Abstract
A series of fluorine-substituted monomeric and dimeric cRGD peptide derivatives, such as cRGD-ADIBOT-F (ADIBOT = azadibenzocyclooctatriazole), di-cRGD-ADIBOT-F, cRGD-PEG5-ADIBOT-F, and di-cRGD-PEG5-ADIBOT-F, were prepared by strain-promoted alkyne azide cycloaddition (SPAAC) reaction of the corresponding aza-dibenzocyclooctyne (ADIBO) substituted peptides with a fluorinated azide 3. Among these cRGD derivatives, di-cRGD-PEG5-ADIBOT-F had the highest binding affinity in a competitive binding assay compared to other derivatives and even the original cRGDyk. On the basis of the in vitro study results, di-cRGD-PEG5-ADIBOT-18F was prepared from a SPAAC reaction with 18F-labeled azide and subsequent chemo-orthogonal scavenger-assisted separation without high performance liquid chromatography (HPLC) purification in 92% decay-corrected radiochemical yield (dcRCY) with high specific activity for further in vivo positron emission tomography (PET) imaging study. In vivo PET imaging study and biodistribution data showed that this radiotracer allowed successful visualization of tumors with good tumor-to-background contrast and significantly higher tumor uptake compared to other major organs.
Keywords: F-18 labeling method, copper-free click chemistry, fluorine-18, radiopharmaceuticals, RGD peptide, PET molecular imaging, tumor angiogenesis
Positron emission tomography (PET) is a noninvasive molecular imaging modality and a powerful tool in oncology for examining fundamental biological processes and disease pathologies in living subjects.1,2 For these purposes, a range of bioactive molecules has been labeled with positron emitting radionuclides to be used as PET radiopharmaceuticals.3,4 Despite the short half-life of fluorine-18 (F-18, t1/2 = 109.8 min), it is the most popular positron-emitter for PET among various radioisotopes owing to its excellent chemical, physiological, and nuclear properties.3−7 The short half-life of F-18, however, requires rapid, simple and reliable F-18 labeling strategies to obtain new PET molecular imaging probes.8,9
Because angiogenesis associated with the integrin αvβ3 receptor is a key process in tumor growth and metastasis, PET molecular imaging probes for tumor angiogenesis have potential for early tumor diagnosis and monitoring of the therapeutic effects of various treatments in both preclinical and clinical studies.10−14 Over the past decade, a series of small peptides based on the Arg-Gly-Asp (RGD) sequence labeled with F-18 have been developed to visualize tumor angiogenesis using PET by targeting the integrin αvβ3 receptor in a range of tumor models.15−17 Moreover, diversely modified multimeric cyclic RGD derivatives have been developed successfully to increase renal excretion and binding affinity for this integrin.18,19 In particular, F-18 labeled PEGylated or glycosylated dimeric cyclic RGD tracers show good performance in the PET imaging of tumor angiogenesis.18,20−23 Generally, these F-18 labeled RGD-peptide based probes are prepared using a range of conjugation methods between F-18 labeled prosthetic groups and peptides.15,24 However, their complicated multistep radiosynthesis has limited their widespread use.24,25 In particular, because F-18 labeled multimeric RGD peptide tracers frequently show similar retention times to an excess of unlabeled peptide precursors in high performance liquid chromatography (HPLC) purification, their radiosynthesis requires a time-consuming purification process to avoid relatively low specific activity. Specific activity is one of the most important factors for determining PET imaging quality and the toxicity or side-effects of these radiotracers.3,4,26 In this regard, the development of a more reliable F-18 labeling protocol remains an interesting research topic for the preparation of F-18 labeled RGD peptide-based probes.
Recently, copper-free “click chemistry” based on strain-promoted alkyne azide cycloaddition (SPAAC) has been used in radiolabeling studies as a fast and bioorthogonal conjugation method between the radiolabeled prosthetic group and bioactive molecules, such as peptides, protein and nanoparticles.26−31 In particular, aza-dibenzocyclooctyne (ADIBO) derivatives can act as effective azide acceptors to produce corresponding azadibenzocyclooctatriazoles (ADIBOTs) using the SPAAC reaction.32−34 In a recent study, we reported that 18F-labeled peptides could be prepared almost quantitatively by the SPAAC conjugation reaction between ADIBO-tethered peptides and a 18F-labeled azide. Moreover, in that radiosynthetic method, 18F-labeled peptides were efficiently purified by solid phase extraction using an azide-substituted resin as an ADIBO precursor-scavenger without an HPLC purification step.26 This letter introduces the straightforward synthesis of fluorine (including F-18) substituted monomeric and dimeric RGD peptides based on the SPAAC conjugation reaction and reports the results of in vitro and in vivo evaluation of the PET imaging of integrin expression.
