Abstract
Background
Measuring rodent facial movements is a reliable method for studying recovery from facial nerve manipulation, and for examining the behavioral correlates of aberrant regeneration. We quantitatively compared recovery of vibrissal and ocular function following three types of clinically relevant nerve injury.
Methods
178 adult rats underwent facial nerve manipulation and testing. In the experimental groups, the left facial nerve was either crushed, transected and repaired epineurially, or transected and the stumps suture-secured into a tube with a 2 mm gap between them. Facial recovery was measured for the ensuing 1–4 months. Data were analyzed for whisking recovery. Previously developed markers of co-contraction of the upper and midfacial zones (possible synkinesis markers) were also examined.
Results
Animals in the crush groups recovered nearly normal whisking parameters within 25 days. The distal branch crush group showed improved recovery over the main trunk crush group for several days during early recovery. By week 9, the transection/repair groups showed evidence of recovery that trended further upward throughout the study period. The entubulation groups followed a similar recovery pattern, though they did not maintain significant recovery levels by the study conclusion. Markers of potential synkinesis increased in selected groups following facial nerve injury.
Conclusions
Rodent vibrissial function recovers in a predictable fashion following manipulation. Generalized co-contraction of the upper and midfacial zones emerges following facial nerve manipulation, possibly related to aberrant regeneration, polyterminal axons, or hypersensitivity of the rodent to sensory stimuli following nerve manipulation.
Keywords: rat vibrissal function, whisking, facial nerve repair, synkinesis
Introduction
Facial paralysis can be a devastating clinical entity, associated with severe functional impairments and obvious aesthetic issues (1–5). Potential periocular difficulties associated with the condition include an inability to protect and lubricate the cornea, a visual field deficit from brow ptosis, and lack of proper lacrimal drainage based upon paralytic ectropion. In the midface, nasal valve collapse and nasolabial fold malposition are common, and decreased or absent oral commissure excursion leads to inability to express emotion on the face. Oral competence and the articulation of certain sounds may also be impaired, particularly when the lower face and lip are involved.
Despite its clinical importance, facial paralysis has been both an understudied and an under-managed clinical entity. Most investigations have addressed the management of Bell’s Palsy (6–8), and/or described grading scales of facial function for comparative purposes (9–12). Surgical and medical approaches have not been standardized, and only over the past decade have clinical pathways and algorithms begun to emerge that address both acute and longstanding facial nerve conditions (13–15).
A dominant problem following facial nerve injury and recovery involves aberrant regeneration, also termed synkinesis (16,17). This phenomenon arises when fibers intended for one region of the face innervate either an inappropriate target, or provide dual innervation to both the appropriate and inappropriate targets. The facial nerve is predisposed to such aberrant regeneration because its microanatomy changes significantly as it courses through the temporal bone into the face, from having very little fascicular organization proximally, to having well-defined site-specific organization distally (figure 1). The facial expressions generated by patients with marked synkinesis can be as functionally devastating as those with flaccid paralysis (figure 2), and highlight the need for focused investigation of both inadequate and misguided neural regeneration in facial nerve models.
Figure 1.
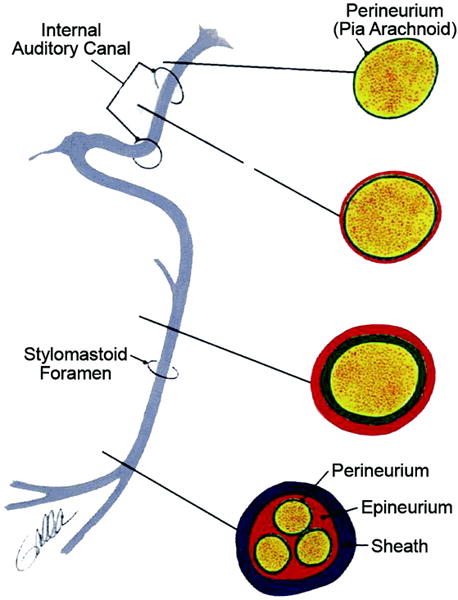
Microanatomy of the facial nerve. Note the emergence of fascicular architecture in the more distal aspects.
Figure 2.
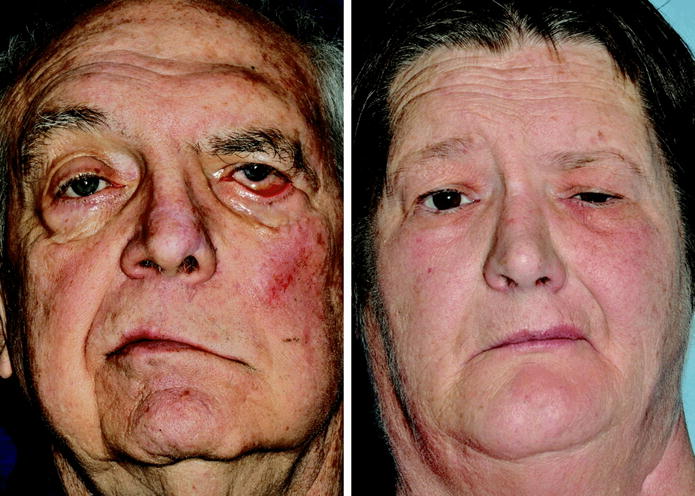
Examples of flaccidity and hypertonicity in facial paralysis. Both patients demonstrate left facial paralysis. A. The flaccid state. Note pan-facial ptosis and a widened palpebral fissure. B. The hypertonic state. Note pan-facial contracture and a narrowed palpebral fissure.
