Abstract
C-reactive protein (CRP) is an inflammatory biomarker of inflammation and may reflect progression of vascular disease. Conflicting evidence suggests CRP may be a prognostic biomarker of ischemic stroke outcome. Most studies that have examined the relationship between CRP and ischemic stroke outcome have used mortality or subsequent vascular event as the primary outcome measure. Given that nearly half of stroke patients experience moderate to severe functional impairments, using a biomarker like CRP to predict functional recovery rather than mortality may have clinical utility for guiding acute stroke treatments. The primary aim of this study was to systematically and critically review the relationship between CRP and long-term functional outcome in ischemic stroke patients to evaluate the current state of the literature. PubMed and MEDLINE databases were searched for original studies which assessed the relationship between acute CRP levels measured within 24 hours of symptom onset and long-term functional outcome. The search yielded articles published between 1989 and 2012. Included studies used neuroimaging to confirm ischemic stroke diagnosis, high-sensitivity CRP assay, and a functional outcome scale to assess prognosis beyond 30 days after stroke. Study quality was assessed using the REMARK recommendations. Five studies met all inclusion criteria. Results indicate a significant association between elevated baseline high sensitivity CRP and unfavorable long-term functional outcome. Our results emphasize the need for additional research to characterize the relationship between acute inflammatory markers and long-term functional outcome using well-defined diagnostic criteria. Additional studies are warranted to prospectively examine the relationship between high sensitivity CRP measures and long-term outcome.
Keywords: C-reactive protein, CRP, Functional recovery, Ischemic stroke, Neuroimaging, Outcome after stroke, Prognosis
1. Introduction
Stroke is the fourth leading cause of death and leading cause of adult disability in the USA (1). Long-term disability is a significant problem amongst survivors, but nonetheless much of the literature has emphasized mortality rates as an outcome measure rather than functional status and reduction of morbidity. Studies have shown that nearly 15–30% of stroke survivors are permanently disabled, and 20% of stroke survivors require institutional care 3 months following stroke (2, 3). Given the large burden of disability following stroke, a need exists to identify clinical biomarkers so that individualized post-ischemic stroke treatment regimens can be developed and aimed at maximizing function and quality of life.
Few studies have investigated the relationship between acute biomarkers and functional outcome following stroke. A potential prognostic biomarker of ischemic stroke (IS) is C-reactive protein (CRP), which is currently used for evaluating pathological inflammation and has been extensively studied in relationship to the progression of atherosclerosis (4). Baseline inflammatory levels of CRP can predict patient outcomes in cardiovascular disease (3, 5-7), including myocardial infarction (8, 9). These findings suggest that prolonged inflammation may alter the acute phase inflammatory response (10, 11) and mediate prognosis. Due to the similarities in vessel pathophysiology between myocardial infarction and IS (8), it is likely that acute CRP levels following IS also have prognostic value.
IS patients with increased circulatory inflammatory mediators upon hospital admission have greater post-IS mortality (12, 13). Other studies have shown that a time course of inflammatory cytokines (i.e. interleukin-2, interleukin-10 and tumor necrosis factor-a) can predict outcome (14) and that markers of adaptive immune function also influence stroke outcome (15-17). Baseline innate immune activity may influence adaptive immune mechanisms that guide recovery. Data suggests that the post-stroke immune response occurs in a time-dependent fashion with the innate immune response occurring in the first 24 hours following ischemic injury. Therefore, studies of CRP that have extended the time window beyond 24 hours would not accurately represent baseline inflammatory status.
In 2005, the CRP Pooling Project Committee evaluated the literature to assess the clinical utility of CRP as a prognostic biomarker of IS outcome. The Committee determined that not enough data existed to draw a conclusion and identified a need for further studies (18). Following these recommendations, the aim of this systematic review was to examine the relationship between acute CRP levels and long-term functional outcome. Included studies must have contained an IS patient cohort that followed clinical standards of diagnosis (19) and based enrollment on imaging findings (20). To better characterize long-term disability, a functional scale was needed to evaluate prognosis beyond 30 days (21). Lastly, CRP levels were assessed with a high sensitivity CRP (hs-CRP) assay within 24 hours. A hs-CRP assay quantifies smaller incremental changes of inflammation, which may be more clinically relevant for assessing the relationship between acute inflammation and predicting the degree of long-term disability.