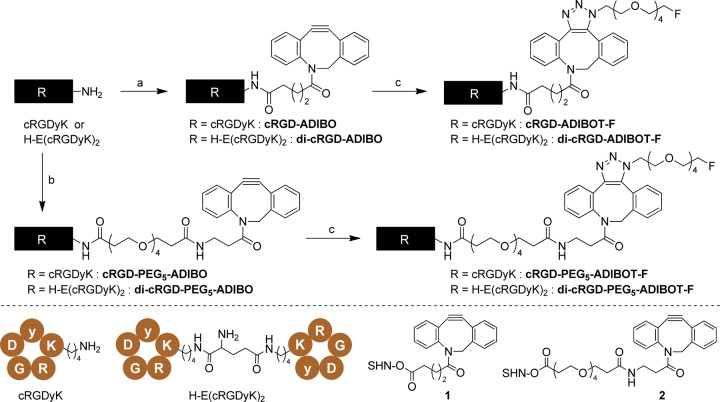
Scheme 1 presents the synthesis of nonradioactive fluorine-substituted monomeric and dimeric RGD peptide derivatives based on the SPAAC conjugation reaction. Treatment of monomeric cRGDyK and dimeric H-E[c(RGDyK)]2 with ADIBO N-succinimidyl ester compound 1 under basic conditions provided the corresponding ADIBO-substituted cRGD compounds, such as cRGD-ADIBO and di-cRGD-ADIBO, respectively. To introduce a polyethylene glycol (PEG) linker between the cRGD peptides and ADIBO moiety, an ADIBO-PEG5 N-succinimidyl ester compound 2 was used in the conjugation reaction of cRGDyK to cRGD-PEG5-ADIBO or H-E[c(RGDyK)]2 to di-cRGD-PEG5-ADIBO. The SPAAC reaction of four ADIBO-substituted cRGD peptide precursors with fluorohexaethylene glycolic azide (3) at 25 °C within 2 h yielded the corresponding fluorine substituted monomeric and dimeric RGD peptide derivatives, including cRGD-ADIBOT-F, di-cRGD-ADIBOT-F, cRGD-PEG5-ADIBOT-F, and di-cRGD-PEG5-ADIBOT-F. The derivatives were characterized by mass spectrometry.
Scheme 1. Preparation of Authentic Nonradioactive cRGD Peptide Derivatives; cRGD-ADIBOT-F, di-cRGD-ADIBOT-F, cRGD-PEG5-ADIBOT-F, and di-cRGD-PEG5-ADIBOT-F.
Reagents and Conditions: (a) 1, DIPEA, DMSO, 25 °C, 12 h; (b) 2, DIPEA, DMSO, 25 °C, 12 h; (c) fluorohexaethylene glycolic azide (3), DMSO, 25 °C, 2 h. NHS = N-succinimidyl.
Figure 1.
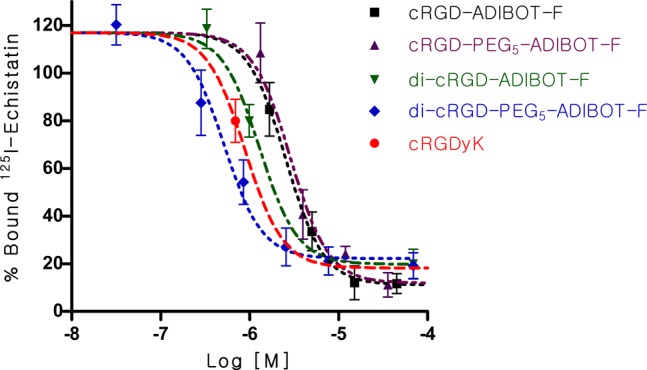
Competitive cell binding assay of cRGD-ADIBOT-F, di-cRGD-ADIBOT-F, cRGD-PEG5-ADIBOT-F, and di-cRGD-PEG5-ADIBOT-F in comparison with unmodified cRGDyK with 125I-echistatin using U87MG cells (n = 3).
