Significance
Protein kinase A (PKA) complexes are versatile signaling enzymes controlling homeostasis in eukaryotes. This enzyme is involved in multiple functions under physiological and pathological conditions in humans and governs the virulence of many pathogenic fungi. Here we systematically identify PKA regulators in yeast. Notably, we describe signaling to PKA that involves feedback from the cellular recycling process, autophagy. We also uncover a posttranslational modification, acetylation, that regulates PKA activity in both yeast and mammals, and we show that this mechanism impacts aging. Thus, we identify what regulates PKA as a first step toward the ability to cure diseases and infections, for instance, by providing new candidate genes for drug targeting in health research and antifungals for agricultural and medical purposes.
Keywords: Ras/cAMP/PKA pathway, acetylation, methionine, autophagy, TOR
Abstract
Cellular processes and homeostasis control in eukaryotic cells is achieved by the action of regulatory proteins such as protein kinase A (PKA). Although the outbound signals from PKA directed to processes such as metabolism, growth, and aging have been well charted, what regulates this conserved regulator remains to be systematically identified to understand how it coordinates biological processes. Using a yeast PKA reporter assay, we identified genes that influence PKA activity by measuring protein–protein interactions between the regulatory and the two catalytic subunits of the PKA complex in 3,726 yeast genetic-deletion backgrounds grown on two carbon sources. Overall, nearly 500 genes were found to be connected directly or indirectly to PKA regulation, including 80 core regulators, denoting a wide diversity of signals regulating PKA, within and beyond the described upstream linear pathways. PKA regulators span multiple processes, including the antagonistic autophagy and methionine biosynthesis pathways. Our results converge toward mechanisms of PKA posttranslational regulation by lysine acetylation, which is conserved between yeast and humans and that, we show, regulates protein complex formation in mammals and carbohydrate storage and aging in yeast. Taken together, these results show that the extent of PKA input matches with its output, because this kinase receives information from upstream and downstream processes, and highlight how biological processes are interconnected and coordinated by PKA.
Cells constantly adjust to internal and environmental fluctuations via a tight homeostasis control achieved through the coordination of biological processes (1, 2). Modification of this coordination is believed to be central to many complex diseases, such as cancer and Alzheimer’s disease (2). Restoring homeostasis through pharmacological intervention thus requires a deep understanding of how biological processes are interconnected and form a highly complex and dynamic equilibrium (3, 4). To understand how molecular networks regulate the balance among biological processes, we need to move from a static and qualitative identification of their molecular interactions to a dynamic and quantitative description (5, 6). At the center of cellular equilibrium are signaling hubs that coordinate biological processes by integrating inbound signals from a diversity of upstream processes and in turn regulate the activity of a variety of downstream processes. For a given hub, upstream and downstream processes overlap considerably, resulting in the monitoring and feedback control of their activity. Among the conserved and best-known signaling hubs are the target of rapamycin (TOR) kinase, the AMP-activated kinase (AMPK), and the cAMP-dependent protein kinase A (PKA). Because we place these regulators upstream of models of signaling cascades, we know much more about the signaling to processes downstream of these regulators than about the upstream signals.
PKA is one of the first kinases to have been identified and dissected at the molecular level (7). This kinase controls cellular homeostasis by coordinating energy metabolism, cellular growth, autophagy, differentiation, aging, stress response, and responses to many external stimuli in fungi and mammals (8–12). Improper regulation of the enzyme is associated with severe phenotypes and diseases in humans (13) and loss of virulence in pathogenic fungi (14). In addition to its importance because of its specific functions, PKA serves as a prototype of an entire superfamily of protein kinases and their regulation (7). However, the inbound and feedback signals this kinase integrates are not entirely known or well understood beyond the canonical Ras/cAMP and G protein-coupled receptor GPCR pathways (15), particularly in fungi. Direct mechanisms of PKA regulation include cAMP binding to the R subunits (8), phosphorylation (16), and subcellular localization of its subunits (17). In mammalian cells, there is extensive evidence for compartmentalization of cAMP signaling in which A kinase-anchoring proteins (AKAPs) play a major role by coupling PKA to a variety of other enzymes (9). AKAPs contain a targeting domain that specifically directs them to cytoskeletal or membrane elements. This targeting implies that the AKAP-PKA complexes in mammals can integrate locally and modulate distinct intracellular signaling pathways (9, 17, 18). No ortholog has been identified for AKAPs in Saccharomyces cerevisiae, but PKA localization appears to be regulated in a condition-dependent manner (19, 20), at least partly through the phosphorylation of its N terminus (20).
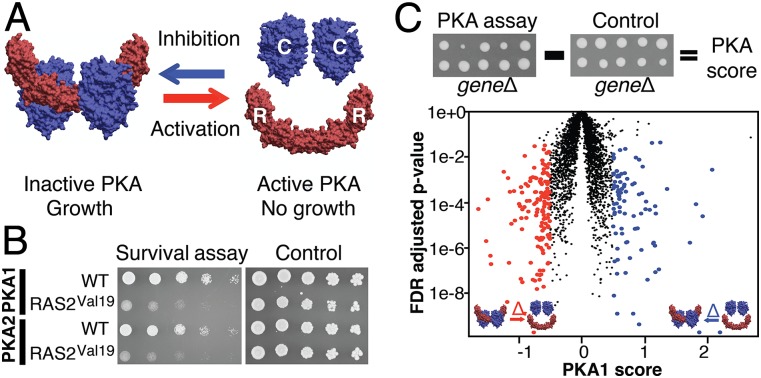
Although yeast is the eukaryote for which we probably have the most extensive knowledge about the functional organization of the cell, we still have a limited understanding of the regulation of PKA isoforms in this model organism. To address this issue, we used a systematic reporter assay to map positive and negative regulators of two PKA isoforms in living yeast cells. PKA is a conserved holoenzyme of two homodimers: a regulatory subunit (R, Bcy1 in yeast) that sequesters and inhibits the catalytic subunit (C, Tpk1, 2, and 3 in yeast) (Fig. 1A) (7). Upon PKA activation, C dissociates from R to phosphorylate its substrates. PKA activity in response to external signals or changes in the activity of its upstream regulators and second messengers can be monitored quantitatively by following the RC assembly and disassembly by survival and bioluminescent protein-fragment complementation assay (PCA) (21, 22). We used a PCA reporter to measure the assembly of this complex in yeast deletion strains to identify systematically genes whose products directly or indirectly regulate the formation of the PKA complex.
