Abstract
Chronic heart failure (CHF) is predominantly seen in older patients, and therefore real life medicine often requires the extrapolation of findings from trials conducted in much younger populations. Prescribing patterns and potential benefits in the elderly are heavily influenced by polypharmacy and co-morbid pathologies. Increasing longevity may become less relevant in the frail elderly, whereas improving quality of life (QoL) often becomes priority; the onus being on improving wellbeing, maintaining independence for longer, and delaying institutionalisation. Specific studies evaluating elderly patients with CHF are lacking and little is known regarding the tolerability and side-effect profile of evidence based drug therapies in this population. There has been recent interest on the impact of heart rate in patients with symptomatic CHF. Ivabradine, with selective heart rate lowering capabilities, is of benefit in patients with CHF and left ventricular systolic dysfunction in sinus rhythm, resulting in reduction of heart failure hospitalisation and cardiovascular death. This manuscript will focus on CHF and the older patient and will discuss the impact of heart rate, drug therapies and tolerability. It will also highlight the unmet need for specific studies that focus on patient-centred study end points rather than mortality targets that characterise most therapeutic trials. An on-going study evaluating the impact of ivabradine on QoL that presents a unique opportunity to evaluate the tolerability and impact of an established therapy on a wide range of real life, older patients with CHF will be discussed.
Keywords: Heart failure, Heart rate, Quality of life, The elderly
1. Heart failure and the elderly
The incidence and prevalence of chronic heart failure (CHF) increase with age, due to a combination of the physiological and anatomical changes associated with ageing, and the increasing frequency of co-morbid conditions, which predispose to CHF. This is exemplified by the finding that the mean age at diagnosis of CHF in the UK is around 77 years.[1] Yet, the average age seen in most landmark CHF trials is somewhere in the 60's (Table 1) and subsequently, the evidence base for treating elderly subjects with CHF is generally extrapolated from cohorts up to 2 decades younger. The reasons for this are multi-fold. Clinical trials require clear pre-determined eligibility criteria and rigorous follow-up. While some trials like SOLVD[2] and MERIT-HF[3] excluded those > 80 years of age, other key trials (without age as a specific exclusion criterion) also failed to recruit significant numbers of older patients. This may be due to the presence of other co-morbidities (such as chronic kidney disease) being exclusion criteria, a refusal of elderly patients committing to multiple study visits over several years of follow-up (limited mobility and functional impairment) or even investigator bias (deeming patients unsuitable or unlikely to participate).
Table 1. Mean age of patients in land mark heart failure trials.[54].
| Trial | Year | Study treatment | No. of patients | Mean age in years (approximate) |
| SOLVD* | 1991 | Enalapril | 2569 | 61 |
| DIG (main trial) | 1997 | Digoxin | 6800 | 63 |
| RALES | 1999 | Spironolactone | 1663 | 65 |
| CIBIS II* | 1999 | Bisoprolol | 2647 | 61 |
| ATLAS | 1999 | Low-dose vs. high-dose lisinopril | 3793 | 64 |
| COPERNICUS | 2001 | Carvedilol | 2289 | 63 |
| BEST | 2001 | Bucindolol | 2706 | 60 |
| EPHESUS | 2001 | Eplerenone | 6632 | 64 |
| Val-HeFT | 2002 | Valsartan | 5010 | 65 |
| MADIT II | 2002 | ICD | 1232 | 64 |
| COMET | 2003 | Carvedilol vs. metoprolol | 3029 | 62 |
| CARE HF | 2005 | CRT vs. medical therapy alone | 813 | 67 |
| MADIT-CRT | 2009 | CRT-defibrillator vs. ICD | 1820 | 65 |
| SHIFT | 2010 | Ivabradine | 6558 | 60 |
| EMPHASIS | 2011 | Eplerenone | 2737 | 69 |
*SOLVD and CIBIS II had age < 80 years in the inclusion criteria. CRT: cardiac resynchronization therapy; ICD: internal cardioverter defibrillator;
2. Heart rate and heart failure
Elevated resting heart rate has been associated with increased risk of all-cause mortality and adverse cardiovascular (CV) outcomes in subjects with hypertension and CV diseases such as coronary artery disease (CAD) and CHF.[4] The strength of this association has been documented in both epidemiological studies and clinical trials: subsequently heart rate has been incorporated into clinical risk prediction tools such as the Global Registry of Acute Coronary Events (GRACE) scoring system for patients presenting with myocardial infarction.[5] Resting heart rate (in sinus rhythm) of greater than 70 beats per minute (bpm) in stable patients with CAD and left-ventricular dysfunction is associated with an increased risk of CV death (34%) and heart failure hospitalisations (53%) compared to those with heart rate of ≤ 70 bpm.[6] An elevated heart rate in the presence of left ventricular systolic dysfunction without co-existent coronary artery disease is also directly related to the risk of death, CV death, or hospitalisation for heart failure.[7] A similar association has been seen in patients with acute heart failure decompensation. Habal, et al.[8] in their study of hospitalised patients with heart failure demonstrated that heart rate at discharge (especially if > 80 bpm) was an important predictor of all-cause mortality, CV mortality and heart failure hospitalisations.
