Abstract
Peptide nucleic acid scaffolds represent a promising tool to interrogate the multivalent effects of ligand binding to a membrane receptor. Dopamine D2 receptors (D2R) are a class of G-protein coupled receptors (GPCRs), and the formation of higher-ordered structures of these receptors has been associated with the progression of several neurological diseases. In this Letter, we describe the synthesis of a library of ligand-modified PNAs bearing a known D2R agonist, (±)-PPHT. The D2R activity for each construct was assessed, and the multivalent effects were evaluated.
Keywords: Peptide nucleic acid, multivalent display, dopamine D2 receptor, self-assembly
Dopamine receptors are a class of G protein-coupled receptors (GPCRs) known to modulate cognitive function associated with several disease states and have been identified as drug targets for Parkinson’s disease,1 schizophrenia,2,3 drug abuse,4 and obesity.5−7 Recent studies have shown that dopamine D2 receptors (D2R) are expressed as dimers in cell lines and brain tissue;8 and higher order oligomeric structures have been observed in mammalian cells.9,10 Moreover, evidence suggests that the dimerization and/or oligomerization of D2Rs may play an important role in the pathophysiology of neurological diseases.3,4,11−15 Thus, the development of new pharmacological probes is crucial to understanding the mechanism and regulation of D2R dimerization/oligomerization and its role in disease pathophysiology.
Bivalent ligands have emerged as a valuable tool to interrogate proposed higher-ordered GPCRs.16−19 This strategy, pioneered by Portoghese,20,21 typically consists of two discrete pharmacophores covalently linked by a spacer of an appropriate length and can lead to higher affinity, potency, and/or selectivity. While many bivalent ligands have been developed to investigate D2R dimerization/oligomerization, as well as to improve the affinity and potency of known D2R pharmacophores,22−26 multivalent (>2 ligands) species targeting dopamine receptors remain unexplored. Multivalent ligand systems allow for multiple simultaneous interactions with binding sites or receptors and can therefore dramatically enhance binding affinity, avidity, and/or specificity.27,28 Until recently, multivalent ligand systems for the interrogation of biological systems were constructed on flexible scaffolds, polymers, dendrimers, and nanoparticles.27,29,30 A major drawback of these constructs has been the poorly defined spatial arrangement and/or ligand density, which can complicate data interpretation. To overcome this limitation, self-organized scaffolds, using DNA or PNA templates, have been developed by us31−33 and others27,34−39 to assemble highly defined supramolecular structures in a rapid and reproducible manner. This method offers significant advantages over previous constructs, including the ability to synthesize monodisperse materials, control the ligand valency, and precisely position functional groups.27
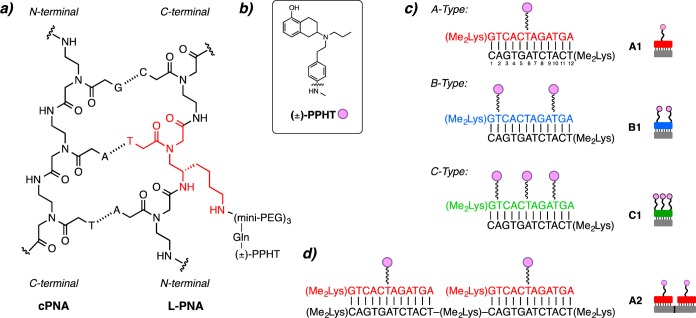
In our quest to interrogate the multivalent effects of peptide nucleic acid (PNA) scaffolds on GPCRs, we have developed a multivalent scaffold system based on peptide nucleic acid duplexes that display ligands in a well-defined, versatile manner.31,33 In general, these duplexes contain a sequence of peptide nucleic acid residues where select residues are replaced with a synthetically modified monomer derived from lysine, LKγ-PNA (Figure 1a).32,40 A ligand can be conjugated to the side-chain of the LKγ-PNA residue, and the resulting ligand-modified PNA (L-PNA) can be organized onto complementary nucleic acid oligomers to generate nanostructures that display ligands in a highly controlled, spatially defined manner.41,42 The programmable nature of the L-PNA:PNA scaffold allows for precise changes in ligand spacing and orientation without altering the scaffold backbone, the number of rotatable bonds, or the number of atoms. This key feature of PNA scaffolds is unique among bi- and multivalent ligand systems and can provide a more detailed depiction of the effect of multivalent displays on GPCR activity. Recently, we utilized this multivalent approach to interrogate the A2A adenosine receptor (A2AAR), a GPCR that is a drug target for neurodegenerative diseases, and observed a multivalent effect indicating the assembly of high-ordered receptor oligomers.31 In this Letter, we expand the utility of our multivalent scaffold system by generating a library of L-PNA:PNA complexes that display a known D2R agonist to probe the effects of multivalent ligand display on D2R activity.