We believe that to truly assess the relationship between baseline inflammatory status and long-term functional outcome in IS patients, all of these parameters are critical. Based upon these inclusion criteria, only five of 597 studies have been included in this review. Interestingly, all five of the included studies discuss a significant relationship between acute hs-CRP levels and long-term functional outcome, suggesting that CRP has clinical utility as a biomarker for predicting post-IS outcome. The fact that so few studies met our inclusion criteria emphasizes the need for additional research to characterize the relationship between acute inflammatory markers and long-term functional outcome using well-defined diagnostic criteria.
2. Methods
2.1. Study identification
PubMed and MEDLINE databases were searched for original studies using the medical subject heading search terms “C-reactive protein” and “stroke”. The terms “C-reactive protein” and “ischemic stroke” were used to search the SCOPUS database and Cochrane Library for relevant conference abstracts and systematic reviews, respectively. The search yielded articles published between 1989 and June 2012. Articles published in languages other than English, reviews, commentaries, editorials and case studies were excluded. However, these types of papers were examined in addition to the reference lists of relevant studies to identify other potential articles for inclusion.
2.2. Study inclusion
Articles presenting original data assessing the relationship between acute CRP levels measured within 24 hours of symptom onset and long-term functional outcome (>30 days) in patients experiencing acute IS were included. Studies were eligible for inclusion if: (1) the study population presented to the hospital within 24 hours of stoke symptom onset and infarct was confirmed on neuroimaging, (2) CRP levels were measured within 24 hours of stroke symptom onset using a hs-CRP assay, and (3) studies included a measure of long-term (>30 days) functional outcome, such as the Barthel Index (BI), modified Rankin Scale (mRS), Glasgow outcome scale (GOS), or National Institutes of Health Stroke Scale (NIHSS). Studies that included mortality and/or a subsequent cerebrovascular event as the measure of prognosis without an assessment of functional outcome were excluded. Studies were limited to those that confirmed stroke infarct with either a follow-up CT scan or MRI, as outlined in the Acute Ischemic Cerebrovascular Syndrome criteria (19) to ensure IS patient cohorts were not biased with transient ischemic attack (TIA), stroke mimics (such as hypoglycemia, complicated migraine) or hemorrhagic stroke patients. Studies that grouped TIA and/or hemorrhagic stroke with IS patients for analyses involving CRP measurements or long-term functional outcome were excluded. Because infection prior to IS is related to a worse stroke outcome and CRP elevates in response to infection, studies included in this review had to possess an internal method for excluding patients with prior infection. Study methods used to identify patients with infection include chest radiograph, routine urine analysis, complete physical exam findings diagnostic of infection, or initially high CRP levels that were deemed outliers. Articles that used a patient population from clinical trials were excluded because investigational therapies introduce a confounding variable.
2.3. Data extraction
Abstracts and titles of articles identified in the electronic database search were reviewed by two authors (E.H. and R.V.) and were selected for possible inclusion. If the abstract was deemed relevant, the corresponding full-text articles were retrieved and independently reviewed by four authors (R.V., D.D., K.S. and K.W.). Data were extracted from each eligible full-text article and authors (R.V., D.D., K.W., K.S., T.B. and J.H.) independently determined whether studies were to be included or excluded and recorded the reason for exclusion. The following information was abstracted from each article: citation, sample size, study setting, study design, definition of stroke, type and timing of any imaging performed, exclusion criteria, control population, measurement and timing of prognostic assessment, CRP measurement and timing of measurement, data analysis techniques, and study findings. Discussion among authors was undertaken to resolve any disagreements. Quality of the included studies was assessed using the study design and assay guidelines of the REMARK recommendations for prognostic tumor markers (22), which have been previously used to evaluate prognostic biomarker studies of IS (20, 23) and cancer (24, 25).