Whilst there is consistent evidence that elevated resting heart rate is independently associated with increased CV risk and mortality across a spectrum of pathologies, including patients with CHF, it has been less clear whether it is a marker of advanced disease or alternatively a modifiable risk factor in its own right. Since heart rate may be influenced by smoking, body mass index, diabetes and physical conditioning,[9] it is possible that elevated heart rate identifies patients with co-morbid pathologies when evaluated in a large cohort (risk marker). There are a number of potential mechanisms by which heart rate per se may affect CV outcomes i.e., contribute to disease progression/expression (risk factor). For example, elevated heart rate is associated with altered myocardial metabolism and impaired efficiency and reflects a sympathovagal imbalance, a potential trigger for inflammation and atherosclerosis.[10] Hemodynamic differences have been demonstrated across a range of heart rates and it has been postulated that these may translate to raised arterial stiffness, higher blood pressure, persistent myocardial strain, increased cardiac workload and adverse left ventricular remodelling over time. Activation of endothelial mechanoreceptors can additionally trigger a complex network of several intracellular pathways that promote atherogenesis and risk of plaque rupture.
Treatments that result in heart rate reduction are associated with better outcomes in patients with CHF, adding some support to heart rate being a modifiable risk factor. Beta-blockers have been extensively used in CHF and although several of their beneficial effects are thought to be secondary to the inhibition of the deleterious effects of adrenergic receptor stimulation (impairment of cardiac myocyte function and survival, myocardial ischaemia, arrhythmogenesis, and renin secretion) the magnitude of heart rate reduction achieved may play an equally important role.[11]–[15] A meta-regression analysis of patients treated with carvedilol, bisoprolol, metoprolol, bucindolol, or nebivolol demonstrated that the achieved resting heart rate (RHR) correlated strongly with all-cause mortality (adjusted R2 = 0.51, P < 0.005; nine trials, n = 19,537) and the magnitude of change in RHR correlated with change in left ventricular ejection fraction (LVEF) (adjusted R2 = 0.48, P < 0.005; 26 trials, n = 3389).[16] Cullington, et al.[17] evaluated 654 patients with LVEF ≤ 40% on echocardiography and in sinus rhythm attending a community heart failure clinic and analyzed the effects of heart rate reduction and percentage of target doses of beta-blockers achieved on all-cause mortality. Their findings suggested that beta-blockers exert benefits through dual mechanisms: adrenergic receptor protection, (that may only require a low dose), and heart rate reduction (utilizing whatever beta-blocker dose is required to achieve a target heart rate).
Ivabradine, a selective and specific inhibitor of the cardiac pacemaker If current, that results in pure heart rate reduction without adrenergic inhibition, offers a unique opportunity to test the hypothesis ‘does HR lowering improve outcomes in patients with CHF and left ventricular systolic dysfunction (LVSD)?’ Data from the Systolic Heart failure treatment with the If inhibitor ivabradine Trial (SHIFT, n = 6505), a multicentre, randomised, double-blind study comparing ivabradine with placebo on outcomes in patients with symptomatic CHF [New York Heart Association ( NYHA) class II – IV], in sinus rhythm with RHR ≥ 70 bpm and LVEF ≤ 35% have provided further insight.[18] The primary composite end point was CV death or hospitalisation for worsening heart failure; 24% patients in the ivabradine group and 29% of those taking placebo had a primary endpoint event [hazard ratio (HR): 0.82, 95% confidence interval (CI): 0.75–0.90, P < 0.0001]. These benefits were mainly driven by reduction in hospital admissions for worsening heart failure (21% placebo vs. 16% ivabradine; HR: 0.74, 95%CI: 0.66–0.83; P < 0.0001) and deaths due to heart failure (5% vs. 3%; HR: 0.74, 0.58–0.94, P = 0.014).
As such elevated resting heart rate, at least in patients in sinus rhythm, appears to be a modifiable risk factor in patients with CHF and LVSD. Ivabradine is incorporated into the European Society of Cardiology (ESC) guidelines for the management of heart failure and is licenced as an additional drug or as an alternative to beta-blockers (if not tolerated) when there sting heart rate remains ≥ 75 bpm.[19] Further data are however still required, particularly in respect of specific patients groups. For example, aggressive HR reduction may not be prognostically beneficial in the presence of pre-existing atrial fibrillation.[20] In addition, the tolerability and symptomatic benefit of this strategy in the elderly patient with CHF remains uncertain, when there is often, concern regarding associated co-morbidities and multiple drug therapies.
3. Heart failure therapy in the elderly–what is known?
Key trial data and thereby evidence-based therapy for patients with CHF have generally been established in much younger patient cohorts than those encountered in day-to-day clinical practice.
