i increase, reflecting the effect of P2-R expression on the cells. However, in T. gondii-pretreated cells, the elevation of [Ca2+]i showed a very highly significant attenuation (P < 0.001, Fig. 3). The results show that Ca2+ responses in T. gondii-pretreated cells are functionally reduced compared with those in normal cells.
Fig. 2.
Effect of P2 receptor blocker suramin on intracellular Ca2+ induced by ATP in 16HBE cells. Intracellular Ca2+ measurement in single 16HBE cells using Fluo 3/AM as a probe. (A) The addition of ATP (10 μM) significantly increased the intracellular Ca2+, which was inhibited in the presence of the P2-R antagonist, suramin (100 μM); the addition of 1 μM Thapsigargin (Tg, an inducer of stored Ca2+ release) was done to check the activity of cells. (B) A summary of the effects of suramin on ATP-induced calcium elevation. Data are presented as mean of fluorescence intensity ± SEM (n = 4) and are based on four independent experiments. **P < 0.01.
Fig. 3.
Effect of T. gondii on intracellular Ca2+ induced by ATP in 16HBE cells. Intracellular Ca2+ measurement in single 16HBE cells using Fluo 3/AM as a probe. (A) Addition of ATP (10 μM) significantly increased the intracellular Ca2+ inhibited after T. gondii pretreatment for 3 h or 6 h. (B) A summary of the effects of T. gondii on ATP-induced calcium elevation. Data are presented as mean fluorescence intensity ± SEM (n = 4) and are based on four independent experiments. ***P < 0.001; NS, P > 0.05.
The Effect of T. gondii on Expression of P2Y2-Rs.
Several studies have demonstrated that P2Y2-R is expressed in epithelial cells, smooth muscle cells, endothelial cells, leukocytes, and cardiomyocytes (27–31). Particularly, mice deficient in P2Y2-R lost 85–95% of the nucleotide-stimulated Cl− secretion in mouse tracheal epithelium (18). To investigate the change in the mRNA expression level of the P2Y2-R in T. gondii-infected cells, quantitative real-time PCR analysis was carried out in mouse tracheal tissue that had been infected with T. gondii for 3 d. The mean value of the P2Y2-R mRNA expression level in normal tissue was normalized to 1. After infecting the mouse with T. gondii, the relative levels of the P2Y2-R mRNA increased to 1.90 (normalized by GAPDH; Fig. 4) and 1.55 (normalized by beta-actin; Fig. S4), which was significantly higher than that in the normal tissue (P < 0.05). These results indicate that the mRNA expression of the P2Y2-R increased in T. gondii-infected tracheal tissue.
Fig. 4.
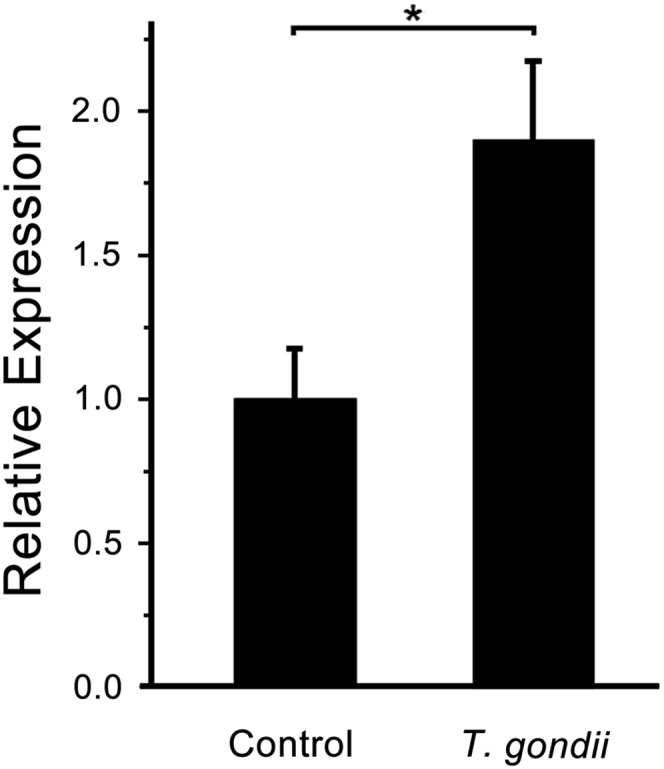
T. gondii infection results in increased P2Y2-R expression. Quantitative real-time PCR analysis of mRNA expression of P2Y2-R in mice trachea treated without or with T. gondii for 3 d. Absolute values were corrected using GAPDH as a reference gene. Gene expression was reported as the relative variation to unstimulated control tissue mRNA levels. Bars represent means ± SEM (n = 3) and are based on three independent experiments. *P < 0.05.