3. Results
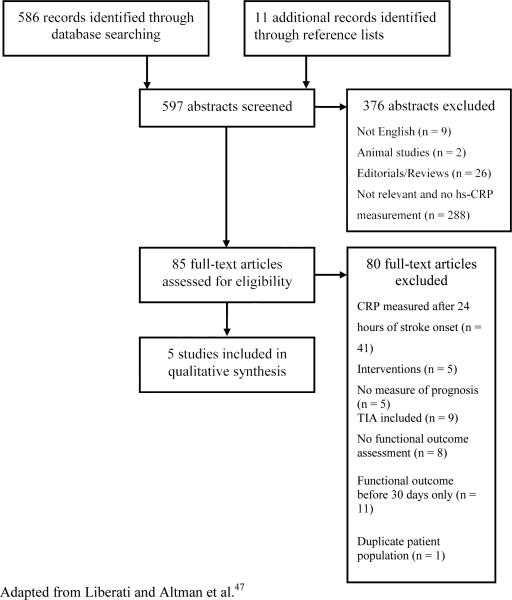
The search strategy revealed 586 articles plus 11 additional studies that were identified from reference lists. The abstracts of each record were screened, and 79 were eligible for full-text review (Fig. 1). Of these, five met the criteria for inclusion in the review. Studies were from Germany (n = 2), India (n = 1), Spain (n = 1), and South Korea (n = 1).
Fig. 1.
Search strategy for a systematic review of C-reactive protein and ischemic stroke prognosis
3.1. Quality assessment
Study sample size ranged from 50 patients (26) to 581 patients (21). None of the studies provided a justification for sample size (Table 1). All five studies were prospective, precisely defined all clinical outcomes at the beginning of the study, and adequately described and referenced hs-CRP measurements. Two of the five studies used multivariate analysis to determine whether CRP was independently predictive of long-term functional outcome while controlling for confounding variables (21, 27). Remaining studies used correlation to assess the relationship between CRP and long-term functional outcome. Four studies mentioned blinding between CRP levels and patient outcome, but blinding was not evident in one study (27). Three studies provided start and end dates of patient enrollment (Table 1).
Table 1.
Assessment of study quality using modified REMARK recommendationsa
| Study quality question/Item | Marquardt et al., 2005 [26] | Montaner et al., 2006 [28] | Song et al., 2010 [29] | Winbeck et al., 2002 [27] | Rajeshwar et al., 2012 [21] |
|---|---|---|---|---|---|
| 1. Was the study prospective? | Yes | Yes | Yes | Yes | Yes |
| 2. Was the evaluation of prognostic marker blinded to patient outcome? | Yes | Yes | Yes | Unclear | Yes |
| 3. Was there a defined time period during which patients were enrolled? | No | No | Yes | No | Yes |
| 4. Were there precisely defined clinical outcomes at the beginning of the study? | Yes | Yes | Yes | Yes | Yes |
| 5. Did the study provide a rational for sample size? | No | No | No | No | No |
| 6. Did the study provide a list of candidate variables? | N/Ab | N/Ab | N/Ab | Yes | Yes |
| 7. Were the methods for measuring the prognostic marker adequately described and referenced? | Yes | Yes | Yes | Yes | Yes |
| 8. Were patients unselected/unbiased? | Yes | Yes | Yes | Yes | Yes |
Adapted from McShane et al.22 and Whiteley et al.23
Study did not use multivariate modeling.
N/A = not available.
