Abstract
Background
A close association between periodontitis and diabetes has been demonstrated in human cross-sectional studies, but an exact relationship between periodontitis and prediabetes has not been established. Previous studies using animal model systems consistently have shown that hyperinsulinemia occurs in animals with periodontitis compared to animals with healthy periodontium (while maintaining normoglycemia). Because bacterial lipopolysaccharide (LPS) plays an important role in the pathogenesis of periodontitis, we hypothesized that LPS may stimulate insulin secretion through a direct effect on β cell function. To test this hypothesis, pancreatic β cell line MIN6 cells were used to determine the effect of Porphyromonas gingivalis (Pg) LPS on insulin secretion. Furthermore, expression of genes altered by Pg LPS in innate immunity and insulin-signaling pathways was determined.
Methods
MIN6 cells were grown in medium with glucose concentration of normoglycemia (5.5 mM). Pg LPS was added to each well at final concentrations of 50, 200, and 500 ng/mL. Insulin secretion was measured using enzyme-linked immunosorbent assay. Gene expression levels altered by Pg LPS were determined by polymerase chain reaction (PCR) array for mouse innate and adaptive immunity response and mouse insulin-signaling pathways, and results were confirmed for specific genes of interest by quantitative PCR.
Results
Pg LPS stimulated insulin secretion in the normoglycemic condition by ≈1.5- to 3.0-fold depending on the concentration of LPS. Pg LPS treatment altered the expression of several genes involved in innate and adaptive immune response and insulin-signaling pathway. Pg LPS upregulated the expression of the immune response–related genes cluster of differentiation 8a (Cd8a), Cd14, and intercellular adhesion molecule-1 (Icam1) by about two-fold. LPS also increased the expression of two insulin signaling–related genes, glucose-6-phosphatase catalytic subunit (G6pc) and insulin-like 3 (Insl3), by three- to four-fold.
Conclusions
We have demonstrated for the first time that Pg LPS stimulates insulin secretion by pancreatic β cell line MIN cells. Pg LPS may have significant implications on the development of β cell compensation and insulin resistance in prediabetes in individuals with periodontitis.
Keywords: Gene expression, insulin, insulin resistance, insulin-secreting cells, lipopolysaccharides, Porphyromonas gingivalis.
A close association between periodontitis and diabetes has been demonstrated in cross-sectional studies1,2 as well as prospective studies,3,4 but the exact impact of periodontitis on the development and progression of diabetes is not well understood in human studies.
It has been shown that periodontitis is associated with ulceration of the epithelium surrounding the teeth.5,6 Thus, it is speculated that bacteria/lipopolysaccharide (LPS) enter the systemic circulation through these micro-ulcerations and cause bacteremia or induce endotoxemia.7,8 Therefore, periodontal pathogens or their products, such as LPS, may influence the physiology of insulin target organs, such as liver, skeletal muscle, and adipose tissue, and may alter glucose homeostasis by promoting insulin resistance (IR).
It is known that the prediabetic condition typically presents with IR, while the fasting glucose level is within a normal range (normoglycemia) due to increased insulin secretion (hyperinsulinemia) from pancreatic β cells. This β cell compensation9 is associated with β cell hyperplasia. However, detailed mechanisms by which hyperinsulinemia occurs are still not clear.
Results from the authors' previous studies10,11 using an animal model indicate that IR develops in the setting of periodontitis induced by ligature placement and application of LPS around the gingival sulcus. IR that developed in these animals was associated with a reduced phosphorylated Akt (p-Akt)/Akt ratio in the liver upon stimulation with exogenous insulin.10 The authors have also shown that insulin levels increase during IR/prediabetic development in this animal model.10,11 Although LPS can stimulate the production of cytokines that promote IR in insulin target organs,12,13 it has been shown that hyper-insulinemia drives the development of peripheral IR.14 Thus, LPS may have direct effects on insulin secretion and contribute to the development of hyperinsulinemia and, thereby, promote IR by this additional mechanism. Thus, the authors hypothesized that LPS from periodontal pathogens stimulates β cells to increase insulin secretion. To test this hypothesis, insulin secretion by MIN cells, which are a pancreatic islet β cell line, was measured following introduction of periodontal pathogen Porphyromonas gingivalis (Pg) LPS.
Materials and Methods
As a first step to understand possible mechanisms by which LPS influences insulin secretion from pancreatic β cells, expression patterns of genes that are modulated by Pg LPS were determined using polymerase chain reaction (PCR) arrays. PCR arrays‖ take advantage of the combination of sensitive and reliable PCR performance with the ability of the microarrays to analyze the expression of many genes simultaneously. The authors selected PCR arrays specific for genes related to two pathways: 1) mouse innate and adaptive immune response; and 2) mouse insulin signaling.
MIN6 Cell Culture
MIN6 cells were grown in Dulbecco's modified Eagle's medium¶ (DMEM) (25 mM glucose, 4 mM L-glutamine, 1 mM of 0.1 mg/mL sodium pyruvate) supplemented with penicillin/streptomycin, 2 μL/L 2-mercaptoethanol, and 10% final concentration of fetal bovine serum (FBS).# Cells were incubated at 37°C in a 5% CO2 humidified incubator. The culture medium was changed every 3 to 4 days and also passaged once a week.