Discussion
Acute infection with T. gondii during pregnancy and its potentially tragic outcome for the fetus and newborn continue to occur worldwide (21), with a significant proportion of pregnancies being at risk (32, 33). Toxoplasmosis is being recognized with increased frequency, especially in patients with AIDS (34). The mechanism of pathogenesis for this disease is complex, although some results from ours and other laboratories indicate that it might be linked with NO production by the host (35–37) and transepithelial migration of T. gondii (38). Little is known about the pathogenesis of host lung infection with T. gondii. In this study, we have examined the role of the predominant subtype P2Y2-R purinoceptors that are expressed in the lungs.
Airway epithelial cells are traditionally known as barrier cells that line the surface of the airways. The apical membrane faces the lumen of the airways, which in turn are in direct contact with the external environment. The fluid-covered microenvironment allows autocrine or paracrine mediators to diffuse easily and rapidly. This provides an ideal setting for autocrine and paracrine purinergic signaling, and this has been implicated in the regulation of airway epithelial cell functions including transepithelial ion transport. In general, extracellular nucleotides and nucleosides, through P2Y, P2X, and P1 receptors, stimulate secretory Cl− and H2O transport (39). Our results demonstrate that ATP could stimulate Cl− secretion via a P2Y2-R–mediated PLC–Ca2+ pathway across mice tracheal epithelia, but that the secretion was attenuated in the epithelia of T. gondii-infected mice. The [Ca2+]i elevation was suppressed in T. gondii-pretreated 16HBE cells. Moreover, the mRNA expression level of P2Y2-R was abnormal in the T. gondii-infected mice trachea.
Abnormal ion transport and mucociliary clearance in airway epithelia is one of the general features of respiratory infection. Extracellular ATP is considered a key signaling molecule to maintain the normal homeostasis of these processes in airway epithelia (40, 41). Our studies in mouse trachea epithelia show that the response of the ATP-stimulated Cl− secretion consisted of an initial spike and a long-term component, which is consistent with previous studies in various organs (23–25). P2Y2-R is a Gq-coupled protein that is activated by extracellular ATP to promote Gq-dependent activation of PLC, which in turn regulates calcium flux and protein kinase C (PKC) activity, by an IP3 and diacylglycerol (DAG)-dependent mechanism, in Schwann cells and eye suprachoroid (42, 43). Based on this fact, in our study, the suppression of the ATP-induced Isc increase by a P2-R inhibitor, suramin, revealed that transepithelial Cl− transport induced by ATP was mediated through the P2-R. Similarly, suppression by a PLC inhibitor, U73122, indicated that PLC activation participated in the response and triggered downstream signaling, in which [Ca2+]i played a major role in stimulation of Cl− secretion. This can be concluded because pretreatment of tracheal epithelia with apical addition of the specific inhibitors BAPTA-AM, a [Ca2+]i chelator, and 2-APB, an inhibitor of [Ca2+]i release, prevented the ATP-induced Isc response. These results demonstrated the role of ATP on the P2-R to stimulate the transfer of signals through the PLC–Ca2+ pathway.