The receptor-binding assays for these modified fluorinated cRGD compounds (cRGD-ADIBOT-F, di-cRGD-ADIBOT-F, cRGD-PEG5-ADIBOT-F, and di-cRGD-PEG5-ADIBOT-F) and the unmodified cRGDyk were determined by competitive displacement studies using 125I-echistatin and integrin αvβ3-expressing U87MG cells. In this in vitro assay, binding of 125I-echistatin to the U87MG cells was inhibited by all of the synthesized fluorinated cRGD peptides in a concentration-dependent manner. As expected, the dimeric cRGD peptides exhibited better binding affinities than the corresponding monomeric RGD peptides. In particular, the mini-PEG linker between the cRGDs and ADIBOT-F helped increase their binding affinities. The longer distance between the RGDs and bulk ADIBOT-F moiety by the PEG linker was expected to provide easier accessibility to integrin αvβ3. Consequently, di-cRGD-PEG5-ADIBOT-F, which has both dimeric cRGD and the PEG linker, exhibited the highest binding affinity compared to the other RGD derivatives, even the original cRGDyk. These in vitro results showed similar patterns to previous binding affinity studies by other research groups.16−20 On the basis of the in vitro study results, di-cRGD-PEG5-ADIBOT-F was selected as the compound of choice for radiosynthesis of di-cRGD-PEG5-ADIBOT-18F and a further in vivo PET imaging study.
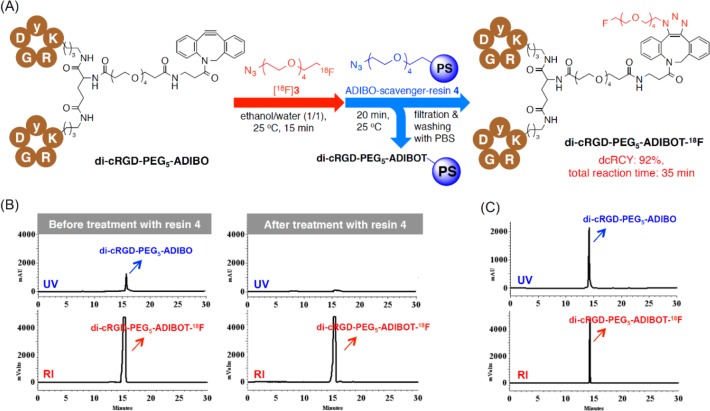
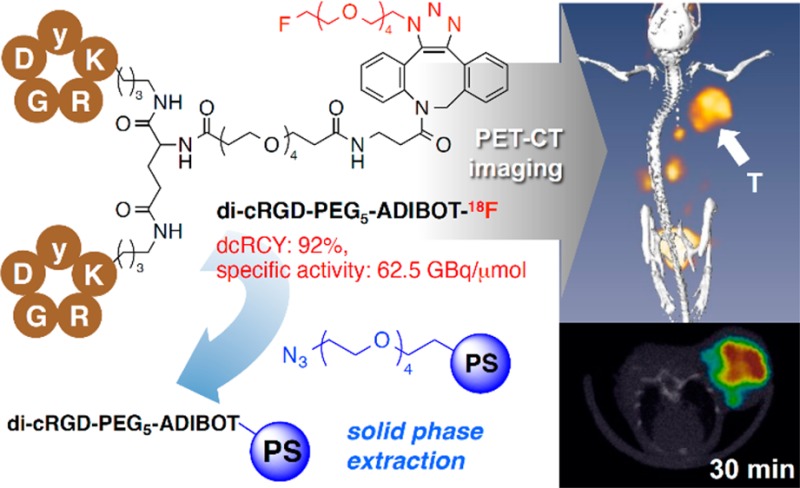
As depicted in Figure 2A, The SPAAC reaction of the di-cRGD-PEG5-ADIBO precursor (0.87 μmol) as an azide acceptor using 18F-labeled azide [18F]3 in an aqueous solution proceeded quantitatively within 15 min, affording the 18F-labeled cRGD dimer compound (di-cRGD-PEG5-ADIBOT-18F) with an excess of unreacted di-cRGD-PEG5-ADIBO precursor. This unreacted ADIBO precursor was removed by the subsequent treatment of this crude solution with an azide resin 4 as an ADIBO-scavenger for 20 min, as observed by HPLC analysis of the reaction mixture before and after treatment with resin 4 (Figure 2B). In particular, although the di-cRGD-PEG5-ADIBOT-18F tracer showed a similar retention time to its precursor on HPLC, it was successfully produced with a reasonably high specific activity (62.5 GBq/μmol) in 92% decay-corrected radiochemical