Fig. 1.
Systematic PKA assay in yeast. (A) RC association is monitored by DHFR-PCA. Activation of PKA (RC dissociation) reduces growth, whereas growth is promoted when PKA is repressed (RC association). Shown is the murine PKA complex structure from Protein Data Bank ID code 3TNP. (B) Activation of PKA by the hyperactive RAS2Val19 (pPHY921) compared with wild type (WT, pRS316). (C) The PKA assay was performed in the yeast deletion collection. The PKA score is negative when gene deletion decreases growth and is positive when growth increases relative to a constitutive interaction (SI Methods). Red dots indicate genes that are candidate negative regulators, and blue dots indicate genes that are candidate positive regulators. Thresholds were set to a PKA score ≤0.5 or >0.5 and an FDR-adjusted P < 0.05 (Welch’s test). Results are shown for the PKA1 assay in glucose. See Fig. S2 for other assays.
Results and Discussion
Systematic Identification of PKA Regulators.
As illustrated in Fig. 1B, enhanced activity of a well-known PKA upstream activator, Ras2, decreases both Tpk1–Bcy1 and Tpk2–Bcy1 interactions (referred to as “PKA1” and “PKA2,” respectively), and this decrease translates into a reduced growth in a quantitative PCA (qPCA) based on the dihydrofolate reductase (DHFR) enzyme as a reporter (21). Using a variant of the high-throughput method we reported in ref. 23 (SI Methods and Fig. S1), we measured the effect of 3,726 individual deletions of nonessential genes on PKA1 and PKA2 complex formation (Fig. 1C). To maximize the number of positive and negative regulators identified, we used either glucose or galactose as the carbon source, because PKA generally is activated in glucose and repressed in galactose (21).
We identified 494 high-confidence candidate regulators, including 80 core candidates that were candidates in the four PKA assays, at thresholds corresponding to an average empirical false-positive rate of 5% (Fig. 1C, Fig. S2, and Dataset S1). We confirmed 20 of 24 candidate regulators (83%) qualitatively by spot dilution assays of reconstructed strains (Fig. S3 and Dataset S1). In addition, we tested high- and low-confidence candidates by liquid quantitative growth assays (Fig. 2A) and confirmed 9 of 11 (82%) high-confidence candidates and two of four low-confidence candidates. We further confirmed a positive regulator SAP30 and a negative regulator, the guanine nucleotide exchange factor CCZ1, using a direct measurement of protein complex formation (Fig. S3). Finally, we validated functional links between candidates and the PKA pathway by showing that gene deletion of a subset of candidate regulators alleviates or aggravates PKA hyperactivity in various conditions (Fig. S4).
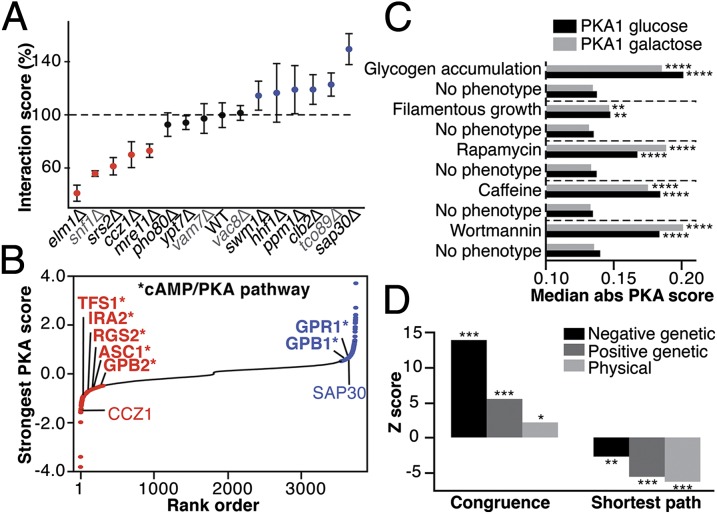
Fig. 2.
Confirmation of PKA assays. (A) PKA2 assay in glucose by high-resolution growth profiling. Dots indicate average interaction score. Error bars indicate SD. n = 10. Colored dots are significantly different from the wild-type strain (P < 0.05; Dunnett’s method). Gray labels indicate low-confidence candidates. (B) Ranked distribution of the strongest effect of each strain. Known cAMP/PKA pathway members are labeled with *. (C) PKA score enrichments for genes with phenotypes related to PKA in yeast. “No phenotype” indicates genes not reported to have the specified phenotype (**P < 0.01, ****P < 0.0001; Wilcoxon test). Bars show median absolute PKA score. (D) Congruence and shortest path among candidate regulators in the genetic and physical interaction networks. Congruence reflects the extent to which genetic interaction profiles are correlated, and the shortest path is a measure of proximity in the network. *P < 0.05, **P < 0.01, ***P < 0.001.