Glucose-Stimulated Insulin Secretion and Determination of Insulin Levels
Mid-log MIN6 cells (passages 23 to 25) were plated in 24-well plates at 2 × 105 cells in DMEM described above, except with 5.5 mM glucose and incubated at 37°C and 5% CO2. After 24 hours, media were replenished with DMEM with 5.5 mM glucose. Pg LPS at final concentrations of 0, 50, 200, and 500 ng/mL was added to each well, and plates were incubated for an additional 16 hours. The wells were washed twice with Krebs-Ringer bicarbonate HEPES (KRBH) 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (119 mM NaCl, 2.54 mM CaCl20.2H2O, 1.19 mM KH2PO4, 4.74 mM KCl, 25 mM NaHCO3, MgCl20.6H2O, 10 mM HEPES) plus 0.5% bovine serum albumin (BSA).** KRBH-0.5% BSA plus 2.8 mM glucose was added to each well, and cells were incubated for 30 minutes. This minimal glucose solution was removed and replaced with KRBH-0.5% BSA plus 5 mM glucose (normoglycemia concentration) or 25 mM glucose (hyperglycemia concentration) with the same LPS concentration, and the cells were reincubated for 2 hours. Supernatants (700 μL) were collected from each well and spun briefly to remove cell debris. The insulin concentrations in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA).††
Total RNA Extraction and Reverse Transcription
The remaining buffer was removed from the 24-well plates. Cells were lysed immediately, and total RNA was isolated using acid guanidinium thiocyanate-phenol-chloroform extraction‡‡ according to the manufacturer's protocol. Total RNA was further purified using a kit§§ along with DNase I treatment, according to the manufacturer's protocol. The integrity of the RNA was ascertained using screen tape–based capillary electrophoresis.‖‖
Genomic DNA elimination and cDNA synthesis were carried out using a kit,¶¶ according to the manufacturer's protocol. Briefly, 0.5 μg purified RNA was incubated with genomic DNA elimination mix (10 μL) for 5 minutes at 42°C and then chilled on ice. Reverse-transcription mix (10 μL) was added and incubated at 42°C for 15 minutes. The reaction was stopped immediately by incubating tubes at 95°C for 5 minutes, tubes were placed on ice, and the cDNA synthesized was either used immediately or stored at −20°C and then used for quantitative PCR (qPCR).
PCR Array
PCR arrays were used to compare the messenger RNA (mRNA) expression levels in RNAs from untreated and Pg LPS–treated MIN6 cells. The authors selected arrays for two pathways: 1) mouse innate and adaptive immune response; and 2) mouse insulin signaling. Briefly, the PCR master mix was prepared using qPCR solution,## cDNA, and RNase-free water, and the mix was dispensed into the wells of PCR arrays. The thermal cycler was programmed to carry out amplification as follows: one cycle of 10 minutes at 95°C and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. After the amplification, the baselines were defined by choosing the automated baseline option. The threshold values were defined manually, ensuring that they were the same across all PCR arrays. Dissociation curve analysis was performed to verify PCR specificity. Cycle threshold (CT) values were analyzed using software.***
Validation of PCR Array
The cDNAs obtained from purified RNAs of untreated and Pg LPS–treated MIN6 cells were amplified using the same reagents and the same cycling parameters as the PCR array described above. Fold changes in mRNA levels were calculated using the ΔΔCT method. Four independent experiments were performed for each gene. The catalog number or specific sequences of primers††† used for the various genes are shown in Table 1.
Table 1. Catalog Number or Custom-Made Specific Sequences of Primers Used for the Various Genes.
| Gene | Catalog Number or Sequence |
|---|---|
|
| |
| Cd8a | PPM04031A |
| G6pc | PPM05087F |
| Cd14 | PPM06249G |
| Icam1 | PPM03196A |
| Insl3 | PPM41817B |
| β2-m | PPM03562A |
| Ins1 | CCTGTTGGTGCACTTCCTA and TCTGAAGGTCCCCGGGGCT |
| Ins2 | TGGCTTCTTCTACACACCCAAG and ACAATGCCACGCTTCTGCC |
Statistical Analyses
Statistical analysis was performed using a Mann-Whitney U test for between-group comparisons with a significance level of P <0.05.
Results
Treatment With Pg LPS Augments Glucose-Stimulated Insulin Secretion in MIN6 Cells in Normoglycemic but not Hyperglycemic Condition
MIN6 cells were treated with 0, 50, 200, and 500 ng/mL Pg LPS for 16 hours in DMEM containing 5.5 mM glucose and 10% FBS, then treated with 5 mM or 25 mM glucose for 2 hours, and the supernatant was collected to determine secreted insulin levels. As shown in Figure 1, Pg LPS significantly augmented basal insulin secretion by MIN6 cells. Whereas 50 ng/mL LPS increased the insulin secretion by 1.5-fold, higher concentrations of LPS induced a two- to three-fold increase in insulin secretion. However, LPS treatment did not alter stimulation of insulin secretion by high concentrations of glucose (25 mM) (Fig. 1).
