Significance
Signals through the T-cell receptor (TCR) activate T lymphocytes and promote protective immunity. Antigen binding to the TCR triggers both signaling through the receptor and its internalization from the cell surface. Continuous TCR signaling is required for T-lymphocyte proliferation, yet how signaling is maintained for many hours despite TCR internalization is unknown. In this report we show that genetically blocking TCR internalization inhibits T-lymphocyte proliferation. TCR internalization was required for sustained signaling and the activation of key pathways that promote T-lymphocyte metabolism. Together these results may have implications for T-lymphocyte–based immunotherapies.
Keywords: T-cell receptor, endocytosis, metabolism, proliferation
Abstract
Prolonged T-cell receptor (TCR) signaling is required for the proliferation of T lymphocytes. Ligation of the TCR activates signaling, but also causes internalization of the TCR from the cell surface. How TCR signaling is sustained for many hours despite lower surface expression is unknown. Using genetic inhibition of endocytosis, we show here that TCR internalization promotes continued TCR signaling and T-lymphocyte proliferation. T-cell–specific deletion of dynamin 2, an essential component of endocytosis, resulted in reduced TCR signaling strength, impaired homeostatic proliferation, and the inability to undergo clonal expansion in vivo. Blocking endocytosis resulted in a failure to maintain mammalian target of rapamycin (mTOR) activity and to stably induce the transcription factor myelocytomatosis oncogene (c-Myc), which led to metabolic stress and a defect in cell growth. Our results support the concept that the TCR can continue to signal after it is internalized from the cell surface, thereby enabling sustained signaling and cell proliferation.
Signals through the T-cell receptor (TCR) direct the function of T lymphocytes. In quiescent naive T cells, TCR ligation triggers intracellular signaling pathways that result in proliferation and differentiation into effector cells. T cells are distinguished by an exceptionally high rate of proliferation, and accordingly, the transition from quiescence to proliferation is a metabolically demanding process. T-cell proliferation is strictly preceded by a growth phase that lasts about ∼24 h. During this growth phase, T cells increase their size and prepare for cell division. To meet the increased metabolic demands, T-cell metabolism must be reprogrammed from a mainly catabolic to an anabolic state. This involves a switch from oxidative phosphorylation to glycolysis to provide fuel and metabolites for nucleic acid, protein, and lipid biosynthesis. T-cell metabolism is controlled by thymoma viral proto-oncogene (Akt), mammalian target of rapamycin (mTOR), and ERK signaling pathways as well as by the transcription factors myelocytomatosis oncogene (c-Myc), hypoxia-inducible factor-1 alpha (HIF-1α), and estrogen-related receptor alpha (ERR) (1–3). Induction of c-Myc by TCR and cytokine signals is particularly critical for the metabolic reprogramming and the proliferation of naive T cells (4, 5).
Previous studies have shown that 10–24 h of continuous TCR signaling are required for the proliferation of CD4 T cells in vitro (6, 7) and for CD8 T-cell proliferation in vivo (8). How TCR signaling is maintained for this extended period remains unclear because the initiation of TCR signaling causes TCR down-modulation from the cell surface. After ligation, the TCR is internalized via endocytosis and sorted to lysosomes where it is degraded, leading to reduced amounts on the cell surface (9, 10). Despite lower TCR surface expression, TCR signaling is sustained for many hours (7). This raises the important question of how TCR signaling is maintained when the TCR is removed from the cell surface.
One potential mechanism to explain the sustained signaling observed after the TCR is removed from the cell surface is that the TCR continues to signal after it is internalized. The concept of continued signaling from internalized receptors is supported by the finding that endocytosis cannot only limit, but also can promote signaling from G protein-coupled receptors (GPCRs) and transmembrane receptor tyrosine kinases (RTKs) (11). The TCR is structurally related to RTKs and, therefore, continued signaling from internalized receptors represents an attractive possibility to explain how TCR signaling is maintained. This possibility is supported by several observations. An early report described colocalization of the TCR with active signaling molecules in endosomes (12). This was confirmed by a recent study in Jurkat cells, which found evidence for continued signaling from the TCR after receptor internalization (13). Further support comes from work in B lymphocytes, which demonstrated that the B-cell receptor continues to signal from endosomal compartments (14). However, the functional consequence and physiological relevance of TCR signaling from endosomes is unknown.
We hypothesized that prolonged TCR signaling is sustained by signaling from within endosomes, thereby promoting T-cell proliferation. To test this hypothesis, we used a genetic approach to inhibit TCR internalization in vivo and to disable signaling from internalized receptors. Specifically, we used T cells deficient in dynamin 2 because dynamins are an essential part of the molecular machinery required for endocytosis (15). We report here that dynamin 2-dependent endocytosis is critical for maintaining mTOR complex 1 (mTORC1) activity and expression of the transcription factor c-Myc and for the subsequent metabolic switch required for T-cell growth. We propose that continued signaling from internalized TCRs represents a mechanism that promotes T-cell metabolism and proliferation.
Results
Dynamin 2 Is Required for TCR Internalization.
We used genetic inhibition of endocytosis in T lymphocytes as a physiologically relevant system to interrogate the role of TCR internalization in vivo. TCR internalization can occur via clathrin-dependent and clathrin-independent endoctytic pathways that are both dependent on dynamin (16). We therefore used mice deficient in dynamin 2, the only dynamin isoform present in T lymphocytes (17), to inhibit TCR internalization. Mice with a conditional allele of the dynamin 2 (Dnm2) gene were bred to Cd4-cre transgenic mice to generate Cd4-creDnm2flox/flox mice (“Dnm2 KO”) and to ablate dynamin 2 expression specifically in T cells (17). Littermate Cd4-creDnm2flox/+ mice (“Dnm2 HET”) were used as controls. To confirm that dynamin 2-dependent endocytosis is required for TCR internalization, we stimulated T cells from Dnm2 KO and control mice with plate-bound α-CD3 antibody (Ab) to cross-link the TCR. Flow cytometric analysis revealed a time-dependent down-modulation of the TCR from the cell surface in T cells with a peak at 8 h (Fig. S1). Importantly, ligand-stimulated TCR down-modulation was largely abolished in dynamin 2-deficient T cells (Fig. S1). These data demonstrate that dynamin 2 is necessary for TCR internalization, thereby validating our experimental system to investigate the function of TCR internalization in T lymphocytes.