3.2. Association between CRP and long-term functional outcome
Acutely elevated CRP showed positive, significant associations with long-term (>30 days) unfavorable outcome in all five studies (Table 2). One study reported a significant positive correlation between CRP and stroke severity at 90 days using the NIHSS but did not report the correlation coefficient (26); therefore, no information on the strength of association can be inferred. Another study found a significant, positive correlation between elevated baseline hs-CRP levels and high mRS scores at a 3 month follow-up (28). Since the mRS was combined into a categorical variable and scores for severe disability and death were not separated for analyses, it is difficult to determine if this finding represents the relationship between CRP and functional outcome or between CRP and mortality. One study observed that CRP levels were significantly and positively correlated with mRS scores at 1, 3, 6, and 12 month follow-up, with progressively stronger associations observed with increasing time (29). A noteworthy finding is that CRP measurements at 7 days after admission had stronger statistical correlation with mRS scores at 12 months than CRP levels measured within 24 hours of admission (29). Using multiple logistic regression, Rajeshwar et al. demonstrated that higher hs-CRP levels were significantly associated with poor outcome in their sample of 581 patients after controlling for several confounding variables (21). Similarly, the study by Winbeck et al. used logistic regression analysis to predict long-term functional outcome and found that CRP levels taken 12 to 24 hours within stroke symptom onset were independently predictive of unfavorable functional outcome at 1 year follow-up (27). CRP levels measured 24 to 48 hours after symptom onset were even stronger predictors, but the time frame did not meet inclusion criteria because it is outside the time window for assessing acute inflammatory status.
Table 2.
Included studies assessing association between C-reactive protein and long-term functional outcome after ischemic stroke
| Reference | Population | Excludes infection? | Measurement of long-term functional outcome | CRP measurement | Results |
|---|---|---|---|---|---|
| Marquardt et al., 2005 [26] | 100 subjects included: 50 stroke patients, 30 healthy control subjects; 20 risk factor control subjects | Yes - Any symptoms of infection were assessed during the study to recognize influence of infections on variables | NIHSS; 3 months after stroke | hs-CRP turbidimetrical test based on latex beads, threshold of detectability = 0.01 mg/dL. CRP measured within 24 h ours | NIHSS at 90 days was positively correlated with CRP levels (p < 0.05) |
| Montaner et al., 2006 [28] | 143 acute stroke patients involving the MCA territory | Yes - CRP >6 mg/dL were excluded due to likely infection before stroke | mRS; 90 days after stroke | hs-CRP via nephelometry using Behring Nephelometer Analyzera within 3 hours (before tPA administration). CRP cutoff level was set at 0.77 mg/dL | A significant, positive correlation between CRP level and mRS was found (r = 0.24, p = 0.02) |
| Song et al., 2010 [29] | 309 first-ever ischemic stroke patients | Yes - Previous infections were excluded by medical history, chest radiograph, routine urinalysis, and complete physical exam. Patients that developed an infection within 7 days of stroke onset were excluded | mRS; 1, 3, 6, and 12 months after stroke | hs-CRP was measured within the first 24 hours and at day 7 | CRP levels were significantly and positively correlated with mRS scores at 1, 3, 6, and 12 months follow-up (r = 0.29, 0.30, 0.31, and 0.34, respectively, p < 0.01) |
| Winbeck et al., 2002 [27] | 127 first-ever ischemic stroke patients | Yes - Exclusion criteria included history of recent infection, obvious signs of acquired infection before stroke onset, and an initial CRP level >10 mg/dL due to presumed infection | BI and mRS; 1 year after stroke | CRP was measured on admission (within 12 hours) using a Dimension RxL analyzer.a A hs-CRP analysis was done for measurements taken within a 12-24 hour period and within a 24-48 hour period using a Tina-quantb hs-CRP assay | CRP levels from 12-24 hours after symptom onset independently predicted unfavorable outcome (BI < 85; OR = 2.3, 95% CI 1.3-4.8; p = 0.03) |
| Rajeshwar et al., 2012 [21] | 1157 subjects included: 581 stroke patients and 576 controls | Yes | mRS and GOS-E; 3, 6, and 12 months after hospital discharge | CRP was collected on admission (within 24 hours after qualifying stroke) and levels were determined using ELISA from Diagnostics Biochem Canadac | High CRP levels associated with poor outcome (adjusted OR = 3.50, 95% CI 1.312-6.365, p < 0.001) |
BI = Barthel index; CI = confidence interval, ELISA = enzyme-linked immunosorbent assay, GOS-E = Glasgow outcome scale - extended, hs = high sensitivity, MCA = middle cerebral artery, mRS = modified Rankin Scale, NIHSS = National Institutes of Health stroke scale, OR = odds ratio.