Figure 1.
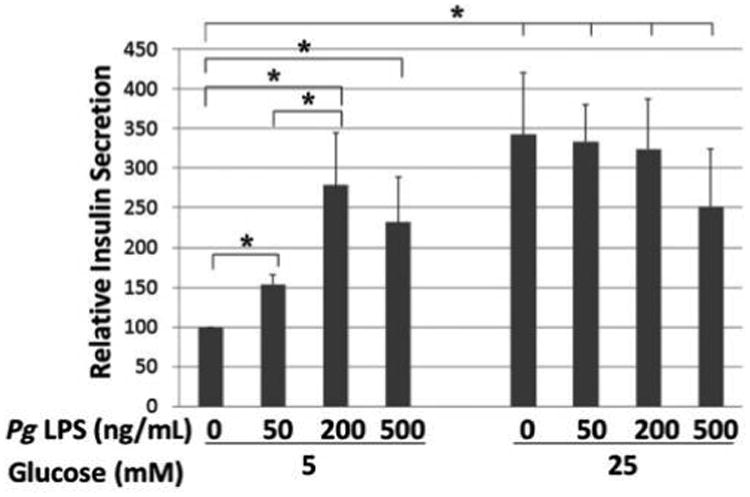
Effect of Pg LPS treatment on insulin secretion in MIN6 cells stimulated with 5 or 25 mM glucose. The insulin secretion assay (glucose-stimulated insulin secretion) outcome was analyzed by ELISA. x-Axis: concentration of Pg LPS; y-axis: relative insulin secretion. Mean ± SD for three independent experiments. * P <0.05.
Pg LPS Upregulates the Expression of Several Genes Involved in Immune Response and Insulin-Signaling Pathway in MIN6 Cells
Genes whose expression was upregulated more than two-fold in PCR arrays are shown in Table 2. Of these genes, several of interest were selected for validation by performing real-time qPCR with gene-specific primers. As shown in Figure 2, treatment with Pg LPS at 200 ng/mL upregulated the expression of the immune response–related genes cluster of differentiation 8a (Cd8a), Cd14, and intercellular adhesion molecule-1 (Icam1) by approximately two-fold (P <0.05). LPS also increased the expression of two insulin-signaling pathway genes, glucose-6-phosphatase catalytic subunit (G6pc) and insulin-like 3 (Insl3), by three- to four-fold (P <0.05). Increased expression of signal transducer and activator of transcription protein 4 (Stat4) was suggested by the array, but the increase was not significant using qPCR (P >0.05) (data not shown). The authors further investigated insulin 1 (ins1) and ins2 gene expression because insulin secretion was upregulated upon Pg LPS addition. The result indicates that LPS treatment did not significantly alter the expression of the two mouse insulin genes, ins1 and ins2, in MIN6 cells (Fig. 3).
Table 2. Change in Mouse Gene Expression Following Incubation With Pg LPS.
| Gene | Fold change |
|---|---|
| Immune response genes | |
| Increased expression | |
| Stat4 | +19.63 |
| Cd8a | +7.23 |
| Cd4 | +6.59 |
| Itgam | +5.44 |
| Foxp3 | +4.19 |
| Icam1 | +4.16 |
| C5ar1 | +3.73 |
| Cd80 | +3.05 |
| Fasl | +3.02 |
| Cd14 | +2.43 |
| II4 | +2.30 |
| Tbx21 | +2.34 |
| Decreased expression | |
| Inb1 | −23.01 |
| IL6 | −11.94 |
| Csf2 | −11.80 |
| Gata3 | −9.10 |
| Il5 | −8.78 |
| Tlr7 | −5.55 |
| Nlrp3 | −4.94 |
| Ifna2 | −4.80 |
| Ccr4 | −4.51 |
| Tlr6 | −4.34 |
| Cd40lg | −3.40 |
| Tlr9 | −2.29 |
| Il10 | −2.21 |
|
| |
| Insulin-signaling genes | |
| Increased expression | |
| Insl3 | +4.89 |
| G6pc | +4.00 |
| Igfbp1 | +3.89 |
| Nos2 | +3.64 |
| Retn | +3.49 |
| Hk2 | +2.11 |
| Serpine1 | +2.09 |
| Decreased expression | |
| Tg | −34.84 |
| Lep | −9.09 |
| Dok1 | −2.26 |
| Grb10 | −2.21 |
Figure 2.