PKA regulators acting via the Ras/cAMP pathway (for instance, the Ras GTPase inhibitor IRA2, its interaction partner GPB2, and the nutritional sensor GPR1) were candidates (Fig. 2B). Interestingly, many candidates with effects stronger than these canonical regulators were observed, illustrating that the inbound PKA signaling space is still largely unexplored. We recovered nine transcriptional regulators of the PKA and 11 known or putative kinases that phosphorylate PKA subunits (Fig. S5, Table S1, and Dataset S2), including Mck1, Pkh1, Cdc28, and members of the Pho85 complex. Furthermore, genes involved in glycogen accumulation and filamentous growth, which are two PKA-regulated phenotypes (15, 24), showed significantly stronger PKA scores (Fig. 2C and Fig. S5). Several genes coding for PKA substrates were also found (Table S1), revealing an extensive level of feedback mechanisms on PKA complex formation. The 494 candidate regulators are strongly connected with each other physically and genetically (Fig. 2D), showing that signals regulating PKA are channeled along specific genetic and physical pathways. In addition, candidate PKA regulators were enriched in genes that alleviate or enhance bcy1Δ or tpk2Δ phenotypes (Fisher’s test; P value = 7e-4 and 0.014, respectively), and in Tpk2 and Tpk3 protein-interaction partners (Fisher’s test; P value = 0.018 and 0.049, respectively) (Table S1), emphasizing that the PKA assay identifies both direct and indirect regulators. To uncover the relative participation of direct regulation, we compiled physical interaction data for PKA from the BioGRID (25) and from experiments we performed (Datasets S1, S3, and S4). We identified 64 candidate regulators (13%) that may regulate PKA complex formation through physical association. Of these, 59 are located in the cytoplasm, including six that localize at the cytoskeleton, three that bind to the peroxisome-targeting sequence, and two that bind to phosphatidylinositol-3-phosphate. These candidates thus might act as scaffold and have functions analogous to mammalian AKAPs that target the PKA to specific compartments (9) but for which there is no formal evidence in yeast. Taken together, these results demonstrate that the reporter assay covers a wide range of possible regulatory mechanisms affecting the amount of PKA complex formed via protein–protein interactions, which may result partly from changes in transcriptional levels of the subunits. Thus, our results support the central position of PKA in the eukaryotic cellular network.
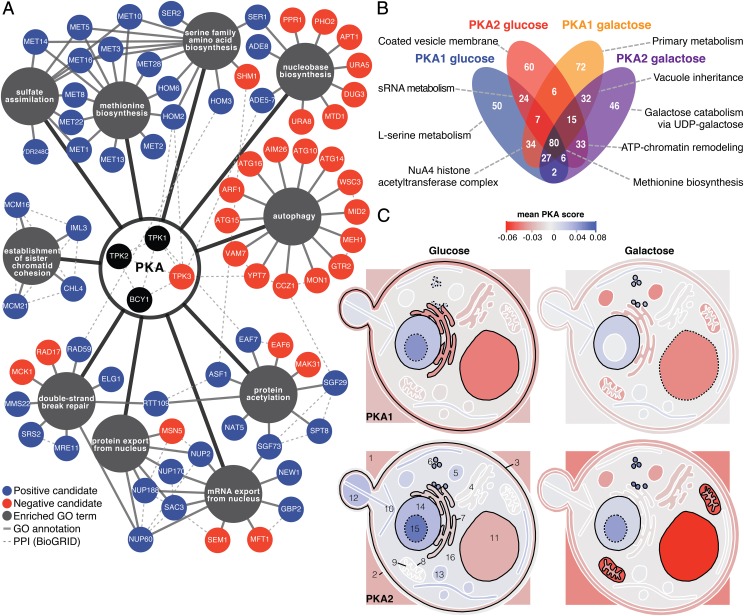
We found that PKA receives positive and negative inputs that correspond to specific processes. Positive candidates were enriched for amino acid biosynthesis, protein acetylation, DNA repair, export from the nucleus, and chromatid cohesion; negative candidates were enriched for autophagy and nucleobase biosynthesis in both carbon source conditions (Fig. 3A; see Dataset S5 for the complete list). Overall, the PKA1 and PKA2 assays were highly correlated (r = 0.79–0.85) (Fig. S2), as were the PKA assays in the two carbon sources (r = 0.77–0.79). A total of 80 candidates overlapped in all four PKA assays (Fig. 3B), reflecting the largely overlapping regulation of Tpk1 and Tpk2 under these conditions. However, several candidate regulators were specific to one of the two isoforms and/or to one of the two conditions, highlighting their potential specialized and condition-dependent functions. Tpk1 and Tpk2 are considered to have only partially overlapping substrates (26). Our results suggest that their regulation by Bcy1 is also partly specific. For instance, our assay identified PKH1 as a PKA2-specific candidate, in agreement with evidence that Pkh1 preferentially phosphorylates Tpk2 over its paralogs (27). Moreover, our results follow patterns of compartmentalized activation and inactivation (Fig. 3C), overlapping with previous results showing condition-dependent localization of yeast PKA subunits (28).
Fig. 3.
Inbound PKA signals come from specific processes. (A) Network of biological processes enriched among positive and negative candidates in glucose and galactose assays (P < 0.001; Fisher’s test; n > 3). PPI, protein–protein interactions. (B) Venn diagram of the 494 candidates in the four PKA assays. Strongest enrichment is indicated for each subset (P < 0.01; Fisher’s test) (Dataset S5). (C) Average PKA score per cellular localization in each assay. Localizations with a stronger score than expected by chance are outlined (solid black line: P < 0.05; dotted line: P < 0.1) were 1, extracellular region; 2, cell wall; 3, plasma membrane; 4, Golgi apparatus; 5, peroxisome; 6, ribosome; 7, endoplasmic reticulum; 8, mitochondrial envelope; 9, mitochondrion; 10, cytoskeleton; 11, vacuole; 12, cellular bud; 13, vesicle; 14, nucleus; 15, nucleolus; 16, cytoplasm (not to scale).