Siemens AG, Munich, Germany.
Roche Diagnostics, Basel, Switzerland.
Diagnostics Biochem Canada, Ontario, Canada.
4. Discussion
Of the 455 studies reviewed, only five studies met the inclusion criteria for this systematic review. Results of the included studies suggest a significant association between elevated CRP within 24 hours of IS onset and unfavorable long-term functional outcome. This article discusses the clinical utility of hs-CRP measures as a prognostic biomarker for IS and identifies gaps in published studies such as inclusion criteria that neglect imaging findings for defining IS and a lack of studies that assess long-term functional outcome.
4.1. Importance of neuroimaging for IS biomarker studies
Results of the included studies suggest a significant association between elevated CRP within 24 hours of IS onset and unfavorable long-term outcome. Many studies that were reviewed were excluded due to a lack of neuroimaging using CT scan or MRI for confirmation of IS. Clinical evaluation of stroke symptoms may not always be reliable for differentiating diagnosis of IS from TIA or mimic. Neuroimaging confirms both perfusion deficits and/or the presence of ischemic infarct and differentiates stroke-like from pathologies with similar symptomatic presentation, such as TIA/ hemorrhagic stroke or stroke mimic (30). While clinical evidence supports that imaging with CT-perfusion or MRI is essential for identifying patients for acute IS research studies (30), much of the IS literature studying CRP lacks imaging criteria to identify IS patients. Studies neglecting positive imaging findings for patient enrollment may have resulted in including TIA patients in an IS cohort. In this review, two studies were found that grouped TIA and IS together and subsequently did not find a significant correlation between acute CRP levels and outcome (31, 32). This disparity may reflect an altered inflammatory response between a resolved ischemic event and an ischemic event that results in brain tissue damage. Other studies examining acute blood levels of inflammatory mediators including CRP have shown different protein levels between intracerebral hemorrhage and IS (33, 34). These findings emphasize the importance of using neuroimaging findings in biomarker studies to define IS patient cohorts. Combining other neuropathologies in an IS patient study population makes it difficult to decipher the diagnostic or prognostic potential of a biomarker. Future IS biomarker studies in patient cohorts should adhere to including only patients with imaging findings of ischemia or infarct.
4.2. CRP measurements
A majority of the studies reviewed used hs-CRP assays, which more accurately measure subtle changes in CRP as compared to conventional CRP assays. Hs-CRP assays typically have detection limits around 0.1 mg/L (18), providing the ability to analyze smaller changes in CRP that may be clinically relevant, unlike conventional CRP assays with detection limits of around 3–6 mg/L (18). This may reflect why Winbeck and colleagues noted a correlation between hs-CRP and outcome levels between 12–24 hours, but not with conventional CRP levels between 0–12 hours of IS onset (27). Even though all studies showed a significant correlation between acute hs-CRP and long-term prognosis, no specific measurement of hs-CRP predicted prognosis between the studies included in this review. Arbitrary standards grouping hs-CRP levels potentially introduce bias when being compared to outcome (26). A more thorough body of literature is needed to standardize the correlation between precise hs-CRP blood levels with specific outcome measures before usage ensues in routine clinical practice. Interestingly, two of the studies show stronger associations of poor outcome with hs-CRP measurements at 24–48 hours and 7 days (27, 29). These data may reflect that prolonged inflammation after IS impairs the recovery process. Further investigation of inflammatory processes following stroke may provide insight to molecular mechanisms that influence stroke outcome.