Dynamin 2 Deficiency Causes Reduced TCR Signal Strength.
We next wished to establish whether inhibition of TCR internalization raises TCR signal strength by prolonging signaling from the plasma membrane, or alternatively, whether it lowers TCR signal strength due to reduced signaling from intracellular compartments. To distinguish between these possibilities, we determined expression of the cell surface protein CD5 because it has been shown to correlate with the strength of the TCR signal in vivo. We found that the absence of dynamin 2 did not alter CD5 expression in double-positive thymocytes (Fig. S2A). In contrast, CD5 surface levels were markedly lower in mature (TCRβhiCD24lo) thymocytes and naive T cells in the periphery (Fig. S2A). We have previously shown that Cd4-creDnm2flox/flox mice are lymphopenic due to impaired thymic egress (17). To exclude any potential confounding effect of lymphopenia, we examined CD5 surface levels in mixed bone marrow chimeras. This revealed that reduced CD5 expression in the absence of dynamin 2 was cell intrinsic and not due to lymphopenia because T cells derived from dynamin 2-deficient bone marrow cells had lower CD5 expression than cells derived from control bone marrow (Fig. S2B). Impaired egress also increases the residence time of mature T cells in the thymus and we wished to rule out that this was the cause for the reduced TCR signal strength in dynamin 2-deficient T cells. To exclude this possibility, we treated wild-type mice with the high-affinity sphingosine-1-phosphate (S1P) agonist FTY720 to block egress (Fig. S3 A–C). CD5 expression was not perturbed in mature thymocytes from FTY720-treated mice (Fig. S3D), demonstrating that blocked thymic egress does not affect TCR signal strength. Thus, reduced TCR signal strength in dynamin 2-deficient T cells was not caused by impaired egress from the thymus. We conclude that dynamin 2-deficiency lowers TCR signaling strength in vivo, suggesting an essential role for TCR internalization in maintaining responsiveness to TCR signals.
Dynamin 2 Is Critical for T-Cell Proliferation in Vivo.
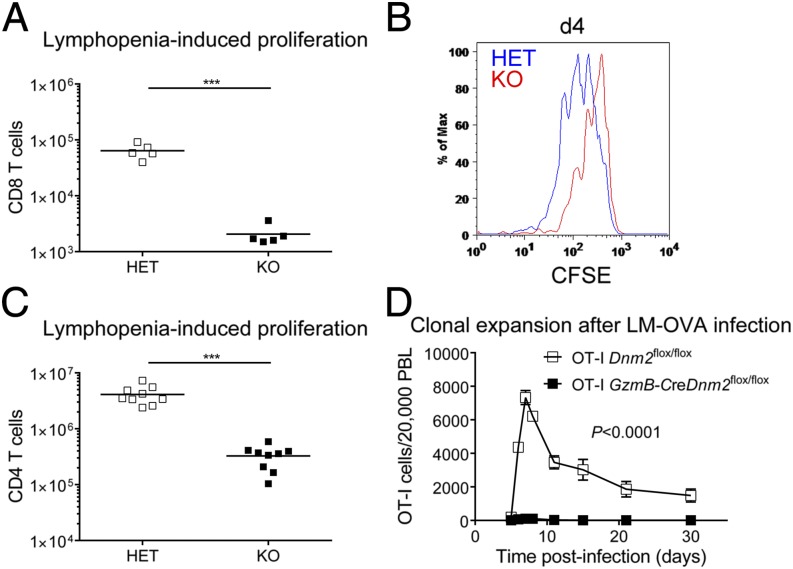
Our observation that dynamin-dependent TCR internalization ensures proper TCR signaling strength predicted that dynamin 2 deficiency should perturb TCR-dependent events that regulate the function of T lymphocytes. Moreover, the observed reduction of TCR signaling strength in postselection T cells (Fig. S2) raised the possibility that dynamin 2-dependent TCR internalization was primarily important in peripheral T cells. In support of this possibility, the number of peripheral T cells was reduced in Dnm2 KO mice (Fig. S4). Dnm2 KO mice have normal T-cell development (17), which suggests that dynamin 2 promotes peripheral T-cell homeostasis. However, loss of naive T cells in Dnm2 KO mice could be exclusively due to impaired thymic exit of mature T cells (17). To assess T-cell homeostasis without this confounding effect, we adoptively transferred dynamin 2-deficient naive T cells into new host mice, thereby bypassing T-cell egress from the thymus. Using this system, we first asked whether homeostatic T-cell proliferation is dependent on dynamin-dependent TCR internalization. To address this question, we cotransferred congenically marked naive T cells from Dnm2 HET and KO mice at a 1:1 ratio into recombination activating gene 1 (Rag1)-deficient mice that lack lymphocytes. One week after transfer, control CD8 T cells outnumbered dynamin 2-deficient cells by a factor of greater than 100-fold (Fig. 1A). To measure cell division directly, T cells were labeled with carboxyfluorescein succinimidyl ester (CFSE) before transfer and CFSE dilution was assessed by flow cytometry. This analysis demonstrated that Dnm2 KO cells had undergone fewer cell divisions than their HET counterparts (Fig. 1B). Dynamin 2-deficient naive CD4 T cells were also defective in their ability to expand in a lymphopenic host (Fig. 1C). Dynamin 2-deficient naive T cells did not have an intrinsic defect in their ability to survive because they showed similar persistence as control cells after cotransfer into mice with a full lymphocyte compartment (Fig. S5A). Lymphopenia-induced proliferation is not only dependent on TCR ligation, but also on the cytokine IL-7. To exclude that defective T-cell proliferation in the absence of dynamin 2 was secondary to impaired IL-7 signaling, we stimulated naive T cells with recombinant IL-7 in vitro. Dynamin 2-deficient T cells showed normal survival in response to IL-7 (Fig. S5B) and IL-7 