Regulation via TOR, Autophagy, and Methionine.
PKA candidate regulators are enriched in protein complexes and phenotypes that point toward strong connections with TOR, a well-conserved regulator involved in nutrient sensing and autophagy regulation (29). Among the four PKA assays, 225 protein complexes have an average PKA score greater or lower than expected by chance alone (P value < 0.05) (Dataset S6). Among the most significant ones (Fig. S5) is a complex involved in the regulation of translational elongation (YC1126) that comprises regulators of Gcn2 (GCN1 and GCN20), a downstream effector of the rapamycin and nutrient-sensitive TOR complex (TORC1). We also find the vacuolar membrane-associated EGO complex (30), a protein complex composed of Gtr1, Gtr2, Meh1, and Slm4, which regulates autophagy via TORC1. Furthermore, PKA scores are stronger for genes with phenotypes that are associated with TOR1, such as response to rapamycin and caffeine and sensitivity to wortmannin, an autophagy inhibitor (Fig. 2C and Fig. S5) (16, 29). Our data underline functional connections between two critical signaling hubs, PKA and TOR.
Consistent with the connection with TOR, we found that genes involved in each step of autophagy, from induction (PHO80) to vesicle breakdown (ATG15), were candidate PKA negative regulators (Fig. 4A). Because PKA is known to inhibit the initiation of autophagy (11), these results point toward feedback regulation. A bidirectional regulation between TOR and autophagy was shown recently (31). Inhibition of TOR lifts the repression of autophagy, and recycled amino acids reactivate TOR, which in turns attenuates autophagy. The TOR and PKA pathways are considered to work cooperatively in some studies (11) but to be antagonistic in others (32), and the mechanistic bases of their crosstalk are still largely unknown (29). Our finding that a nonessential subunit of TORC1 (TCO89) was a marginally significant candidate [PKA score: 0.86; false discovery rate (FDR): 0.06] motivated us to examine further the relationship between PKA, TOR, and autophagy. We first confirmed that TCO89 modulates PKA complex formation (Fig. 2A) and found that inhibition of TOR signaling by rapamycin leads to PKA inactivation (Fig. 4B). Because it was demonstrated recently that methionine inhibits autophagy via PPM1, the NPR complex, and TORC1 (33), which are candidate PKA regulators (Fig. 4A), we hypothesized that methionine released from autophagy could mediate the feedback to PKA via either a TOR-dependent or -independent pathway. We found that methionine, which delays autophagy under the conditions of the PKA assays (Fig. S6), leads to PKA complex dissociation (Fig. 4B). Furthermore, in a background where a gene essential for vesicle docking and autophagy completion (CCZ1) is deleted, methionine partially restored PKA complex formation (Fig. 4C and Fig. S6). Methionine and rapamycin antagonize each other’s effects on PKA activity (Fig. 4B), suggesting that methionine could regulate PKA partly in a TOR-independent manner. To explore pathways by which methionine could signal to PKA, we tested several protein interactions using DHFR-PCA in vivo in the presence of methionine and/or rapamycin (Dataset S7). We found that Bcy1 interacts with Mck1, a downstream TOR kinase (16), and with Meh1, an EGO complex member, and that these interactions are modulated when methionine and rapamycin are present (Fig. S6 and Dataset S7). Taken together, these results support a model in which PKA is regulated by methionine through both a TOR-independent pathway and a TOR-dependent pathway, likely via the EGO complex (Fig. 4A), which may sense cytoplasmic and intravacuolar amino acid levels (29).
Fig. 4.
Regulation of PKA by autophagy, methionine, TOR, and EGO. (A) Model of regulatory relationships between autophagy, methionine, the TOR/EGO complex, and PKA in yeast. (B) DHFR-PCA showing PKA1 and PKA2 complex formation in presence of methionine (Met) and/or rapamycin (Rap) in the control strain (hoΔ). Complex formation, i.e., growth, is expressed as a ranked score to allow nonparametric comparison among conditions. n = 8. (C) Spot assay of PKA1 and PKA2 in the ccz1Δ strain showing that methionine alleviates the effect of ccz1Δ on PKA.
PKA Regulation by Lysine Acetylation.
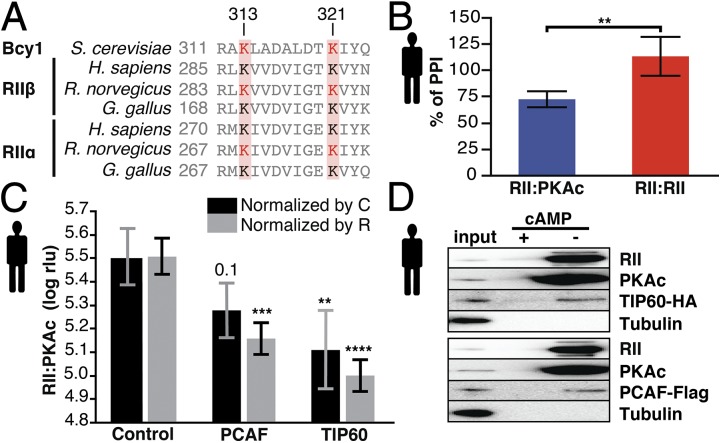
Dynamic posttranslational modifications of signaling hubs are critical mechanisms for the precise integration of input and output signals from various cellular processes. Our data highlight connections between protein acetylation and PKA complex formation. We found strong enrichment for protein acetylation (Fig. 3A and Dataset S5) among PKA regulators, and acetylated proteins had significantly stronger PKA scores (Fig. S5). Five residues are acetylated in Bcy1 (34, 35), and at least two of them are conserved and acetylated in mammals (Fig. 5A) (36). Using a deacetylase inhibitor and acetyltransferase overexpression, we investigated whether PKA complex formation in mammalian cells is also regulated by acetylation. We overexpressed P300/CBP-associated factor (PCAF) and 60-kDa Tat-interactive protein (TIP60), two acetyltransferase homologs of acetylation complexes from the identified PKA regulators (SAGA and NuA4, respectively) (Dataset S5). PKA bioluminescent assays showed that drug inhibition of deacetylases, as well as PCAF and TIP60 overexpression, reduce the formation of mammalian RII:PKAc complexes compared with the PKA regulatory homodimer RII:RII and mock control, respectively (Fig. 5 B and C), mirroring the effects seen in yeast. Moreover, we showed that overexpressed PCAF and TIP60 are specifically copurified with macromolecular PKA complexes (Fig. 5D).