Figure 1.
Ligand modified PNAs. (a) Chemical structure of L-PNA:PNA duplex containing the lKγ-PNA side chain highlighted in red. (b) Chemical structure of D2R agonist (±)-PPHT (represented as pink circles). (c) L-PNA oligomer bound to complementary PNA with one (±)-PPHT ligand (A-type, red), two (±)-PPHT ligands (B-type, blue), and three (±)-PPHT ligands (C-type, green) per PNA. (d) Each L-PNA sequence is identified by its constituent parts; for example, an A2 complex contains 2 A-type L-PNA units annealed along a 24-residue cPNA.
A library of ligand-modified peptide nucleic acids bearing a known D2R agonist, (±)-2-(N-phenethyl-N-propyl)amino-5-hydroxytetralin, (±)-PPHT22,43−45 (Figure 1b), was generated by systematic insertion of synthetic LKγ monomers into a 12-residue PNA oligomer (Figure 1a). To attach the ligand, the lysine moiety of the incorporated LKγ monomer was extended from the main PNA backbone using three mini-PEG (8-amino-3,6-dioxaoctanoic acid) linkers. A glutamic acid modified (±)-PPHT was then conjugated to the mini-PEG N-terminus to generate the desired L-PNA. The ligand valency of L-PNAs was varied from one ligand per L-PNA (A-type), to two (B-type), and three (C-type) ligands per L-PNA by incorporating one, two, or three LKγ-PNA monomers, respectively (Figure 1c). In the A-type L-PNA constructs, the ligand was attached to the central residue, while in the B-type the ligands were attached at residues 2 and 10. The C-type constructs contained 3 ligands that were attached at residues 2, 6, and 10 (Figure 1c). The L-PNAs were then annealed to complementary PNA oligomers (cPNA) in accordance with traditional Watson–Crick base pairing41,46,47 to provide a library of multivalent scaffolds with defined valency, ligand spacing, and orientation (Figure 1a). In an earlier report, we demonstrated that L-PNA:PNA duplexes are preferred to L-PNA:DNA when targeting membrane proteins such as GPCRs.31 This preference is likely due to the minimization of the charge repulsion forces that exist between the anionic DNA backbone and the cell surface in the case of L-PNA:DNA. To identify the library constructs, we refer to each L-PNA sequence according to the constituent parts; for example, a single A-type L-PNA annealed to its 12-residue cPNA is referred to as A1 (Figure 1c). Similarly, an A2 complex contains two A-type L-PNA units annealed along a 24-residue cPNA (Figure 1d). In total, 15 unique L-PNA:PNA complexes were generated systematically and span a valency of 1–15 ligands (Figure 2). For longer cPNAs, an N,N-dimethyl lysine was incorporated after each 12-residue sequence to maintain aqueous solubility at longer PNA lengths.
Figure 2.