Masoudi, et al.[21] recently applied the inclusion criteria of three major heart failure drug trials (angiotensin-converting-enzyme inhibitor: SOLVD;[2] beta-blocker: MERIT-HF;[14] and spironolactone: RALES[22]) to a typical heart failure population (mean age 78 years) in the US to ascertain what proportion of real life patients would have been included in these trials. Patients were identified from the medical records of Medicare beneficiaries (aged ≥ 65 years) who had been hospitalised between 1998 and 1999 with a primary diagnosis of heart failure. Only 25% would have been deemed eligible for inclusion in RALES (spironolactone), 17% for SOLVD (ACE inhibitor) and 13% for MERIT-HF (beta-blocker). More than 40% of the reference population would have been excluded from SOLVD and MERIT on the basis of age alone. Although the RALES trial did not exclude people on the basis of age, only 16% of women and 24% of men aged greater than 85 years would have been eligible due to other exclusion criteria.
Given the changes in the demography of the population, there is a pressing need to conduct drug trials in older patients. Only two trials have specifically targeted an older CHF population. The SENIORS study was a randomised trial to determine the effect of nebivolol on mortality and CV hospitalisation in CHF patients ≥ 70 years of age.[15] PEP-CHF (perindopril in elderly people with CHF) compared the effect of perindopril versus placebo in patients > 70 years with CHF and preserved ejection fraction on a composite of all-cause mortality and unplanned heart failure related hospitalisation.[23]
Sub-group analyses of landmark studies retaining the original primary endpoints have been conducted in attempts to ascertain the benefits in older patients. For example, 22.8% of the CHARM study participants were aged 75 years or older (mean age of original cohort was 66 years); treatment with candesartan showed a similar benefit on the primary endpoint of CV death or heart failure hospitalisation in older patients to that seen in the whole study group.[24] Subgroup analysis of the Cardiac Resynchronization-Heart Failure study (CARE-HF, mean age: 65 years, n = 813) showed that cardiac resynchronization therapy reduced the risk of the primary endpoint of all-cause mortality or CV hospitalization in both younger (< 66 years; n = 406) and older (≥ 66 years; n = 407) patients. However only 40 patients were 80–90 years of age, and none were ≥ 90 years.[25]
An age specific analysis of the SHIFT study evaluated the effects of ivabradine on heart rate, CV outcomes, as well as adverse events (in particular bradycardia).[26] Patients were grouped according to quartiles of age (< 53 years, n = 1522; 53 to < 60 years, n = 1521; 60 to < 69 years, n = 1750; ≥ 69 years, n = 1712). Ivabradine (2.5 to 7.5 mg bid) reduced heart rate in all age groups (by around 11 bpm) with associated reductions in the relative risk of the combined primary endpoint of CV death and heart failure hospitalisation: for example, 38% (P < 0.001) in patients < 53 years and 16% (P = 0.035) in the oldest patients (≥ 69 years). Cardiovascular and heart failure deaths were also significantly (P < 0.05) reduced with ivabradine in a subgroup of older patients (≥ 75 years, n = 722). The incidence of adverse events increased with age, but no substantial differences between ivabradine and placebo were noted in any of the age groups. Bradycardia occurred more frequently with ivabradine irrespective of age but there were no episodes of severe bradycardia or pathological pauses occurring in any age group.
4. How important is quality of life for older patients with chronic heart failure?
The main objectives of treating patients with heart failure are to relieve symptoms, improve exercise capacity, reduce hospitalisations and prolong life and these goals can be applied to patients of all ages. As patients grow older, develop more co-morbidities and become frailer, for many there comes a tipping point where QoL and functional capacity become of greater importance.
The impact of co-morbidities in the management of the older patient with heart failure cannot be under estimated.[27] A United States based study of Medicare beneficiaries with CHF (hence > 65 years of age) demonstrated that about 40% of subjects had ≥ 5 co-morbidities and this group of patients accounted for 81% of total inpatient hospital days.[28] In an attempt to curtail the burgeoning numbers of new heart failure diagnoses, aggressive preventive measure strategies targeted at populations identified as high risk have been proposed. Risk prediction models incorporate risk factors such as age, coronary heart disease, impaired kidney function and impaired glucose tolerance. It is now accepted that additionally, in older patients, more complex factors such as social isolation,[29] dispositional optimism or the lack of it,[30] and poor drug compliance add significantly to the risk of developing heart failure.
Social isolation is a key concept and can arise from lifestyle changes, medication regimens and drug side effects, combined with physical restrictions and limited ability to participate in social events.[31] Patients are frequently anxious and live in fear of pain, of the future and of death. Patients also lose a sense of control over their lives and develop feelings of helplessness. Functional impairments and the loss of self-sufficiency also worsen short-term prognosis in the older patient admitted with ADHF.[32] It is thus hardly surprising then that QoL is much worse in CHF than in many other comparable chronic conditions and is itself increasingly recognised as a useful risk stratification tool.[33] Poor QoL scores predict higher mortality, worsening NYHA class and the presence of comorbidities such as chronic kidney disease. For many frailer older patients, improving QoL, optimising physical and cognitive function and maintaining independence are equally if not more important than prolongation of life – ‘the quality versus quantity debate’. Stevenson examined how patient preferences for survival versus QoL change after a hospitalisation for advanced heart failure; the median time that patients would “trade” in terms of survival time for fewer symptoms was 3 months.[34]
Targeting these issues, needless to say, is complex and beyond the limited physician–patient contact that occurs in real life clinical practice. While the importance of QoL, particularly for older patients is irrefutable, the efficacy of heart failure therapy has in general been assessed in large scale studies with robust endpoints such as death and hospitalisation. These are reproducible and relatively easy to measure and are of course fundamental to establishing benefit and safety of a specific therapy. In contrast, assessment of QoL or functional capacity is much more challenging and has historically been rather perfunctory. Ameta analysis by Chang, et al.[35] utilized the minimum standards criteria for health related quality of life (HRQoL) assessment recommended in oncology studies[36] to assess the validity of heart failure studies where QoL was the primary end point. Of the 136 articles identified, only 19 heart failure studies between 1990 and 2009 were considered to be ‘probably robust’ in terms of methodological and reporting rigor. The mean age of subjects seen in these trials was generally in the 60s, again under representing the older heart failure patients where improving QoL may be the greatest priority (Table 2).
