Abstract
Background
No-reflow is associated with an adverse outcome and higher mortality in patients with ST-segment elevation acute myocardial infarction (STEMI) who undergo percutaneous coronary intervention (PCI) and is considered a dynamic process characterized by multiple pathogenetic components. The aim of this study was to investigate the effectiveness of a combination therapy for the prevention of no-reflow in patient with acute myocardial infarction (AMI) undergoing primary PCI.
Methods
A total of 621 patients with STEMI who underwent emergency primary PCI were enrolled in this study. Patients with high risk of no-reflow (no-flow score ≥ 10, by using a no-flow risk prediction model, n = 216) were randomly divided into a controlled group (n = 108) and a combination therapy group (n = 108). Patients in the controlled group received conventional treatment, while patients in combination therapy group received high-dose (80 mg) atorvastatin pre-treatment, intracoronary administration of adenosine (140 µg/min per kilogram) during PCI procedure, platelet membrane glycoprotein IIb/IIIa receptor antagonist (tirofiban, 10µg/kg bolus followed by 0.15 µg/kg per minute) and thrombus aspiration. Myocardial contrast echocardiography was performed to assess the myocardial perfusion 72 h after PCI. Major adverse cardiac events (MACE) were followed up for six months.
Results
Incidence of no-reflow in combination therapy group was 2.8%, which was similar to that in low risk group 2.7% and was significantly lower than that in control group (35.2%, P < 0.01). The myocardial perfusion (A × β) values were higher in combination therapy group than that in control group 72 h after PCI. After 6 months, there were six (6.3%) MACE events (one death, two non-fatal MIs and three revascularizations) in combination therapy group and 12 (13.2%) (four deaths, three non-fatal MIs and five revascularizations, P < 0.05) in control group.
Conclusions
Combination of thrombus aspiration, high-dose statin pre-treatment, intracoronary administration of adenosine during PCI procedure and platelet membrane glycoprotein IIb/IIIa receptor antagonist reduce the incidence of no-reflow after primary PCI in patients with acute myocardial infarction who are at high risk of no-reflow.
Keywords: Acute myocardial infarction, Myocardial contrast echocardiography, No-reflow phenomenon, Percutaneous coronary intervention, ST-elevation myocardial infarction
1. Introduction
Primary percutaneous coronary intervention (pPCI) is currently the most effective treatment strategy in ST-segment elevation acute myocardial infarction (STEMI).[1] A considerable number of patients, however, develop no-reflow phenomenon during pPCI.[2],[3] Compared to similar patients with adequate reflow, those with the no-reflow phenomenon have a higher incidence of death, myocardial infarction and heart failure.[5],[6]
No-reflow is considered a dynamic process characterized by multiple pathogenetic components including distal atherothrombotic embolization, ischemic injury, reperfusion injury, and susceptibility of coronary microcirculation to injury, and current ways of treatment are limited.[7] We have established a risk prediction model of no-reflow in our previous studies,[8],[9] through which we were able to find out patients at high risk of no-reflow. The aim of this study was to investigate the effectiveness of a combination therapy for the prevention of no-reflow in patient with STEMI undergoing primary PCI.
2. Methods
2.1. Study population
Patients with chest pain were enrolled between May 2009 and May 2013 at the Chinese PLA General Hospital. The criteria for inclusion were patients diagnosed with STEMI for the first time and treated with emergency pPCI. STEMI was defined as chest pain suggestive of myocardial ischemia for at least 30 min before hospital admission, and serial changes on electrocardiogram: ST elevation > 2 mm in ≥ 2 precordial leads, ST elevation > 1 mm in ≥ 2 limb leads, or a new onset left bundle branch block. A concomitant increase of at least one cardiac enzyme was also needed. The criteria for exclusion included previous history of myocardial infarction, atrial fibrillation and frequent premature beats, mechanic complications, previous coronary artery bypass grafting (CABG) or lesion needed CABG, malignant tumour.
Before PCI procedure, study patients were assessed for their risk of no-reflow by using a no-flow risk prediction model. High risk (no-reflow score ≥ 10) patients were randomly divided into a controlled group and a combination therapy group. Patients with low risk and those in the controlled group received conventional treatment. Use of adenosine, thrombus aspiration or treatment of IIb/IIIa receptor antagonists were based on the treating physicians' decision in low risk group and high risk controlled group, while patients in the combination therapy group received high-dose (80 mg) atorvastatin pre-treatment, intracoronary administration of adenosine (140 µg/min per kilogram) during PCI procedure, platelet membrane glycoprotein IIb/IIIa receptor antagonist (tirofiban, 10 µg/kg bolus followed by 0.15 µg/kg per minute) and thrombus aspiration. And 40 mg atorvastatin daily for seven days followed by 20 mg daily for six months at follow-up post PCI.
This study was approved by the Ethics Committee of the Chinese PLA General Hospital, and all patients gave their written informed consent before PCI treatment. The study was conducted according to the principles of the Declaration of Helsinki, 2008 version.
2.2. PCI procedure and medication
PPCI was performed by one of four interventionists, using standard techniques. Unfractionated heparin was administered intravenously as 50−70 IU/kg bolus with subsequent boluses to achieve an activated clotting time of 200−250 s. Patients chewed 300 mg aspirin in the emergency department, followed by 75−100 mg orally daily indefinitely thereafter. A clopidogrel loading dose (300 mg) was administered before catheterization, followed by 75 mg orally daily for at least 12 months. Drug-eluting stents were implanted as the first-line choice of stents (Table 1).