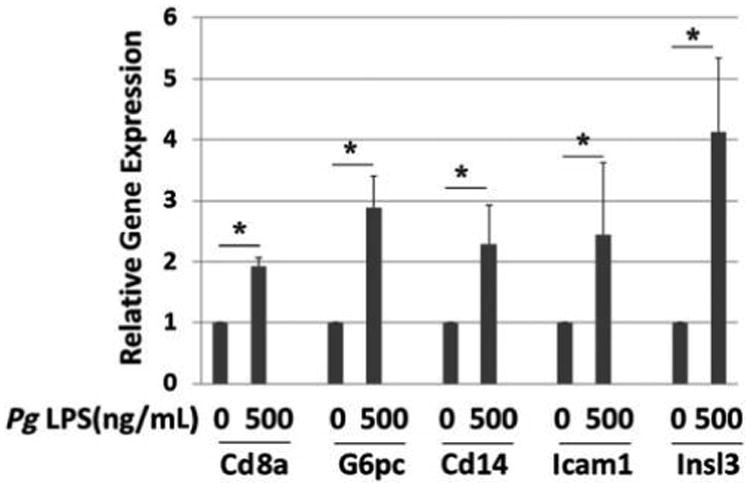
Effect of Pg LPS on gene expression by MIN6 cells. Relative gene expression determined by qPCR. x-Axis: genes and concentration of Pg LPS; y-axis: relative gene expression. Mean ± SD for three independent experiments. * P <0.05.
Figure 3.
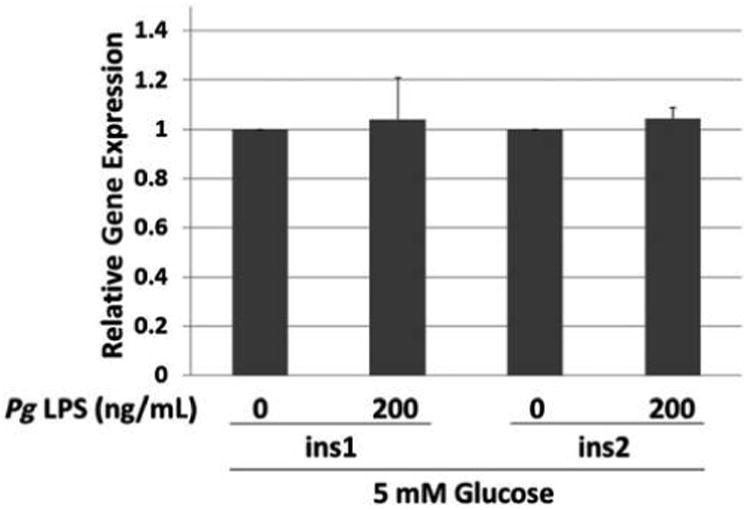
Effect of Pg LPS on expression of ins1 and ins2 genes by MIN6 cells. Relative gene expression determined by qPCR. x-Axis: genes and concentration of Pg LPS. Mean ± SD of three independent experiments. All comparisons P >0.05.
Discussion
Results from epidemiologic studies15,16 suggest that IR before the onset of type 2 diabetes may be associated with periodontitis. Insulin resistance/prediabetes can exist while blood glucose levels are still in the normal or impaired glycemic range and, thus, occur well before the onset of diabetes. In addition, IR occurs when insulin signaling is impaired in insulin target organs. During the development of IR, β cells undergo compensatory alterations to increase insulin secretion (hyperinsulinemia) in an early stage of IR followed by declining insulin secretion resulting from β cell apoptosis in type 2 diabetes.9
Previous studies indicated that hyperinsulinemia occurs in animals with periodontitis compared to animals with healthy gingiva.10,11 However, whether this upregulated insulin secretion was in response to impaired insulin signaling on insulin target organs, which in turn resulted in β cell compensation, and/or some other factor stimulating insulin secretion was not determined. These two different pathways of how hyperinsulinemia occurs may be crucial in understanding how periodontitis influences the development of prediabetes and diabetes. Therefore, the current study investigates whether LPS might exert direct effects on insulin secretion by β cells. The concentration range of Pg LPS investigated (0 to 500 ng/mL) was at the low end of the range used in in vivo and in vitro studies (0 to 50,000 ng/mL).17-19 It is noteworthy that the concentration of Pg LPS in the systemic circulation in humans with periodontitis is not known.
These results indicate that Pg LPS is able to stimulate insulin secretion from the β cell line MIN6 at normoglycemic levels. However, the authors did not observe a significant effect of LPS at hyperglycemic levels. Because hyperglycemia induces high levels of insulin secretion using 25 mM glucose concentration without LPS (Fig. 1), LPS-stimulated insulin secretion might have been masked. It has been reported that Escherichia coli LPS at normoglycemia does not influence insulin secretion from rat islet cells during a 36-hour incubation.20 Thus, it is possible that hyperinsulinemia observed in the setting of periodontitis10,11 may be due, at least in part, to direct effects of Pg LPS on β cells. However, it is not known how LPS is transported to the β cells, and the concentration of LPS in the pancreas needs to be determined. Studies to identify pathways of LPS to the pancreas would be technically very challenging, and they are beyond the scope of this initial report. Altogether, it is important to note that other mechanisms may also contribute to this effect of periodontitis/LPS on β cell function in vivo, and that additional studies are needed to establish the relative importance of the direct versus indirect effect of LPS in mediating the impact of periodontitis on β cell function in vivo.