Table 2. Trials in heart failure with quality of life as primary end point.
| Ref. | Main objective | Inclusion criteria | Sample size | Mean age in years (approximate) | Follow up period | QoL instrument | Findings |
| Rogers, et al.[46] | Assess QoL in patients with LVSD after randomization to enalapril or placebo | EF ≤ 0.35 | 5025 | 57 | 104 weeks | POM, functional status questionnaire, SF-36 | Modest benefits in QoL for ≥ 1 year in enalapril treated symptomatic CHF patients with LVSD. |
| Cohn, et al.[47] | Describe QoL response to carvedilol vs. placebo in a subset of patients with the most severe impairment of exercise capacity | NYHA III–IV | 131 | 58 | 26 weeks | MLWHF | QoL improved similarly in the carvedilol and placebo groups, global assessment by the physicians and the patient exhibited a better response to carvedilol (P < 0.05). |
| Sanderson, et al.[48] | Compare the long-term clinical efficacy of treatment with metoprolol versus carvedilol | NYHA II–IV | 51 | 60 | 12 weeks | MLWHF | Beneficial improvement in symptoms and exercise capacity seen for both with no significant difference between drugs. |
| Cowley,et al.[49] | Measure QoL in elderly symptomatic heart failure patients following treatment with losartan vs. captopril | NYHA II–IV | 203 | 74 | 48 weeks | MLWHF SIP | Significant improvements in QoL were observed with losartan and captopril long-term. Losartan was better tolerated than captopril (significantly fewer losartan patients discontinued therapy, 19.6% vs. 10.9%, P = 0.038). |
| Fung, et al.[50] | Compare effectiveness of beta blockade in patients with heart failure and AF using MLWHF (Metoprolol 50 mg twice daily or carvedilol 25 mg twice) | NYHA II–IV | 63 | 58 | 12 weeks | MLWHF | Significant improvement in symptoms (P < 0.001) and exercise capacity (P < 0.001) observed in sinus rhythm but not in the AF group despite a significant improvement in LVEF. |
| Lader et al.[51] | Evaluate effect of digoxin therapy on QoL (sub study of DIG trial) | NYHA I–IV | 589 | 65 | 52 weeks | SF-36 Ladder of life CES-D state anxiety inventory state anger inventory MLWHF | No effect on QoL in patients with heart failure in sinus rhythm. |
| Majani et al.[52] | Examine the effect on QoL of valsartan 80 mg bid vs. placebo administered in addition to prescribed background heart failure therapy | NYHA II–IV | 3010 | 63 | 156 weeks | MLWHF | Valsartan had a significant beneficial effect on change in overall MLWHF score from baseline to study endpoint compared with placebo (0.19 ± 0.47 vs. 1.94 ± 0.48; P = 0.005, respectively). |
| Veazie et al.[53] (MADIT-CRT) | Compare QoL of patients with CRT-D to patients with an ICD only. | Ischemic cardiomyopathy (NYHA I/II) or non-ischemic cardiomyopathy (NYHA II), sinus rhythm, LVEF ≤ 30% and QRS duration of ≥ 130 ms | 1820 | 64 | 2.4 years | KCCQ | CRT-D group had greater improvement than the ICD-only group on all KCCQ measures (P < 0.05). This was seen in patients with left bundle branch block conduction disturbance (n = 1,204, P < 0.01), but not among patients without left bundle branch block (n = 494). |
CRT: cardiac resynchronisation therapy; CRT-D: cardiac resynchronisation therapy-defibrillator; ICD: implantable cardioverter defibrillator; LVEF: left ventricular ejection fraction; LVSD: left ventricular systolic dysfunction; MLHFQ: Minnesota living with heart failure questionnaire; NYHA: New York Heart Association; POM: personal outcome measures; QoL: quality of Life; SIP: sickness impact profile.