However, the physical Cl− secretion in mouse tracheal epithelia evoked by the apical administration of ATP was impaired in the mice infected with T. gondii for 3 d. In a Ca2+ measurement experiment, it was shown that ATP could raise cytosolic Ca2+ in control cells. Pretreatment of the cells with the P2-R antagonist could block this [Ca2+]i elevation response. However, this ATP-induced intracellular Ca2+ increase was attenuated in T. gondii-infected cells, showing that the interference by Ca2+-dependent signals may account for the inhibited Cl− current in the T. gondii-infected epithelial cells. The experiments traced the effects back to the function of the P2Y receptor, which acts upstream of the Ca2+ signal. Moreover, the P2Y2-R mediates the majority of epithelial Cl− secretory responses (18). From this, we deduce that T. gondii can inhibit the activation of purinergic receptors to reduce Cl− secretion. We hypothesize that T. gondii infection can result in abnormal airway function by controlling the function and expression of purinergic receptors. In T. gondii-infected mice trachea, the mRNA expression levels of P2Y2-R were compared with the control using real-time PCR. However, the mRNA expression in T. gondii-infected mice trachea was higher than that in normal trachea. The abnormal expression level may be caused by the dysfunction of P2Y2-R. The cells need higher receptor mRNA expression to counteract the functional decline in the P2Y2-R. Despite the receptor mRNA expression being increased, it could not rescue the activity of P2Y2-R to exploit the downstream pathways. These results show that T. gondii infection has had a negative potential impact on the cell membrane P2Y2-R, which plays an important role in regulation of airway water homeostasis. These results were in line with the suppressed [Ca2+]i and Isc increase when T. gondii-infected epithelial cells were stimulated by the addition of ATP and the P2-R antagonist. Because the extracellular ATP (and its analog), secreted from airway tissue, can modulate Cl− secretion in airway epithelial cells, the deficiency in the P2Y2-R can result in the decrease of Cl− secretion. This, in turn, can lead to an unusual ASL height as well as poor mucociliary clearance and then to a weakening of the airway innate immune system in fighting against T. gondii infection.
A series of studies have demonstrated that P2Y2-R has important functions in different tissues. The activation of P2Y2-R can promote neuroprotective responses in glial cells (44), mediate cytoprotection in alveolar epithelial cells (45), inhibit inducible NO synthase in cultured rat mesangial cells (46), regulate expression of cyclooxygenase-2 and release of prostaglandin E2 in airways (47), and exert a protective role against infection of the lungs by Pseudomonas aeruginosa (48). Moreover, our studies reveal that T. gondii could result in the abnormal expression and function of P2Y2-R in airways of the infected host. This evidence suggests that T. gondii, by means of the effect on P2Y2-R, could lead to various abnormal changes in the physiological and immune functions involved in the pathological mechanism underlying T. gondii-determined pulmonary infection. This could provide new insights for better understanding the pathogenesis, the prevention, and the therapy of pulmonary toxoplasmosis.
This investigation addressed the questions of whether the ion transport induced by ATP is shifted in T. gondii-infected mammalian hosts, including human tracheal epithelia, and whether purinergic signaling is potentially involved in the mechanisms underlying the disease caused by T. gondii infection in airway epithelial cells. This research may widen our view not only on the physiological functions of purinergic signaling in the airway epithelia but also on the pathophysiology and the pathogenesis of T. gondii infection.
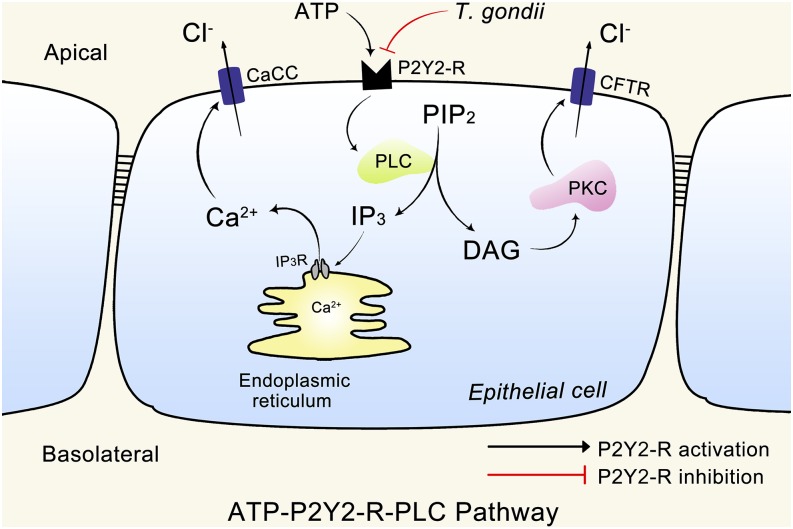
In summary, our results demonstrate that T. gondii can reduce ATP-induced Cl− secretion in airway epithelium by inhibiting the function of the P2Y2-R. The impairment action of T. gondii on chloride secretion was related to its ability to inhibit P2Y2-Rs that are activated by extracellular ATP to promote the Gq-dependent activation of the PLC/IP3/Ca2+- and PLC/DAG/PKC-sensitive Cl− channel pathway (summarized in Fig. 5). The finding that the P2Y2-R can be an essential target blocked by T. gondii provides previously unidentified insights into the mechanism underlying host impairment caused by the infection with T. gondii.