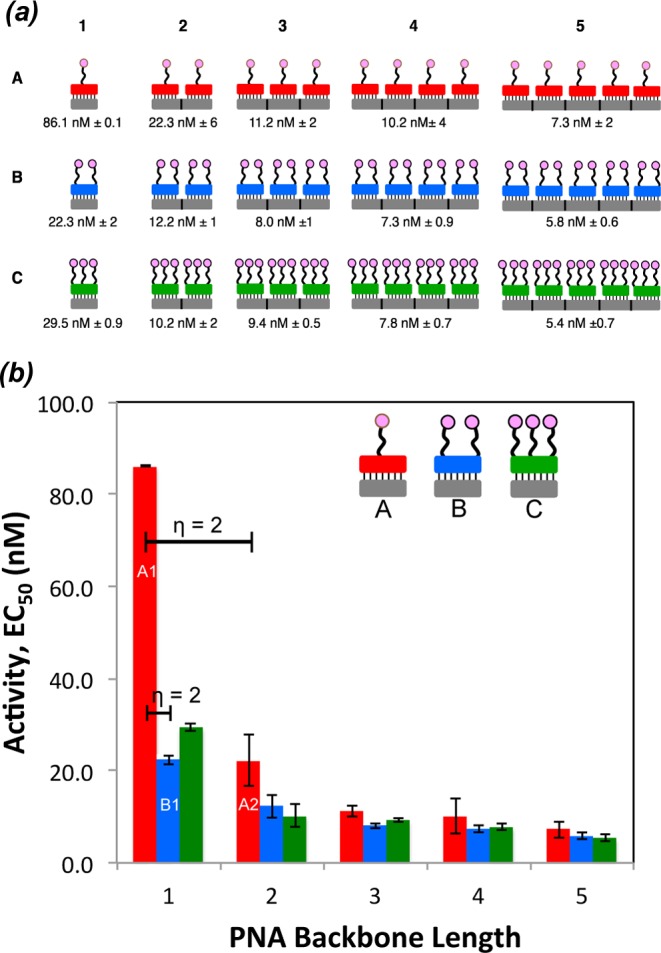
L-PNA:PNA multivalent library and landscape. (a) L-PNA:PNA multivalent library and the associated EC50 values for D2R activity. (b) Multivalent landscape highlighting the relationships between the A (red), B (blue), and C (green) type L-PNA constructs when annealed to various lengths of DNA. Key η values are included and indicate an increase in the individual ligand binding affinity.
Each member of the L-PNA:PNA library was tested for D2R activity using a whole cell β-arrestin recruitment assay,48,49 and the data are summarized in Figure 2a,b. Overall, attaching linkers to (±)-PPHT decreases D2R activation by about one order of magnitude relative to the free ligand, which is consistent with previous reports.22 Importantly, the data demonstrate that an increase in ligand valency is associated with improved EC50 values. Of particular interest was the dramatic change in the EC50 values when the valency was increased from one to two ligands, specifically in going from A1 to A2 and A1 to B1. These data were further analyzed using η values, a term that we recently introduced,31 to evaluate the change in D2R activity between L-PNA:PNA complexes of the same type when the change in ligand valency is normalized (i.e., comparing sequential A-type L-PNA:PNA complexes). For the purpose of this Letter, an η value of approximately one indicates that improvement in D2R activity is proportional to the increase in ligand valency. Alternatively, η values greater than two suggest that the incorporation of additional ligands results in an increase in D2R activation that cannot be attributed solely to increased ligand content. Using the η parameter to analyze D2R activity, we obtained an η value of two in the transition from one to two ligands for both the A1 to A2 and A1 to B1 transitions (Figure 2b). This indicates that increasing the valency from one to two ligands significantly enhances the D2R activity. Interestingly, the ligand spacing in both the A2 and B1 constructs did not impact D2R activity. In contrast, the addition of a third ligand to the 12-residue L-PNA C1 had a slightly detrimental effect on D2R activation. This is likely due to steric crowding, which does not allow for favorable ligand–receptor interactions. The η values for the remaining constructs are close to one, indicating that an increase in ligand valency beyond two ligands marginally improves D2R activity. We also examined the nonspecific binding effects using an acetylated A type PNA that did not contain the (±)-PPHT ligand. We did not observe any nonspecific binding for this construct. Additionally, shorter linkers were considerably less active compared to the three mini-PEG linker. Taken together, these data demonstrate that the most significant activation of D2R is observed when the ligand valency is increased from one to two and that additional ligands only slightly improve activity.
