Abstract
Objective
To establish whether nimodipine, a calcium channel blocker, accelerates or otherwise improves functional recovery of whisking after facial nerve crush injury in the rat.
Methods
Thirty rats underwent exposure of the left main trunk of the facial nerve followed by a standard crush injury, and subsequent quantitative facial movement testing. Animals were randomized into an experimental group (n=15) and a control group (n=15 each). Four days prior to facial nerve manipulation, experimental animals underwent subcutaneous implantation of a nimodipine-secreting pellet. All animals were tested pre-operatively and on post-crush days 2, 8–17, 20, 22, 24, and 31, using a validated, quantitative whisking kinematics apparatus. Whisks were analyzed for amplitude, velocity, and acceleration.
Results
Animals receiving nimodipine demonstrated significantly better whisking on days 9, 11–13, and 20 compared with control animals (p<.05). Overall, the nimodipine-treated animals showed earlier recovery as compared to the untreated animals.
Conclusions
We demonstrate that nimodipine improves recovery of whisking after facial nerve crush. This finding corroborates the semi-quantitative findings of others, and provides complete whisking kinematic data on its effects. Given the low side effect profile of nimodipine, there may be clinical implications in its administration in patients experiencing facial nerve injury.
Keywords: Facial Nerve, facial nerve crush injury, drug therapy
Introduction
Facial nerve injury carries significant adverse social and functional consequences, including decreased ability to communicate using facial expression, synkinesis, incomplete eye closure, external nasal valve collapse, and oral incompetence. Our laboratory is focused upon the development and study of interventions designed to accelerate and improve recovery from these sequelae.
A variety of pharmacologic agents, including FK-506 1, TJ-23 (Tokishakuyakusan)2, angiotensin II (ANG II)3, and nitric oxide (NO)4,5 have been shown to improve the functional recovery of peripheral nerves after injury, however, none are in clinical use. Nimodipine, a calcium channel blocker, is an FDA-approved drug used to reduce the morbidity and mortality associated with delayed ischemic deficits in patients with subarachnoid hemorrhage. In addition to its activity in the CNS, nimodipine has shown promise in multiple rodent models as a possible pharmacologic treatment for peripheral nerve injury 6–9.
Many researchers have used qualitative and semiquantitative methods to examine recovery of the facial nerve after injury 1,10,11. Our laboratory has recently developed a quantitative system to measure the return of facial nerve function after injury in a rat model 12,13. This apparatus accurately measures the amplitude, velocity, and acceleration of whisks, and provides a useful tool for precise observations of timing and completeness of facial nerve recovery after injury. The aim of this study was to evaluate the effect of nimodipine on whisking kinematics after facial nerve crush injury, using a quantitative instrument. We hypothesized that treatment with nimodipine would accelerate and/or improve overall recovery from facial nerve crush, compared with placebo. If effective, nimodipine might provide a clinically useful treatment for the management of facial nerve crush injuries.
METHODS
HEAD FIXATION AND BEHAVIORAL ADAPTION
Thirty female Wistar-Hannover rats (Charles River Laboratories, Wilmington, Massachusetts), weighing 200 to 250g, were handled daily for 2 weeks prior to surgery to condition them for behavior testing. Subsequently, rats underwent surgical insertion of a light-weight titanium head implant that provided a set of four external attachment points for rigid head fixation, as previously described 12,13. One week after head-fixation device implantation, rats were conditioned to a body restraint apparatus by brief daily placements into a snuggly fitting sack. In the third week, head restraint was added to the daily conditioning regimen. After the third week, the rats were sufficiently conditioned to undergo head/body restraint without struggling or signs of stress, and baseline testing was performed. All experimentation was conducted under protocols approved by the Massachusetts Eye and Ear Infirmary Animal Care and Use Committee and conducted in accordance with international standards on animal welfare as well as local and national regulations.