Fig. 5.
Mechanisms underlying the impairment action of T. gondii on chloride secretion in airway epithelia. The schematic model shows that the impairment action of T. gondii on chloride secretion was related to its ability to inhibit P2Y2-Rs. Black arrows represent the P2Y2-R activation signaling pathways, or cellular events identified in the present and previous studies (42, 43), and a red block symbol denotes the P2Y2-R inhibition signaling pathways and cellular events described in airway epithelia [PIP2, phosphatidylinositol (4,5) bisphosphate].
Materials and Methods
Chemicals.
Dulbecco’s modified Eagle medium/nutrient mixture F12 (DMEM/F12), FBS, penicillin/streptomycin, Hank’s Balance Salt Solution, and trypsin were purchased from Gibco Laboratories. ATP disodium salt, amiloride, DPC, DIDS, CFTRinh-172, suramin sodium salt, U73122, BAPTA-AM, and 2-APB were from Sigma Chemical Co. Fluo 3/AM was obtained from Molecular Probes, Inc. ATP and suramin were dissolved in water. The other chemical stocks were dissolved in dimethyl sulfoxide (DMSO).
Animals and T. gondii Infection.
Swiss mice (local name, Kunming mice) were purchased from the Animal Center of Sun Yat-Sen University. Animals were housed and fed according to the guidelines of the Sun Yat-Sen University Animal Use Committee, and all procedures were approved before each experiment. Animals were hosted in a constant-temperature room (25 °C) with a 12 h light/dark photoperiod and were allowed food and water ad libitum. Mice were infected for 3 d with tachyzoites from the T. gondii RH strain by peritoneal injection (5 × 105 per mouse).
Cell Culture and Stimulation of Cells with T. gondii.
The HBE cell line, 16HBE (a gift from J. Xu, Guangzhou Institute of Respiratory Disease, Guangzhou Medical University, Guangzhou, China) was cultured in DMEM/F12 with 10% (vol/vol) FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin at 37 °C, 5% CO2, in a humidified atmosphere.
16HBE cells were seeded at a density of 1 × 105 cells per well in six-well tissue culture plates with medium. Cells were cultured for 48–72 h with a medium change after 24 h, and then incubated with T. gondii at a ratio of 5:1 (parasites:cells, 5:1) in medium without serum and antibiotics for the duration of the experiment.
Tissue Preparation and Short-Circuit Current (Isc) Measurement.
Mice were anesthetized using CO2, and the proximal tracheas were dissected out and put in K–H solution. After carefully removing the blood vessels and connective tissue, the trachea was longitudinally cut open with fine scissors under a stereo microscope and clamped between the two halves of an Ussing Chamber as previously described (49).
Measurement of Intracellular Calcium.
The cells were grown in culture medium on six-well tissue plates with a piece of cover glass at 37 °C. After 2 d of culture and T. gondii infection for 3 h and 6 h, the cells were prepared and loaded with the fluorescence dye Fluo 3/AM, as previously described (50). Data analysis was processed with Origin 8.0. The change of fluorescence intensity after the drug treatments was normalized with the initial intensity.
Total RNA Extraction, Reverse Transcription (RT), and Quantitative Real-Time PCR.
The total RNA was extracted by TRIzol reagents (Invitrogen). The RT reaction of RNA was performed using PrimeScrip First Strand cDNA Synthesis Kit (Takara Bio Inc.) according to the manufacturer’s protocol. The P2Y2-R (forward primer, 5′-TGCCGCTGCTGGTCTATT-3′, and reverse primer, 5′-GGAGCGCAGAGGTCGTAA-3′) and GAPDH (forward primer, 5′-ACATCATCCCTGCATCCACTG-3′, and reverse primer, 5′-TCATTGAGAGCAATGCCAGC-3′) primer sequences were designed using Primer 5.0 software and synthesized by Sangon. The reaction was performed in an iQ5 Gradient Real Time PCR Amplification System (Bio-Rad) using the SYBR Premix Ex Taq (Takara Bio Inc.). After the optimization of RT-PCR conditions, the reaction was conducted with SYBR Green master mix, 10 μM forward/reverse primers, and 100 ng of each cDNA sample. The P2Y2-R primers’ amplification curve was carried out using the following parameters: a hot start at 95 °C for 30 s and then 40 cycles of the 95 °C denaturing step for 5 s and 30 s annealing at 60 °C. The products generated were confirmed by melting curves. The results were analyzed by the comparative threshold cycle (CT) method, and the mRNA expression of P2Y2-R was normalized by GAPDH. Each experiment was carried out in triplicate.