signaling was intact as determined by STAT5 phosphorylation (Fig. S5C). Overall, these results suggest that dynamin 2-dependent endocytosis promotes homeostatic T-cell proliferation induced by self-peptide–TCR interactions. We next asked whether dynamin 2-dependent TCR internalization was also required for T-cell proliferation in response to foreign antigen. To address this question, we used infection with Listeria monocytogenes, which express the ovalbumin (OVA) peptide (LM-OVA) that is recognized by the transgenic OT-I TCR. We first crossed Dnm2flox/flox mice to GzmB-cre transgenic mice, which results in dynamin 2 ablation in effector T cells, and then generated GzmB-creDnm2flox/flox mice on the OT-I TCR transgenic background. To study T-cell proliferation, we transferred naive OT-I GzmB-creDnm2flox/flox and OT-I Dnm2flox/flox T cells at a ratio of 1:1 into B6 recipient mice that were then infected with LM-OVA. As expected, OT-I Dnm2flox/flox T cells underwent clonal expansion after infection (Fig. 1D). In contrast, OT-I GzmB-creDnm2flox/flox T cells failed to proliferate (Fig. 1D). Collectively, these data show that dynamin 2 is critical for T-cell proliferation in response to self and foreign antigen.
Fig. 1.
Dynamin 2 promotes T-cell proliferation in vivo. (A) Naive CD8 T cells from Dnm2 HET (CD45.1.2+) and KO (CD45.2+) mice were mixed 1:1 and injected into Rag1 KO recipients (CD45.1+). Number of CD8 T cells in spleen/lymph nodes recovered from Rag1 KO recipients 8 d after cotransfer is shown (n = 5). (B) Naive CD8 T cells from Dnm2 HET and KO mice were labeled with CSFE, mixed 1:1, and cotranferred into Rag1 KO mice. CFSE dilution was measured by flow cytometry 4 d after transfer. (C) Number of Dnm2 HET and KO CD4 T cells in spleen and lymph nodes recovered from Rag1 KO recipients 14 d after cotransfer (n = 9). A 1:1 mixture of naive Dnm2 HET and KO CD4 T cells was cotransferred into Rag1 KO recipients. (D) Number of Dnm2 WT and KO OT-I T cells in the blood of B6 recipient mice after infection with LM-OVA (n = 6–8). Naive CD8 T cells from OT-I Dnm2flox/flox (CD45.1.2+) and OT-I GzmB-creDnm2flox/flox (CD45.2+) mice were mixed 1:1 and injected into B6 recipients (CD45.1+). One day after transfer, recipient mice were infected with Listeria monocytogenes expressing OVA peptide (LM-OVA). All error bars represent SEM. ***P < 0.001 by unpaired Student's t test (A and C) or one-way ANOVA (D). Results are representative of or combined from two (A, B, and D), or three (C) experiments.
Dynamin 2 Promotes T-Cell Growth.
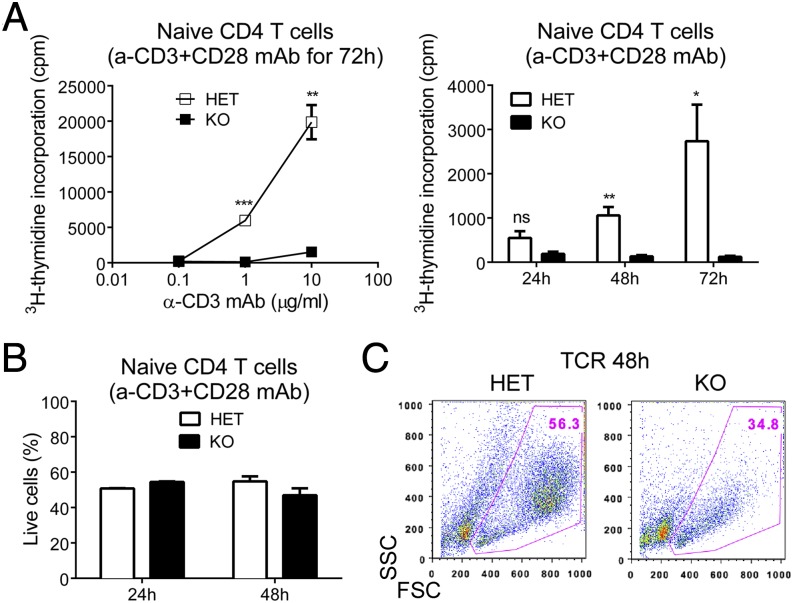
We next wished to dissect the requirement of dynamin 2 for T-cell proliferation and for this we turned to in vitro studies. To study T-cell proliferation in vitro, we purified naive T cells and stimulated them with plate-bound α-CD3 Ab to cross-link the TCR and with soluble α-CD28 Ab to provide a costimulatory signal. As an overall measure of proliferation, we determined TCR-induced DNA synthesis by 3H-thymidine incorporation. Consistent with the requirement of dynamin 2 for T-cell proliferation in vivo, we found that dynamin 2-deficient naive CD4 T cells were unable to proliferate in response to varying doses of α-CD3 Ab in vitro (Fig. 2A). Similar results were obtained with naive CD8 T cells (Fig. S6A). The proliferation defect was not caused by excessive cell death early after TCR ligation because survival of dynamin 2-deficient cells was normal within the first 24–48 h of stimulation (Fig. 2B and Fig. S6B). Next, we wished to address at which stage of TCR stimulation the defect in proliferation occurs. T-cell proliferation is preceded by a ∼24-h period of activation and cell growth, during which biosynthesis of cell components and a subsequent increase in cell size takes place. Dynamin 2-deficient T cells showed up-regulation of the activation marker CD69 after TCR stimulation (Figs. S6C and S7A), consistent with normal initial T-cell activation. However, closer inspection revealed lower CD25 (IL-2Rα) surface expression in dynamin 2-deficient CD4 T cells at 20 h of TCR stimulation (Fig. S7B), whereas CD25 expression was not reduced in dynamin 2-deficient CD8 T cells (Fig. S6C). As expected, control T cells had the typical “blast” appearance 48 h after TCR stimulation due to increased cell size (Fig. 2C and Fig. S6D). In contrast, most dynamin 2-deficient T cells did not blast and were smaller than their dynamin 2-sufficient counterparts (Fig. 2C and Fig. S6D). Together, our results establish that dynamin 2 is essential for T-cell growth.