Table 1. Treatment of patients in different groups.
| Low-risk (n = 405) | Control (n = 108) | Combination therapy (n = 108) | P value | |
| Large doses of statin | ||||
| Before-PCI | 0 (0) | 0 (0) | 108 (100%) | 0.001 |
| After-PCI | 215 (53.1%) | 75 (69.4%) | 108 (100%) | 0.024 |
| Adenosine | 40 (10.0%) | 23 (21.3%) | 108 (100%) | 0.001 |
| Thrombus aspiration | 12 (1.8%) | 46 (42.6%) | 089 (82.4%) | 0.001 |
| Platelet membrane glycoprotein IIb/IIIa receptor antagonist | 17 (4.2%) | 23 (21.2%) | 067 (62.0%) | 0.03 |
PCI: percutaneous coronary intervention.
2.3. Endpoints
Study endpoints were the incidence of no-reflow during PCI procedure. Angiographic no-reflow was defined as significant coronary flow decrease [thrombolysis in myocardial infarction (TIMI) flow grade < 3] without noticed mechanical obstruction on final angiograms obtained at the completion of PCI.[10]
The secondary end points of this study were myocardial perfusion at 72 h after PCI, and major adverse cardiovascular events (MACE) occurred during six months after PCI. MACE was defined as cardiac death, non-fatal MI, and ischemia driven target lesion revascularization (TLR).
2.4. Clinical data collection
Creatine kinase (CK) and CK-MB, troponin T, blood glucose, Cr, markers of inflammation [high sensitivity C reactive protein (hs-CRP)] and brain natriuretic peptide (BNP) were tested before PCI. CK and CK-MB, troponin as well as hs-CRP were also tested at 6 h, 12 h, 24 h after PCI.
2.5. Myocardial perfusion assessment
Myocardial contrast echocardiography (MCE) was done 72 h after PCI in order to assess the myocardial perfusion. Apical 4-chamber, 2-chamber and 3-chamber views were acquired for myocardial perfusion imaging. RANK 512 ultrasonic diagnostic apparatus (Siemens Acuson Sequoia) were switched to low power real time perfusion imaging (MI < 0.2) with adjustment of transmit power, gain, dynamic range and line density to achieve optimal and artefact-free visualisation of myocardial perfusion. Infusion rate of SonoVue® was adjusted for optimal visualisation of myocardial perfusion. Myocardial perfusion was assessed on a segmental level using a 16-segment model, then analyzed off line using auto-tracking contrast quantification. A value represents the vascular bed area, β value represents the flow velocity, A × β values represents myocardial perfusion.
2.6. Statistical analysis
Continuous variables were presented as mean ± SD and categorical variables as frequencies and percentages. Data were statistically analyzed with SPSS version 17. Continuous variables were compared using One-way ANOVA. Categorical variables were compared using chi-square statistics. P < 0.05 was considered significant.
3. Results
3.1. Study population and baseline characteristics
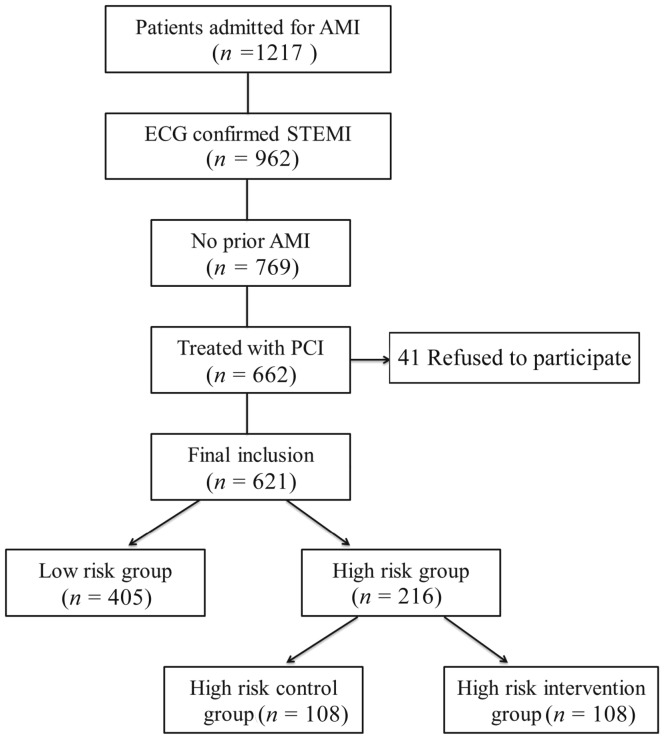
A total of 1217 patients were admitted to our hospital during the enrolment for AMI; 962 (79%) were considered to have STEMI by 12-leads electrocardiography and 769 had no documented or self-reported prior AMI. Of the first STEMI, 17 died and 15 were transferred to other hospitals before a decision on whether or not to undergo PCI was made. Sixty-four were excluded due to contraindications to reperfusion and 41 patients refused to participate in this study. Finally, 621 patients were enrolled (Figure 1), Among which, 216 (34.8%) high risk patients of no-reflow were selected by no-reflow risk prediction model.[8],[9] Patients demographics, angiography and procedural data examined in different group are shown in Table 2.