Thus while some clinical trials over the last decade have included measures of QoL, there is a need to develop more standardised methodology for measuring and reporting HRQoL in studies that genuinely reflect clinical practice, and optimise their interpretation and applicability to real-life patient management. Data regarding the tolerability of guideline recommended therapy in elderly patients with CHF and its impact on QoLis limited. Why or by whom potentially lifesaving medications are stopped remains uncertain. In UK primary care for example, around one third of patients with CHF are no longer receiving beta-blocker prescription at 1 year.[37] It is encouraging to note the increasing emphasis on improving QoL in heart failure- the National Institute of Clinical Excellence (NICE) in the UK now recommends the use of QoL instruments as a means of assessing the overall health of a patient. The ESC has also recently stated that all studies examining treatment of heart failure should include QoL as an endpoint. These kinds of trials would help to establish whether specific therapies are truly safe, well tolerated and benefit QoL in elderly patients with CHF.
The SHIFT trial did evaluate QoL in a sub-study of 1944 symptomatic patients (with CHF and LVSD) treated with recommended background therapy and randomised to ivabradine (n = 968) or placebo (n = 976).[38] Mean age of the study population was younger than in clinical practice at 60.7 years. Health-related QoL was assessed by the Kansas City Cardiomyopathy Questionnaire (KCCQ) containing the following dimensions: overall summary score (OSS) and clinical summary score (CSS) at baseline, and 4, 12, and 24 months. The incidence of clinical events (CV death or hospital admission for heart failure) was inversely associated with KCCQ scores. Treatment with ivabradine was associated with improved KCCQ, by 1.8 for CSS and 2.4 for OSS (placebo-corrected, P = 0.02 and P < 0.01, respectively); these changes were associated with the magnitude of heart rate reduction for both CSS (P < 0.001) and OSS (P < 0.001).
Initiation and adjustment of drug therapy and enrolment in a heart failure management programme form the mainstay of management for older patients with heart failure and requires full engagement of both patients and carers. Discussion around potential benefits (hard endpoints and QoL), likely tolerability potential adverse effects combined with an assessment of comorbidity underpins an agreed individualised management plan. It would be extremely valuable to know whether or not the improvement in QoL seen in SHIFT can be replicated in a much older, frailer “real life” population of patients with heart failure, and this forms the basis for the recently initiated UK based Live: Life study.
5. Live: Life—a prospective observational study of the effects of ivabradine on quality of life in older patients in the UK
Live: Life is a multi-centre, open-label, prospective, observational, cohort study specifically designed to assess the impact of ivabradine on QoL and functional endpoints in an older cohort of patients. We believe the study presents a refreshing opportunity to recruit a wide range of real life, older patients who fulfill the indications for treatment with ivabradine. The Live: Life study will recruit patients over the age of 70 years (often the lower age limit for admission to specialist services for the treatment of older people within the UK), who have been identified to be initiated on ivabradine according to the licenced criteria in CHF. Patients will be recruited across a range of clinical services: specialist cardiology, medicine for the elderly, and primary care. As ivabradine is already licensed for management of CHF and recommended by NICE and ESC guidelines, it was felt that it would be unethical to conduct a randomised control trial in which patients could be randomised to placebo. A nested control of similarly matched individuals would have marked limitations, as by their very nature patients in whom ivabradine is indicated constitute a subset of patients with CHF and have specific characteristics (driven by a resting heart rate in sinus rhythm ≥ 75 bpm).Whilst the open label observational design of Live: Life, with the absence of a placebo group, has potential limitations and may lead to an overestimation of treatment effects, it will in contrast also permit evaluation of treatment effects in a population that is more reflective of routine clinical practice than that seen in landmark outcome studies. Controlled studies often restrict the inclusion of frailer and older patients with multiple co-morbidities due to strict inclusion and exclusion criteria. In Live: Life, for example, there are thus no significant inclusion/exclusion criteria other than actually receiving ivabradine within two weeks of recruitment.
Consent will also be gained to contact general practitioners one year after the patient completes the six month study to determine if the patient is still alive, assess number of hospitalisations, current medications and the reasons behind medication changes. This should provide data on longer-term tolerability of drug therapy, and provide insight as to why adjustments have been made.
QoL will be assessed utilizing well-validated tools; both generic and disease specific to allow a clear understanding of all influences on a patient's health at the time they complete the questionnaire. The primary end point will involve assessing the effect on ivabradine therapy on change in QoL score between baseline and final visits. The study will use the Minnesota Living with Heart Failure Questionnaire (MLHFQ) as a disease specific measure of heart failure. The MLHFQ is a user-friendly QoL measure consisting of 21 items focusing on patient perception of the effect of their heart failure on their physical, psychological, emotional and socio-economic functioning. The MLHFQ has been shown to be very sensitive to change and has a rich track record in heart failure clinical trials.[12],[39] Based on two previous studies with ivabradine and MLHFQ we have calculated a sample size of 500 patients to allow for a significant change in QoL score to be seen.[40],[41] The main analysis will focus on the change in QoL score between the baseline and final visit. Figure 1 provides an overview of the study and the various QoL assessments that are being utilized at different time points.
Figure 1. Proposed visit schedule for “Live: Life” participants.