The highly programmable and versatile nature of the PNA scaffold lends itself to the rapid assembly of multivalent tools in a predictable manner. The ability to rigorously and precisely control the ligand content, density, and spatial orientation of the PNA scaffold represents a clear advantage over traditional bi- and multivalent approaches to investigate GPCRs. In this work, a multivalent scaffold system based on L-PNA:PNA duplexes was used to explore the effects of multivalency on D2R activity. A library of 15 unique L-PNA:PNA complexes bearing a known D2R agonist, (±)-PPHT, was prepared, and the D2R activity was evaluated. A significant increase in D2R activity was observed when the valency was increased from one to two ligands in both the A1 to A2 and A1 to B1 constructs. Using η values to further examine the A1 to A2 or B1 transitions, we conclude that the substantial increase in D2R activity is due to a multivalent effect that cannot be attributed solely to the change in ligand valency. The incorporation of additional ligands in the remaining constructs improved activity proportionally to the increase in ligands. These data suggest that the formation of discrete receptor dimers may be responsible for the enhanced D2R activity when comparing constructs with one versus two ligands, which would agree with previous observations of dimeric receptors in cell lines and brain tissue.8,9 It is important to note that the presence of the PNA construct could drive dimer formation, and the receptor does not associate in the absence of ligand. It is also possible that ligand rebinding effects could account for the increases in activity with additional ligands, although the high affinity of the ligands for the receptor likely minimize significant contribution from rebinding.51 With mounting evidence suggesting the importance of oligomeric GPCRs in disease pathophysiology, the L-PNA scaffold represents an important pharmacological tool to probe the effects of multivalent ligand displays on GPCR activity. In future work, we intend to probe the multivalent profiles of other ligand–receptor systems.
Experimental Procedures
PNA Oligomer Synthesis
Commercial-grade reagents and solvents were used without further purification unless indicated. The resin (MBHA, 100–200 mesh, 1% divinylbenzene, 0.3 mmol g–1, Advanced Chemtech) was prepared by swelling in CH2Cl2 and downloading the resin with N,N-dimethyl lysine to 0.1 mmol g–1 capacity. Boc-protected aegPNA monomers were purchased from PolyOrg. PNA oligomer synthesis was carried out on a 5 μmol scale on an Applied BioSystems 433A Automated Peptide Synthesizer. The resin was swelled with CH2Cl2 for 105 min before synthesis. The LKγ-PNA monomer was synthesized according to published procedures.32,40 Activated LKγ-PNA monomer was allowed 90 min to couple. A further treatment of trifluoroacetic acid deprotection solution was also used to remove the N-Boc protecting group from LKγ-PNA residues. The lysine side chains of LKγ-PNA monomers (Fmoc) were orthogonally deprotected with 20% piperidine in DMF. When multiple LKγ-PNA residues were present in the PNA oligomer (PNA-B and PNA-C), the primary amines on the side chains were deprotected and coupled to mini-PEG residues in tandem, followed by coupling to (±)-PPHT. Purification of PNA oligomers was carried out using an XBridge Prep BEH 130 C18 5 μm (10 mm × 250 mm) column on an Agilent 1100 HPLC. In all cases, 0.1% aqueous trifluoroacetic acid and acetonitrile were used as solvents. Additional information can be found in the Supporting Information.
General Annealing Conditions for Formation of L-PNA:PNA Duplexes
In RNA/DNAase free microfuge tubes, L-PNA, cPNA, and PBS buffer were combined at room temperature. Equivalents of PNA were calculated based on the number or repeating 12-residue sequences in the PNA. For example, to generate L-PNA:PNA multi5, a 5:1 molar ratio of L-PNA:cPNA was used. The solution was heated to 90 °C, held for 5 min, then slowly allowed to cool down to 25 °C over a period of 3 h. Additional information can be found in the Supporting Information.
β-Arrestin Recruitment Assay
Agonist-mediated recruitment of β-arrestin-2 was determined using the DiscoveRx PathHunter complementation assay (DiscoveRx Inc., Fremont, CA), as previously described.49,50 Briefly, CHO-K1 cells stably expressing the D2R were seeded in cell plating (CP) media (DiscoveRx) at a density of 2625 cells/well in 384-well black, clear-bottom plates. Following 24 h of incubation, the cells were treated with multiple concentrations of compound in PBS buffer containing 0.2 mM sodium metabisulfite and incubated at 37 °C for 90 min. DiscoveRx reagent was then added to cells according to the manufacturer’s protocol followed by a 60 min incubation in the dark at room temperature. Luminescence was measured on a Hamamatsu FDSS μ-cell reader (Hamamatsu, Bridgewater, NJ), and data was collected using the FDSS software.