No significant effort has been channeled toward the development of a model for measuring the effect of mechanism of injury or repair method on the phenomenon of synkinesis. Because the many common clinical scenarios requiring facial nerve reconstruction involve the main trunk of the facial nerve, thus predisposing to synkinesis, a model to study regeneration across lesions of the main trunk would be of significant value.
The development of technology for quantitatively tracking whisking movement for studies of learning behavior in the rat has had an unexpected benefit for facial nerve investigators; it has produced the tools to precisely examine vibrissal motor recovery. It is now possible to compare recovery from various facial nerve lesions and repair methods in an efficient, precise, and quantitative manner. This technology, which has now been tailored to study zonal facial movement in the rat (18,19), offers improvement over the prior state of the art in rodent facial function measurements, which involved videographic assessments, did not permit digitally controlled stimuli to elicit and record behaviors, and do not provide simultaneous, high resolution, quantitative ocular and vibrissal motion analysis.
In this study, the objective was to quantify vibrissal recovery following clinically relevant facial nerve injury mechanisms: crush, transection with epineurial repair, and transection with entubulation of the proximal and distal stumps across a 2 mm gap (level 2 injury through a level 5 injury, according to the Sunderland classification) (20). Our second objective was to discern the differences in both rate and completeness of vibrissal motor recovery following lesions either proximal or distal to the trifurcation of the facial nerve, under the hypothesis that lesions proximal to the trifurcation would be synkinesis-producing, and thus yield delayed and less complete recovery than lesions occurring distal to the trifurcation.
Our final objective was to determine whether evidence of synkinetic co-contractions emerged following nerve injury, and whether it differed according to lesion location, either proximal to or distal to the trifurcation.
Methods
187 adult female Wistar rats, 250–300 grams, underwent head fixation, followed by conditioning to the facial nerve testing apparatus, then facial nerve manipulation, and finally periodic quantitative assessments of vibrissal function, according to a schedule appropriate to the degree of injury and the expected time frame of recovery. At the conclusion of the study, animals were euthanized according to NIH and the American Veterinary Medical Association guidelines, and the data analyzed for statistical differences.
Head Fixation
Animals underwent implantation of a titanium head fixation device, according to previously described methods (21). Briefly, each animal was anesthetized with ketamine (50mg/kg) and medetomodine (0.5mg/kg) IM. A midline incision was made, the cranium exposed, and the titanium head fixation device secured to the cranium with titanium screws. Each fixation device had four posts, providing points of attachment with an external head fixation apparatus. The midline incision was closed, and the anesthetic reversed with a subcutaneous injection of Atipamezole (2.5 mg/kg). The animals were treated daily with oral Cephalexin and topical antibacterial ointment, for the ensuing week.
Rodent Conditioning
Animals were conditioned to the testing apparatus according to previously described methods (18,19). Briefly, they were handled on a daily basis for approximately 2 weeks prior to implantation of the devices, and then after implantation up to the day of testing. Once accustomed to being securely held for 10 minutes, animals were placed in a fitted cloth sack for brief periods, where the limbs had minimal ability to move (see reference 19 for thorough description). For the five days prior to testing, animals were head-fixed in the testing apparatus, beginning for 30 seconds, and progressing to 10 minutes.
Facial Nerve Manipulations
Figure 3 shows the facial nerve manipulations. All nine groups (n = 18–21) underwent left facial nerve exposure under anesthesia identical to that described above for head fixation. A left pre-auricular incision was made, and the parotid gland was removed, exposing the main trunk of the facial nerve. In the sham group, the wound was closed. In the experimental groups, the nerve was either crushed, transected and repaired with two or three 10-0 nylon epineurial sutures, or transected and the proximal and distal stumps suture-secured into a silicone tube slightly larger than the nerve diameter, with a 2 mm gap between stumps (ID 1.5 mm for main trunk conduits, 0.6 mm for peripheral branch conduits). Crush injuries were created using smooth-surfaced jeweler’s forceps, compressing the nerve twice in each crush location for 30 seconds per compression. For each injury/repair method, lesions were made in the main trunk in 20 animals (main trunk lesion groups), and in each of the three distal branches in another 20 animals (peripheral lesion groups). In the two denervation control groups, the nerve was transected, and the proximal stump(s) buried to prevent sprouting. In the first denervation control group, the nerve was transected at the main trunk, and in the second denervation control group, it was made in the three distal facial nerve branches. In all animals, wounds were closed and animals reversed from anesthesia as described above. Animals were housed separately for the first two postoperative days, and monitored for weight gain, cage behavior, and wound problems.
Figure 3.
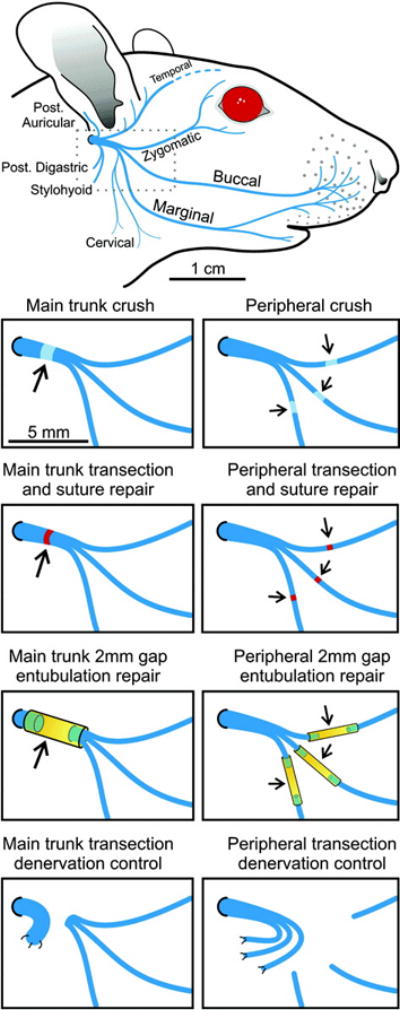
Facial nerve manipulations. Top panel: typical rodent facial nerve anatomy. Middle panels: experimental crush, transection/repair, and entubulation groups at either main trunk or distal to the trifurcation. Bottom panel: Denervation control groups, at either main trunk (left) or distal branches (right).