Using PCR arrays, genes whose expression is altered by Pg LPS were further determined. Genes altered by Pg LPS included those for mouse innate and adaptive immunity response and mouse insulin signaling. The authors focused on upregulated genes based on the results from PCR arrays. Four independent experiments and qPCR were then performed for all genes listed in Table 1. Cd8a, G6pc, Cd14, Icam1, and Insl3 were reproducibly upregulated by LPS. Cd8a, Cd14, and Icam1 belong to innate and adaptive immunity response genes, and G6pc and Insl3 belong to insulin-signaling pathway genes.
G6pc codes for the catalytic subunit of glucose-6-phosphatase, which catalyzes glucose-6-phosphate to glucose and is involved in gluconeogenesis and glycogenolysis. Changes in G6pc expression in liver, one of the major insulin target organs, has been investigated by others. For example, treatment with E. coli LPS (10 mg/kg) induced increased G6pc activity in hepatocytes of Sprague-Dawley rats,21 and E. coli LPS (20 mg/mL) in rats increased hepatic G6pc mRNA.22 In the current study, overnight treatment with Pg LPS resulted in a three-fold upregulation of G6pc mRNA, which was concomitant with a two-fold increase in glucose-stimulated insulin secretion from pancreatic MIN6 cells. Thus, it appears that LPS influences G6pc expression in both liver and β cells of the pancreas.
Another gene for which expression was altered by Pg LPS is Cd14, which functions in LPS binding to Toll-like receptor (TLR2 and -4) at the surface of innate immune cells such as monocytes, macro-phages, and neutrophils. For LPS to exert activation of innate immunity, CD14 is required.23,24 Activation of TLR2 and -4 by CD14 and LPS triggers the secretion of proinflammatory cytokines via the myeloid differentiation primary response 88 (MyD88)-dependent pathway, and some of these cytokines interfere with insulin signaling and thus contribute to IR. Cani et al.25 have shown that Cd14 mutant mice fed a normal diet are hypersensitive to insulin (i.e., delayed IR) and are resistant to most of the LPS-induced and/or high fat diet–induced metabolic indicators of endotoxemia. A two-fold increase was observed in Cd14 mRNA in MIN6 cells treated with Pg LPS. This upregulated expression of Cd14 by Pg LPS parallels the upregulation of insulin secretion by MIN cells, suggesting that CD14 may function in binding LPS to activate β cells. Increased levels of soluble CD14 were found in the serum of patients with periodontitis compared with individuals without periodontitis.26 Thus, in the setting of periodontitis, CD14 in the systemic circulation as well as upregulated expression of Cd14 by β cells may both be evident.
Another gene that was altered and its upregulation confirmed by qPCR is Cd8a. Cd8a codes for the a-chain isoform of the CD8 cell surface glycoprotein found in most cytotoxic T cells. In addition to cytotoxic T cells, CD8 is also expressed in natural killer cells, cortical thymocytes, and dendritic cells. In association with the T cell receptor, CD8 recognizes antigen displayed on the surface of antigen-presenting cells via major histocompatibility complex class I.27 In type 1 autoimmune diabetes, CD8+ T lymphocytes are cytotoxic for pancreatic β cells.28 It has been reported that CD8+ cells react with a number of auto-antigens related to diabetes, such as proinsulin29 and G6pc-related protein (islet-specific G6pc subunit– related protein [IGRP]),30 in non-obese diabetic (NOD) mouse models. Thus far, there is no report of Cd8a expression in pancreatic β cells or MIN cells. However, a two-fold increase was observed in Cd8 mRNA in response to treatment with Pg LPS in MIN6 cells.
Expression of Icam1 is also upregulated two- to three-fold in Pg LPS–stimulated MIN cells. ICAM1 is a cell surface glycoprotein that is a member of the immunoglobulin gene family and is expressed on many cell types including those of the immune system such as macrophages, neutrophils, and lymphocytes.31,32 ICAM1 is a ligand for lymphocyte function–associated antigen-1 (LFA1) found on leukocytes; when these cells are activated, they bind to endothelial cells via ICAM1/LFA-1 and then migrate into tissues.33 In several other cells types, ICAM1 functions as a signal-transducing molecule associated with proinflammatory pathways.34 ICAM1 binding induces cyclic adenosine monophosphate (cAMP) accumulation and activation of extracellular signal-regulated kinase, protein kinase A, and protein kinase C (but not c-Jun N-terminal kinase) and cAMP response element–binding protein phosphorylation, leading to the induction of tumor necrosis factor-a in rat astrocytes. ICAM1 was also found to be upregulated by Pg, mediated via nucleotide-binding oligomerization domain-containing protein 1/2 in cultured gingival fibroblasts.35 Thus, ICAM1 appears to have different signaling pathways in different cell types. The significance of upregulated Icam1 expression in β cells/MIN6 cells has yet to be determined.