Acknowledgments
We gratefully acknowledge Dr. John Lloyd and the mass spectrometry core facility of NIDDK for analysis of all PNA samples.
Glossary
Abbreviations
- GPCR
G protein-coupled receptors
- D2R
D2 receptor
- PNA
peptide nucleic acid
- L-PNA
ligand modified PNA
- A2AAR
A2A adenosine receptor
- PPHT
2-(N-phenethyl-N-propyl)amino-5-hydroxytetralin
- cPNA
complementary PNA
Supporting Information Available
Experimental procedures and characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
A.V.D. participated in research design and execution (chemistry) and contributed to writing the manuscript; J.L.C. participated in research design and execution (biology); K.M.G.R. participated in research execution (chemistry) and contributed to writing the manuscript; D.R.S. participated in research design (biology), acquired funding, and contributed to the writing of this manuscript; D.H.A. participated in research design (chemistry), acquired funding, and contributed to the writing of the manuscript.
This research was supported by the Intramural Research Programs (IRPs) of NIDDK and NINDS at NIH.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Hisahara S.; Shimohama S. Dopamine Receptors and Parkinson’s Disease. Int. J. Med. Chem. 2011, 2011, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P.; Kapur S. Schizophrenia: More Dopamine, More D2 Receptors. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 7673–7675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.; Pei L.; Fletcher P. J.; Kapur S.; Seeman P.; Liu F. Schizophrenia, Aphetamine-Induced Sensitized State and Acute Amphetamine Exposure All Show a Common Alteration: Increased Dopamine D2 Receptor Dimeration. Mol. Brain 2010, 25, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M. L.; O’Dowd B. F.; George S. R. Dopamine D1-D2 Receptor Heteromer Regulates Signaling Cascades Involved in Addiction: Potential Relevance to Adolescent Drug Susceptibility. Dev. Neurosci. 2014, 36, 287–296. [DOI] [PubMed] [Google Scholar]
- Wang G.-J.; Volkow N. D.; Logan J.; Pappas N. R.; Wong C. T.; Zhu W.; Netusll N.; Fowler J. S. Brain Dopamine and Obesity. Lancet 2001, 357, 354–357. [DOI] [PubMed] [Google Scholar]
- Johnson P. M.; Kenny P. J. Dopamine D2 Receptors in Addiction-Like Reward Dysfunction and Compulsive Eating in Obese Rats. Nat. Neurosci. 2010, 13, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J. M.; Gainetdinov R. R. The Physiology, Signaling, and Pharmacology of Dopamine Receptors. Pharmacol. Rev. 2011, 63, 182–217. [DOI] [PubMed] [Google Scholar]
- Zawarynski P.; Tallerico T.; Seeman P.; Lee S. P.; O’Dowd B. F.; George S. R. Dopamine D2 Receptor Dimers in Human and Rat Brain. FEBS Lett. 1998, 441, 383–86. [DOI] [PubMed] [Google Scholar]
- Lee S. P.; O’Dowd B. F.; NG G. Y. K.; Varghese G.; Akil H.; Mansour A.; Nguyen T.; George S. R. Inhibition of Cell Surface Expression by Mutan Receptors Demonstrates that D2 Dopamine Receptors Exist as Oligomers in the Cell. Mol. Pharmacol. 2000, 58, 120–128. [DOI] [PubMed] [Google Scholar]