Facial Movement Testing
All animals underwent baseline facial nerve testing to verify normal whisking and blinking. Thereafter, facial recovery was measured on a scheduled basis for 1–4 months. For animals undergoing crush injury, testing was performed on POD 2, to establish a baseline for complete loss of function. Daily testing for recovery began on POD 7, and continued nearly daily through day 21, at which point a twice weekly schedule was employed for the ensuing two weeks. Animals that underwent transection and repair began bi-weekly testing during postoperative week three, which continued for four months. The entubulation groups underwent weekly testing, starting on postoperative week four and continuing for four months.
During each testing session, movement of the tagged C1 whisker on each side of the head was independently tracked using two sets of commercial laser micrometers (MetraLight, San Mateo CA)(figure 4). Animals underwent a five minute recording session during which both C1 whiskers were continuously monitored over the 5 minute testing run, and eyelid closure was continuously monitored via a pair of IR emitter/detectors maintained in a fixed location 3.5 – 4 mm from the ocular surfaces (see 19 for details).
Figure 4.
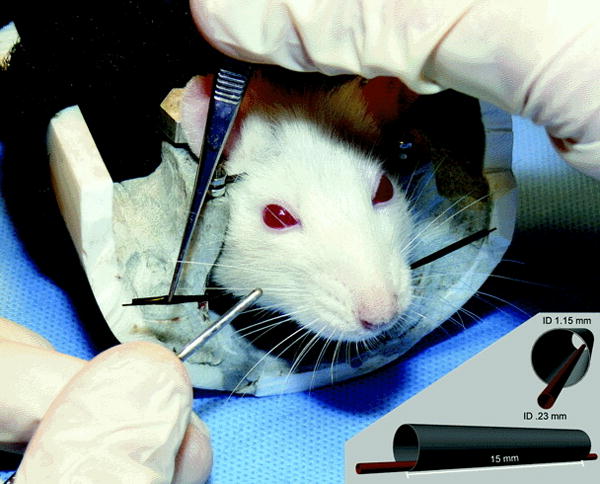
Photograph of rat in head-fixed position, with C1 whiskers entubulated to permit tracking via the laser micrometers.
For inducing whisking during recording sessions, three randomly delivered 10 second flows (1.8 mL/sec) of scented air, from chambers containing either peanut butter or isopropyl alcohol (both found to be effective in eliciting whisking), were directed toward the snout through a glass pipette (see 19 for details)(figure 5A).
Figure 5.
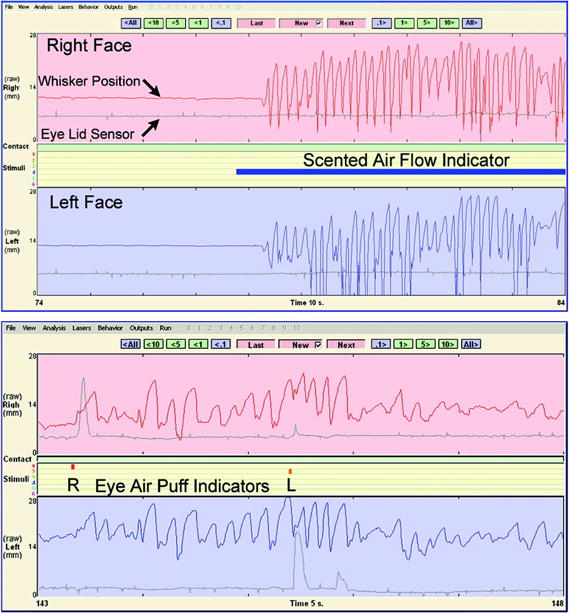
Output generated from the facial movement tracking program of Bermejo et al (22). Red and blue tracings indicate right and left C1 whisker position, respectively. Gray tracings correspond to degree of right (top) and left (bottom) eye closure. A. Vigorous whisking induced by the delivery of scented air. B. Blinking induced by ocular air puff delivery.
In order to trigger eyelid movements, three separate 20 millisecond air puffs were delivered to each eye at random to elicit a blink (figure 5B); for each side independently, the ocular behavior occurring in the 100 msec following the conclusion of the air puff was examined.
Data Analysis
For whisking recovery analysis, all whisks greater than 3 degrees were counted and analyzed in an automated fashion, using previously published criteria (22–24). The three whisks of largest amplitude were further analyzed by computing average whisking amplitude, velocity, and acceleration from these three whisks, using Whisking software developed by Bermejo et al (22). The C1 whisking data from the operated side were compared amongst groups, using two-tailed Student’s t-tests for comparisons, with p<0.05 achieving significance.
For observations regarding the emergence of potential synkinetic behavior, the incidence of scent-induced blinking (SIB) was utilized as a measure of vibrisso-ocular co-contraction (VOC), and the incidence of ocular air puff-induced whisking (PIW) was utilized as a measure of oculo-vibrissal co-contraction (OVC). Each testing session was analyzed as follows: During the scent delivery periods, blinks were recorded as yes/no phenomena, according to the following criteria: a blink was considered present only when there was at least a 100 mV change from baseline or trough to peak activity, and the rapid component of the ocular movement had a slope of at least 5 mv/ms (normal fast blink slope was in the 5–15 mV/msec range). This represented approximately 10% of the change in mV detected by the IR detector during the average complete eye closure in an unmanipulated animal. If these criteria were not met, then no blink was recorded. All criteria-meeting blinks occurring during the scent delivery period (10 sec) and ensuing 0.5 seconds were counted as scent-induced phenomena. The frequency of SIB pre- and post-manipulation was compared using two-tailed Student’s t-tests, with p<0.05 achieving significance.