ADMINSTRATION OF COMPOUNDS
Animals were randomized into experimental (n=15) and control groups (n=15). Four days prior to facial nerve injury, the experimental animals were implanted with a subcutaneous pellet of nimodipine 14 (n=15) (40mg, 21 day sustained release pellet, Innovative research of America, Sarasota, FL) and control animals were implanted with a subcutaneous inert pellet (n=5) (Innovative research of America, Sarasota, FL) or received no pellet (n=10).
SURGICAL PROCEDURE
Rats were anesthetized with an intramuscular injection of ketamine (50 mg/kg) (Fort Dodge Animal Health, Fort Dodge, Iowa) and dexadetomidine hydrochloride (0.25 mg/kg) (Orion Corporation, Espoo, Finland) mixed in saline. The left infraauricular area was shaved and sterilely prepared. Left facial nerve exposure involved a pre-auricular incision, reflection of the parotid gland, and visual identification of the main trunk of the facial nerve as it emerged anteriorly to the posterior belly of the digastric muscle. The common trunk was electrically stimulated with a nerve stimulator (Montgomery Nerve Stimulator; Boston Medical Products, Westford, Massachusetts) at a setting of 1mV to verify complete hemifacial movement. The nerve was then crushed for 30 seconds using a jeweler’s microforceps, and the crush injury was repeated for an additional 30 seconds in the same location. The loss of electrical conductivity was verified by stimulating the proximal nerve at a setting of 2mV and observing an absence of facial movement. The wound was closed, and the anesthetic was reversed with a subcutaneous injection of 0.05mg/kg of atipamezole hydodrochloride (Orion Corporation, Espoo, Finland). Rats were allowed to recover on a warming pad, and were monitored post-operatively for signs of discomfort, including changes in grooming, social interaction, and for maintenance of normal body weight.
FUNCTIONAL RECOVERY TESTING
Baseline whisking testing was performed pre-operatively, and obtained on postoperative day 2. Daily post-operative testing of the animals began on day 8, and continued through day 17, with additional testing on day 20, 22, 24, and 31. Whisking recovery was measured using our previously described testing apparatus 12,13. Briefly, on the day of testing, animals were placed in the body restraint device, their right and left C-1 whiskers were marked using polyimide tubes (SWPT-045, SWPT-008, Small Parts Inc.), and placed into the monitoring apparatus. The horizontal movement of the marked C-1 whiskers was independently tracked using commercial laser micrometers (MetraLight, Santa Mateo CA) and a data acquisition computer 13. A computer-controlled air valve was used to deliver 10 second sustained flows of scented air toward the snout in order to elicit whisking behavior at two random time points during each 5-minute data recording session per animal.
DATA ANALYSIS
The three largest amplitude whisks were detected and analyzed in an automated fashion for each rat on each day of recording using software adapted from Bermejo et al., 1998, 2002. The data was normalized within each animal across the two sides of the face by dividing the amplitude on the injured side by the amplitude on the uninjured side, giving the relative recovery of function. This is based on prior observations that right/left whisking is generally symmetrical and to account for behavioral changes in whisking effort 15,16. A group average for relative recovery of amplitude was calculated for each testing day. Independent two sample, one tailed t-tests were then performed for postoperative days 8–17, 20, 22, 24, and 31 between the experiment group and the control group. The same data analysis was performed for the three whisks with the largest velocity and the three whisks with the greatest acceleration for post-operative days 10 – 14, the anticipated window of accelerated recovery.
RESULTS
All animals in both groups exhibited normal cage behavior throughout the study. They had normal weight gain and did not exhibit aggressive behavior. There were no post-operative wound infections after the head mount, pellet implantation, or facial nerve crush procedures. One animal in the control group did not condition appropriately to the testing apparatus, and was therefore not tested and not included in the study. All other animals tolerated testing throughout the duration of the study. No increased morbidity was noted in the drug treatment group.