Statistical Analysis.
For Isc measurements, the changes in ion transport (Delta Isc) were given as peak values. All of the results were expressed as mean ± SEM. Statistical comparisons were carried out using the Student’s two-tailed t test or ANOVA where appropriate. Differences were considered statistically significant at P < 0.05.
Supplementary Material
Acknowledgments
The authors thank Dr. Min-Hui Chen, Prof. Jun Xu, and Prof. Ya-Xia Tan of the Guangzhou Institute of Respiratory Disease, Guangzhou Medical University, China, for their critical comments and suggestions. This work was supported by the Natural Science Foundation of China (30770817) and The National Basic Research Program of China (973 Program, 2009CB522102 and 2010CB530000).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1503474112/-/DCSupplemental.
References
- 1.Chan HC, Wang ZD, Yang GQ. Exocrine Physiology: Basic Theories and Clinical Aspects. Science Press; Beijing: 2002. [Google Scholar]
- 2.Tarran R, Trout L, Donaldson SH, Boucher RC. Soluble mediators, not cilia, determine airway surface liquid volume in normal and cystic fibrosis superficial airway epithelia. J Gen Physiol. 2006;127(5):591–604. doi: 10.1085/jgp.200509468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spring KR. Epithelial fluid transport—A century of investigation. News Physiol Sci. 1999;14:92–98. doi: 10.1152/physiologyonline.1999.14.3.92. [DOI] [PubMed] [Google Scholar]
- 4.Boucher RC. Human airway ion transport. Part one. Am J Respir Crit Care Med. 1994;150(1):271–281. doi: 10.1164/ajrccm.150.1.8025763. [DOI] [PubMed] [Google Scholar]
- 5.Stutts MJ, et al. Regulation of Cl- channels in normal and cystic fibrosis airway epithelial cells by extracellular ATP. Proc Natl Acad Sci USA. 1992;89(5):1621–1625. doi: 10.1073/pnas.89.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucher RC. Airway surface dehydration in cystic fibrosis: Pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 7.Saano V, et al. The effect of ATP on the ciliary activity of normal and pathological human respiratory mucosa in vitro. Acta Otolaryngol. 1991;111(1):130–134. [PubMed] [Google Scholar]
- 8.Davis CW, Lazarowski E. Coupling of airway ciliary activity and mucin secretion to mechanical stresses by purinergic signaling. Respir Physiol Neurobiol. 2008;163(1-3):208–213. doi: 10.1016/j.resp.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal. 2005;1(4):311–328. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Button B, Boucher RC. University of North Carolina Virtual Lung Group Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163(1-3):189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devor DC, Bridges RJ, Pilewski JM. Pharmacological modulation of ion transport across wild-type and DeltaF508 CFTR-expressing human bronchial epithelia. Am J Physiol Cell Physiol. 2000;279(2):C461–C479. doi: 10.1152/ajpcell.2000.279.2.C461. [DOI] [PubMed] [Google Scholar]
- 12.Mall M, et al. Inhibition of amiloride-sensitive epithelial Na(+) absorption by extracellular nucleotides in human normal and cystic fibrosis airways. Am J Respir Cell Mol Biol. 2000;23(6):755–761. doi: 10.1165/ajrcmb.23.6.4207. [DOI] [PubMed] [Google Scholar]
- 13.Mason SJ, Paradiso AM, Boucher RC. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br J Pharmacol. 1991;103(3):1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke LL, Boucher RC. Chloride secretory response to extracellular ATP in human normal and cystic fibrosis nasal epithelia. Am J Physiol. 1992;263(2 Pt 1):C348–C356. doi: 10.1152/ajpcell.1992.263.2.C348. [DOI] [PubMed] [Google Scholar]
- 15.Di Virgilio F, et al. Nucleotide receptors: An emerging family of regulatory molecules in blood cells. Blood. 2001;97(3):587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- 16.Sak K, Webb TE. A retrospective of recombinant P2Y receptor subtypes and their pharmacology. Arch Biochem Biophys. 2002;397(1):131–136. doi: 10.1006/abbi.2001.2616. [DOI] [PubMed] [Google Scholar]