Figure 1. Selection of study patients.
AMI: acute myocardial infarction; STEMI: ST-segment elevation acute myocardial infarction; PCI: percutaneous coronary intervention.
Table 2. Baseline clinical data.
| Characteristics | Low-risk (n = 405) | Control group (n = 108) | Combination therapy group (n = 108) | P value |
| Age, yrs | 62 ± 12 | 64 ± 9 | 64 ± 13 | 0.885 |
| Male | 297 (73.3%) | 77 (71.3%) | 78 (72.2%) | 0.067 |
| Hypertension | 252 (62.2%) | 65 (60.2%) | 63 (58.3%) | 0.130 |
| Diabetes mellitus | 111 (27.4%) | 46 (42.6%) | 48 (44.4%) | 0.035* |
| Smoking status | ||||
| Current smoker | 194 (47.9%) | 49 (45.4%) | 54 (50.0%) | 0.052 |
| Ex-smoker | 49 (12.1%) | 12 (11.1%) | 10 (9.3%) | 0.461 |
| Never smoker | 162 (40.0%) | 43 (39.8%) | 40 (37.0%) | 0.132 |
| Pre-infarction angina | 352 (86.9%) | 96 (88.9%) | 101 (93.5%) | 0.052 |
| Medication usage before myocardial infarction | ||||
| Aspirin | 48 (11.9%) | 16 (14.8%) | 14 (13.0%) | 0.401 |
| Angiotension-converting enzyme inhibitors | 34 (8.4%) | 8 (7.4%) | 10 (9.3%) | 0.102 |
| β-blocker | 149 (36.8%) | 20 (18.5%) | 21 (19.4%) | 0.010* |
| Calcium channel blocker | 115 (28.4%) | 36 (33.3%) | 31 (28.7%) | 0.328 |
| Statin | 104 (25.7%) | 29 (26.9%) | 33 (30.6%) | 0.446 |
| Time-to-hospital admission, h | 6.5 ± 14.0 | 9.1 ± 16.8 | 8.6 ± 12.2 | 0.021* |
| Physical findings on admission | ||||
| Systolic blood pressure, mmHg | 121 ± 25 | 119 ± 23 | 123 ± 21 | 0.583 |
| Diastolic blood pressure, mmHg | 72 ± 12 | 75 ± 17 | 73 ± 16 | 0.165 |
| Heart rate, beats/min | 76 ± 16 | 75 ± 8 | 73 ± 17 | 0.213 |
| Plasma glucose, mmol/L | 10.3 ± 3.5 | 14 ± 4.2 | 13.1 ± 4.4 | 0.04* |
| CK, U/L | 254 ± 12.1 | 271 ± 14.5 | 268 ± 20.6 | 0.067 |
| CK-MB, ng/mL | 16.5 ± 3.1 | 13.5 ± 2.2 | 15.3 ± 1.7 | 0.31 |
| Hs-CRP, mmol/L | 4.7 ± 4.5 | 4.6 ± 3.8 | 4.5 ± 4.0 | 0.434 |
| BNP, mmol/L | 1041 ± 1021 | 914 ± 1007 | 1110 ± 987 | 0.165 |
| Neutrophil count, ×109/L | 9.3 ± 3.0 | 12.1 ± 8.0 | 11.9 ± 3.1 | 0.001* |
| Low-density lipoprotein cholesterol, mmol/L | 2.83 ± 1.00 | 2.94 ± 1.03 | 2.91 ± 1.2 | 0.069 |
| Triglycerides, mmol/L | 1.58 ± 1.12 | 1.63 ± 1.11 | 1.60 ± 1.21 | 0.139 |
| Killip classes | ||||
| 1 | 46 (11.4%) | 8 (7.4%) | 14 (13.0%) | 0.322 |
| 2 | 160 (39.5%) | 20 (18.5%) | 19 (17.6%) | 0.427 |
| 3 | 103 (25.4%) | 34 (31.4%) | 32 (29.6%) | 0.053 |
| 4 | 96 (23.7%) | 46 (42.6%) | 43 (39.8%) | 0.043* |
| Angiography | ||||
| Multivessel disease | 240 (59.3%) | 66 (63.0%) | 63 (60.2%) | 0.753 |
| Infarct-related artery | ||||
| Left main artery | 4 (1.0%) | 1 (1.0%) | 1 (1.0%) | 0.861 |
| Left anterior descending artery | 234 (57.8%) | 61 (56.5%) | 62 (57.4%) | 0.352 |
| Left circumflex artery | 35 (8.6%) | 11 (10.2%) | 8 (7.4%) | 0.427 |
| Right coronary artery | 116 (28.6%) | 30 (27.8%) | 29 (26.9%) | 0.198 |
| Procedure | ||||
| Direct stenting | 19 (4.7%) | 5 (4.6%) | 60 (5.6%) | 0.146 |
| After dilation | 237 (58.5%) | 61 (56.5%) | 60 (55.6%) | 0.453 |
| Maximal inflation pressure, atmosphere | 14.6 ± 3.4 | 15.7 ± 3.9 | 15.7 ± 4.1 | 0.218 |
| Stent diameter, mm | 3.01 ± 0.61 | 3.02 ± 0.72 | 3.01 ± 0.68 | 0.343 |
| Total stent length, mm | 24.7 ± 6.1 | 25.7 ± 9.1 | 25.5 ± 6.7 | 0.199 |
Data are presented as mean ± SD or n (%). *Compared with control group (P < 0.05). Hypertension is defined as physician office systolic BP level of ≥= 140 mmHg and diastolic BP level of ≥ 90 mmHg. Diabetes: FPG ≥ 7.0 mmol/L or 2 h PG ≥ 11.1 mmol/L. Diabetes was defined as a self-reported diabetes with a validated history or newly diagnostic diabetes by OGTT. BNP: brain natriuretic peptide; BP: blood pressure; CK: creatine kinase; CK-MB: creatine kinase-MB; hs-CRP: high sensitivity C reactive protein; FPG: fasting plasma glucose; PG: plasma glucose.