Fig. 2.
Dynamin 2 is essential for T-cell growth. Naive CD4 T cells from Dnm2 HET and KO mice were stimulated with plate-bound α-CD3 Ab and soluble α-CD28 Ab in vitro. (A) DNA synthesis was measured in Dnm2 HET and KO cells by 3H-thymidine incorporation as cpm. Graph shows DNA synthesis in naive CD4 T cells stimulated for 72 h with various concentrations of α-CD3 Ab and with 5 μg/mL α-CD28 Ab (Left) or stimulated for the indicated times with 10 μg/mL α-CD3 Ab and 5 μg/mL α-CD28 Ab (Right). (B) Frequency of live Dnm2 HET and KO CD4 T cells after stimulation with 10 μg/mL α-CD3 Ab and 5 μg/mL α-CD28 Ab. Live cells were gated as 7-AAD−annexinV− cells. (C) Representative dot plots showing forward scatter (FSC) and side scatter (SSC) profiles of naive CD4 T cells from Dnm2 HET and KO mice stimulated for 48 h with α-CD3 and α-CD28 Abs. All error bars represent SEM. P > 0.05; *P < 0.05; **P < 0.01; ***P < 0.001 by unpaired Student's t test. Results are representative of or combined from three (B), four (A), or more than five (C) experiments.
Dynamin 2 Sustains mTORC1 Signaling and Limits Autophagy.
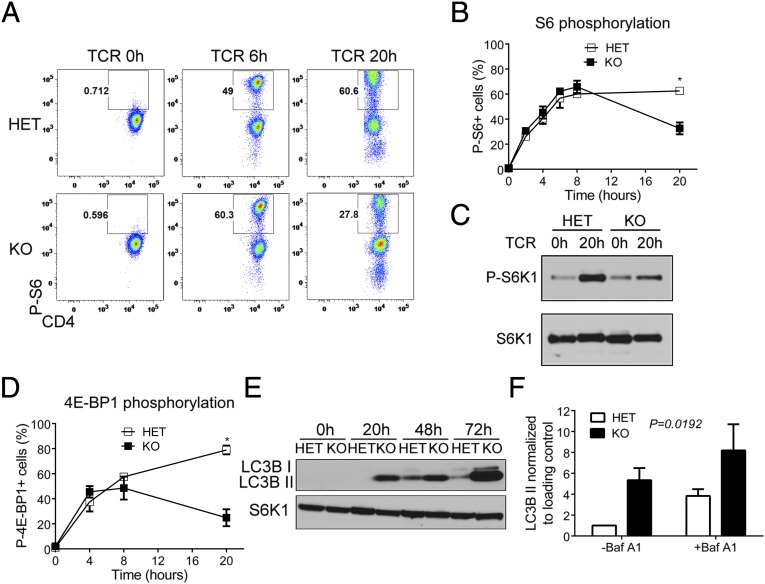
We next wanted to define how dynamin 2 regulates T-cell growth. T-cell growth is dependent on metabolic reprogramming, which predicts that signaling pathways regulating cell metabolism are defective in T cells lacking dynamin 2. To test this prediction, we examined TCR-induced activation of Akt, mTOR, and MAP kinase pathways. Compared with control cells, ERK phosphorylation was enhanced in dynamin 2-deficient T cells after TCR stimulation (Fig. S8 A and B), possibly due to prolonged TCR signaling from the plasma membrane, whereas amounts of phospho-JNK (Fig. S8C) and phospho-p38 (Fig. S8D) were similar. We also determined the kinetics of tyrosine kinase signaling in dynamin 2-deficient T cells. Whereas phosphorylation of Lck was not affected by the absence of dynamin 2 (Fig. S8E), the amount of phospho-ZAP70 was lower at 20 h of TCR stimulation (Fig. S8F), which supports our concept that TCR signaling is not sustained in the absence of dynamin 2. These results suggested that defects in other pathways are responsible for impaired T-cell growth in dynamin 2 KO T cells. To test this notion, we measured phosphorylation of ribosomal protein S6 as a readout for mTORC1 activation. Whereas TCR ligation activated mTORC1 signaling in both control and dynamin 2-deficient T cells to a similar extent during the first 8 h of stimulation, KO cells were unable to sustain S6 phosphorylation at 20 h of stimulation (Fig. 3 A and B). We confirmed that dynamin 2 is required for sustained mTORC1 signaling by measuring phosphorylation of the direct mTORC1 substrates Ribosomal protein S6 kinase 1 (S6K1) and eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1), which was lower in the absence of dynamin 2 (Fig. 3 C and D). We next asked whether the failure to maintain mTORC1 activity was caused by altered upstream signaling inputs (3). To address this possibility, we first examined TCR-induced phosphorylation of Akt because PI3K-Akt signaling is thought to induce mTORC1 activation. However, we found that Akt phosphorylation on threonine 308, which activates Akt kinase activity, was similar in Dnm2 HET and KO T cells (Fig. S9A). In addition, Akt phosphorylation on serine 473, which is mediated by mTOR complex 2, was not affected by the lack of dynamin 2 (Fig. S9B). These data indicate that PI3K-Akt signaling is intact in dynamin 2-deficient T cells. Accordingly, phosphorylation of the mTORC1 inhibitor and Akt substrate tuberous sclerosis complex 2 (Tsc2) was not affected by the absence of dynamin 2 (Fig. S9C). mTORC1 activity is negatively regulated by AMP-activated protein kinase alpha (AMPKa), a kinase-sensing cellular energy stress (3). However, AMPKα activity, as measured by threonine 172 phosphorylation (Fig. S9D) and by phosphorylation of the AMPKα substrates Tsc2 (Fig. S9E) and Raptor (Fig. S9F), was decreased rather than increased in Dnm2 KO cells. Availability of nutrients is another critical requirement for mTORC1 activation in T cells. TCR signaling increases amino acid uptake, which is essential for mTORC1 activation (18). We therefore determined surface expression of the amino acid transporter CD98. Flow cytometric analysis revealed that, similar to control T cells, dynamin 2-deficient cells up-regulated CD98 in response to TCR stimulation (Fig. S9G). Thus, upstream regulators of mTORC1 seem to be intact in T cells lacking dynamin 2, which suggests that reduced mTORC1 activity in the absence of dynamin 2 is caused by the failure to activate a signaling pathway that cross-talks with mTORC1.