Fig. 5.
Acetylation regulates PKA in yeast and humans. (A) Sequence alignment of the PKA regulatory subunits. The displayed region contains two conserved (red boxes) and acetylated lysine (red residues) positions. (B) HDAC inhibition using trichostatin A (TSA) (1 µM, 3 h) affects PKA type II complex formation in mammalian cells as quantified using the Rluc PCA (22). Treatment with TSA diminishes complex formation of RII:PKAc, but the RII:RII interaction is not affected. **P < 0.01; Welch’s test. Bars indicate average percent of PPI, and error bars indicate SD; n = 5. (C) Mammalian PKA complex formation decreases by up to 55% and 69% upon PCAF or TIP60 overexpression, respectively. The signal (expressed in relative light units, rlu) was normalized by the expression of either the R (RII-F1) or the C (PKAc-F2) subunit (Fig. S7) and was log transformed. **P < 0.01, *** P < 0.001, **** P < 0.0001; blocked Dunnett’s test. Bars show average log rlu; error bars indicate SD. n = 5. Control, mock treatment. (D) Precipitation of cAMP agarose protein from HEK293 cells transiently overexpressing TIP60 (n = 3) or PCAF (n = 2). Tubulin was used as a negative control.
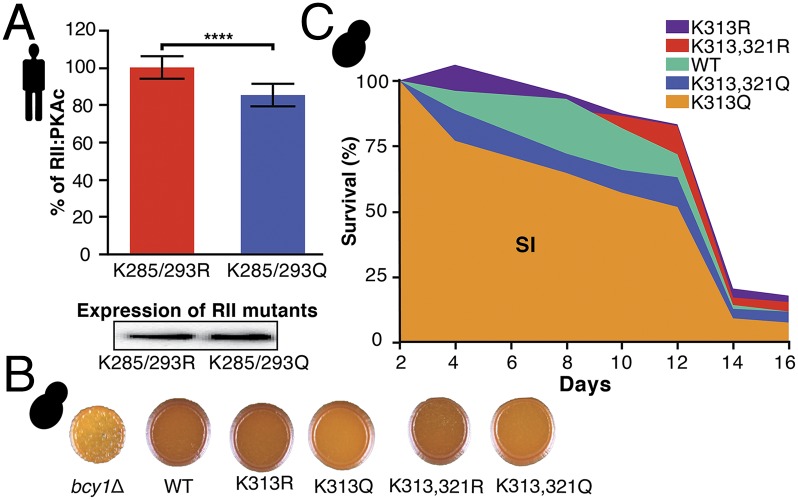
To examine further whether acetylation directly regulates PKA complex formation and activity, we constructed mutants of the regulatory subunit that mimic an acetylated state (K→Q) (37) as well as mutants that prevent acetylation (K→R) in both yeast and mammalian cells. In mammalian cells, K285/293R and K285/293Q mutants of RII showed differences in the heterodimeric complex formation with PKAc (Fig. 6A). In yeast, we found that the acetylated mimetic K313Q mutant accumulated less glucose reserve (glycogen) than the wild type and its K313R counterpart (Fig. 6B and Fig. S7). Similarly, we found that the mutant mimicking K313 acetylation affected chronological life span (Fig. 6C), suggesting that the coupling between carbohydrate storage and aging (38) is mediated in part by acetylation of the PKA at a conserved residue. Although significant, the effects measured with both mammalian and yeast mutants are small, suggesting that other acetylation sites reported in both the regulatory and catalytic subunits (34–36) or other indirect mechanisms may contribute to PKA regulation.
Fig. 6.
Functional impact of K acetylation mutations in human and yeast cells. (A) Difference in RII:PKAc formation between RII-F1 mutants K285/293R (preventing acetylation) and K285/293Q (mimicking acetylation) measured by Rluc PCA in HEK293 cells. Immunoblotting confirmed equal expression levels. ****P < 0.0001; blocked Welch’s test. Bars indicate average percent of PPI; error bars indicate SD. n = 10. (B) Glycogen staining of Bcy1 acetylation site mutants compared with wild type (pJS11) and bcy1Δ (p415). Dark color indicates glycogen accumulation. n = 3. (C) Chronological lifespan corresponding to the area under the survival curve (SI) is decreased in Bcy1 K313Q compared with K313R strains [P = 0.007; Tukey–Kramer honestly significant difference (HSD) test]. n = 6.