3.2. Incidence of no-reflow
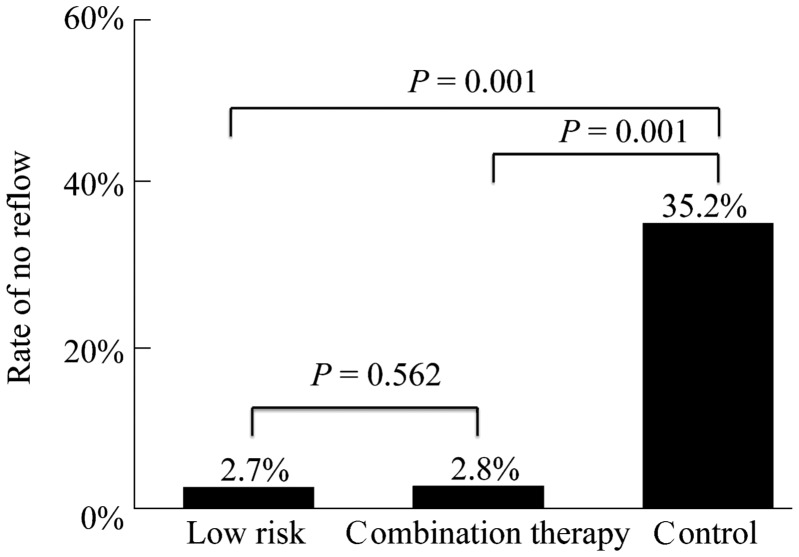
No-reflow occurred in 11 cases (11/405, 2.7%) in low risk patients, 38 cases (38/108, 35.2%) in controlled group and 3 cases (2.8%) in combination therapy group (Figure 2).
Figure 2. Rates of no-reflow in patients with low risk score and high risk score.
*Compared with control group (P < 0.05).
3.3. Myocardial perfusion
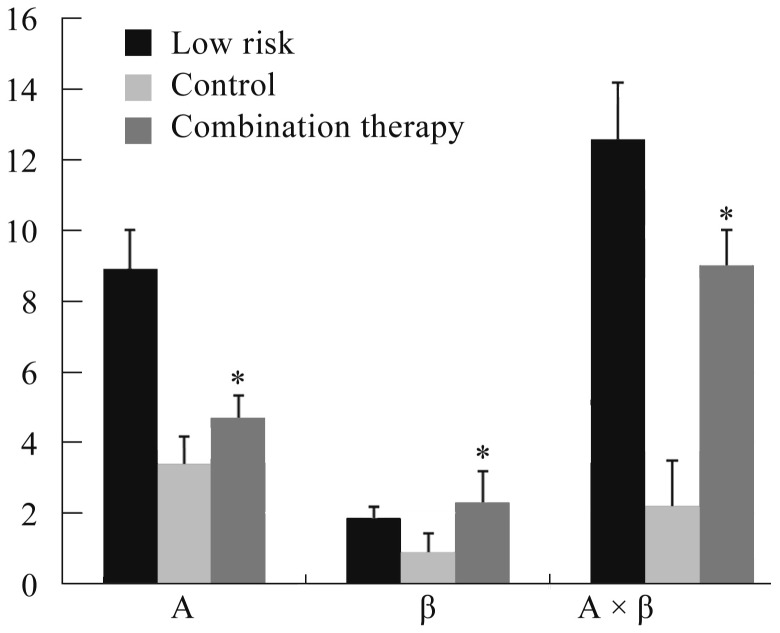
MCE at 72 h after PCI procedure suggested a higher A × β value in combination therapy group than that of controlled group (Figure 3 & 4).
Figure 3. Myocardial contrast echocardiography in patients.
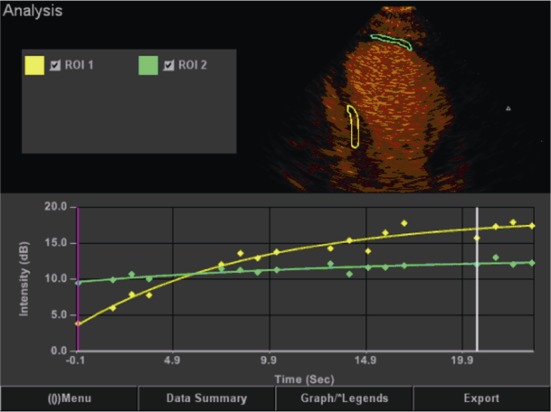
The green curve represents the perfusion is poor, yellow represents normal perfusion segments. Larger slope indicates better myocardial perfusion.