It has been reported that both Icam1-immunoglobulin fusion proteins and recombinant soluble ICAM1 can prevent the proliferation of T cells to pancreatic islet cells in patients with type 1 diabetes as well as in individuals at risk for developing the disease. Thus, circulating soluble forms of ICAM1 (sICAM1) may downregulate inflammation in patients prone to type 1 diabetes.36 Increased Icam1 expression may thus relate to sICAM1, and this possibility needs to be investigated.
Another gene expression altered by Pg LPS is Insl3. The authors observed four- to five-fold increase in Insl3 expression in MIN6 cells treated with Pg LPS. Insl3 is a hormone secreted in the gonadal tissues of males and females. Insl3 secreted by Leydig cells in the testes is responsible for testicular descent.37 In men with type 2 diabetes, an early impairment of overall Leydig cell function with concomitant decrease in Insl3 expression is reported.38 However, there are no reports on the effect of LPS on Insl3 expression in other cell types, including b cells, MIN cells, or hepatocytes. Exact function of this protein in insulin secretion needs to be investigated.
The authors also investigated whether the observed increase in insulin secretion by Pg LPS is due to upregulation of its transcription. Unlike other mammals, rats and mice possess two non-allelic but structurally similar insulin genes, termed ins1 and ins2, that are located on different chromosomes.39 Whereas ins2, which has two introns, is an ortholog to the insulin genes in other mammals, ins1 has only one intron and originates from partially processed mRNA of ins2. Abrogation of ins2 leads to IR in NOD mice, but no such effect was observed in ins1-knockout NOD mice.40 No significant effect was observed for Pg LPS on mRNAs of either mouse insulin gene, suggesting that the observed increase in insulin secretion occurs after transcription of insulin genes. Genes known to be involved in insulin secretion were not included in the PCR array; thus, no data are available on the expression level of these genes.
Although primary pancreatic β cells are ideal in biochemical and molecular studies, the difficulty of isolating and maintaining islets is a major drawback. To overcome this difficulty, several β cell lines have been developed. Among them, cell lines such as MIN6, obtained from insulinoma developed by targeted expression of Simian virus 40 large T antigen in transgenic mice, have been well characterized.41 MIN6 cells transport and metabolize glucose similarly to primary b cells and secrete insulin in an appropriate glucose-dependent manner.42-44 These characteristics make the MIN6 cell line especially useful to study the modulation of glucose-stimulated insulin secretion in mice. However, high-passage (>40) MIN6 cells have been shown to have impaired glucose-stimulated insulin secretion and impaired glucose and lipid metabolism as well as changes in morphology.45 Therefore, the authors used MIN6 cells of low passage number (23 to 25) in all of the experiments.
Conclusions
We have demonstrated for the first time that Pg LPS stimulates insulin secretion by the pancreatic β cell line MIN6. Pg LPS may have significant implications on the development of β cell compensatory response and IR in prediabetes in individuals with periodontitis. Several genes are involved in this process, and functions and contributions of these gene products need to be further investigated.
Acknowledgments
This study was supported by the NIH R01DE021405 (KW). MIN6 cells were a kind gift from Dr. Donald Steiner, A.N. Pritzker Distinguished Service Professor, Department of Biochemistry and Molecular Biology and Professor, Department of Medicine, Section of Endocrinology, Diabetes & Metabolism, The University of Chicago, Chicago, Illinois. The authors report no conflicts of interest related to this study.
Footnotes
RT2 Profiler PCR arrays, Qiagen, Valencia, CA.
Life Technologies, Thermo Fisher Scientific, Waltham, MA.
Atlanta Biologicals, Lawrenceville, GA.
Sigma, St. Louis, MO.
Mouse Insulin High Range ELISA kit, ALPCO, Salem, NH.
Trizol, Life Technologies, Thermo Fisher Scientific.
RNeasy Mini kit from Qiagen.
Agilent 2200 TapeStation, Agilent Technologies, Santa Clara, CA.
RT2 First Strand Kit (cat# 330401), Qiagen.
RT2 SYBR Green ROX qPCR Mastermix (cat # 330520), Qiagen.
SABioscience/Qiagen PCR Array Data Analysis software, Qiagen.
RT2 qPCR Primer Assays, Qiagen.