Conclusion
The 494 candidate PKA regulators we identified show that PKA receives inputs from various cellular processes, notably through multiple feedback loops. The interplay between TOR, autophagy, and methionine on PKA regulation illustrates this type of signal integration. Methionine is considered a regulatory metabolite that coordinates diverse cellular functions in yeast (39). Connections with the cAMP/PKA pathway exist in other fungi (40) and more distant eukaryotes (41), hinting that this signaling may be evolutionarily conserved and thus may play important roles. In addition to this metabolic connection, we also identified protein acetylation as a conserved posttranslational modification regulating PKA activity in yeast and mammalian cells. Because protein acetylation plays a broad role in the regulation of cell metabolism (42), PKA acetylation may be a key mechanism for this enzyme to monitor the overall metabolic status of the cell. Interestingly, acetylation recently has been reported to regulate autophagy in yeast via the Rpd3 deacetylase complex (43), which once again highlights the high level of interconnection between the processes that regulate PKA. More generally, signal processing has long been regarded as a set of unidirectional, linear pathways. The extensive range of inbound and feedback signals that PKA integrates exemplifies that the coordination of cellular processes takes place in a complex network centered on a few interconnected regulator hubs (1, 2).
Methods
Strains and Media.
The PKA reporter strains used in this study (BCY1-DHFR F[3], TPK1-DHFR F[1,2], and TPK2-DHFR F[1,2]) (44) were purchased from Open Biosystems and confirmed by sequencing. Strains and oligonucleotides are described in Datasets S8 and S9. Plasmids, culture media, and antibiotics are described in Tables S2–S4.
PKA Assay with Hyperactive Ras.
Plasmids pRS316 (empty vector) and pPHY921 (kindly provided by Paul Herman, Department of Molecular Genetics, Ohio State University, Columbus, OH) containing the allele RAS2Val19 (32) were each transformed into strains JFL001 and JFL002, constructed by mating strain BCY1-DHFR F[3] with TPK1-DHFR F[1,2] and TPK2-DHFR F[1,2], respectively. Four microliters were spotted on MTX/Glu and DMSO/Glu (control medium; DMSO is the methotrexate solvent). Pictures were taken after 3 d of growth at 30 °C.
Candidate PKA Regulator Identification.
PKA complex formation was measured by DHFR-PCA using the PKA reporter strains (44, 45). The reporter constructs were introduced in the yeast deletion collection using Synthetic Genetic Array technology (46) as described in ref. 23. For further details, see SI Methods.
Confirmation of Candidates by Spot Dilution Assay and High-Resolution Growth Monitoring in Liquid Cultures.
Confirmation strains were reconstructed manually by PCR-mediated gene deletion. For further details, see SI Methods. Spot dilution assays were performed as previously described (45). High-resolution growth profiling was performed for some strains using qPCA as described by Freschi et al. (21).
Coimmunoprecipitation and Western Blotting.
For details on coimmunoprecipitation and Western blotting, see SI Methods.
Genetic Interactions Between the Ras/cAMP/PKA Pathway and PKA Regulator Candidates.
Interactions between the Ras/cAMP/PKA pathway and PKA regulators candidates were tested by overexpressing a hyperactive allele of RAS2 (32) in strains from the deletion collection (47). For further details, see SI Methods.
Physical Interaction Network of PKA Subunits in Yeast.
The standard DHFR-PCA screens for Tpk1, Tpk2, and Bcy1 against the DHFR collections were performed in glucose and galactose conditions. To map the interactome of Bcy1 by an orthogonal method, Bcy1 interaction partners were assessed by affinity purifications (AP) followed by MS experiments of Bcy1 tagged with two different tags, a tandem affinity purification (TAP) tag (48) and a GFP tag (49). For further details, see SI Methods.
Comparison of Autophagy Induction in Different Media.
Comparison of autophagy induction in different media was performed as previously described (12). For further details, see SI Methods.
PKA Assays and DHFR-PCA with Methionine and Rapamycin.
A small-scale PKA assay for selected manually reconstructed strains was performed with methionine, rapamycin, or both. Reported protein interactions involving Bcy1 and other interactions relevant to autophagy and methionine signaling were also tested by DHFR-PCA on the same media (Dataset S7). For further details, see SI Methods.
Phylogenetic Analysis of Bcy1 Acetylation Sites.
Sequences were obtained from the Ensembl database and aligned in Mega 5.0 (50). Acetylation sites are reported in refs. 34 and 35 for S. cerevisiae and in ref. 36 for Rattus norvegicus.
Renilla Luciferase PCA in Mammalian Cells and cAMP Agarose Protein Precipitation Assay.
Measurements of protein–protein interactions (PPIs) using the Renilla luciferase (Rluc)-based PCA PKA reporter were performed with wild-type PKAc and wild-type and mutated RII subunits (K mutants) according to previously described protocols (22, 51). Macromolecular PKA complexes were precipitated from HEK293 cells transiently expressing either TIP60-HA or PCAF-flag with PKA-selective cAMP agarose resin (52–54). For further details, see SI Methods.
Bcy1 Acetylation Mutants.
Mutants of BCY1 were constructed by directed mutagenesis of pJS11 (55) following the manufacturer’s protocol (Stratagene). Glycogen accumulation was measured by staining strains with iodine vapor. The chronological lifespan of yeast strain was measured as described in ref. 56. For further details, see SI Methods.
Data Analysis.
Data analysis is described in SI Methods. Statistical tests were performed with JMP10. Error bars in bar plots are constructed with 1 SD from the mean. Two-tailed P values are reported for Fisher’s exact test.
Supplementary Material
Acknowledgments
We thank S. W. Michnick and P. K. Herman for their comments. This work was supported by Canadian Institute of Health Research (CIHR) Grants 324265 and 299432 (to C.R.L.) and MOP-14308 (to J.C.), Fonds de Recherche Québec-Santé (FRQ-S) and Human Frontier Science Program Grant RGY0073/2010 (to C.R.L.), and Austrian Science Fund Grants P22608 and P27606 (to E.S.). G.D. and F.T.-Q. were supported by a fellowship from the Québec Research Network on Protein Function, Structure and Engineering. N.B. is an FRQ-S/Québec Breast Cancer Foundation Junior 1 investigator. C.R.L. is a CIHR New Investigator and J.C. holds a Canada Research Chair.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409938112/-/DCSupplemental.