WHISKING RECOVERY
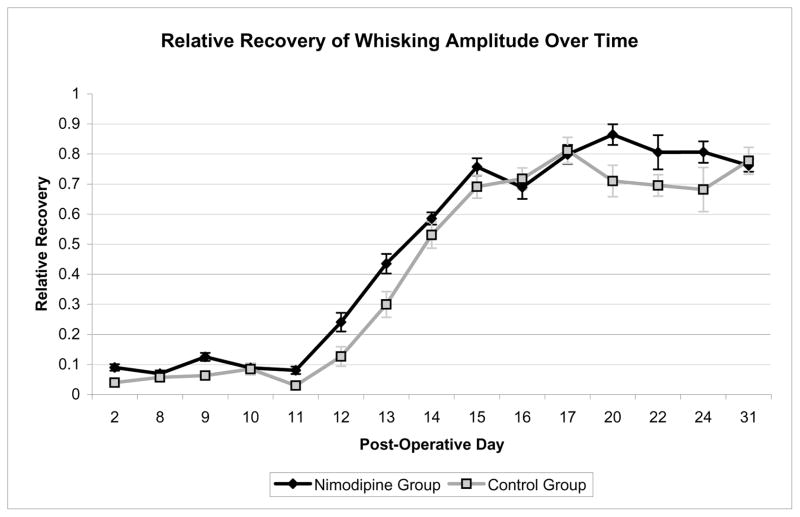
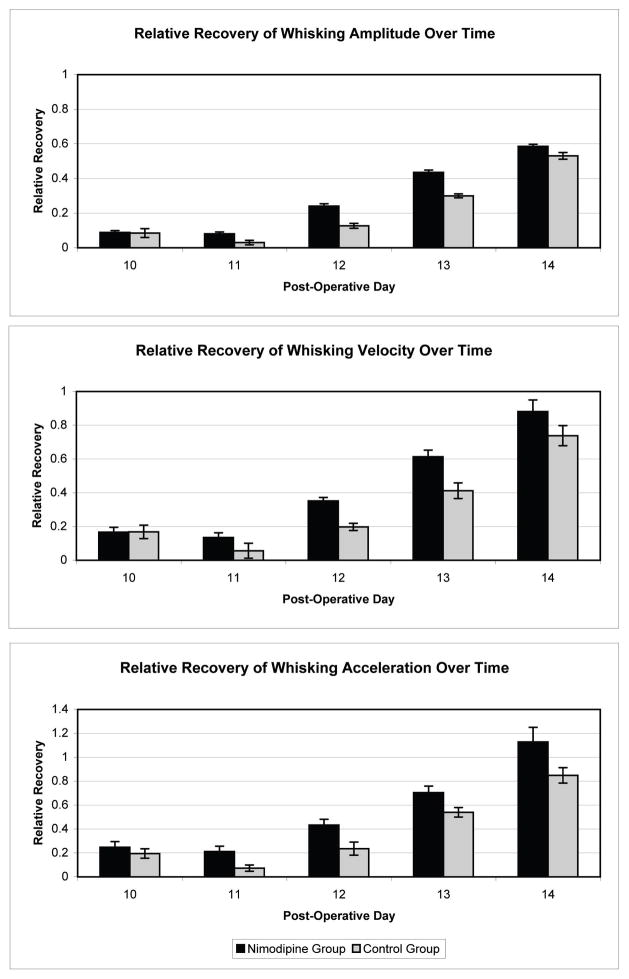
On pre-operative testing the experimental and control animals demonstrated symmetric whisking with a relative amplitude of 1.00 (standard error .023) and 1.08 (standard error .025), respectively. All animals had absence of whisking function on POD 2 with a relative amplitude in the experimental group of .09 (standard error .011) and a relative amplitude in the control group of .04 (standard error .013). Animals were noted to have return of whisking function starting on POD 9. Return of function followed a sigmoid curve, with rapid recovery of function between PODs 11 and 17. The average amplitude, velocity, and acceleration were calculated for each group. The nimodipine treated group showed a statistically significant improvement in amplitude on days 9, 11–13, and 20 as compared with the controls (p<.05, one-tailed t-test). A plateau of recovery was achieved between PODs 17 and 31, as expected. At POD 31 both groups continued to demonstrate a statistically significant difference in amplitude, velocity, and acceleration between the operated and non-operated sides (p<.05, one-tailed t-test) (Figure 1). The nimodipine-treated group also showed significantly better whisking velocity on days 11–13 and acceleration on days 11–14 as compared with the controls (p<.05, one-tailed t-test) (Figure 2).