- 17.Weisman GA, et al. Molecular determinants of P2Y2 nucleotide receptor function: Implications for proliferative and inflammatory pathways in astrocytes. Mol Neurobiol. 2005;31(1-3):169–183. doi: 10.1385/MN:31:1-3:169. [DOI] [PubMed] [Google Scholar]
- 18.Cressman VL, et al. Effect of loss of P2Y(2) receptor gene expression on nucleotide regulation of murine epithelial Cl(-) transport. J Biol Chem. 1999;274(37):26461–26468. doi: 10.1074/jbc.274.37.26461. [DOI] [PubMed] [Google Scholar]
- 19.Brown HA, Lazarowski ER, Boucher RC, Harden TK. Evidence that UTP and ATP regulate phospholipase C through a common extracellular 5′-nucleotide receptor in human airway epithelial cells. Mol Pharmacol. 1991;40(5):648–655. [PubMed] [Google Scholar]
- 20.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965–1976. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 21.Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis. 2008;47(4):554–566. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- 22.Lahiri K. Parasitic infections of the respiratory tract (diagnosis and management) J Postgrad Med. 1993;39(3):144–148. [PubMed] [Google Scholar]
- 23.Chan HC, Zhou WL, Fu WO, Ko WH, Wong PY. Different regulatory pathways involved in ATP-stimulated chloride secretion in rat epididymal epithelium. J Cell Physiol. 1995;164(2):271–276. doi: 10.1002/jcp.1041640207. [DOI] [PubMed] [Google Scholar]
- 24.Chan HC, et al. Regulation of Cl− secretion by extracellular ATP in cultured mouse endometrial epithelium. J Membr Biol. 1997;156(1):45–52. doi: 10.1007/s002329900186. [DOI] [PubMed] [Google Scholar]
- 25.Kanoh S, et al. Differential regulations between adenosine triphosphate (ATP)- and uridine triphosphate-induced Cl(-) secretion in bovine tracheal epithelium. Direct stimulation of P1-like receptor by ATP. Am J Respir Cell Mol Biol. 2001;25(3):370–376. doi: 10.1165/ajrcmb.25.3.4382. [DOI] [PubMed] [Google Scholar]
- 26.Werry TD, Wilkinson GF, Willars GB. Mechanisms of cross-talk between G-protein-coupled receptors resulting in enhanced release of intracellular Ca2+ Biochem J. 2003;374(Pt 2):281–296. doi: 10.1042/BJ20030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lustig KD, et al. Mechanisms by which extracellular ATP and UTP stimulate the release of prostacyclin from bovine pulmonary artery endothelial cells. Biochim Biophys Acta. 1992;1134(1):61–72. doi: 10.1016/0167-4889(92)90028-a. [DOI] [PubMed] [Google Scholar]
- 28.Kim KC, Park HR, Shin CY, Akiyama T, Ko KH. Nucleotide-induced mucin release from primary hamster tracheal surface epithelial cells involves the P2u purinoceptor. Eur Respir J. 1996;9(7):1579. [PubMed] [Google Scholar]
- 29.Kunapuli SP, Daniel JL. P2 receptor subtypes in the cardiovascular system. Biochem J. 1998;336(Pt 3):513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pillois X, et al. Nucleotide receptors involved in UTP-induced rat arterial smooth muscle cell migration. Circ Res. 2002;90(6):678–681. doi: 10.1161/01.res.0000013700.98464.8e. [DOI] [PubMed] [Google Scholar]
- 31.Seye CI, et al. Functional P2Y2 nucleotide receptors mediate uridine 5′-triphosphate-induced intimal hyperplasia in collared rabbit carotid arteries. Circulation. 2002;106(21):2720–2726. doi: 10.1161/01.cir.0000038111.00518.35. [DOI] [PubMed] [Google Scholar]
- 32.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: Global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39(12):1385–1394. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Gao XJ, et al. Toxoplasma gondii infection in pregnant women in China. Parasitology. 2012;139(2):139–147. doi: 10.1017/S0031182011001880. [DOI] [PubMed] [Google Scholar]