References
- 1.Landry CR, Levy ED, Abd Rabbo D, Tarassov K, Michnick SW. Extracting insight from noisy cellular networks. Cell. 2013;155(5):983–989. doi: 10.1016/j.cell.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Vidal M, Cusick ME, Barabási AL. Interactome networks and human disease. Cell. 2011;144(6):986–998. doi: 10.1016/j.cell.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacunski A, Tatonetti NP. Connecting the dots: Applications of network medicine in pharmacology and disease. Clin Pharmacol Ther. 2013;94(6):659–669. doi: 10.1038/clpt.2013.168. [DOI] [PubMed] [Google Scholar]
- 4.Lee MJ, et al. Sequential application of anticancer drugs enhances cell death by rewiring apoptotic signaling networks. Cell. 2012;149(4):780–794. doi: 10.1016/j.cell.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ideker T, Krogan NJ. Differential network biology. Mol Syst Biol. 2012;8:565. doi: 10.1038/msb.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diss G, et al. Integrative avenues for exploring the dynamics and evolution of protein interaction networks. Curr Opin Biotechnol. 2013;24(4):775–783. doi: 10.1016/j.copbio.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Taylor SS, Ilouz R, Zhang P, Kornev AP. Assembly of allosteric macromolecular switches: Lessons from PKA. Nat Rev Mol Cell Biol. 2012;13(10):646–658. doi: 10.1038/nrm3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vandamme J, Castermans D, Thevelein JM. Molecular mechanisms of feedback inhibition of protein kinase A on intracellular cAMP accumulation. Cell Signal. 2012;24(8):1610–1618. doi: 10.1016/j.cellsig.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Wong W, Scott JD. AKAP signalling complexes: Focal points in space and time. Nat Rev Mol Cell Biol. 2004;5(12):959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 10.Fontana L, Partridge L, Longo VD. Extending healthy life span—from yeast to humans. Science. 2010;328(5976):321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, Herman PK. The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy. Proc Natl Acad Sci USA. 2009;106(40):17049–17054. doi: 10.1073/pnas.0903316106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z, Klionsky DJ. An overview of the molecular mechanism of autophagy. Curr Top Microbiol Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beuschlein F, et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med. 2014;370(11):1019–1028. doi: 10.1056/NEJMoa1310359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuller KK, Rhodes JC. Protein kinase A and fungal virulence: A sinister side to a conserved nutrient sensing pathway. Virulence. 2012;3(2):109–121. doi: 10.4161/viru.19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cullen PJ, Sprague GF., Jr The regulation of filamentous growth in yeast. Genetics. 2012;190(1):23–49. doi: 10.1534/genetics.111.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soulard A, et al. The rapamycin-sensitive phosphoproteome reveals that TOR controls protein kinase A toward some but not all substrates. Mol Biol Cell. 2010;21(19):3475–3486. doi: 10.1091/mbc.E10-03-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scott JD, Pawson T. Cell signaling in space and time: Where proteins come together and when they’re apart. Science. 2009;326(5957):1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feliciello A, Gottesman ME, Avvedimento EV. cAMP-PKA signaling to the mitochondria: Protein scaffolds, mRNA and phosphatases. Cell Signal. 2005;17(3):279–287. doi: 10.1016/j.cellsig.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Griffioen G, Anghileri P, Imre E, Baroni MD, Ruis H. Nutritional control of nucleocytoplasmic localization of cAMP-dependent protein kinase catalytic and regulatory subunits in Saccharomyces cerevisiae. J Biol Chem. 2000;275(2):1449–1456. doi: 10.1074/jbc.275.2.1449. [DOI] [PubMed] [Google Scholar]
- 20.Griffioen G, et al. Nucleocytoplasmic distribution of budding yeast protein kinase A regulatory subunit Bcy1 requires Zds1 and is regulated by Yak1-dependent phosphorylation of its targeting domain. Mol Cell Biol. 2001;21(2):511–523. doi: 10.1128/MCB.21.2.511-523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freschi L, Torres-Quiroz F, Dubé AK, Landry CR. qPCA: A scalable assay to measure the perturbation of protein-protein interactions in living cells. Mol Biosyst. 2013;9(1):36–43. doi: 10.1039/c2mb25265a. [DOI] [PubMed] [Google Scholar]
- 22.Stefan E, et al. Quantification of dynamic protein complexes using Renilla luciferase fragment complementation applied to protein kinase A activities in vivo. Proc Natl Acad Sci USA. 2007;104(43):16916–16921. doi: 10.1073/pnas.0704257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diss G, Dubé AK, Boutin J, Gagnon-Arsenault I, Landry CR. A systematic approach for the genetic dissection of protein complexes in living cells. Cell Reports. 2013;3(6):2155–2167. doi: 10.1016/j.celrep.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 24.Wilson WA, Wang Z, Roach PJ. Systematic identification of the genes affecting glycogen storage in the yeast Saccharomyces cerevisiae: Implication of the vacuole as a determinant of glycogen level. Mol Cell Proteomics. 2002;1(3):232–242. doi: 10.1074/mcp.m100024-mcp200. [DOI] [PubMed] [Google Scholar]
- 25.Chatr-Aryamontri A, et al. The BioGRID interaction database: 2013 update. Nucleic Acids Res. 2013;41(Database issue):D816–D823. doi: 10.1093/nar/gks1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ptacek J, et al. Global analysis of protein phosphorylation in yeast. Nature. 2005;438(7068):679–684. doi: 10.1038/nature04187. [DOI] [PubMed] [Google Scholar]