Figure 1.
Relative recovery of whisking amplitude over time. Curves represent the calculated average relative amplitude of the 3 largest amplitude whisks for each animal on each day of testing. The nimodipine treated group showed a statistically significant improvement on post-operative days 9, 11–13, and 20 as compared with the controls (p<.05, one-tailed t-test). Error bars indicate 2-tailed standard error.
Figure 2.
Demonstrates the relative recovery of facial function for post-operative days 10–14, the days of rapid recovery of function. (Top) The nimodipine treated group showed a statistically significant improvement in relative amplitude on days 11–13 as compared with the controls (p<.05, one-tailed t-test). The nimodipine-treated group also showed significantly better relative whisking velocity on days 11–13 (middle) and relative acceleration on days 11–14 (bottom) as compared with the controls (p<.05, one-tailed t-test)
COMMENT
In this study, we administered nimodipine to facial nerve-crushed rodents, under the hypothesis that it would improve functional recovery. This hypothesis was based on literature showing that nimodipine, a calcium channel antagonist, improves the electrophysiologic recovery of the recurrent laryngeal nerve, and the functional recovery of the sciatic nerve after peripheral nerve crush 7,17. This study corroborates the previously reported benefits of nimodipine in peripheral nerve recovery, and provides quantitative functional data that demonstrates a statistically significant improvement in the relative recovery of whisking amplitude, velocity, and acceleration during the period of rapid recovery after facial nerve crush injury.
Nimodipine has demonstrated a functional benefit after both nerve crush and nerve transection/repair; however, functional analysis after extracranial facial nerve crush injury has not been previously studied. After sciatic nerve crush injury, nimodipine leads to earlier onset of functional recovery in a dose-dependent manner 17. This hastening of recovery by approximately 1–2 days after nimodipine treatment in the sciatic nerve is similar to our present findings in the facial nerve. In the rat laryngeal nerve, improved neuromuscular function has also been reported after nimodipine administration 7. In a case report, nimodipine was thought to improve vocal cord function after transection and repair of the recurrent laryngeal nerve 18. Nimodipine has also been shown in an intracranial facial nerve transection/repair model to decrease neuronal cell death 8, and in a peripheral nerve transection/repair model to increase axonal sprouting and decrease the polyneuronal innervation of target muscles 6; however, functional analysis was not performed. In an intracranial facial nerve crush model, nimodipine did not attenuate the modest (13%) facial motor nucleus cell loss caused by axonotmesis, but did accelerate the onset of axonal growth and functional recovery 9. Visual assessment of whisking after intracranial facial nerve crush identified the initiation of movement as occurring approximately 6 days sooner for nimodipine-treated rats than controls. This more pronounced hastening of whisking recovery than found in the present report may be due to differences between intra versus extracranial nerve crush locations in regeneration length from the point of injury and cellular milieu at the point of injury.
Investigators have previously shown in rats that subcutaneous pellet administration of nimodipine is safe, enhances spatial learning, and decreases the age-related decline in performance on behavioral tasks 14,19,20. These studies also proved the effectiveness of subcutaneous nimodipine pellets in providing dose-dependent levels of nimodipine in both plasma and brain 14,20. Because subcutaneous pellets obviate daily drug injections, they reduce the neurophysiological trauma that can be detrimental to behavioral testing 14, and eliminate the intake and bioavailability issues associated with oral dosing regimens 21.