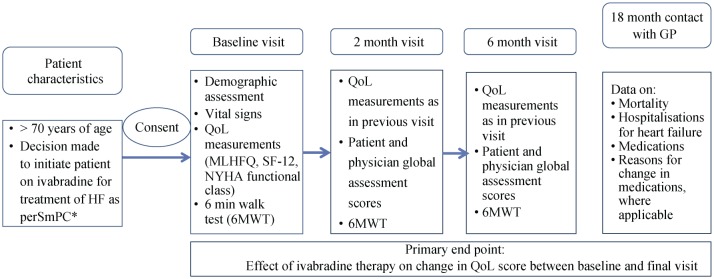
*Ivabradine is indicated: Chronic heart failure NYHA Class II to IV with systolic dysfunction; In sinus rhythm with resting HR ≥ 75 bpm; In combination with standard therapy including beta blockers or when beta blockers are contraindicated or not tolerated. QoL: quality of life; MLHFQ: Minnesota Living with Heart Failure Questionnaire; NYHA: New York Heart Association; 6MWT: 6 minute walk test.
SF-12 provides a more generic assessment, and has demonstrated a strong correlation with the MLHFQ physical and total scores.[42] Additionally, global assessment scores (by both patients and investigator) will be evaluated. If there is a perceived change, then the direction of change and estimate the magnitude of that change will be defined as previously described. Global assessment scores are more likely than NYHA classification to detect a meaningful change in clinical status of the patient, but it is accepted that they can be influenced by knowledge about the perceived benefits or side effects of medications. The incorporation of a 6 minute walk test (6MWT) fits with current guidelines for CHF management,[19],[43] and the recommendation within clinical practice to determine functional capacity. It is a simple and low-cost method for estimating exercise capacity with applicability to a wide population of heart failure patients, and only requires a pre-measured distance over a flat surface and a timing device. Various studies have shown the 6MWT results to correlate with mortality and morbidity,[44],[45] and in the older population; change in 6MWT results appears to correlate to change in self-perceived symptoms.
6. Conclusions
The last three decades have seen significant advances in the management of CHF, which has translated into better outcomes for patients. The data behind these advances is generally derived from studies in younger subjects. Yet with the changing demographics of our population, patients with CHF are often older with complex co-morbidities. As such patients who are the largest users of health and social service resources are those for whom we have less evidence on which to base our treatment. Clinical trials have generally focused on important outcomes such as avoiding death or hospitalisation – these outcomes are crucial to demonstrate benefit without evidence of harm. Improved longevity may be less relevant as patients get a lot older; QoL and maintained independence become the priorities. The Live: Life Study is unique for several reasons. It is a collaboration between physicians in cardiology, medicine for the elderly and general practice that will recruit a cohort of patients who are much more reflective of day-to-day clinical practice. Although there are inevitable limitations that are associated with an observational study, it is anticipated it will yield important data which otherwise would be difficult to obtain, with the focus being on symptoms, tolerability of drugs, QoL and function. This trial shifts the focus to patients' needs and for older patients even small changes in these parameters can have a dramatic impact on day to day living.
Conflicts of interest
Zachariah D, Taylor J, Rowell N declare that there is no conflict of interest. Spooner C is an employee of Servier Laboratories Ltd (UK). Kalra PR reports research grants, consultancy fees/honoraria from Servier Laboratories Ltd (UK).
Acknowledgments
Writing of the manuscript and critical review for important intellectual content was carried out by all authors. The Live Life study is funded by Servier Laboratories Ltd (UK).
References
- 1.National Heart Failure Audit UK electronic version accessible from http://www.ucl.ac.uk/nicor/audits/heartfailure/additionalfiles/pdfs/annualreports/NHFA13medium.pdf.
- 2.The SOLVD Investigators Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 3.Gullestad L, Wikstrand J, Deedwania P, et al. What resting heart rate should one aim for when treating patients with heart failure with a beta-blocker? Experiences from the metoprolol controlled release/extended release randomized intervention trial in chronic heart failure (MERIT-HF) J Am Coll Cardiol. 2005;45:252–259. doi: 10.1016/j.jacc.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Cooney MT, Vartiainen E, Laatikainen T, et al. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am Heart J. 2010;159:612–619. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Fox KA, Dabbous OH, Goldberg RJ, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE) BMJ. 2006;333:1079–1080. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox K, Ford I, Steg PG, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): A subgroup analysis of a randomised controlled trial. Lancet. 2008;372:817–821. doi: 10.1016/S0140-6736(08)61171-X. [DOI] [PubMed] [Google Scholar]
- 7.Castagno D, Skali H, Takeuchi M, et al. Association of heart rate and outcomes in a broad spectrum of patients with chronic heart failure: results from the CHARM Program. JAMA. 2012;59:1785–1795. doi: 10.1016/j.jacc.2011.12.044. [DOI] [PubMed] [Google Scholar]