References
- 1.Tsai C, Hayes C, Taylor GW. Glycemic control of type 2 diabetes and severe periodontal disease in the US adult population. Community Dent Oral Epidemiol. 2002;30:182–192. doi: 10.1034/j.1600-0528.2002.300304.x. [DOI] [PubMed] [Google Scholar]
- 2.Shlossman M, Knowler WC, Pettitt DJ, Genco RJ. Type 2 diabetes mellitus and periodontal disease. J Am Dent Assoc. 1990;121:532–536. doi: 10.14219/jada.archive.1990.0211. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay D, Marlow NM, Fernandes JK, Leite RS. Periodontal disease progression and glycaemic control among Gullah African Americans with type-2 diabetes. J Clin Periodontol. 2010;37:501–509. doi: 10.1111/j.1600-051X.2010.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demmer RT, Desvarieux M, Holtfreter B, et al. Periodontal status and A1C change: Longitudinal results from the study of health in Pomerania (SHIP) Diabetes Care. 2010;33:1037–1043. doi: 10.2337/dc09-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller-Glauser W, Schroeder HE. The pocket epithelium: A light- and electronmicroscopic study. J Periodontol. 1982;53:133–144. doi: 10.1902/jop.1982.53.3.133. [DOI] [PubMed] [Google Scholar]
- 6.Davenport RH, Jr, Simpson DM, Hassell TM. Histometric comparison of active and inactive lesions of advanced periodontitis. J Periodontol. 1982;53:285–295. doi: 10.1902/jop.1982.53.5.285. [DOI] [PubMed] [Google Scholar]
- 7.Castillo DM, Sánchez-Beltrán MC, Castellanos JE, et al. Detection of specific periodontal microorganisms from bacteraemia samples after periodontal therapy using molecular-based diagnostics. J Clin Periodontol. 2011;38:418–427. doi: 10.1111/j.1600-051X.2011.01717.x. [DOI] [PubMed] [Google Scholar]
- 8.Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(Suppl. 11):2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 9.Rafacho A, Cestari TM, Taboga SR, Boschero AC, Bosqueiro JR. High doses of dexamethasone induce increased beta-cell proliferation in pancreatic rat islets. Am J Physiol Endocrinol Metab. 2009;296:E681–E689. doi: 10.1152/ajpendo.90931.2008. [DOI] [PubMed] [Google Scholar]
- 10.Watanabe K, Iizuka T, Adeleke A, et al. Involvement of toll-like receptor 4 in alveolar bone loss and glucose homeostasis in experimental periodontitis. J Periodontal Res. 2011;46:21–30. doi: 10.1111/j.1600-0765.2010.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe K, Petro BJ, Shlimon AE, Unterman TG. Effect of periodontitis on insulin resistance and the onset of type 2 diabetes mellitus in Zucker diabetic fatty rats. J Periodontol. 2008;79:1208–1216. doi: 10.1902/jop.2008.070605. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 13.Feinstein R, Kanety H, Papa MZ, Lunenfeld B, Karasik A. Tumor necrosis factor-a suppresses insulin-induced tyrosine phosphorylation of insulin receptor and its substrates. J Biol Chem. 1993;268:26055–26058. [PubMed] [Google Scholar]
- 14.Rizza RA, Mandarino LJ, Genest J, Baker BA, Gerich JE. Production of insulin resistance by hyperinsulinaemia in man. Diabetologia. 1985;28:70–75. doi: 10.1007/BF00279918. [DOI] [PubMed] [Google Scholar]
- 15.Choi YH, McKeown RE, Mayer-Davis EJ, Liese AD, Song KB, Merchant AT. Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care. 2011;34:381–386. doi: 10.2337/dc10-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zadik Y, Bechor R, Galor S, Levin L. Periodontal disease might be associated even with impaired fasting glucose. Br Dent J. 2010;208:E20. doi: 10.1038/sj.bdj.2010.291. [DOI] [PubMed] [Google Scholar]
- 17.Deleon-Pennell KY, de Castro Bras LE, Lindsey ML. Circulating Porphyromonas gingivalis lipopolysaccharide resets cardiac homeostasis in mice through a matrix metalloproteinase-9-dependent mechanism. Physiol Rep. 2013:e00079. doi: 10.1002/phy2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung SL, Lee NG, Chang LY, Chen YT, Lai YL. Stimulatory effects of glucose and Porphyromonas gingivalis lipopolysaccharide on the secretion of inflammatory mediators from human macrophages. J Periodontol. 2014;85:140–149. doi: 10.1902/jop.2013.130009. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa T, Asai Y, Yamamoto H, et al. Immunobiological activities of a chemically synthesized lipid A of Porphyromonas gingivalis. FEMS Immunol Med Microbiol. 2000;28:273–281. doi: 10.1111/j.1574-695X.2000.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 20.Vives-Pi M, Somoza N, Fernández-Alvarez J, et al. Evidence of expression of endotoxin receptors CD14, toll-like receptors TLR4 and TLR2 and associated molecule MD-2 and of sensitivity to endotoxin (LPS) in islet beta cells. Clin Exp Immunol. 2003;133:208–218. doi: 10.1046/j.1365-2249.2003.02211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang YH, McKenna T, Watson LP, Williams R, Holt M. Cytochemical changes in hepatocytes of rats with endotoxemia or sepsis: Localization of fibronectin, calcium, and enzymes. J Histochem Cytochem. 1988;36:665–678. doi: 10.1177/36.6.2835411. [DOI] [PubMed] [Google Scholar]