- Guo W.; Urizar E.; Kralikova M.; Mobarec J. C.; Shi L.; Filizola M.; Javitch J. A. Dopamine D2 Receptors Form Higher Order Oligomers at Physiological Expression Levels. EMBO J. 2008, 27, 2293–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M. L.; Hasbi A.; O’Dowd B. F.; George S. R. Heteromeric Dopamine Receptor Signaling Complexes: Emerging Neurobiology and Disease Relevance. Neuropsychopharmacology 2014, 39, 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R. Neurotransmitter Receptor Heteromers in Neurodegenerative Diseases and Neural Plasticity. J. Neural. Transm. 2009, 116, 983–987. [DOI] [PubMed] [Google Scholar]
- Fuxe K.; Marcellino D.; Rivera A.; Diaz-Cabiale Z.; Filip M.; Gago B.; Roberts D. C.; Langel U.; Genedani S.; Ferraro L.; de la Calle A.; Narvaez J.; Tanganelli S.; Woods A.; Agnati L. F. Receptor-Receptor Interactions Within Receptor Mosaics. Impact on Neuropsychopharmacology. Brain Res. Rev. 2008, 58, 415–452. [DOI] [PubMed] [Google Scholar]
- Kearn C. S.; Blake-Palmer K.; Daniel E.; Mackie K.; Glass M. Concurrent Stimulation of Cannabinoid CB1 and Dopamine D2 Receptors Enhances Heterodimer Formation: A Mechanism for Receptor Cross-Talk?. Mol. Pharmacol. 2005, 67, 1697–1704. [DOI] [PubMed] [Google Scholar]
- George S. R.; O’Dowd B. F.; Lee S. P. G-Protein-Coupled Receptor Oligomerization and Its Potential for Drug Discovery. Nat. Rev. Drug Discovery 2002, 1, 808–820. [DOI] [PubMed] [Google Scholar]
- Ferré S.; Navarro G.; Casadó V.; Cortés A.; Mallol J.; Canela E. I.; Lluís C.; Franco R. G Protein-Coupled Receptor Heteromers as New Targets for Drug Development. Prog. Mol. Biol. Transl. Sci. 2010, 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer W. S. J. Bivalent Ligands for G Protein-Coupled Receptors. Curr. Pharm. Des. 2004, 10, 2015–2020. [DOI] [PubMed] [Google Scholar]
- Berque-Bestel I.; Lezoualc’h F.; Jockers R.; Gainetdinov R. R. Bivalent Ligands as Specific Pharmacological Tools for G Protein-Coupled Receptors. Curr. Drug Discovery Technol. 2008, 5, 312–318. [DOI] [PubMed] [Google Scholar]
- Hiller C.; Kuhhorn J.; Gmeiner P.; Class A. G-Protein-Coupled Receptor (GPCR) Dimers and Bivalent Ligands. J. Med. Chem. 2013, 56, 6542–6559. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S.; Ronsisvalle G.; Larson D. L.; Yim C. B.; Sayre L. M.; Takemori A. E. Opiod Agonist and Antagonist Bivalent Ligands as Receptor Probes. Life Sci. 1982, 31, 1283–1286. [DOI] [PubMed] [Google Scholar]
- Erez M.; Takemori A. E.; Portoghese P. S. Narcotic Antagonistic Potency of Bivalent Ligands Which Contain B-Naltrexamine. Evidence for Bridging Between Proximal Recognition Sites. J. Med. Chem. 1982, 25, 847–849. [DOI] [PubMed] [Google Scholar]
- Soriano A.; Ventura R.; Molero A.; Hoen R.; Casadó V.; Cortés A.; Fanelli F.; Albericio F.; Lluís C.; Franco R.; Royo M. Adenosine A2A Receptor-Antagonist/Dopamine D2 Receptor-Agonist Bivalent Ligands as Pharmacological Tools to Detect A2A-D2 Receptor Heteromers. J. Med. Chem. 2009, 52, 5590–5602. [DOI] [PubMed] [Google Scholar]
- Kuhhorn J.; Hubner H.; Gmeiner P. Bivalent Dopamine D2 Receptor Ligands: Synthesis and Binding Properties. J. Med. Chem. 2011, 54, 4896–4903. [DOI] [PubMed] [Google Scholar]