- 27.Haesendonckx S, et al. The activation loop of PKA catalytic isoforms is differentially phosphorylated by Pkh protein kinases in Saccharomyces cerevisiae. Biochem J. 2012;448(3):307–320. doi: 10.1042/BJ20121061. [DOI] [PubMed] [Google Scholar]
- 28.Tudisca V, et al. Differential localization to cytoplasm, nucleus or P-bodies of yeast PKA subunits under different growth conditions. Eur J Cell Biol. 2010;89(4):339–348. doi: 10.1016/j.ejcb.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189(4):1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smets B, et al. Life in the midst of scarcity: Adaptations to nutrient availability in Saccharomyces cerevisiae. Curr Genet. 2010;56(1):1–32. doi: 10.1007/s00294-009-0287-1. [DOI] [PubMed] [Google Scholar]
- 31.Shin CS, Huh WK. Bidirectional regulation between TORC1 and autophagy in Saccharomyces cerevisiae. Autophagy. 2011;7(8):854–862. doi: 10.4161/auto.7.8.15696. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran V, Herman PK. Antagonistic interactions between the cAMP-dependent protein kinase and Tor signaling pathways modulate cell growth in Saccharomyces cerevisiae. Genetics. 2011;187(2):441–454. doi: 10.1534/genetics.110.123372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutter BM, Wu X, Laxman S, Tu BP. Methionine inhibits autophagy and promotes growth by inducing the SAM-responsive methylation of PP2A. Cell. 2013;154(2):403–415. doi: 10.1016/j.cell.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henriksen P, et al. Proteome-wide analysis of lysine acetylation suggests its broad regulatory scope in Saccharomyces cerevisiae. Mol Cell Proteomics. 2012;11(11):1510–1522. doi: 10.1074/mcp.M112.017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weinert BT, et al. Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol Syst Biol. 2014;10:716. doi: 10.1002/msb.134766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundby A, et al. Proteomic analysis of lysine acetylation sites in rat tissues reveals organ specificity and subcellular patterns. Cell Reports. 2012;2(2):419–431. doi: 10.1016/j.celrep.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim SC, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23(4):607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 38.Samokhvalov V, Ignatov V, Kondrashova M. Reserve carbohydrates maintain the viability of Saccharomyces cerevisiae cells during chronological aging. Mech Ageing Dev. 2004;125(3):229–235. doi: 10.1016/j.mad.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Petti AA, Crutchfield CA, Rabinowitz JD, Botstein D. Survival of starving yeast is correlated with oxidative stress response and nonrespiratory mitochondrial function. Proc Natl Acad Sci USA. 2011;108(45):E1089–E1098. doi: 10.1073/pnas.1101494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li DD, et al. ECM17-dependent methionine/cysteine biosynthesis contributes to biofilm formation in Candida albicans. Fungal Genet Biol. 2013;51:50–59. doi: 10.1016/j.fgb.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 41.Liu WJ, et al. Inhibition of hepatic glycogen synthesis by hyperhomocysteinemia mediated by TRB3. Am J Pathol. 2011;178(4):1489–1499. doi: 10.1016/j.ajpath.2010.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao S, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi C, et al. Function and molecular mechanism of acetylation in autophagy regulation. Science. 2012;336(6080):474–477. doi: 10.1126/science.1216990. [DOI] [PubMed] [Google Scholar]
- 44.Tarassov K, et al. An in vivo map of the yeast protein interactome. Science. 2008;320(5882):1465–1470. doi: 10.1126/science.1153878. [DOI] [PubMed] [Google Scholar]
- 45.Gagnon-Arsenault I, et al. Transcriptional divergence plays a role in the rewiring of protein interaction networks after gene duplication. J Proteomics. 2013;81:112–125. doi: 10.1016/j.jprot.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 46.Costanzo M, et al. The genetic landscape of a cell. Science. 2010;327(5964):425–431. doi: 10.1126/science.1180823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giaever G, et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature. 2002;418(6896):387–391. doi: 10.1038/nature00935. [DOI] [PubMed] [Google Scholar]
- 48.Ghaemmaghami S, et al. Global analysis of protein expression in yeast. Nature. 2003;425(6959):737–741. doi: 10.1038/nature02046. [DOI] [PubMed] [Google Scholar]
- 49.Huh WK, et al. Global analysis of protein localization in budding yeast. Nature. 2003;425(6959):686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 50.Tamura K, et al. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bachmann VA, et al. Reciprocal regulation of PKA and Rac signaling. Proc Natl Acad Sci USA. 2013;110(21):8531–8536. doi: 10.1073/pnas.1215902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Legube G, et al. Tip60 is targeted to proteasome-mediated degradation by Mdm2 and accumulates after UV irradiation. EMBO J. 2002;21(7):1704–1712. doi: 10.1093/emboj/21.7.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382(6589):319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 54.Stefan E, et al. PKA regulatory subunits mediate synergy among conserved G-protein-coupled receptor cascades. Nat Commun. 2011;2:598. doi: 10.1038/ncomms1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Searle JS, Wood MD, Kaur M, Tobin DV, Sanchez Y. Proteins in the nutrient-sensing and DNA damage checkpoint pathways cooperate to restrain mitotic progression following DNA damage. PLoS Genet. 2011;7(7):e1002176. doi: 10.1371/journal.pgen.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murakami C, Kaeberlein M. 2009. Quantifying yeast chronological life span by outgrowth of aged cells. JoVE (27):e1156.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.