yield (dcRCY) within a total reaction time of approximately 35 min without any HPLC purification; rather, resin 4 was merely filtered and washed (total radiosynthetic time of approximately 80 min including the radiosynthesis of 18F-labeled azide [18F]3 from 18F–). Considering the specific activity of the tracer, at least 0.85 μmol of the precursor could be removed by this chemo-orthogonal solid phase extraction process using resin 4. This radiosynthetic method was highly efficient for preparation of a 18F-labeled dimeric RGD tracer compared with conventional radiosynthesis.17,22,35,36 This radiotracer was characterized by HPLC coinjected with its nonradioactive di-cRGD-PEG5-ADIBOT-F (Figure 2C).
Figure 2.
(A) Schematic diagram of the procedure for the radiosynthesis of di-cRGD-PEG5-ADIBOT-18F with [18F]3 and the subsequent chemo-orthogonal scavenger-assisted separation using a polystyrene-supported azide resin 4. (B) HPLC chromatograms of the di-cRGD-PEG5-ADIBOT-18F reaction mixture before and after treatment with the ADIBO-precursor scavenger-resin 4. (C) HPLC chromatograph of di-cRGD-PEG5-ADIBOT-18F coinjected with its nonradioactive di-cRGD-PEG5-ADIBOT-F.
After radiolabeling, in vivo evaluation of the di-cRGD-PEG5-ADIBOT-18F tracer was performed on U87MG tumor bearing mice using a micro-PET-CT system. Figure 3A shows the representative three-dimensional reconstruction, coronal, and transverse images at 30, 60, 90, and 120 min intervals after intravenous injection of the radiotracer (1.8 MBq). This radiotracer was used to visualize the tumor region successfully with good tumor-to-background contrast within 30 min postinjection. Most activity in other organs and nontargeted tissues was cleared within 1 h after injection. In a mouse model coinjected with radiotracer and nonradiolabeled di-cRGD-PEG5-ADIBOT-F (10 mg/kg), tumor uptake was negligible, demonstrating the specificity of di-cRGD-PEG5-ADIBOT-18F for in vivo imaging of the integrin αvβ3 expressing tumor using the micro-PET-CT system, as shown in Figure 3B. The results indicated that the di-cRGD-PEG5-ADIBOT-18F produced by the chemo-orthogonal F-18 labeling protocol can act as a promising molecular PET imaging probe for cancer diagnosis.
Figure 3.