Ocular air-puff induced whisking was analyzed as follows: Puff-induced ipsilateral and contralateral whisks were examined in binary fashion, where the criteria utilized for this binary yes/no analysis involved any distinct change in whisking function, of greater than 12 degrees, with onset within 100 msec of administration of the ocular air puff (for detailed description see 18). Data were statistically analyzed as described above for SIB data.
Results
A total of 187 animals underwent implantation of the titanium head fixation devices, and achieved adequate stability of the device to undergo baseline head-fixed facial nerve testing. There were nine animals excluded from facial nerve manipulation, based upon either early loosening of the head fixation device (3 animals), poor conditioning to the testing apparatus (5 animals), or recognized iatrogenic facial nerve injury during exposure (1 animal). Of the 178 animals that underwent facial nerve manipulation and testing, 144 remained testable throughout the study period. In two of the nine groups, higher than expected head fixation device failure rates were recorded (8/21 failures and 10/20 failures), and were thought to be related to screw extrusion from the cranium, plate exposure, and subsequent infection. In the remaining seven groups, there were 16 head fixation device failures in 137 implantations (11.7%), commensurate with a prior investigation using this head fixation device (21). Animals with device instability were removed from the study and euthanized.
Whisking Recovery
Control Animals
Animals undergoing sham surgery exhibited normal whisking function at postoperative days 10 and 20, and were not tested further. Denervation control animals experienced complete ipsilateral loss of whisking, and did not recover any significant whisking function for the duration of the 4 month study. Based upon lack of meaningful recovery, they were not assessed for the presence of synkinetic behavior.
Crush-Injured Animals
Animals that underwent facial nerve crush injury all experienced total loss of ipsilateral vibrissal movement. By postoperative day 11, the first signs of recovery were evident, and a plateau of recovery was evident by days 19–28. There was a statistically significant improvement in recovery of whisking amplitude in the animals with peripheral branch crushes over the main trunk-injured group during a three day period centered around day 14 (p < 0.05), though this difference disappeared by day 16 (figure 6). Complete whisking kinematics are presented in Table I.
Figure 6.
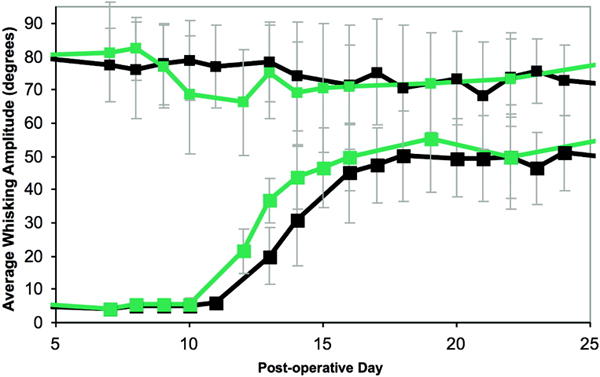
Graph illustrating the recovery of whisking amplitude over time, following crush injury. Black line represents main trunk crush, and gray line represents peripheral branch crush. Large squares represent manipulated side, small squares represent unmanipulated side. Error bars represent standard deviation. Note the slightly earlier recovery in the distally-lesioned group.
Table 1.
Kinematics of Whisking 5 Weeks after Crush Injury to the Left Facial Nerve*
| Injury Type | Amplitude (degrees)
|
Velocity (degrees/sec)
|
Acceleration (degrees/sec2)
|
|||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |
| Main trunk crush | 73.70 ± 14.74 | 58.56 ± 20.47 | 1666.67 ± 376.01 | 1387.56 ± 544.59 | 169.54 ± 66.74 | 130.46 ± 70.08 |
| Peripheral crush | 82.91 ± 7.95 | 61.82 ± 13.75 | 1981.92 ± 441.02 | 1539.25 ± 525.87 | 160.81 ± 47.26 | 142.51 ± 55.85 |
Right-sided (unmanipulated) whisking kinematics are shown for comparisons.
Transection Groups, with Epineurial Repair or Entubulation Repair
Animals that underwent transection and repair (either epineurial suture or entubulation) all experienced total ipsilateral loss of whisking post-operatively. Four months postoperatively, both main trunk- and peripheral-lesioned groups repaired via direct epineurial suture techniques experienced statistically significant whisking recovery above baseline, and there were no between-group differences based upon lesion location (figure 7A). The main trunk entubulation group trended toward recovery, though neither entubulation group achieved significant whisking function over the study period (figure 7B). As expected, recovery followed a protracted pattern, rather than the sinusoidal recovery seen following crush. Table II shows all final whisking recovery parameters for all four groups.
Figure 7.