Fig. 3.
Dynamin 2 sustains mTORC1 signaling and limits autophagy. Naive CD4 T cells from Dnm2 HET and KO mice were activated with α-CD3 and α-CD28 mAbs for the indicated time periods. (A and B) Phosphorylated ribosomal protein S6 (P-S6) was detected in CD4 T cells from Dnm2 HET and KO mice by intracellular flow cytometry. Representative dot plots (A) and frequency (B) of P-S6+ Dnm2 HET and KO cells are shown. (C) Phosphorylation of S6K1 was measured in Dnm2 HET and KO CD4 T cells by Western blot. (D) Phosphorylated 4E-BP1 was detected in CD4 T cells from Dnm2 HET and KO mice by intracellular flow cytometry. (E) LC3B protein expression in activated CD4 T cells from Dnm2 HET or KO mice was measured by Western blot. The nonlipidated (I) and lipidated (II) isoforms of LC3B are indicated. S6K1 was used as a loading control. (F) Dnm2 HET and KO CD4 T cells were activated for 48 h in the absence or presence of the lysosomal inhibitor bafilomycin A1 (Baf A1) for the last 4 h of culture and LC3B II amounts quantified by densitometry (n = 3). All error bars represent SEM. *P < 0.05 by unpaired Student's t test (B and D) or one-way ANOVA (F). Results are representative of or combined from two (A, B, and D), three (C and F), or five (E) experiments.
Because mTORC1 signaling promotes anabolic pathways, while repressing catabolic pathways, such as autophagy, we hypothesized that reduced mTORC1 activity would lead to up-regulation of autophagy in dynamin 2-deficient T cells. In agreement with our hypothesis, Western blotting of lysates from Dnm2 KO T cells demonstrated increased amounts of light chain 3 beta (LC3B) II (Fig. 3E), the lipidated form of LC3B that is found in autophagosomes. More LC3B II could be due to increased autophagosome synthesis, or alternatively, due to a block in autophagosome degradation (19). To distinguish between these possibilities, we measured LC3B II in the presence of bafilomycin A1 (20), which inhibits lysosomal degradation of LC3B II. We found that LC3B II amounts increased in both Dnm2 HET and KO cells in the presence of bafilomycin A1 (Fig. 3F), demonstrating that autophagosome synthesis and autophagic flux is higher in T cells lacking dynamin 2. These results collectively indicate that lack of dynamin 2 causes reduced mTORC1 activation and, subsequently, uncontrolled autophagy in T cells.
Reprogramming of T-Cell Metabolism Through c-Myc Is Dependent on Dynamin 2.