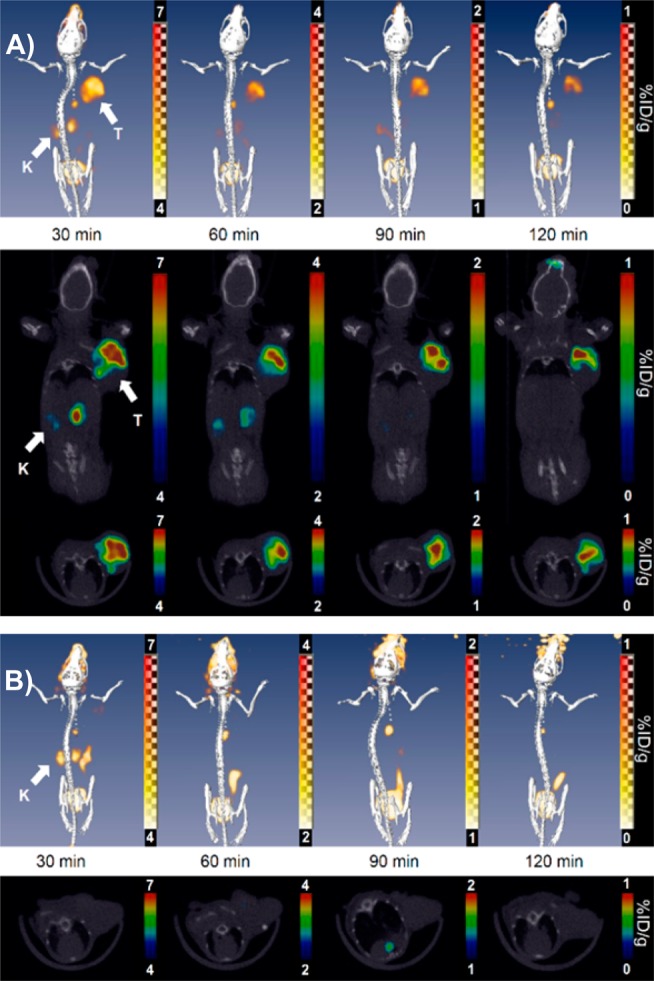
(A) In vivo evaluation of di-cRGD-PEG5-ADIBOT-18F; three-dimensional (3D) reconstruction (upper), coronal (middle), and transverse section (lower) combined PET-CT images of the U87MG tumor-bearing mice at 30, 60, 90, and 120 min postinjection of di-cRGD-PEG5-ADIBOT-18F (1.8 MBq). (B) “Blocking” images with a coinjection of nonradioactive di-cRGD-PEG5-ADIBOT-F (10 mg/kg). T = tumor, K = kidney.
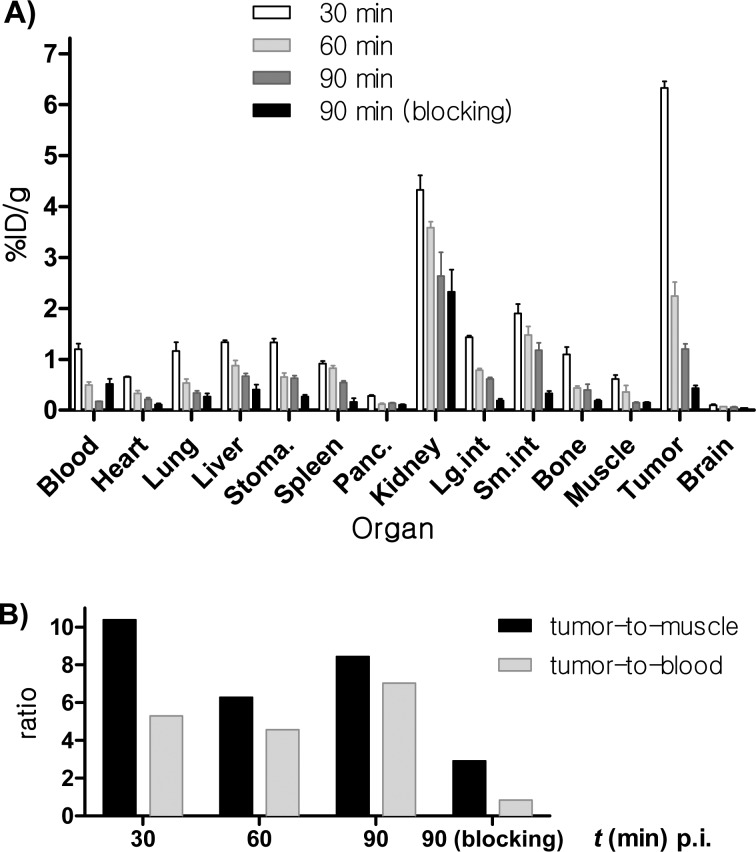
To further confirm the relationships derived from PET imaging results, the tissue distribution of di-cRGD-PEG5-ADIBOT-18F with and without a blocking dose was evaluated in the U87MG tumor bearing mice immediately after PET imaging (Figure 4A). The tumor uptake values of di-cRGD-PEG5-ADIBOT-18F at 30, 60, and 90 min postinjection (p.i.) were 6.3 ± 0.1, 2.2 ± 0.3, and 1.2 ± 0.1%ID/g, respectively (n = 4), showing significantly higher tumor uptake compared to the other major organs, including the liver, spleen, blood, and muscle. In particular, our radiotracer exhibited higher tumor uptake compared (in particular, at 30 min p.i.) with previous dimeric RGD radiotracers. It is supposed that the efficient removal of nonlabeled peptide precursor by chemo-orthogonal scavenger-assisted separation using the azide-resin enabled enhancement of the tumor uptake of the tracer.17,22,35,36 However, this tracer may be rapidly washed out considering the highest tumor-to-muscle ratio occurs at 30 min p.i. (Figure 4B). In terms of the tumor-to-blood ratio, the highest tumor-to-muscle ratio was observed at 90 min p.i. because the tracer showed a relatively high blood uptake initially and was rapidly cleared of blood. In addition to tumor uptake, this radiotracer exhibited relatively high kidney uptake, indicating its predominant renal clearance. A blocking experiment where the tracer was coinjected with the nonradiolabeled compound also confirmed the specific tumor uptake of di-cRGD-PEG5-ADIBOT-18F. At 90 min postinjection, the uptake of a tumor coinjected with the nonradioactive RGD peptide was 0.4 ± 0.05%ID/g, which is significantly lower than that in the unblocked mouse model. Consequently, these biodistribution results were well-matched with the PET imaging study.
Figure 4.