- 34.Pomeroy C, Filice GA. Pulmonary toxoplasmosis: A review. Clin Infect Dis. 1992;14(4):863–870. doi: 10.1093/clinids/14.4.863. [DOI] [PubMed] [Google Scholar]
- 35.Scharton-Kersten TM, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185(7):1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z, et al. Differences in iNOS and arginase expression and activity in the macrophages of rats are responsible for the resistance against T. gondii infection. PLoS ONE. 2012;7(4):e35834. doi: 10.1371/journal.pone.0035834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao ZJ, et al. Lower expression of inducible nitric oxide synthase and higher expression of arginase in rat alveolar macrophages are linked to their susceptibility to Toxoplasma gondii infection. PLoS ONE. 2013;8(5):e63650. doi: 10.1371/journal.pone.0063650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barragan A, Sibley LD. Transepithelial migration of Toxoplasma gondii is linked to parasite motility and virulence. J Exp Med. 2002;195(12):1625–1633. doi: 10.1084/jem.20020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwiebert EM, Zsembery A. Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta. 2003;1615(1-2):7–32. doi: 10.1016/s0005-2736(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 40.Donaldson SH, et al. Basal nucleotide levels, release, and metabolism in normal and cystic fibrosis airways. Mol Med. 2000;6(11):969–982. [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi T, et al. ATP regulation of ciliary beat frequency in rat tracheal and distal airway epithelium. Exp Physiol. 2005;90(4):535–544. doi: 10.1113/expphysiol.2004.028746. [DOI] [PubMed] [Google Scholar]
- 42.Sugamoto Y, Hirai K, Tokoro T. P2Y2 receptor elevates intracellular calcium concentration in rabbit eye suprachoroid. J Med Dent Sci. 1999;46(2):83–92. [PubMed] [Google Scholar]
- 43.Liu GJ, Werry EL, Bennett MR. Secretion of ATP from Schwann cells in response to uridine triphosphate. Eur J Neurosci. 2005;21(1):151–160. doi: 10.1111/j.1460-9568.2004.03831.x. [DOI] [PubMed] [Google Scholar]
- 44.Peterson TS, et al. P2Y2 nucleotide receptor-mediated responses in brain cells. Mol Neurobiol. 2010;41(2-3):356–366. doi: 10.1007/s12035-010-8115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belete HA, Hubmayr RD, Wang S, Singh RD. The role of purinergic signaling on deformation induced injury and repair responses of alveolar epithelial cells. PLoS ONE. 2011;6(11):e27469. doi: 10.1371/journal.pone.0027469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mohaupt MG, Fischer T, Schwöbel J, Sterzel RB, Schulze-Lohoff E. Activation of purinergic P2Y2 receptors inhibits inducible NO synthase in cultured rat mesangial cells. Am J Physiol. 1998;275(1 Pt 2):F103–F110. doi: 10.1152/ajprenal.1998.275.1.F103. [DOI] [PubMed] [Google Scholar]
- 47.Marcet B, Libert F, Boeynaems JM, Communi D. Extracellular nucleotides induce COX-2 up-regulation and prostaglandin E2 production in human A549 alveolar type II epithelial cells. Eur J Pharmacol. 2007;566(1-3):167–171. doi: 10.1016/j.ejphar.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Geary C, et al. Increased susceptibility of purinergic receptor-deficient mice to lung infection with Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L890–L895. doi: 10.1152/ajplung.00428.2004. [DOI] [PubMed] [Google Scholar]
- 49.Du JY, et al. Cellular mechanisms of carbachol-stimulated Cl− secretion in rat epididymal epithelium. Biol Reprod. 2006;75(3):407–413. doi: 10.1095/biolreprod.106.052316. [DOI] [PubMed] [Google Scholar]
- 50.Ruan YC, et al. Regulation of smooth muscle contractility by the epithelium in rat vas deferens: Role of ATP-induced release of PGE2. J Physiol. 2008;586(Pt 20):4843–4857. doi: 10.1113/jphysiol.2008.154096. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.