- 8.Habal MV, Liu PP, Austin PC, et al. Association of heart rate at hospital discharge with mortality and hospitalizations in patients with heart failure. Circ Heart Fai. 2013 Dec 2; doi: 10.1161/CIRCHEARTFAILURE.113.000429. [DOI] [PubMed] [Google Scholar]
- 9.Menown IB, Davies S, Gupta S, et al. Resting heart rate and outcomes in patients with cardiovascular disease: where do we currently stand? CardiovascTher. 2013;31:215–223. doi: 10.1111/j.1755-5922.2012.00321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang CC, Gupta S, Kalra P, et al. Elevated heart rate and cardiovascular outcomes in patients with coronary artery disease: Clinical evidence and pathophysiological mechanisms. Atherosclerosis. 2010;212:1–8. doi: 10.1016/j.atherosclerosis.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 11.Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 12.Packer M, Bristow MR, Cohn JN. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 13.The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 14.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL randomised intervention trial in congestive heart failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 15.Flather MD, Shibata MC, Coats AJ, et al. Randomised trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26:215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 16.Flannery G, Gehrig-Mills R, Billah B, et al. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta-blockers. Am J Cardiol. 2008;101:865–869. doi: 10.1016/j.amjcard.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Cullington D, Goode KM, Clark AL, et al. Heart rate achieved or beta-blocker dose in patients with chronic heart failure: which is the better target? Eur J Heart Fail. 2012;4:737–747. doi: 10.1093/eurjhf/hfs060. [DOI] [PubMed] [Google Scholar]
- 18.Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 19.European Society of Cardiology (ESC) guidelines for the diagnosis and treatment of acute and chronic heart failure2012: The task force for the diagnosis and treatment of acute and chronic heart failure2012 of the European Society of Cardiology. ESC Committee for practice guidelines. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 20.Van Gelder IC, Groenveld HF, Crijns HJ, et al. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337. [DOI] [PubMed] [Google Scholar]
- 21.Masoudi FA, Havranek EP, Wolfe P, et al. Most hospitalized older persons do not meet the enrolment criteria for clinical trials in heart failure. Am Heart J. 2003;146:250–257. doi: 10.1016/S0002-8703(03)00189-3. [DOI] [PubMed] [Google Scholar]
- 22.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]) Am J Cardiol. 1996;78:902–907. doi: 10.1016/s0002-9149(96)00465-1. [DOI] [PubMed] [Google Scholar]
- 23.Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 24.Cohen-Solal A, McMurray JJ, Swedberg K, et al. Benefits and safety of candesartan treatment in heart failure are independent of age: insights from the candesartan in heart failure-assessment of reduction in mortality and morbidity programme. Eur Heart J. 2008;29:3022–3028. doi: 10.1093/eurheartj/ehn476. [DOI] [PubMed] [Google Scholar]
- 25.Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 26.Tavazzi L, Swedberg K, Komajda M, et al. Efficacy and safety of ivabradine in chronic heart failure across the age spectrum: insights from the SHIFT study. Eur J Heart Fail. doi: 10.1093/eurjhf/hft102. Published Online First: 2013 Jun 26. [DOI] [PubMed] [Google Scholar]
- 27.Abete P, Testa G, Della-Morte D, et al. Treatment for chronic heart failure in the elderly: current practice and problems. Heart Fail Rev. 2013;18:529–551. doi: 10.1007/s10741-012-9363-6. [DOI] [PubMed] [Google Scholar]
- 28.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 29.Cené CW, Loehr L, Lin FC, et al. Social isolation, vital exhaustion, and incident heart failure: findings from the Atherosclerosis Risk in Communities Study. Eur J Heart Fail. 2012;14:748–753. doi: 10.1093/eurjhf/hfs064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim ES, Smith J, Kubzansky LD. Prospective study of the association between dispositional optimism and incident heart failure. Circ Heart Fail. 2014;7:394–400. doi: 10.1161/CIRCHEARTFAILURE.113.000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bentley B, De Jong MJ, Moser DK, et al. Factors related to nonadherence to low sodium diet recommendations in heart failure patients. Eur J Cardiovasc Nurs. 2005;4:331–336. doi: 10.1016/j.ejcnurse.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Le Corvoisier P, Bastuji-Garin S, Renaud B, et al. Functional status and co-morbidities are associated with in-hospital mortality among older patients with acute decompensated heart failure: a multicentre prospective cohort study. Age Ageing. 2015;44:225–231. doi: 10.1093/ageing/afu144. [DOI] [PubMed] [Google Scholar]
- 33.Lupón J, Gastelurrutia P, de Antonio M, et al. Quality of life monitoring in ambulatory heart failure patients: temporal changes and prognostic value. Eur J Heart Fail. 2013;15:103–109. doi: 10.1093/eurjhf/hfs133. [DOI] [PubMed] [Google Scholar]