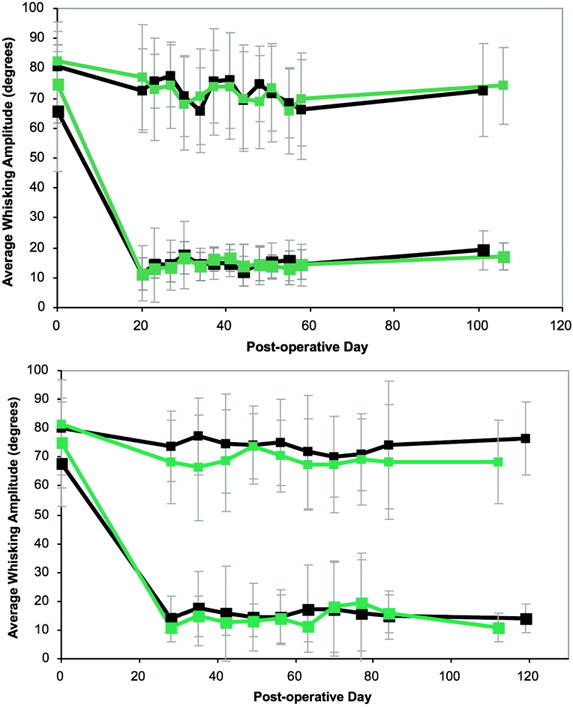
Recovery of whisking amplitude following transection and repair. A. Epineurial repair. Black line represents main trunk injury/repair, and gray line represents peripheral branch injury/repair. Large squares represent manipulated side, small squares represent unmanipulated side. Error bars represent standard deviation. B. Entubulation repair; Black line represents main trunk injury/repair, and gray line represents peripheral branch injury/repair. Large squares represent manipulated side, small squares represent unmanipulated side. Error bars represent standard deviation.
Table 2.
Kinematics of Whisking 4 Months after Transection Injury to the Left Facial Nerve*
| Injury/Repair Type | Amplitude (degrees)
|
Velocity (degrees/sec)
|
Acceleration (degrees/sec2)
|
|||
|---|---|---|---|---|---|---|
| Right | Left | Right | Left | Right | Left | |
| Main trunk transection repair | 72.65 ± 15.62 | 19.25 ± 6.43 | 1746.73 ± 598.16 | 693.58 ± 219.0 | 163.56 ± 59.32 | 70.31 ± 19.16 |
| Peripheral transection/repair | 74.10 ± 12.85 | 17.23 ± 4.53 | 1546.78 ± 427.02 | 591.89 ± 69.51 | 139.00 ± 50.03 | 69.51 ± 17.31 |
| Main trunk entubulation | 76.53 ± 12.78 | 13.97 ± 5.09 | 1724.43 ± 570.75 | 532.78 ± 202.31 | 149.00 ± 52.37 | 57.44 ± 19.55 |
| Peripheral entubulation | 68.18 ± 14.52 | 10.91 ± 4.90 | 1786.45 ± 624.01 | 394.39 ± 109.77 | 170.33 ± 64.51 | 49.35 ± 14.21 |
Data for epineurial repair and entubulation groups are shown. Right-sided (unmanipulated) whisking kinematics are shown for comparisons.
Parameters of potential synkinesis
Vibrisso-ocular Co-Contraction: Scent-Induced Blinking
The incidence of scent-induced blinking (SIB) was assessed at each experiment’s final time point. For crush injuries, this was POD 42–43, though for transection injuries, it occurred after 4 months. Data are reported in table III. In the crushed groups, the peripheral crush-injured animals experienced a decline in incidence of SIB compared with preoperative values, indicating less ocular activity during whisking; neither group developed changes in the incidence of SIB on their healthy sides, and the main trunk-injured animals did not demonstrate measurable left-sided vibrisso-ocular co-contraction (VOC) based upon these criteria.
Table 3.
Frequency of Left-Sided Scent-Induced Blinking for All Experimental Groups*
| Injury and Repair Type | Preoperative | Final Recovery |
|---|---|---|
| Main trunk crush | 7.14 ± 18.16 | 21.43 ± 37.80 |
| Peripheral crush | 35.7 ± 28.03 | 14.28 ± 28.03 |
| Main trunk transection/repair | 2.38 ± 8.91 | 31.82 ± 40.45 |
| Peripheral transection/repair | 18.18 ± 22.92 | 45.24 ± 45.96 |
| Main trunk entubulation | 25.93 ± 29.27 | 6.67 ± 25.82 |
| Peripheral entubulation | 31.11 ± 29.46 | 46.15 ± 43.12 |
Data are represented as the mean ± SD frequency of occurrence of blinking behavior during scent delivery.
In the transected groups, the degree of synkinesis correlated with lesion location. Main trunk-injured groups, both those who underwent epineurial repair and those who underwent entubulation, developed significantly different incidences of SIB postoperatively. The epineurial repair group had an increased incidence of SIB, whereas the entubulation group experienced a decline in SIB, perhaps related to overall poorer recovery across the gap. In contrast, neither peripherally injured transection group experienced different rates of SIB compared with preoperative values.
Oculo-vibrissal Co-Contraction: Ocular Air Puff-Induced Whisking
All crush injured animals remained stable with respect to the incidence of ocular air puff-induced whisks (PIW), on both operated and unoperated sides. The transection-injured groups showed mixed results (Table IV). The main trunk epineurial repair group showed a modest but statistically significant increase in incidence of PIW on the unoperated side, but no change on the left. The peripheral epineurial repair group showed no changes between pre- and post-operative incidence of right or left PIW.
Table 4.
Frequency of Puff-Induced Whisking for Crush and Transection/Epineurial Repair Groups*
| Injury Location and Repair Type | Change between Preoperative and Final Postoperative Value
|
|
|---|---|---|
| Right (Unoperated) |
Left (Operated) |
|
| Main trunk crush | −4.11 | 4.03 |
| Peripheral crush | 3.38 | −3.44 |
| Main trunk transection/repair | 18.89† | 3.98 |
| Peripheral transection/repair | 20.83 | −18.83 |
Data are presented as the frequency of occurrence of whisking behavior during ocular air puff delivery.
Statistically significant change.