Nimodipine acts by blocking L-type voltage-gated calcium channels; however, the precise mechanism of action by which nimodipine exerts its neuroprotective effects is still unknown. It has been theorized that it may enhance the supply of oxygen and nutrients to the injured region 17. Other experiments indicate that nimodipine may act by blocking L-type calcium channels to prevent intracellar accumulation of calcium, which leads to cell death 6. In addition, it may exert a positive affect on the calcium levels in nerve growth cones, increasing axonal spouting 9.
Nimodipine is an FDA-approved, orally available drug with a low side effect profile. It is the only available therapy to treat subarachnoid hemorrhage-associated vasospasm, and has been proven to reduce the morbidity and mortality associated with delayed ischemic deficits 22. Aside from nimodipine, there is no other pharmacologic treatment in clinical use shown to improve facial nerve function after injury. Herein, we demonstrate a statistically significant functional improvement after facial nerve crush injury. Given that nimodipine is an FDA-approved drug with a low side effect profile, this represents a critical step in bringing us closer to a clinical treatment for patients after peripheral nerve crush.
CONCLUSIONS
The present study demonstrates accelerated functional recovery associated with nimodipine treatment after facial nerve crush injury. These results are consistent with prior findings of enhanced peripheral nerve recovery in rats, and further indicate that nimodipine treatment may have clinical utility for patients after facial nerve injury.
Acknowledgments
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Massachusetts Eye and Ear Infirmary
Dr. Robin Lindsay and Dr. Tessa Hadlock had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. This work was supported by NIH grant number K-08 DEO15665-01 A2
References
- 1.Yeh C, Bowers D, Hadlock TA. Effect of FK506 on functional recovery after facial nerve injury in the rat. Arch Facial Plast Surg. 2007;9:333–339. doi: 10.1001/archfaci.9.5.333. [DOI] [PubMed] [Google Scholar]
- 2.Ito M, Ohbayashi M, Furukawa M, Okoyama S. Neuroprotective effects of TJ-23 (Tokishakuyakusan) on adult rat motoneurons following peripheral facial nerve axotomy. Otolaryngol Head Neck Surg. 2007;136:225–230. doi: 10.1016/j.otohns.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Reinecke K, Lucius R, Reinecke A, Rickert U, Herdegen T, Unger T. Angiotensin II accelerates functional recovery in the rat sciatic nerve in vivo: role of the AT2 receptor and the transcription factor NF-kappaB. Faseb J. 2003;17:2094–2096. doi: 10.1096/fj.02-1193fje. [DOI] [PubMed] [Google Scholar]
- 4.Hindley S, Juurlink BH, Gysbers JW, Middlemiss PJ, Herman MA, Rathbone MP. Nitric oxide donors enhance neurotrophin-induced neurite outgrowth through a cGMP-dependent mechanism. J Neurosci Res. 1997;47:427–439. [PubMed] [Google Scholar]
- 5.Gonzalez-Hernandez T, Rustioni A. Nitric oxide synthase and growth-associated protein are coexpressed in primary sensory neurons after peripheral injury. J Comp Neurol. 1999;404:64–74. [PubMed] [Google Scholar]
- 6.Angelov DN, Neiss WF, Streppel M, Andermahr J, Mader K, Stennert E. Nimodipine accelerates axonal sprouting after surgical repair of rat facial nerve. J Neurosci. 1996;16:1041–1048. doi: 10.1523/JNEUROSCI.16-03-01041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hydman J, Remahl S, Bjorck G, Svensson M, Mattsson P. Nimodipine improves reinnervation and neuromuscular function after injury to the recurrent laryngeal nerve in the rat. Ann Otol Rhinol Laryngol. 2007;116:623–630. doi: 10.1177/000348940711600811. [DOI] [PubMed] [Google Scholar]