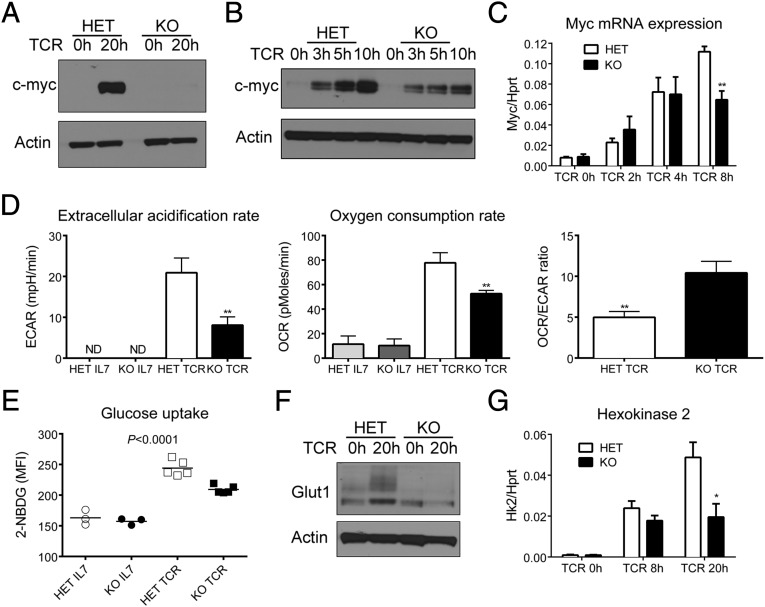
Our observation that mTORC1 activity is reduced rather than abolished and that it is normal during the first 8 h of TCR stimulation suggested that perturbed mTORC1 signaling may not be the main cause for the profound proliferation defect in T cells lacking dynamin 2. This possibility is also supported by the observation that, in contrast to dynamin 2, mTOR is dispensable for the clonal expansion of T cells (21). We therefore explored other pathways that regulate cell growth in T cells. In this respect, the transcription factor c-Myc was particularly interesting because it is induced by TCR and cytokine signals, represents a nonredundant regulator of T lymphocyte metabolism, and T cells lacking c-Myc cannot proliferate. Moreover, mTORC1 activity is lower in myc-deficient T cells, whereas Akt/ERK phosphorylation and up-regulation of the activation marker CD69 is normal (4). Because of the similarity between dynamin 2- and myc-deficient T cells, we hypothesized that dynamin 2 regulates T-cell growth through c-Myc. To test this hypothesis, we determined c-Myc expression in dynamin 2-deficient T cells after TCR ligation. Consistent with our hypothesis, control T cells showed robust c-Myc protein expression at 20 h of stimulation, whereas dynamin 2-deficient T cells failed to express c-Myc protein (Fig. 4A). Western blot analysis showed that Dnm2 KO cells were capable of inducing c-Myc after TCR stimulation, but failed to sustain high c-Myc expression (Fig. 4B). Because c-Myc protein, but not mRNA, expression in activated T cells is dependent on mTORC1 (18), we asked whether lack of c-Myc protein in Dnm2 KO cells was due to impaired Myc mRNA transcription or translation. In control cells, we observed the peak of Myc mRNA expression at 8 h post-TCR stimulation (Fig. 4C), in line with published results (4). Interestingly, Dnm2 KO T cells showed similar Myc mRNA induction at 4 h, whereas Myc mRNA expression at 8 h was reduced by 50% compared with HET cells (Fig. 4C). This result suggests that lack of c-Myc induction in dynamin 2-deficient cells is not due to reduced mTORC1 activity. The failure to sustain c-Myc expression and the finding that the defect in c-Myc expression occurs already at 8 h poststimulation when mTORC1 activity is still normal (Fig. 3 B and D) suggests that dynamin 2 promotes T-cell growth primarily through c-Myc.
Fig. 4.
Reprogramming of T-cell metabolism through myc is dependent on dynamin 2. Naive CD4 T cells from Dnm2 HET and KO mice were activated with α-CD3 and α-CD28 mAbs. (A and B) Myc protein expression in Dnm2 HET and KO CD4 T cells. Actin was used as a loading control. (C) Myc mRNA expression in CD4 T cells from Dnm2 HET and KO mice (n = 4). mRNA expression was normalized to Hprt. (D) Metabolic analysis using the Seahorse extracellular flux analyzer. The extracellular acidification rate (ECAR), oxygen consumption rate (OCR), and OCR/ECAR ratio were determined in CD4 T cells cultured in IL-7 or stimulated for 20 h (TCR). (E) Glucose uptake in CD4 T cells cultured in IL-7 or stimulated for 20 h through the TCR. Graph shows the mean fluorescence intensity (MFI) of 2-NBDG staining. (F) Glut1 protein expression in activated CD4 T cells from Dnm2 HET or KO mice was measured by Western blot. (G) Hexokinase 2 (Hk2) mRNA expression in CD4 T cells from Dnm2 HET and KO mice (n = 5). All error bars represent SEM. *P < 0.05, **P < 0.01 by unpaired Student's t test (C, D, and G) or one-way ANOVA (E). Results are representative of or combined from two (D–F), three (B and C), four (G), or five (A) experiments.
A critical function of c-Myc in T lymphocytes is the switch to anabolic metabolism after TCR stimulation by promoting glycolysis (4). To directly test whether dynamin 2 is required for this switch, we performed metabolic studies using the Seahorse analyzer. The Seahorse analyzer measures the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) as surrogates of oxidative phosphorylation and glycolysis, respectively. Using this method, we found that the ECAR of dynamin 2-deficient cells was only ∼50% of that of control T cells after TCR stimulation (Fig. 4D), consistent with reduced glycolysis. The OCR was also lower in T cells lacking dynamin 2 (Fig. 4D), likely reflecting overall reduced metabolic fitness. The OCR/ECAR ratio was significantly higher in Dnm2 KO T cells (Fig. 4D), which suggests that dynamin 2-deficient T cells primarily rely on oxidative phosphorylation instead of glycolysis. Myc supports the switch to glycolytic metabolism by increasing glucose uptake and by activating the transcription of key glycolytic enzymes (4). Compared with resting T cells, TCR stimulation increased glucose uptake in both Dnm2 KO and control cells (Fig. 4E). However, TCR-induced glucose uptake was suboptimal in dynamin 2-deficient T cells (Fig. 4E). Glucose uptake in T cells is mainly dependent on glucose transporter 1 (Glut1) (2). As expected, control T cells up-regulated Glut1 protein expression in response to TCR stimulation (Fig. 4F). Consistent with impaired c-myc expression, the amount of Glut1 protein was lower in dynamin 2-deficient than in control cells after 20 h of TCR stimulation (Fig. 4F). Moreover, mRNA expression of hexokinase 2 (Hk2), a rate-limiting enzyme catalyzing the first step of glycolysis, was lower in Dnm2 KO compared with control cells (Fig. 4G). Induction of pyruvate kinase (Pkm) and lactate dehydrogenase A (Ldha), enzymes regulating late steps of glycolysis, was slightly lower in dynamin 2-deficient T cells after TCR stimulation (Fig. S10A). Finally, expression of phosphoribosyl pyrophosphate amidotransferase (Ppat) and glutamic-oxaloacetic transaminase 1 (Got1), two myc-dependent enzymes required for glutaminolysis (4), was reduced in Dnm2 KO compared with control cells after TCR stimulation (Fig. S10B), suggesting reduced glutaminolysis in dynamin 2-deficient cells. In summary, we demonstrate that dynamin 2 maintains c-Myc expression, which enables the metabolic switch to glycolysis.