Discussion
While investigators have long been interested in facial function, until recently there was no precise, quantitative method of assessing the kinematics of whisking. Investigators relied heavily upon videographic recordings of whisking and blinking (25–29), sometimes in sedated animals (30). The recent development of a device that provides zonal facial movement recovery data, examining whisking and blinking simultaneously yet independently, on both sides of the face (19) offers an excellent tool for the study of facial co-contraction. The apparatus has provided us with a tool to study normal and recovering facial function in the rat, and to examine the incidence of potentially synkinetic behaviors following facial nerve manipulation.
Our recovery data following crush injury agree with published literature using videography for calculations of whisking amplitude (30,31), and we determined that whisking amplitude lags behind velocity and acceleration in recovery to normal values.
Crush injuries recovered more quickly when the injury occurred distal to the trifurcation, as expected. Final recovery parameters were equivalent in the two groups, as expected since in a rodent nerve crush model complete or near-complete recovery is the norm (30–32).
In the crush-injured rodents, there was no increase in either SIB or PIW, as expected. Because crush injuries represent Sunderland level 2 injuries, which do not involve endoneurial disruption, one would not expect any increase in these behavioral parameters. The decline in SIB in the peripherally crushed animals postoperatively is likely a reflection of the relative imprecision of the functional assay, though might represent a behavioral trend toward less co-contraction of the orbicularis oculi with scent-induced whisking in animals who underwent testing on a daily basis for prolonged periods. Ocular air puff-induced blinking, known to occur 99% of the time in non-lesioned animals, recovered fully in both crush-injured groups, indicating that orbicularis oculi response to stimulus did not diminish despite daily testing.
The transection-injured animals recovered poorly after four months. While there was a statistically significant recovery from the denervation control group by week 16 in the epineurial repair groups, the gains were modest, the whisking disorganized, and of generally low amplitude. It is possible that re-innervation (arrival of axons to target musculature) was fairly complete in the transection-injured animals, but that functional outcome was poor due to peripheral misrouting of axons among facial zones, and reduction of brainstem synaptic connection with facial motoneurons.
Entubulation with a 2 mm gap between the two stumps proved to be the least favorable injury/repair method. Neither animals lesioned at the main trunk, nor those with distal lesions, recovered meaningful whisking after four months, despite providing a gap and time frame that is ordinarily permissive to axonal traversing (33,34). Post mortem evaluations of these animals revealed visible cables in all but two conduits (both of which appeared to have experienced dislodgement of one of the nerve stumps from the lumen). This observation obligates us to consider that poor function following facial nerve lesioning is related more to severe axonal misrouting and hyperinnnervation from polyterminal axons, than from generalized lack of conduit penetration, a conclusion supported by the work of other facial nerve investigators (26). This poor recovery rendered the parameters of synkinesis uninterpretable, though the unoperated sides of these animals demonstrated stable rates of SIB, and a higher rate of PIW compared with preoperative values.
The fact that incidences of SIB and PIW fluctuated significantly amongst animals, varied between pre- and post-operative states, even on the unmanipulated side, indicates that the data must be interpreted with caution. Myriad factors control the co-contracture of vibrissae and orbicularis oculi musculature, and SIB and PIW are only two of several measures that must be taken together with anatomic evidence of synkinesis (including retrograde tracer data and fluorescently labeled motor axon models (35,36)) to understand the biological processes governing synkinesis.
In addition to more thoroughly undersanding synkinesis, our ability to precisely quantify co-contraction of rodent facial musculature in response to stimuli, as described herin, will permit detailed study of interventions that directly affect the biological phenomena thought to lead to synkinesis, including massive axonal sprouting and polyterminal axons, and hyperinnervation of muscle targets. Potential interventions to curb these phenomena might include the delivery of appropriate inhibitory factors, the focused delivery of cell populations that have negative effects on neural sprouting, and/or carefully designed mechanical stimulation regimens that promote one-to-one axon-motor end plate relationships.
Although many different research approaches (pharmacologic, physical therapy, or electrical stimulation) may appear efficacious for improving facial function versus no intervention (37–41), there is little indication of what particular aspects of treatment are bringing about positive change, and whether aspects of these various approaches can be combined synergistically. Rigorous scientific study of this issue is critical to broaden potential clinical approached to the problem, and the model utilized herein provides a platform for studying the underlying neural and physiological mechanisms for improved functional outcomes following facial nerve injury.
Results in the present report demonstrate the time-course and degree of prolonged functional deficit that can occur following neurotmesis and suture or entubulation repair of the rat facial nerve in contrast to crush (axonotmesis) injury. These findings provide an important benchmark against which to evaluate surgical/pharmacologic interventions, as well as to explore what modes of sensory feedback, exercise, environmental stimuli, and physical manipulations are the most effective in enhancing recovery from cranial nerve injury (42,43).
Acknowledgments
NIDCR K-08 DE015665-01A2
Footnotes
This work was presented in part at the Society for Neuroscience Meetings, Washington D.C., November 2008, and in part at the American Society for Peripheral Nerve Meetings, Maui HI, January 2009
The authors have no financial disclosures to make.