Figure 4. Myocardial contrast echocardiography parameters in patients with low risk score and high risk score.
*Compared with High-risk-control (P < 0.05).
3.4. MACE at six months
Six months clinical follow-up was obtained in 552 patients. Events rates are presented in Table 3. There were 6 (6.3%) events (one death, two non-fatal MIs and three revascularizations) in combination therapy group, significantly lower than 12 (13.2%) events (four deaths, three non-fatal MIs and five revascularizations) in controlled group. Three months later, echocardiogram showed that heart function in combination therapy group significantly better than that of control group ones (Table 4).
Table 3. Major adverse cardiac events of different groups.
| MACE | In-hospital (n = 621) |
Three months (n = 587) |
Six months (n = 552) |
||||||
| Low-risk (n = 405) | Control (n = 108) | Combination therapy (n = 108) | Low-risk (n = 392) | Control (n = 97) | Combination therapy (n = 98) | Low-risk (n = 365) | Control (n = 91) | Combination therapy (n = 96) | |
| Ischaemia driven MACE | 8 (2.0%) | 5 (4.6%) | 2 (1.9%)* | 15 (3.8%) | 7 (7.2%) | 4 (4.1%)* | 20 (5.5%) | 12 (13.2%) | 6 (6.3%)* |
| Cardiac death | 1 (0.2%) | 1 (0.9%) | 0 (0%) | 1 (0.3%) | 3 (3.1%) | 1 (1.0%)* | 2 (0.5%) | 4 (4.4%) | 1 (1.0%)* |
| MI | 2 (0.5%) | 1 (0.9%) | 0 (0%) | 5 (1.3%) | 1 (1.0%) | 1 (1.0%)* | 6 (1.6%) | 3 (3.3%) | 2 (2.1%)* |
| Ischaemia driven TLR | 5 (1.2%) | 3 (2.8%) | 2 (1.9%)* | 9 (2.3%) | 3 (3.1%) | 2 (2.0%)* | 12 (3.3%) | 5 (5.5%) | 3 (3.1%)* |
Data are presented as n (%). *Compared with control, P < 0.05. MACE: major adverse cardiac events; MI: myocardial infarction; TLR: target lesion revascularization.
Table 4. Parameters of echocardiography.
| Characteristics | Low-risk (n = 394) | High-risk-control (n = 103) | High risk-treated (n = 103) |
| EDV, mL | 110 ± 12 | 119 ± 11 | 120 ± 16 |
| ESV, mL | 68 ± 6 | 78 ± 5 | 72 ± 10* |
| LVEF, % | 58 ± 9 | 44 ± 6 | 53 ± 8* |
*Compared with High-risk-control, P < 0.05. EDV: end-diastolic volume; ESV: end-systolic volume; LVEF: left ventricular ejection fraction.
4. Discussion
Our study discovered that using no-flow risk prediction model to screen AMI patients who had been suffered with high risk of no-reflow, and pre-treated them with combination treatment could significantly lower the incidence of no-reflow, and further improved the prognosis. MACE happened in combination treatment group decreased by 55% compared with controlled group.
No-reflow phenomenon remains a serious complication of PCI in patients with AMI,[2],[3] associated with short-term and long-term clinical outcomes.[12]–[17] Therefore, prediction, prevention, and treatment of no-reflow are likely to have an important impact on the outcome of pPCI. Currently, there still lack of effective methods that can classify the no-reflow patients and give a targeted therapy to those ones highly affected by no-reflow. Therefore, we established a risk prediction model of no-reflow in our previous research.[8],[9] In this study, we observed the overall incidence of no-reflow in STEMI patients, which was 16.0% without any interventions, was similar to that of previous reports.[18]–[20] Then we stratified these STEMI patients into low-risk no-reflow and high-risk no-reflow ones through this model,[8],[9] incidences of which were 2.8% and 65.7%, respectively. The accuracy of no-reflow risk model has been well confirmed in this study.[8],[9]
The pathogenesis of no-reflow is complex. Several known causes including embolization, ischemia-reperfusion injury, and individual predisposition of coronary microcirculation to injury,[2] are variable in different patients. Current several monotherapies are reported to have certain effects, but not satisfactory at all. As far as we know, this is the first study that has tested the effectiveness of combined therapy of thrombus aspiration, high-dose statin, adenosine and platelet membrane glycoprotein IIb/IIIa receptor antagonist in reducing the incidence of no-reflow and further improve the myocardial microcirculation perfusion and heart function. Study results shown that reduction by about 62.9% the incidence of no-reflow compared with control group in high risk patients. MCE and echocardiogram showed better perfusion and heart function of high risk intervention group than that of high risk control group ones. Statin treatment is associated with a marked reduction in event rate and mortality after coronary intervention independent of the lipid lowering.[21] Beyond their lipid-lowering effects, statins have favourable effects on platelet adhesion,[22] thrombosis,[23] endothelial function,[24],[25] plaque stability[26],[27] and inflammation.[28] These pleiotropic effects could contribute to the preservation of microvascular function during ischaemia and reperfusion. Results of ARMYDA-ACS trial show good outcome of 80 mg atorvastatin pre-treatment in patients with acute coronary syndromes undergoing early percutaneous coronary intervention.[29] It shown that 80 mg atorvastatin dramatically decreased 88% risk of adverse cardiac events and 70% incidence of myocardial infarction within perioperative period. The ARMYDA-RECAPTURE trial indicates that a short-term pre-treatment with high-dose atorvastatin load before PCI improves outcome in patients already receiving chronic statin therapy.[30] In particular, an acute bolus of 80 mg atorvastatin given 12 h before intervention followed by a further 40 mg pre-procedural dose was associated with 50% relative risk reduction of MACE at 30 days versus placebo. Iwakura, et al.[31] have demonstrated that chronic statin therapy in patients with or without hypercholesterolemia is associated with lower prevalence of no-reflow and better functional recovery. In this study, patients in high risk group received 80 mg atorvastatin before operation, with a further 40 mg pre-procedural in order to prevent occurring of no reflow and improve microcirculation of STEMI patient with pPCI.