(A) Biodistribution of di-cRGD-PEG5-ADIBOT-18F in U87MG tumor bearing mice at 30, 60, and 90 min time points after microPET scans with or without nonradioactive di-cRGD-PEG5-ADIBOT-F as a blocking agent. The data are expressed as normalized accumulation of activity in %ID/g ± SD (n = 4). (B) Tumor-to-muscle and tumor-to-blood ratio.
In summary, four fluorine-substituted monomeric and dimeric cRGD peptide derivatives were synthesized based on the SPAAC conjugation reaction. In an in vitro evaluation of these cRGD derivatives via competition binding assay, the di-cRGD-PEG5-ADIBOT-F derivative, which has both dimeric cRGD and a mini-PEG linker, exhibited the highest binding affinity compared to the other derivatives and even original cRGDyk. On the basis of these in vitro results, di-cRGD-PEG5-ADIBOT-18F tracer was prepared via the SPAAC radiolabeling reaction of the ADIBO precursor with a 18F-labeled azide in 95% dcRCY for use in in vivo PET imaging studies. Despite the same retention time as its precursor on HPLC, it could be separated with high specific activity by subsequent chemo-orthogonal scavenger-assisted separation using an azide-resin without the need for HPLC purification. This radiotracer provided successful visualization of a tumor with good tumor-to-background contrast as well as significantly higher tumor uptake compared to other major organs in PET imaging and biodistribution studies. These in vivo study results suggest that the di-cRGD-PEG5-ADIBOT-18F produced by this straightforward 18F-labeling protocol platform based on the SPAAC reaction, can act as a promising PET imaging probe for cancer diagnosis. This 18F-labeling method is also expected to be useful for radiosynthesis of other peptide probes for PET molecular imaging.
Glossary
ABBREVIATIONS
- PET
positron emission tomography
- CT
computed tomography
- HPLC
high performance liquid chromatography
- SPAAC
strain-promoted alkyne azide cycloaddition
- ADIBO
aza-dibenzocyclooctyne
- ADIBOT
azadibenzocyclooctatriazole
- dcRCY
decay-corrected radiochemical yield
Supporting Information Available
All experimental procedure and characterization data of all RGD derivatives. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by Basic Science Research Program (grant code: NRF-2014R1A2A2A03007401 and 2014003870) and Nuclear Research & Development Program (grant code: NRF-2013M2A2A7059471 and 2012M2A2A7035589) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
The authors declare no competing financial interest.