References
- 1.Lee J, Fung K, Lownie SP, Parnes LS. Assessing impairment and disability of facial paralysis in patients with vestibular schwannoma. Arch Otolaryngol Head Neck Surg. 2007 Jan;133(1):56–60. doi: 10.1001/archotol.133.1.56. [DOI] [PubMed] [Google Scholar]
- 2.Ryzenman JM, Pensak ML, Tew JM., Jr Facial paralysis and surgical rehabilitation: a quality of life analysis in a cohort of 1,595 patients after acoustic neuroma surgery. Otol Neurotol. 2005 May;26(3):516–21. doi: 10.1097/01.mao.0000169786.22707.12. discussion 521. [DOI] [PubMed] [Google Scholar]
- 3.Coulson SE, O’dwyer NJ, Adams RD, Croxson GR. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol. 2004 Nov;25(6):1014–9. doi: 10.1097/00129492-200411000-00026. [DOI] [PubMed] [Google Scholar]
- 4.Kahn JB, Gliklich RE, Boyev KP, Stewart MG, Metson RB, McKenna MJ. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope. 2001 Mar;111(3):387–98. doi: 10.1097/00005537-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lohne V, Bjørnsborg E, Westerby R, Heiberg E. I want to smile. How do individuals with facial paralysis resulting from surgical removal of an acoustic neuroma cope with daily living? Vard Nord Utveckl Forsk. 1986 Spring;6(1):311–9. doi: 10.1177/010740838600600104. [DOI] [PubMed] [Google Scholar]
- 6.Salinas RA, Alvarez G, Ferreira J. Corticosteroids for Bell’s palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2004 Oct 18;(4):CD001942. doi: 10.1002/14651858.CD001942.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Allen D, Dunn L. Aciclovir or valaciclovir for Bell’s palsy (idiopathic facial paralysis) Cochrane Database Syst Rev. 2004(3):CD001869. doi: 10.1002/14651858.CD001869.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey MJ, DerSimonian R, Holtel MR, Burgess LP. Corticosteroid treatment for idiopathic facial nerve paralysis: a meta-analysis. Laryngoscope. 2000 Mar;110(3 Pt 1):335–41. doi: 10.1097/00005537-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 9.House JW. Facial nerve grading systems. Laryngoscope. 1983 Aug;93(8):1056–69. doi: 10.1288/00005537-198308000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Coulson SE, Croxson GR, Adams RD, O’Dwyer NJ. Reliability of the “Sydney,” “Sunnybrook,” and “House Brackmann” facial grading systems to assess voluntary movement and synkinesis after facial nerve paralysis. Otolaryngol Head Neck Surg. 2005 Apr;132(4):543–9. doi: 10.1016/j.otohns.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 11.Berg T, Jonsson L, Engström M. Agreement between the Sunnybrook, House-Brackmann, and Yanagihara facial nerve grading systems in Bell’s palsy. Otol Neurotol. 2004 Nov;25(6):1020–6. doi: 10.1097/00129492-200411000-00027. [DOI] [PubMed] [Google Scholar]
- 12.House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985 Apr;93(2):146–7. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- 13.Hadlock TA, Greenfield LJ, Wernick-Robinson M, Cheney ML. Multimodality approach to management of the paralyzed face. Laryngoscope. 2006 Aug;116(8):1385–9. doi: 10.1097/01.mlg.0000225980.38147.c6. [DOI] [PubMed] [Google Scholar]
- 14.Sarkies NJ. A clinical algorithm for the management of facial nerve palsy from an oculoplastic perspective. Eye. 1999 Apr;13(Pt 2):273. doi: 10.1038/eye.1999.72. [DOI] [PubMed] [Google Scholar]
- 15.Gantz BJ, Rubinstein JT, Gidley P, Woodworth GG. Surgical management of Bell’s palsy. Laryngoscope. 1999 Aug;109(8):1177–88. doi: 10.1097/00005537-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Husseman J, Mehta RP. Management of synkinesis. Facial Plast Surg. 2008 May;24(2):242–9. doi: 10.1055/s-2008-1075840. [DOI] [PubMed] [Google Scholar]
- 17.Peitersen E. Bell’s palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl. 2002;(549):4–30. Review. [PubMed] [Google Scholar]
- 18.Hadlock T, Kowaleski J, Lo D, Bermejo R, Zeigler HP, Mackinnon S, Heaton JT. Functional assessments of the rodent facial nerve: a synkinesis model. Laryngoscope. 2008 Oct;118(10):1744–9. doi: 10.1097/MLG.0b013e31817f5255. [DOI] [PubMed] [Google Scholar]
- 19.Heaton JT, Kowaleski JM, Bermejo R, Zeigler HP, Ahlgren DJ, Hadlock TA. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods. 2008 Jun 30;171(2):197–206. doi: 10.1016/j.jneumeth.2008.02.023. Epub 2008 Mar 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951 Dec;74(4):491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 21.Hadlock T, Kowaleski J, Mackinnon S, Heaton JT. A novel method of head fixation for the study of rodent facial function. Exp Neurol. 2007 May;205(1):279–82. doi: 10.1016/j.expneurol.2007.02.014. Epub 2007 Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermejo R, Szwed M, Friedman W, Ahissar E, Zeigler HP. One whisker whisking: unit recording during conditioned whisking in rats. Somatosens Mot Res. 2004;21:183–7. doi: 10.1080/08990220400012430. [DOI] [PubMed] [Google Scholar]
- 23.Bermejo R, Vyas A, Zeigler HP. Topography of rodent whisking–I. Two-dimensional monitoring of whisker movements. Somatosens Mot Res. 2002;19:341–6. doi: 10.1080/0899022021000037809. [DOI] [PubMed] [Google Scholar]