The benefits of the thrombus aspiration were promising. The REMEDIA trial,[32] which was the first randomized trial to assess the role of thrombectomy performed with a simple manual aspiration catheter, as compared with conventional pPCI. The results shown that manual thrombectomy was safe and resulted in better myocardial perfusion indexes as compared with standard pPCI. Incidence of no-reflow was significantly reduced In the MCE sub-study of the same trial.[33] Large trial by Svilaas, et al.[34] confirmed the improvement of reperfusion associated with manual thrombus-aspiration as compared with standard pPCI. TAPAS trial shown that improvement of myocardial perfusion by manual thrombus aspiration translated in a strikingly lower mortality at 12-month follow-up.[35] These studies suggest the benefit was particularly evident in the subset of patients with higher thrombus burden, thus suggesting that the efficacy of thrombectomy might be dependent on individual patient characteristics.[36] Therefore, thrombus aspiration was an important part of interventions during PCI in this study.
Adenosine is an endogenous nucleoside degraded from endogenous ATP,[37]–[39] which has been widely studied for the treatment of no reflow. Among its many beneficial effects, adenosine increases microvascular flow owing to its vasodilator properties, inhibits neutrophil adhesion and migration, exerts antiplatelet effects, and inhibits oxygen free radical formation, which results in decreased cellular acidosis.[40] Adenosine has shown benefits in both intravenous and intracoronary administration. Intravenous adenosine has been tested in two randomized trials: AMISTAD[41] and AMISTAD II.[42] Navarese, et al.,[43] analyzed 10 randomized clinical trials, including the data of coronary flow collected from 3821 patients, which later underwent Meta analysis. Results of which discovered that the usage of adenosine in adjuvant therapy during thrombolytic treatment could significantly improve the coronary blood flow of the patients with acute coronary syndrome and further improved the myocardial perfusion.
There are convincing randomized trial data for hard clinical outcome using IIb/IIIa inhibitors in the setting of ST elevation MI,[44] and its use in this setting is a Class II indication in the current 2011 PCI guidelines.[45] It is possible that one mechanism of benefit is the reduction in the no-reflow phenomenon in the IIb/IIIa treated patients. Large scaled clinical researches have proven the preventive effects of platelet membrane glycoprotein IIb/IIIa receptor antagonists on the no-reflow events after AMI.[46] The largest randomized trial of mechanical aspiration in the setting of ST elevation MI was the TAPAS trial (n = 1 071, export catheter).[47],[48] This trial utilized myocardial blush grading as the primary endpoint to show that manual aspiration thrombectomy reduced the incidence of no reflow and improved angiographic outcomes.
In conclusion, combination of thrombus aspiration, high-dose statin pre-treatment, intracoronary administration of adenosine during PCI procedure and platelet membrane glycoprotein IIb/IIIa receptor antagonist reduces the incidence of no-reflow after primary PCI in patients with acute myocardial infarction who are at high risk of no-reflow.
Acknowledgments
The authors declare that they have no conflict of interest. This study was supported by Beijing Science and Technology Commission, code: Z121107001012002.