- 22.Maitra SR, Gestring ML, El-Maghrabi MR, Lang CH, Henry MC. Endotoxin-induced alterations in hepatic glucose-6-phosphatase activity and gene expression. Mol Cell Biochem. 1999;196:79–83. [PubMed] [Google Scholar]
- 23.Kitchens RL. Role of CD14 in cellular recognition of bacterial lipopolysaccharides. Chem Immunol. 2000;74:61–82. doi: 10.1159/000058750. [DOI] [PubMed] [Google Scholar]
- 24.Tapping RI, Tobias PS. Soluble CD14-mediated cellular responses to lipopolysaccharide. Chem Immunol. 2000;74:108–121. doi: 10.1159/000058751. [DOI] [PubMed] [Google Scholar]
- 25.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi J, Masaka T, Ishikawa I. Increased levels of soluble CD14 in sera of periodontitis patients. Infect Immun. 1999;67:417–420. doi: 10.1128/iai.67.1.417-420.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao GF, Jakobsen BK. Molecular interactions of coreceptor CD8 and MHC class I: The molecular basis for functional coordination with the T-cell receptor. Immunol Today. 2000;21:630–636. doi: 10.1016/s0167-5699(00)01750-3. [DOI] [PubMed] [Google Scholar]
- 28.Wong FS, Siew LK, Scott G, et al. Activation of insulin-reactive CD8 T-cells for development of autoimmune diabetes. Diabetes. 2009;58:1156–1164. doi: 10.2337/db08-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama M, Abiru N, Moriyama H, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–223. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieberman SM, Evans AM, Han B, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci USA. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Springer TA. Adhesion receptors of the immune system. Nature. 1990;346:425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- 32.Satoh S, Nüssler AK, Liu ZZ, Thomson AW. Proinflammatory cytokines and endotoxin stimulate ICAM-1 gene expression and secretion by normal human hepatocytes. Immunology. 1994;82:571–576. [PMC free article] [PubMed] [Google Scholar]
- 33.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etienne-Manneville S, Chaverot N, Strosberg AD, Couraud PO. ICAM-1-coupled signaling pathways in astrocytes converge to cyclic AMP response element-binding protein phosphorylation and TNF-alpha secretion. J Immunol. 1999;163:668–674. [PubMed] [Google Scholar]
- 35.Liu J, Duan J, Wang Y, Ouyang X. Intracellular adhesion molecule-1 is regulated by Porphyromonas gingivalis through nucleotide binding oligomerization domain-containing proteins 1 and 2 molecules in periodontal fibroblasts. J Periodontol. 2014;85:358–368. doi: 10.1902/jop.2013.130152. [DOI] [PubMed] [Google Scholar]
- 36.Roep BO, Heidenthal E, de Vries RR, Kolb H, Martin S. Soluble forms of intercellular adhesion molecule-1 in insulin-dependent diabetes mellitus. Lancet. 1994;343:1590–1593. doi: 10.1016/s0140-6736(94)93055-4. [DOI] [PubMed] [Google Scholar]
- 37.Ivell R, Anand-Ivell R. Biological role and clinical significance of insulin-like peptide 3. Curr Opin Endocrinol Diabetes Obes. 2011;18:210–216. doi: 10.1097/MED.0b013e3283453fe6. [DOI] [PubMed] [Google Scholar]
- 38.Ermetici F, Donadio F, Iorio L, et al. Peripheral insulinlike factor 3 concentrations are reduced in men with type 2 diabetes mellitus: Effect of glycemic control and visceral adiposity on Leydig cell function. Eur J Endocrinol. 2009;161:853–859. doi: 10.1530/EJE-09-0203. [DOI] [PubMed] [Google Scholar]
- 39.Shiao MS, Liao BY, Long M, Yu HT. Adaptive evolution of the insulin two-gene system in mouse. Genetics. 2008;178:1683–1691. doi: 10.1534/genetics.108.087023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Babaya N, Nakayama M, Moriyama H, et al. A new model of insulin-deficient diabetes: Male NOD mice with a single copy of Ins1 and no Ins2. Diabetologia. 2006;49:1222–1228. doi: 10.1007/s00125-006-0241-4. [DOI] [PubMed] [Google Scholar]
- 41.Skelin M, Rupnik M, Cencic A. Pancreatic beta cell lines and their applications in diabetes mellitus research. ALTEX. 2010;27:105–113. doi: 10.14573/altex.2010.2.105. [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki J, Araki K, Yamato E, et al. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- 43.Ishihara H, Asano T, Tsukuda K, et al. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia. 1993;36:1139–1145. doi: 10.1007/BF00401058. [DOI] [PubMed] [Google Scholar]
- 44.Nakashima K, Kanda Y, Hirokawa Y, Kawasaki F, Matsuki M, Kaku K. MIN6 is not a pure beta cell line but a mixed cell line with other pancreatic endocrine hormones. Endocr J. 2009;56:45–53. doi: 10.1507/endocrj.k08e-172. [DOI] [PubMed] [Google Scholar]
- 45.Cheng K, Delghingaro-Augusto V, Nolan CJ, et al. High passage MIN6 cells have impaired insulin secretion with impaired glucose and lipid oxidation. PLoS ONE. 2012;7:e40868. doi: 10.1371/journal.pone.0040868. [DOI] [PMC free article] [PubMed] [Google Scholar]