- 24.Harvey MA, Bermejo R, Zeigler HP. Discriminative whisking in the head-fixed rat: optoelectronic monitoring during tactile detection and discrimination tasks. Somatosens Mot Res. 2001;18:211–22. doi: 10.1080/01421590120072204. [DOI] [PubMed] [Google Scholar]
- 25.Guntinas-Lichius O, Effenberger K, Angelov DN, Klein J, Streppel M, Stennert E, Neiss WF. Delayed rat facial nerve repair leads to accelerated and enhanced muscle reinnervation with reduced collateral axonal sprouting during a definite denervation period using a cross-anastomosis paradigm. Exp Neurol. 2000;162:98–111. doi: 10.1006/exnr.2000.7309. [DOI] [PubMed] [Google Scholar]
- 26.Guntinas-Lichius O, Irintchev A, Streppel M, Lenzen M, Grosheva M, Wewetzer K, Neiss WF, Angelov DN. Factors limiting motor recovery after facial nerve transection in the rat: combined structural and functional analyses. Eur J Neurosci. 2005;21:391–402. doi: 10.1111/j.1460-9568.2005.03877.x. [DOI] [PubMed] [Google Scholar]
- 27.Tomov TL, Guntinas-Lichius O, Grosheva M, Streppel M, Schraermeyer U, Neiss WF, Angelov DN. An example of neural plasticity evoked by putative behavioral demand and early use of vibrissal hairs after facial nerve transection. Exp Neurol. 2002;178:207–18. doi: 10.1006/exnr.2002.8040. [DOI] [PubMed] [Google Scholar]
- 28.Ferri CC, Ghasemlou N, Bisby MA, Kawaja MD. Nerve growth factor alters p75 neurotrophin receptor-induced effects in mouse facial motoneurons following axotomy. Brain Res. 2002;950:180–5. doi: 10.1016/s0006-8993(02)03035-4. [DOI] [PubMed] [Google Scholar]
- 29.Yeh C, Bowers D, Hadlock TA. Effect of FK506 on functional recovery after facial nerve injury in the rat. Arch Facial Plast Surg. 2007 Sep-Oct;9(5):333–9. doi: 10.1001/archfaci.9.5.333. [DOI] [PubMed] [Google Scholar]
- 30.Hadlock TA, Heaton J, Cheney M, Mackinnon SE. Functional recovery after facial and sciatic nerve crush injury in the rat. Arch Facial Plast Surg. 2005 Jan-Feb;7(1):17–20. doi: 10.1001/archfaci.7.1.17. [DOI] [PubMed] [Google Scholar]
- 31.Knutsen PM, Derdikman D, Ahissar E. Tracking whisker movements in unrestrained behaving rodents. J Neurophysiol. 2005;93(4):2294–301. doi: 10.1152/jn.00718.2004. [DOI] [PubMed] [Google Scholar]
- 32.Vilela DS, Lazarini PR, Da Silva CF. Effects of hyperbaric oxygen therapy on facial nerve regeneration. Acta Otolaryngol. 2008 Mar;10:1–5. doi: 10.1080/00016480701827525. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Brenner MJ, Moradzadeh A, Myckatyn TM, Tung TH, Mendez AB, Hunter DA, Mackinnon SE. Role of timing in assessment of nerve regeneration. Microsurgery. 2008;28(4):265–72. doi: 10.1002/micr.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore AM, Kasukurthi R, Magill CK, Farhadi HF, Borschel GH, Mackinnon SE. Limitations of Conduits in Peripheral Nerve Repairs. Hand (N Y) 2009 Jan 10; doi: 10.1007/s11552-008-9158-3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007 Nov 1;450(7166):56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- 36.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000 Oct;28(1):41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 37.Lal D, Hetzler LT, Sharma N, Wurster RD, Marzo SJ, Jones KJ, Foecking EM. Electrical stimulation facilitates rat facial nerve recovery from a crush injury. Otolaryngol Head Neck Surg. 2008 Jul;139(1):68–73. doi: 10.1016/j.otohns.2008.04.030. [DOI] [PubMed] [Google Scholar]
- 38.Hetzler LE, Sharma N, Tanzer L, Wurster RD, Leonetti J, Marzo SJ, Jones KJ, Foecking EM. Accelerating functional recovery after rat facial nerve injury: Effects of gonadal steroids and electrical stimulation. doi: 10.1016/j.otohns.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Brown TJ, Khan T, Jones KJ. Androgen induced acceleration of functional recovery after rat sciatic nerve injury. Restor Neurol Neurosci. 1999;15(4):289–295. [PubMed] [Google Scholar]
- 40.Mattsson P, Janson AM, Aldskogius H, Svensson M. Nimodipine promotes regeneration and functional recovery after intracranial facial nerve crush. J Comp Neurol. 2001 Aug 13;437(1):106–17. doi: 10.1002/cne.1273. [DOI] [PubMed] [Google Scholar]
- 41.Gilad VH, Tetzlaff WG, Rabey JM, Gilad GM. Accelerated recovery following polyamines and aminoguanidine treatment after facial nerve injury in rats. Brain Res. 1996 Jun 10;724(1):141–4. doi: 10.1016/0006-8993(96)00287-9. [DOI] [PubMed] [Google Scholar]
- 42.Angelov DN, Ceynowa M, Guntinas-Lichius O, Streppel M, Grosheva M, Kiryakova SI, Skouras E, Maegele M, Irintchev A, Neiss WF, Sinis N, Alvanou A, Dunlop SA. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiol Dis. 2007 Apr;26(1):229–42. doi: 10.1016/j.nbd.2006.12.016. Epub 2007 Jan 3. [DOI] [PubMed] [Google Scholar]
- 43.Evgenieva E, Schweigert P, Guntinas-Lichius O, Pavlov S, Grosheva M, Angelova S, Streppel M, Irintchev A, Skouras E, Kuerten S, Sinis N, Dunlop S, Radeva V, Angelov DN. Manual stimulation of the suprahyoid-sublingual region diminishes polyinnervation of the motor endplates and improves recovery of function after hypoglossal nerve injury in rats. Neurorehabil Neural Repair. 2008 Nov-Dec;22(6):754–68. doi: 10.1177/1545968308316387. Epub 2008 Jul 8. [DOI] [PubMed] [Google Scholar]