Discussion
An intimate relationship exists between endocytosis and signaling. Receptor internalization through endocytosis can influence signaling both negatively and positively (11). On the one hand, it can attenuate signaling by removing receptors from the cell surface. On the other, it can support signaling by either continued signaling from internalized receptors or by receptor resensitization during recycling of the receptor back to the cell surface. The physiological significance of TCR internalization has been controversial and TCR internalization mainly viewed as a mechanism to terminate signaling. In this study, we identify dynamin 2-dependent endocytosis as an essential regulator of metabolism and proliferation in T lymphocytes. Absence of dynamin 2 allows initial T-cell activation, but the failure to sustain key signaling pathways, such as c-Myc and mTORC1, causes metabolic stress and a futile attempt to meet the metabolic demands through autophagy.
Our work extends previous in vitro studies that examined the role of antigen receptor internalization on signaling in lymphocytes through the pharmacological inhibition of endocytosis. Treatment of Jurkat cells with the dynamin inhibitor dynasore reduced tyrosine-phosphorylated CD3ζ found in endosomes (13), but the functional significance of decreased endosomal TCR signaling was not examined. Dynasore treatment of B lymphocytes enhanced ERK and attenuated Akt signaling (14), similar to our results in T lymphocytes. This suggests that continued signaling after internalization represents a conserved mechanism shared by both lineages of lymphocytes. Our genetic approach has the advantage of avoiding potential off-target effects of pharmacological dynamin inhibition (22). We propose a model where TCR signaling from the plasma membrane represents “wave 1” of signaling, which is followed by TCR internalization and signaling from intracellular compartments (such as endosomes) that constitutes “wave 2” of signaling. A similar dual mode of signaling has recently been described for GPCRs (23). Signaling from internalized TCRs could either prolong signaling initiated at cell surface or activate signaling pathways distinct from those activated at the plasma membrane, e.g., through signaling molecules that specifically localize to endosomes.
Some pathways downstream of the TCR, such as Akt and ERK, are intact in T cells lacking dynamin 2, despite reduced TCR signal strength. In contrast, other TCR-activated pathways, such as c-Myc and mTORC1, fail to maintain their activity over time when dynamin 2 is absent. The most likely explanation is that pathways required for T-cell proliferation, i.e., c-Myc and mTORC1, have particularly stringent requirements in terms of TCR signaling. T-cell proliferation requires extensive metabolic reprogramming and has a higher TCR signaling threshold than for example cytokine production (5). Consistent with this notion, maintenance of myc expression is dependent on high CD3-immunoreceptor tyrosine-based activation motif (ITAM) multiplicity (5). In contrast, low CD3-ITAM multiplicity is sufficient for ERK signaling, but not for proliferation. Moreover, Akt is not required for the metabolic reprogramming and proliferation of T cells (24).
In addition to their essential role in endocytosis, it has been proposed that dynamins can regulate signaling by acting as scaffold proteins through their proline-rich domain. Related to this notion, it has previously been suggested that dynamin 2 regulates T-cell activation through actin polymerization, but is not required for TCR internalization (25). This study mainly used overexpression and knockdown experiments in Jurkat cells and concluded that dynamin 2 was required for the up-regulation of the activation marker CD69 and for optimal ERK phosphorylation. In contrast, in our study genetic ablation of dynamin 2 in primary T lymphocytes did not affect CD69 up-regulation and caused hyper- instead of hypophosphorylation of ERK. Our finding that initial TCR signaling and activation was normal and only defective at later time points is more consistent with the possibility that dynamin 2 regulates T-cell proliferation through its role in TCR internalization. However, we cannot formally exclude that dynamin 2 mainly functions as an adaptor protein in T cells because it is not possible to separate the endocytic from the adaptor function of dynamins. Finally, lack of dynamin 2 likely affects the internalization of many cell surface receptors. In our experiments we stimulated dynamin 2-deficient T cells specifically through the TCR in vitro, which makes it unlikely that the endocytosis of other surface receptors plays a role in the observed defects in TCR signaling and T-cell proliferation. However, we cannot completely rule out secondary effects due to altered endocytosis of surface proteins that are regulated by TCR signaling, such as cytokine receptors. Expression of c-Myc is induced by TCR and IL-2 signaling (18) and IL-2 receptor recycling could be altered in the absence of dynamin 2. We observed lower IL-2Rα surface expression in dynamin 2-deficient CD4 T cells at 20 h of TCR stimulation, which likely causes reduced responsiveness to IL-2 and may contribute to impaired c-Myc expression and defective proliferation.
In conclusion, the current work supports the concept that endocytosis mediated by dynamin 2 provides a critical signal for the metabolic switch that enables T lymphocyte to undergo proliferation. Understanding how TCR signaling is maintained may open new opportunities to modulate adaptive immune responses and to treat autoimmune disease.
Materials and Methods
Mice.
Cd4-creDnm2flox//flox mice (referred to as Dnm2 KO) (17) and littermate Cd4-creDnm2flox/+ controls (referred to as HET) were used in all experiments apart from Fig. 1D, where Dnm2flox/flox mice were bred to GzmB-cre transgenic mice and OT-I TCR transgenic mice (JAX) to generate OT-I GzmB-creDnm2flox/flox mice. All mouse experiments were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of Yale University.
T-Cell Proliferation in Vivo.
Naive T cells were purified from Dnm2 HET (CD45.1.2+) and KO (CD45.2+) mice and cotransferred into recipient mice (CD45.1+) as described in SI Materials and Methods.
T-Cell Stimulation in Vitro.