- 8.Mattsson P, Aldskogius H, Svensson M. Nimodipine-induced improved survival rate of facial motor neurons following intracranial transection of the facial nerve in the adult rat. J Neurosurg. 1999;90:760–765. doi: 10.3171/jns.1999.90.4.0760. [DOI] [PubMed] [Google Scholar]
- 9.Mattsson P, Janson AM, Aldskogius H, Svensson M. Nimodipine promotes regeneration and functional recovery after intracranial facial nerve crush. J Comp Neurol. 2001;437:106–117. doi: 10.1002/cne.1273. [DOI] [PubMed] [Google Scholar]
- 10.Ferri CC, Moore FA, Bisby MA. Effects of facial nerve injury on mouse motoneurons lacking the p75 low-affinity neurotrophin receptor. J Neurobiol. 1998;34:1–9. [PubMed] [Google Scholar]
- 11.Most SP. Facial nerve recovery in bcl2 overexpression mice after crush injury. Arch Facial Plast Surg. 2004;6:82–87. doi: 10.1001/archfaci.6.2.82. [DOI] [PubMed] [Google Scholar]
- 12.Hadlock T, Kowaleski J, Lo D, et al. Functional Assessments of the Rodent Facial Nerve: A Synkinesis Model. Laryngoscope. 2008 doi: 10.1097/MLG.0b013e31817f5255. [DOI] [PubMed] [Google Scholar]
- 13.Heaton JT, Kowaleski JM, Bermejo R, Zeigler HP, Ahlgren DJ, Hadlock TA. A system for studying facial nerve function in rats through simultaneous bilateral monitoring of eyelid and whisker movements. J Neurosci Methods. 2008;171:197–206. doi: 10.1016/j.jneumeth.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMonagle-Strucko K, Fanelli RJ. Enhanced acquisition of reversal training in a spatial learning task in rats treated with chronic nimodipine. Pharmacol Biochem Behav. 1993;44:827–835. doi: 10.1016/0091-3057(93)90013-j. [DOI] [PubMed] [Google Scholar]
- 15.Bermejo R, Vyas A, Zeigler HP. Topography of rodent whisking--I. Two-dimensional monitoring of whisker movements. Somatosens Mot Res. 2002;19:341–346. doi: 10.1080/0899022021000037809. [DOI] [PubMed] [Google Scholar]
- 16.Bermejo R, Houben D, Zeigler HP. Optoelectronic monitoring of individual whisker movements in rats. J Neurosci Methods. 1998;83:89–96. doi: 10.1016/s0165-0270(98)00050-8. [DOI] [PubMed] [Google Scholar]
- 17.van der Zee CE, Schuurman T, Traber J, Gispen WH. Oral administration of nimodipine accelerates functional recovery following peripheral nerve damage in the rat. Neurosci Lett. 1987;83:143–148. doi: 10.1016/0304-3940(87)90231-x. [DOI] [PubMed] [Google Scholar]
- 18.Mattsson P, Bjorck G, Remahl S, et al. Nimodipine and microsurgery induced recovery of the vocal cord after recurrent laryngeal nerve resection. Laryngoscope. 2005;115:1863–1865. doi: 10.1097/01.mlg.0000177034.51559.50. [DOI] [PubMed] [Google Scholar]
- 19.Ingram DK, Joseph JA, Spangler EL, Roberts D, Hengemihle J, Fanelli RJ. Chronic nimodipine treatment in aged rats: analysis of motor and cognitive effects and muscarinic-induced striatal dopamine release. Neurobiol Aging. 1994;15:55–61. doi: 10.1016/0197-4580(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 20.Kusztos RD, Ingram DK, Spangler EL, London ED. Effects of aging and chronic nimodipine on hippocampal binding of [3H]CGS 19755. Neurobiol Aging. 1996;17:453–457. doi: 10.1016/0197-4580(96)00032-2. [DOI] [PubMed] [Google Scholar]
- 21.Laslo AM, Eastwood JD, Urquhart B, Lee TY, Freeman D. Subcutaneous administration of nimodipine improves bioavailability in rabbits. J Neurosci Methods. 2004;139:195–201. doi: 10.1016/j.jneumeth.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Allen GS, Ahn HS, Preziosi TJ, et al. Cerebral arterial spasm--a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308:619–624. doi: 10.1056/NEJM198303173081103. [DOI] [PubMed] [Google Scholar]