- 34.Stevenson LW, Hellkamp AS, Leier CV, et al. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. 2008;52:1702–1708. doi: 10.1016/j.jacc.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang SW, Davidson PM, Newton PJ, et al. What is the methodological and reporting quality of health related quality of life in chronic heart failure clinical trials? Int J Cardiol. 2013;164:133–140. doi: 10.1016/j.ijcard.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 36.Efficace F1, Bottomley A, Osoba D, et al. Beyond the development of health-related quality-of-life (HRQOL) measures: a checklist for evaluating HRQOL outcomes in cancer clinical trials—does HRQOL evaluation in prostate cancer research inform clinical decision making? J Clin Oncol. 2003;21:3502–3511. doi: 10.1200/JCO.2003.12.121. [DOI] [PubMed] [Google Scholar]
- 37.Kalra PR, Morley C, Barnes S, et al. Discontinuation of beta-blockers in cardiovascular disease: UK primary care cohort study. Int J Cardiol. 2013;167:2695–2699. doi: 10.1016/j.ijcard.2012.06.116. [DOI] [PubMed] [Google Scholar]
- 38.Ekman I, Chassany O, Komajda M, et al. Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study. Eur Heart J. 2011;32:2395–2404. doi: 10.1093/eurheartj/ehr343. [DOI] [PubMed] [Google Scholar]
- 39.Majani G, Giardini A, Opasich C, et al. Effect of valsartan on quality of life when added to usual therapy for heart failure: results from the valsartan heart failure trial. J Card Fail. 2005;11:253–259. doi: 10.1016/j.cardfail.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 40.Mansour S, Youssef A, Rayan M, et al. Efficacy of ivabradine in idiopathic dilated cardiomyopathy patients with chronic heart failure. The Egyptian Heart Journal. 2011;63:79–85. [Google Scholar]
- 41.Sarullo FM, Fazio G, Puccio D, et al. Impact of “off-label” use of ivabradine on exercise capacity, gas exchange, functional class, quality of life, and neurohormonal modulation in patients with ischemic chronic heart failure. J Cardiovasc Pharmacol Ther. 2010;15:349–355. doi: 10.1177/1074248410370326. [DOI] [PubMed] [Google Scholar]
- 42.Ni H, Toy W, Burgess D, et al. Comparative responsiveness of short-form 12 and minnesota living with heart failure questionnaire in patients with heart failure. J Card Fail. 2000;6:83–91. doi: 10.1054/jcaf.2000.7869. [DOI] [PubMed] [Google Scholar]
- 43.NICE (National institute of clinical excellence) chronic heart failure guidelines 2010. https://www.nice.org.uk/guidance/cg108 (accessed March 24, 2015)
- 44.Ingle L, Shelton RJ, Rigby AS, et al. The reproducibility and sensitivity of the 6-min walk test in elderly patients with chronic heart failure. Eur Heart J. 2005;26:1742–1751. doi: 10.1093/eurheartj/ehi259. [DOI] [PubMed] [Google Scholar]
- 45.Shah MR, Hasselblad V, Gheorghiade M, et al. Prognostic usefulness of the six-minute walk in patients with advanced congestive heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2001;88:987–993. doi: 10.1016/s0002-9149(01)01975-0. [DOI] [PubMed] [Google Scholar]
- 46.Rogers WJ, Johnstone DE, Yusuf S, et al. Quality of life among 5,025 patients with left ventricular dysfunction randomized between placebo and enalapril: the studies of left ventricular dysfunction. J Am Coll Cardiol. 1994;23:393–400. doi: 10.1016/0735-1097(94)90426-x. [DOI] [PubMed] [Google Scholar]
- 47.Cohn JN, Fowler MB, Bristow MR, et al. Safety and efficacy of carvedilol in severe heart failure. J Card Fail. 1997;3:173–179. doi: 10.1016/s1071-9164(97)90013-0. [DOI] [PubMed] [Google Scholar]
- 48.Sanderson JE, Chan SKW, Yip G, et al. Beta-blockade in heart failure: a comparison of carvedilol with metoprolol. J Am Coll Cardiol. 1999;34:1522–1528. doi: 10.1016/s0735-1097(99)00367-8. [DOI] [PubMed] [Google Scholar]
- 49.Cowley AJ, Wiens BL, Segal R, et al. Randomised comparison of losartan vs. captopril on quality of life in elderly patients with symptomatic heart failure: the losartan heart failure ELITE quality of life substudy. Qual Life Res. 2000;9:377–384. doi: 10.1023/a:1008948930206. [DOI] [PubMed] [Google Scholar]
- 50.Fung JW, Chan SK, Yeung LY, et al. Is beta-blockade useful in heart failure patients with atrial fibrillation? An analysis of data from two previously completed prospective trials. Eur J Heart Fail. 2002;4:489–494. doi: 10.1016/s1388-9842(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 51.Lader E, Egan D, Hunsberger S, et al. The effect of digoxin on the quality of life in patients with heart failure. J Card Fail. 2003;9:4–12. doi: 10.1054/jcaf.2003.7. [DOI] [PubMed] [Google Scholar]
- 52.Majani G, Giardini A, Opasich C, et al. Effect of valsartan on quality of life when added to usual therapy for heart failure: results from the valsartan heart failure trial. J Card Fail. 2005;11:253–259. doi: 10.1016/j.cardfail.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Veazie PJ, Noyes K, Li Q, et al. Cardiac resynchronization and quality of life in patients with minimally symptomatic heart failure. J Am Coll Cardiol. 2012;60:1940–1944. doi: 10.1016/j.jacc.2012.06.054. [DOI] [PubMed] [Google Scholar]
- 54.Lazzarini Valentina, Mentz Robert J., Fiuzat Mona, et al. Heart failure in elderly patients- distinctive features and unresolved issues. Eur J Heart Fail. 2013;15:717–723. doi: 10.1093/eurjhf/hft028. [DOI] [PMC free article] [PubMed] [Google Scholar]