Supplementary Material
References
- Phelps M. E. Positron emission tomography provides molecular imaging of biological processes. Proc. Natl. Acad. Sci. U.S.A. 2000, 97, 9226–9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud T. F.; Gambhir S. S. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003, 17, 545–580. [DOI] [PubMed] [Google Scholar]
- Vallabhajosula S.Molecular Imaging: Radiopharmaceuticals for PET and SPECT, 1st ed.; Springer: New York, 2009; pp 133–193. [Google Scholar]
- Ametamey S. M.; Honer M.; Schubiger P. A. Molecular imaging with PET. Chem. Rev. 2008, 108, 1501–1516. [DOI] [PubMed] [Google Scholar]
- Gambhir S. S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. 2002, 2, 683–693. [DOI] [PubMed] [Google Scholar]
- Schirrmacher R.; Wängler C.; Schirrmacher S. Recent developments and trends in F-18-radiochemistry: Syntheses and applications. Mini-Rev. Org. Chem. 2007, 4, 317–329. [Google Scholar]
- Kim D. W.; Jeong H.-J.; Lim S. T.; Sohn M.-H. Recent trends in the nucleophilic [18F]-radiolabeling method with no-carrier-added [18F]fluoride. Nucl. Med. Mol. Imaging 2010, 44, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. W.; Ahn D.-S.; Oh Y.-H.; Lee S.; Kil H. S.; Oh S. J.; Lee S. J.; Kim J. S.; Ryu J. S.; Moon D. H.; Chi D. Y. A new class of SN2 reaction catalyzed by protic solvents: facile fluorination for isotopic labeling of diagnostic molecules. J. Am. Chem. Soc. 2006, 128, 16394–16397. [DOI] [PubMed] [Google Scholar]
- Miller P. W.; Long N. J.; Vilar R.; Gee A. D. Synthesis of 11C, 18F, 15O, and 13N radiolabels for positron emission tomography. Angew. Chem., Int. Ed. 2008, 47, 8998–9033. [DOI] [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995, 1, 27–31. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Mechanism of angiogenesis and atheriogenesis. Nat. Med. 2000, 6, 389–395. [DOI] [PubMed] [Google Scholar]
- Schottelius M.; Laufer B.; Kessler H.; Wester H.-J. Ligands for mapping αvβ3-integrin expression in vivo. Acc. Chem. Res. 2009, 42, 969–980. [DOI] [PubMed] [Google Scholar]
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [DOI] [PubMed] [Google Scholar]
- Hwang R.; Varner J. V. The role of integrins in tumor angio-genesis. Hematol. Oncol. Clin. North Am. 2004, 18, 991–1006. [DOI] [PubMed] [Google Scholar]
- Liu S. Radiolabeled cyclic RGD peptides as integrin αvβ3-targeted radiotracers: maximizing binding affinity via bivalency. Bioconjugate Chem. 2009, 20, 2199–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Xiong Z.; Wu Y.; Cai W.; Tseng J. R.; Gambhir S. S.; Chen X. Quantitative PET imaging of tumor integrin αvβ3 expression with 18F-FRGD2. J. Nucl. Med. 2006, 47, 113–21. [PMC free article] [PubMed] [Google Scholar]
- Li Z.-B.; Wu Z.; Chen K.; Chin F. T.; Chen X. Click chemistry for 18F-labeling of RGD peptides and microPET imaging of tumor integrin αvβ3 expression. Bioconjugate Chem. 2007, 18, 1987–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. Radiolabeled multimeric cyclic RGD peptides as integrin αvβ3 targeted radiotracers for tumor imaging. Mol. Pharmaceutics 2006, 3, 472–487. [DOI] [PubMed] [Google Scholar]
- Chen X.; Tohme M.; Park R.; Hou Y.; Bading J. R.; Conti P. S. Micro-PET imaging of αvβ3-integrin expression with 18F-labeled dimeric RGD peptide. Mol. Imaging 2004, 3, 96–104. [DOI] [PubMed] [Google Scholar]
- Haubner R.; Kuhnast B.; Mang C.; Weber W. A.; Kessler H.; Wester H. J.; Schwaiger M. [18F]Galacto-RGD: synthesis, radio-labeling, metabolic stability, and radiation dose estimates. Bioconjugate Chem. 2004, 15, 61–69. [DOI] [PubMed] [Google Scholar]