- Gogoi S.; Biswas S.; Modi G.; Antonio T.; Reith M. E.; Dutta A. K. Novel Bivalent Ligands for D2/D3 Dopamine Receptors: Significant Cooperative Gain in D2 Affinity and Potency. ACS Med. Chem. Lett. 2012, 3, 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobb F. M.; Crosby I. T.; Yuriev E.; Lane J. R.; Capuano B. Homobivalent Ligands of the Atypical Antipsychotic Clozapine: Design, Synthesis, and Pharmacological Evaluation. J. Med. Chem. 2012, 55, 1622–1634. [DOI] [PubMed] [Google Scholar]
- Shonberg J.; Lane J. R.; Scammells P. J.; Capuano B. Synthesis, Functional and Binding Profile of (R)-Apomorphine Based Homobivalent Ligands Targeting the Dopamine D2 Receptor. Med.Chem.Comm 2013, 4, 1290–1296. [Google Scholar]
- Fasting C.; Schalley C. A.; Weber M.; Seitz O.; Hecht S.; Koksch B.; Dernedde J.; Graf C.; Knapp E. W.; Haag R. Multivalency as a Chemical Organization and Action Principle. Angew. Chem., Int. Ed.. 2012, 51, 10472–10498. [DOI] [PubMed] [Google Scholar]
- Mammen M.; Choi S.-K.; Whitesides G. M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem., Int. Ed. 1998, 37, 22754–22794. [DOI] [PubMed] [Google Scholar]
- Barnard A.; Smith D. K. Self-Assembled Multivalency: Dynamic Ligand Arrays for High-Affinity Binding. Angew. Chem., Int. Ed. 2012, 51, 6572–6581. [DOI] [PubMed] [Google Scholar]
- Martos V.; Castreño P.; Valero J.; de Mendoza J. Binding to Protein Surfaces by Supramolecular Multivalent Scaffolds. Curr. Opin. Chem. Biol. 2008, 12, 698–706. [DOI] [PubMed] [Google Scholar]
- Dix A. V.; Moss S. M.; Phan K.; Hoppe T.; Paoletta S.; Kozma E.; Gao Z.-G.; Durell S. R.; Jacobson K. A.; Appella D. H. Programmable Nanoscaffolds That Control Ligand Display to a G-Protein-Coupled Receptor in Membranes To Allow Dissection of Multivalent Effects. J. Am. Chem. Soc. 2014, 12296–12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund E. A.; Appella D. H. γ-Substituted Peptide Nucleic Acids Constructed From l-Lysine are a Versatile Scaffold for Multifunctional Display. Angew. Chem., Int. Ed. 2007, 46, 1414–1418. [DOI] [PubMed] [Google Scholar]
- Englund E. A.; Wang D.; Fujigaki H.; Sakai H.; Micklitsch C. M.; Ghirlando R.; Martin-Manso G.; Pendrak M. L.; Roberts D. D.; Durell S. R.; Appella D. H. Programmable Multivalent Display of Receptor Ligands Using Peptide Nucleic Acid Nanoscaffolds. Nat. Commun. 2012, 3, 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diezmann F.; Seitz O. DNA-Guided Display of Proteins and Protein Ligands for the Interrogation of Biology. Chem. Soc. Rev. 2011, 40, 5789–5801. [DOI] [PubMed] [Google Scholar]
- Abendroth F.; Bujotzek A.; Shan M.; Haag R.; Weber M.; Seitz O. DNA-Controlled Bivalent Presentation of Ligands for the Estrogen Receptor. Angew. Chem., Int. Ed. 2011, 50, 8592–8596. [DOI] [PubMed] [Google Scholar]
- Abendroth F.; Seitz O. Double-Clicking Peptides onto Phosphorothioate Oligonucleotides: Combining Two Proapoptotic Agents in One Molecule. Angew. Chem., Int. Ed. 2014, 53, 10504–10509. [DOI] [PubMed] [Google Scholar]
- Scheibe C.; Bujotzek A.; Dernedde J.; Weber M.; Seitz O. DNA-Programmed Spatial Screening of Carbohydrate–Lectin Interactions. Chem. Sci. 2011, 2, 770–775. [Google Scholar]
- Eberhard H.; Diezmann F.; Seitz O. DNA as a Molecular Ruler: Interrogation of a Tandem SH2 Domain with Self-Assembled, Bivalent DNA-Peptide Complexes. Angew. Chem., Int. Ed. 2011, 50, 4146–4150. [DOI] [PubMed] [Google Scholar]