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 2.Niccoli G, Burzotta F, Galiuto L, et al. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 3.Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: From the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2008;72:950–957. doi: 10.1002/ccd.21715. [DOI] [PubMed] [Google Scholar]
- 4.Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2008;72:950–957. doi: 10.1002/ccd.21715. [DOI] [PubMed] [Google Scholar]
- 5.Brosh D, Assali AR, Mager A, et al. Effect of no-reflow during primary percutaneous coronary intervention for acute myocardial infarction on six-month mortality. Am J Cardiol. 2007;99:442–445. doi: 10.1016/j.amjcard.2006.08.054. [DOI] [PubMed] [Google Scholar]
- 6.Bolognese L, Carrabba N, Parodi G, et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 7.Niccoli G, Burzotta F, Galiuto L, et al. Myocardial no-reflow in humans. J Am Coll Cardiol. 2009;54:281–292. doi: 10.1016/j.jacc.2009.03.054. [DOI] [PubMed] [Google Scholar]
- 8.Wang JW, Chen YD, Wang CH, et al. Development and validation of a clinical risk score p redicting the no-reflow phenomenon in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Cardiology. 2013;124:153–160. doi: 10.1159/000346386. [DOI] [PubMed] [Google Scholar]
- 9.Chen YD, Wang CH, Yang XC, et al. A no-reflow prediction model in patients with ST-elevation acute myocardial infarction and primary drug-eluting stenting. Scandinavian Cardiovascular Journal. 2011;45:98–104. doi: 10.3109/14017431.2011.558209. [DOI] [PubMed] [Google Scholar]
- 10.Morishima I, Sone T, Mokuno S, et al. Clinical significance of no-reflow phenomenon observed on angiography after successful treatment of acute myocardial infarction with percutaneous transluminal coronary angioplasty. Am Heart J. 1995;130:239–243. doi: 10.1016/0002-8703(95)90434-4. [DOI] [PubMed] [Google Scholar]
- 11.Henriques JP, Zijlstra F, van't Hof AW, et al. Angiographic assessment of reperfusion in acute myocardial infarction by myocardial blush grade. Circulation. 2003;107:2115–2119. doi: 10.1161/01.CIR.0000065221.06430.ED. [DOI] [PubMed] [Google Scholar]
- 12.Gibson CM, Cannon CP, Murphy SA, et al. Relationship of the TIMI myocardial perfusion grades, flow grades, frame count, and percutaneous coronary intervention to long-term outcomes after thrombolytic administration in acute myocardial infarction. Circulation. 2002;105:1909–1913. doi: 10.1161/01.cir.0000014683.52177.b5. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin MG, Stone GW, Aymong E, et al. Prognostic utility of comparative methods for assessment of ST-segment resolution after primary angioplasty for acute myocardial infarction: The Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) trial. J Am Coll Cardiol. 2004;44:1215–1223. doi: 10.1016/j.jacc.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 14.Bolognese L, Carrabba N, Parodi G, et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 15.Galiuto L, Garramone B, Scara A, et al. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post-infarct reperfusion in predicting left ventricular remodeling: Results of the multicenter AMICI study. J Am Coll Cardiol. 2008;51:552–559. doi: 10.1016/j.jacc.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 16.Wu KC, Zerhouni EA, Judd RM, et al. Prognostic significance of microvascular obstruction by magnetic resonance imaging in patients with acute myocardial infarction. Circulation. 1998;97:765–772. doi: 10.1161/01.cir.97.8.765. [DOI] [PubMed] [Google Scholar]
- 17.Lee MG, Jeong MH, Ahn Y, et al. Comparison of paclitaxel-, sirolimus-, and zotarolimus-eluting stents in patients with acute ST-segment elevation myocardial infarction and metabolic syndrome. Circ J. 2011;75:2120–2127. doi: 10.1253/circj.cj-11-0263. [DOI] [PubMed] [Google Scholar]
- 18.Choi YH, Suh SH, Choi JS, et al. Triple vs. dual antiplatelet therapy in patients with acute myocardial infarction and renal dysfunction. Circ J. 2012;76:2405–2411. doi: 10.1253/circj.cj-12-0236. [DOI] [PubMed] [Google Scholar]
- 19.Kataoka Y, Yagi N, Kokubu N, et al. Effect of pretreatment with pioglitazone on reperfusion injury in diabetic patients with acute myocardial infarction. Circ J. 2011;75:1968–1974. doi: 10.1253/circj.cj-11-0098. [DOI] [PubMed] [Google Scholar]
- 20.Chan AW, Bhatt DL, Chew DP, et al. Early and sustained survival benefit associated with statin therapy at the time of percutaneous coronary intervention. Circulation. 2002;105:691–696. doi: 10.1161/hc0602.103586. [DOI] [PubMed] [Google Scholar]
- 21.Merten M, Dong JF, Lopez JA, et al. Cholesterol sulfate: a new adhesive molecule for platelets. Circulation. 2001;103:2032–2034. doi: 10.1161/01.cir.103.16.2032. [DOI] [PubMed] [Google Scholar]
- 22.Gaddam V, Li DY, Mehta JL. Anti-thrombotic effects of atorvastatin–an effect unrelated to lipid lowering. J Cardiovasc Pharmacol Ther. 2002;7:247–253. doi: 10.1177/107424840200700408. [DOI] [PubMed] [Google Scholar]
- 23.Beckman JA, Liao JK, Hurley S, et al. Atorvastatin restores endothelial function in normocholesterolemic smokers independent of changes in low-density lipoprotein. Circ Res. 2004;95:217–223. doi: 10.1161/01.RES.0000134628.96682.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakabe K, Fukuda N, Wakayama K, et al. Lipid-altering changes and pleiotropic effects of atorvastatin in patients with hypercholesterolemia. Am J Cardiol. 2004;94:497–500. doi: 10.1016/j.amjcard.2004.04.067. [DOI] [PubMed] [Google Scholar]