- Chen X.; Park R.; Shahinian A. H.; Bading J. R.; Conti P. S. Pharmacokinetics and tumor retention of 125I-labeled RGD peptide are improved by PEGylation. Nucl. Med. Biol. 2004, 31, 11–19. [DOI] [PubMed] [Google Scholar]
- Liu S.; Liu Z.; Chen K.; Yan Y.; Watzlowik P.; Wester H. J.; Chin F. T.; Chen X. 18F-labeled galacto and PEGylated RGD dimers for PET imaging of αvβ3 integrin expression. Mol. Imaging Biol. 2010, 12, 530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschauer S.; Haubner R.; Kuwert T.; Prante O. 18F-Glyco-RGD peptides for PET imaging of integrin expression: efficient radiosynthesis by click chemistry and modulation of biodistribution by glycosylation. Mol. Pharmaceutics 2014, 11, 505–515. [DOI] [PubMed] [Google Scholar]
- Okarvi S. M. Recent progress in fluorine-18 labelled peptide radiopharmaceuticals. Eur. J. Nucl. Med. 2001, 28, 929–938. [DOI] [PubMed] [Google Scholar]
- Lee S.; Xie J.; Chen X. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem. Rev. 2010, 110, 3087–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachin K.; Jadhav V. H.; Kim E. M.; Kim H. L.; Lee S. B.; Jeong H. J.; Lim S. T.; Sohn M. H.; Kim D. W. F-18 Labeling protocol of peptides based on chemically orthogonal strain-promoted cycloaddition under physiologically friendly reaction condition. Bioconjugate Chem. 2012, 23, 1680–1686. [DOI] [PubMed] [Google Scholar]
- Sletten E. M.; Bertozzi C. R. From mechanism to mouse: a tale of two bioorthogonal reactions. Acc. Chem. Res. 2011, 44, 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Verduyn L. S.; Mirfeizi L.; Schoonen A. K.; Dierckx R. A.; Elsinga P. H.; Feringa B. L. Strain-promoted copper-free ″click″ chemistry for 18F radiolabeling of bombesin. Angew. Chem., Int. Ed. 2011, 50, 11117–11120. [DOI] [PubMed] [Google Scholar]
- Carpenter R. D.; Hausner S. H.; Sutcliffe J. L. Copper-free click for PET: rapid 1,3-dipolar cycloadditions with a fluorine-18 cyclooctyne. ACS Med. Chem. Lett. 2011, 2, 885–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet V.; Wuest M.; Wuest F. Copper-free click chemistry with the short-lived positron emitter fluorine-18. Org. Biomol. Chem. 2011, 9, 7393–7399. [DOI] [PubMed] [Google Scholar]
- Lee S. B.; Kim H. L.; Jeong H.-J.; Lim S. T.; Sohn M.-H.; Kim D. W. Mesoporous silica nanoparticle pretargeting for PET imaging based on a rapid bioorthogonal reaction in a living body. Angew. Chem., Int. Ed. 2013, 52, 10549–10552. [DOI] [PubMed] [Google Scholar]
- Ning X.; Guo J.; Wolfert M. A.; Boons G.-J. Visualizing metabolically labeled glycoconjugates of living cells by copper-free and fast huisgen cycloadditions. Angew. Chem., Int. Ed. 2008, 47, 2253–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloukhtine A. A.; Wolfert M. A.; Mbua N. E.; Boons G.-J.; Popik V. V. Selective labeling of living cells by a photo-triggered click reaction. J. Am. Chem. Soc. 2009, 131, 15769–15776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debets M. F.; van Berkel S. S.; Schoffelen S.; Rutjes F. P. J. T.; van Hest J. C. M.; van Delft F. L. Aza-dibenzocyclooctynes for fast and efficient enzyme PEGylation via copper-free (3 + 2) cycloaddition. Chem. Commun. 2010, 46, 97–99. [DOI] [PubMed] [Google Scholar]
- Wu Z.; Li Z. B.; Cai W.; He L.; Chin F. T.; Li F.; Chen X. 18F-labeled mini-PEG spacered RGD dimer (18F-FPRGD2): synthesis and microPET imaging of αvβ3 integrin expression. Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang L.; Li W.; Guo N.; Ma Y.; Zhu L.; Kiesewetter D. O.; Shen B.; Niu G.; Chen X. Comparison study of [18F]FAl-NOTA-PRGD2, [18F]FPPRGD2, and [68Ga]Ga-NOTA-PRGD2 for PET imaging of U87MG tumors in mice. Bioconjugate Chem. 2011, 22, 2415–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.