- Winssinger N. Nucleic Acid-Programmed Assemblies: Translating Instruction into Function in Chemical Biology. Chimia 2013, 67, 340–348. [DOI] [PubMed] [Google Scholar]
- Englund E. A.; Appella D. H. Synthesis of γ-Substituted Peptide Nucleic Acids: A New Place to Attach Fluorophores without Affecting DNA Binding. Org. Lett. 2005, 7, 3465–3467. [DOI] [PubMed] [Google Scholar]
- Egholm M.; Buchardt O.; Christensen L.; Behrens C.; Freier S. M.; Driver D. A.; Berg R. H.; Kim S. K.; Norden B.; Nielsen P. E. PNA Hybridizes to Complementary Oligonucleotides Obeying the Watson-Crick Hydrogen-Bonding Rules. Nature 1993, 365, 566–568. [DOI] [PubMed] [Google Scholar]
- Wittung P.; Nielsen P. E.; Buchardt O.; Egholm M.; Norden B. DNA-Like Double Helix Formed By Peptide Nucleic Acid. Nature 1994, 368, 561–563. [DOI] [PubMed] [Google Scholar]
- Hacksell U.; Svensson U.; Nilsson J. L. G. N-Alkylated 2-Aminotetralins: Central Dopamine-Receptor Stimulating Activity. J. Med. Chem. 1979, 22, 1469–1475. [DOI] [PubMed] [Google Scholar]
- Merali Z.; Piggins H. Effects of Dopamine D1 and D2 Receptor Agonsits and Antagonists on Bombesin-Induced Behaviors. Eur. J. Pharmacol. 1990, 191, 281–293. [DOI] [PubMed] [Google Scholar]
- Bakthavachalam V.; Baindur N.; Madras B. K.; Neumeyer J. L. Fluorescent Probes for Dopamine Receptors: Synthesis and Characterization of Fluorescein and 7-Nitrobenz-2-oxa-1,3-diazol-4-yl Conjugates of D-1 and D-2 Receptor Ligands. J. Med. Chem. 1991, 34, 3235–3241. [DOI] [PubMed] [Google Scholar]
- Nielsen P. E.; Egholm M.; Berg R. H.; Buchardt O. Sequence-Selective Recognition of DNA by Strand Displacement with a Thymine-Substituted Polyamide. Science 1991, 254, 1497–1500. [DOI] [PubMed] [Google Scholar]
- Porcheddu A.; Giacomelli G. Peptide Nucleic Acids (PNAs), A Chemical Overview. Curr. Med. Chem. 2005, 12, 2561–2599. [DOI] [PubMed] [Google Scholar]
- van Der Lee M. M.; Bras M.; van Koppen C. J.; Zaman G. J. β-Arrestin Recruitment Assay for the Identification of Agonists of the Sphingosine 1-Phosphate Receptor EDG1. J. Biomol. Screening 2008, 13, 986–998. [DOI] [PubMed] [Google Scholar]
- Free R. B.; Chun L. S.; Moritz A. E.; Miller B. N.; Doyle T. B.; Conroy J. L.; Padron A.; Meade J. A.; Xiao J.; Hu X.; Dulcey A. E.; Han Y.; Duan L.; Titus S.; Bryant-Genevier M.; Barnaeva E.; Ferrer M.; Javitch J. A.; Beuming T.; Shi L.; Southall N. T.; Marugan J. J.; Sibley D. R. Discovery and Characterization of a G protein-Biased Agonist That Inhibits β-Arrestin Recruitment to the D2 Dopamine Receptor. Mol. Pharmacol. 2014, 86, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J.; Roof R. A.; Furman C. A.; Conroy J. L.; Mello N. K.; Sibley D. R.; Skolnick P. Modification of Cocaine Self-Administration by Buspirone (Buspar®): Potential Involvement of D3 and D4 Dopamine Receptors. Int. J. Neuropsychopharmacol. 2013, 16, 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M.; Bujotzek A.; Haag R. Quantifying the Rebinding Effect in Multivalent Chemical Ligand-Receptor Systems. J. Chem. Phys. 2012, 137, 054111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.