Naive T cells were purified from Dnm2 HET and KO mice and stimulated with plate-bound α-CD3 Ab (10 μg/mL unless indicated otherwise) and 5 μg/mL soluble α-CD28 Ab as described in SI Materials and Methods.
Flow Cytometry.
Phosphorylated proteins were measured by intracellular flow cytometry as described in SI Materials and Methods.
Quantitative RT-PCR and Western Blot.
Total RNA was extracted and cell lysates prepared as described in SI Materials and Methods.
Metabolic Studies.
Analysis of the OCR and ECAR was performed with a Seahorse XF96 extracellular flux analyzer instrument. The same number of control and Dnm2 KO T cells were used for the Seahorse measurements. Glucose uptake was determined by flow cytometry with the fluorescent deoxyglucose analog 2-(N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino)-2-Deoxyglucose (2-NBDG).
Statistical Analysis.
Student's t test was used to determine statistical significance between two groups (α = 0.05). For multigroup comparisons, we applied one-way ANOVA with post hoc testing using Tukey's multiple comparison test (α = 0.05).
Supplementary Material
Acknowledgments
We thank Geoff Holman (University of Bath) for the Glut1 Ab; Adam Williams, Will Bailis, and Ruaidhri Jackson for comments on the manuscript; and Caroline Lieber for help with manuscript submission. This work was supported by an Irvington Institute postdoctoral fellowship from the Cancer Research Institute (to T.W.). R.A.F. and P.D.C. are investigators of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504279112/-/DCSupplemental.
References
- 1.Wang R, Green DR. Metabolic reprogramming and metabolic dependency in T cells. Immunol Rev. 2012;249(1):14–26. doi: 10.1111/j.1600-065X.2012.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navarro MN, Cantrell DA. Serine-threonine kinases in TCR signaling. Nat Immunol. 2014;15(9):808–814. doi: 10.1038/ni.2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang R, et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35(6):871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guy CS, et al. Distinct TCR signaling pathways drive proliferation and cytokine production in T cells. Nat Immunol. 2013;14(3):262–270. doi: 10.1038/ni.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schrum AG, Turka LA. The proliferative capacity of individual naive CD4(+) T cells is amplified by prolonged T cell antigen receptor triggering. J Exp Med. 2002;196(6):793–803. doi: 10.1084/jem.20020158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4(8):749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- 8.van Stipdonk MJ, et al. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4(4):361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 9.Valitutti S, Müller S, Salio M, Lanzavecchia A. Degradation of T cell receptor (TCR)-CD3-zeta complexes after antigenic stimulation. J Exp Med. 1997;185(10):1859–1864. doi: 10.1084/jem.185.10.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Rhodes M, Wiest DL, Vignali DA. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity. 2000;13(5):665–675. doi: 10.1016/s1074-7613(00)00066-2. [DOI] [PubMed] [Google Scholar]
- 11.Sorkin A, von Zastrow M. Endocytosis and signalling: Intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10(9):609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luton F, Legendre V, Gorvel JP, Schmitt-Verhulst AM, Boyer C. Tyrosine and serine protein kinase activities associated with ligand-induced internalized TCR/CD3 complexes. J Immunol. 1997;158(7):3140–3147. [PubMed] [Google Scholar]
- 13.Yudushkin IA, Vale RD. Imaging T-cell receptor activation reveals accumulation of tyrosine-phosphorylated CD3ζ in the endosomal compartment. Proc Natl Acad Sci USA. 2010;107(51):22128–22133. doi: 10.1073/pnas.1016388108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaturvedi A, Martz R, Dorward D, Waisberg M, Pierce SK. Endocytosed BCRs sequentially regulate MAPK and Akt signaling pathways from intracellular compartments. Nat Immunol. 2011;12(11):1119–1126. doi: 10.1038/ni.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 2012;13(2):75–88. doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monjas A, Alcover A, Alarcón B. Engaged and bystander T cell receptors are down-modulated by different endocytotic pathways. J Biol Chem. 2004;279(53):55376–55384. doi: 10.1074/jbc.M409342200. [DOI] [PubMed] [Google Scholar]
- 17.Willinger T, Ferguson SM, Pereira JP, De Camilli P, Flavell RA. Dynamin 2-dependent endocytosis is required for sustained S1PR1 signaling. J Exp Med. 2014;211(4):685–700. doi: 10.1084/jem.20131343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinclair LV, et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol. 2013;14(5):500–508. doi: 10.1038/ni.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140(3):313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willinger T, Flavell RA. Canonical autophagy dependent on the class III phosphoinositide-3 kinase Vps34 is required for naive T-cell homeostasis. Proc Natl Acad Sci USA. 2012;109(22):8670–8675. doi: 10.1073/pnas.1205305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finlay DK, et al. PDK1 regulation of mTOR and hypoxia-inducible factor 1 integrate metabolism and migration of CD8+ T cells. J Exp Med. 2012;209(13):2441–2453. doi: 10.1084/jem.20112607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park RJ, et al. Dynamin triple knockout cells reveal off target effects of commonly used dynamin inhibitors. J Cell Sci. 2013;126(Pt 22):5305–5312. doi: 10.1242/jcs.138578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lohse MJ, Calebiro D. Cell biology: Receptor signals come in waves. Nature. 2013;495(7442):457–458. doi: 10.1038/nature12086. [DOI] [PubMed] [Google Scholar]
- 24.Macintyre AN, et al. Protein kinase B controls transcriptional programs that direct cytotoxic T cell fate but is dispensable for T cell metabolism. Immunity. 2011;34(2):224–236. doi: 10.1016/j.immuni.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez TS, et al. Dynamin 2 regulates T cell activation by controlling actin polymerization at the immunological synapse. Nat Immunol. 2005;6(3):261–270. doi: 10.1038/ni1168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.