- 25.Son JW, Koh KK, Ahn JY, et al. Effects of statin on plaque stability and thrombogenicity in hypercholesterolemic patients with coronary artery disease. Int J Cardiol. 2003;88:77–82. doi: 10.1016/s0167-5273(02)00368-6. [DOI] [PubMed] [Google Scholar]
- 26.Takano M, Mizuno K, Yokoyama S, et al. Changes in coronary plaque color and morphology by lipid-lowering therapy with atorvastatin: serial evaluation by coronary angioscopy. J Am Coll Cardiol. 2003;42:680–686. doi: 10.1016/s0735-1097(03)00770-8. [DOI] [PubMed] [Google Scholar]
- 27.Strandberg TE, Vanhanen H, Tikkanen MJ. Effect of statins on C-reactive protein in patients with coronary artery disease. Lancet. 1999;353:118–119. doi: 10.1016/S0140-6736(05)76154-7. [DOI] [PubMed] [Google Scholar]
- 28.Jialal I, Stein D, Balis D, et al. Effect of hydroxymethyl glutaryl coenzyme a reductase inhibitor therapy on high sensitive C-reactive protein levels. Circulation. 2001;103:1933–1935. doi: 10.1161/01.cir.103.15.1933. [DOI] [PubMed] [Google Scholar]
- 29.Michałek A. Results of ARMYDA-ACS trial show good outcome of 80 mg atorvastatin pretreatment in patients with acute coronary syndromes undergoing early percutaneous coronary intervention. Kardiol Pol. 2007;65:851–852. [PubMed] [Google Scholar]
- 30.Di Sciascio G, Patti G, Pasceri V, et al. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol. 2009;54:558–565. doi: 10.1016/j.jacc.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 31.Iwakura K, Ito H, Kawano S, et al. Chronic pre-treatment of statins is associated with the reduction of the no-reflow phenomenon in the patients with reperfused acute myocardial infarction. Eur Heart J. 2006;27:534–539. doi: 10.1093/eurheartj/ehi715. [DOI] [PubMed] [Google Scholar]
- 32.Burzotta F, Trani C, Romagnoli E, et al. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol. 2005;46:371–376. doi: 10.1016/j.jacc.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 33.Galiuto L, Garramone B, Burzotta F, et al. Thrombus aspiration reduces microvascular obstruction after primary coronary intervention: a myocardial contrast echocardiography substudy of the REMEDIA trial. J Am Coll Cardiol. 2006;48:1355–1360. doi: 10.1016/j.jacc.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 34.Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–567. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 35.Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 36.Burzotta F, Trani C, Romagnoli E, et al. Manual thrombus-aspiration improves myocardial reperfusion: the randomized evaluation of the effect of mechanical reduction of distal embolization by thrombus-aspiration in primary and rescue angioplasty (REMEDIA) trial. J Am Coll Cardiol. 2005;46:371–376. doi: 10.1016/j.jacc.2005.04.057. [DOI] [PubMed] [Google Scholar]
- 37.Aung Naing K, Li L, et al. Adenosine and verapamil for no-reflow during primary percutaneous coronary intervention in people with acute myocardial infarction. Cochrane Database Syst Rev. 2013:CD009503. doi: 10.1002/14651858.CD009503.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Chen YD, Wang J. The beneficial effect on myocardial perfusion of adenosine in patients treated with primary percutaneous coronary intervention for acute myocardial infarction. Clin Exp Pharmacol Physiol. 2012;39:247–252. doi: 10.1111/j.1440-1681.2012.05668.x. [DOI] [PubMed] [Google Scholar]
- 39.Tian F, Chen YD, Lü SZ, et al. Intracoronary adenosine improves myocardial perfusion by myocardial contrast echocardiography in late reperfused myocardial infarction. Chin Med J. 2008;121:195–199. [PubMed] [Google Scholar]
- 40.Marzilli M, Orsini E, Marraccini P, et al. Beneficial effects of intracoronary adenosine as an adjunct to primary angioplasty in acute myocardial infarction. Circulation. 2000;101:2154–2159. doi: 10.1161/01.cir.101.18.2154. [DOI] [PubMed] [Google Scholar]
- 41.Mahaffey KW, Puma JA, Barbagelata NA, et al. Adenosine as an adjunct to thrombolytic therapy for acute myocardial infarction: results of a multicenter, randomized, placebo-controlled trial: the Acute Myocardial Infarction STudy of ADenosine (AMISTAD) trial. J Am Coll Cardiol. 1999;34:1711–1720. doi: 10.1016/s0735-1097(99)00418-0. [DOI] [PubMed] [Google Scholar]
- 42.Ross AM, Gibbons RJ, Stone GW, et al. A randomized, double-blinded, placebo-controlled multicenter trial of adenosine as an adjunct to reperfusion in the treatment of acute myocardial infarction (AMISTAD-II) J Am Coll Cardiol. 2005;45:1775–1780. doi: 10.1016/j.jacc.2005.02.061. [DOI] [PubMed] [Google Scholar]
- 43.Navarese EP, Buffon A, Andreotti F, et al. Adenosine improves post-procedural coronary flow but not clinical outcomes in patients with acute coronary syndrome: a meta-analysis of randomized trials. Atherosclerosis. 2012;222:1–7. doi: 10.1016/j.atherosclerosis.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Montalescot G, Antoniucci D, Kastrati A, et al. Abciximab in primary coronary stenting of ST-elevation myocardial infarction: a European meta-analysis on individual patients' data with long-term follow-up. Eur Heart J. 2007;28:443–449. doi: 10.1093/eurheartj/ehl472. [DOI] [PubMed] [Google Scholar]
- 45.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Magro M1, Springeling T, van Geuns RJ, et al. Myocardial ‘no-reflow’ prevention. Curr Vasc Pharmacol. 2013;11:263–277. [PubMed] [Google Scholar]
- 47.Svilaas T, Vlaar PJ, van der Horst IC, et al. Thrombus aspiration during primary percutaneous coronary intervention. N Engl J Med. 2008;358:557–567. doi: 10.1056/NEJMoa0706416. [DOI] [PubMed] [Google Scholar]
- 48.Vlaar PJ, Svilaas T, van der Horst IC, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]