4.3. Limitations with outcome assessment and future directions
Given the large burden of post-stroke disability, the focus of this review was on studies measuring long-term functional outcome at ≥30 days. Imaging studies have identified that the progression of infarct evolution halts at around 30 days (35), which would reflect the overall disability resulting from IS. Recent evidence suggests that blood biomarkers may be as effective in predicting functional outcome as imaging biomarkers; however future studies should address the efficacy of blood biomarkers in predicting recovery while controlling for imaging findings. Long-term functional outcome scales such as the BI and mRS are commonly used to measure degree of physical dependence (36) and possess high inter-rater reliability when compared to other scales (37). Other scales such as the Scandavian Stroke Scale and the modified NIHSS show similar accuracy for assessing functional outcome (38). However, none of the included studies used these assessments. One included study used the NIHSS as the primary assessment for long-term outcome. While studies suggest that the NIHSS can be used within 7 days as a predictor of long-term outcome (39), few use the NIHSS as the primary measure of functional outcome. However, a greater limitation in current outcome measures is that measures may be more sensitive for assessing outcome at later time points.
Currently, there is no standard definition of “good” versus “unfavorable” outcome using the mRS or BI. Biased interpretation is a concern when subjective standards defining outcome are set by the investigator. Scales like the mRS, which includes a measure of mortality, restrict the ability to assess functional disability from death when both are analyzed as an “unfavorable” outcome. In the study by Montaner and colleagues, it is difficult to determine whether the reported significant correlation between long-term functional outcome and hs-CRP levels would remain if mortality and severe disability were separated in the analysis. Future analyses separating patient groups between disabled and deceased would discern between end points of mortality and severe disability. Moreover, the addition of a more detailed functional scale like the BI, in conjunction with the mRS, would better define the subtleties associated with “mild” and “moderate.” Broader outcome measures that assess the burden of altered psychological states may further elucidate the subtleties of functional deficits. Post-stroke anxiety, depression or altered sleep habits are psychological outcomes that may impact function. An assessment such as the Neuro Quality of Life Scale may help clinicians to better treat IS victims.
However, the larger limitation with the mRS and BI is that post-stroke disability exceeds changes in physical dependence. Altered mental status and co-morbid depression are known to alter quality of life, but are not measured with these scales. Altered mental status and co-morbid depression are significant clinical issues following IS and some studies report correlations between altered mental status after stroke and elevated CRP (40). While a body of literature exists linking increased peripheral vascular disease with clinical depression, there is not a substantial number of studies to evaluate the clinical utility of CRP and altered mental status after stroke. A more comprehensive assessment of outcome using scales that account for both physical and mental well-being may better characterize the overall disability following stroke and may give a better way to interpret acute hs-CRP levels in terms of long-term outcome.
4.4. Clinical utility of CRP as an inflammatory biomarker of IS
Biomarkers can assist with patient care by helping to confirm diagnosis, predicting prognosis, or monitoring response to a therapeutic intervention for both IS treatment and prevention. Currently, neuroimaging modalities such as non-contrast CT scan and diffusion weighted magnetic resonance imaging are the standard clinical tools for IS diagnosis (41) and tissue plasminogen activator (tPA) has been the only Food and Drug Administration approved therapy for IS in the USA for the past 15 years. Lengthy scan times can contribute to the challenge of administering tPA within the approved narrow therapeutic window. A blood biomarker or biomarker panel that provides a definitive IS diagnosis may help to increase utilization of tPA as well as triaging secondary prevention and thus lead to better post-stroke outcomes. In a recent systematic review published by Hasan et. al. CRP was identified as one of three biomarkers that can differentiate between IS and healthy controls (20) and other studies that we reviewed show that acute CRP levels can differentiate between hemorrhagic and IS stroke (33, 34). Due to the complex pathophysiology of stroke, it is likely that a panel of blood biomarkers will be necessary for diagnoses (42, 43), and CRP could be included in this panel.
While most IS biomarker studies emphasize diagnosis, few focus on prognostic prediction beyond mortality. Assessing CRP as a biomarker of non-fatal IS prognosis establishes a foundation for using baseline inflammatory status as a predictor of stroke functional outcome. In addition, assessing acute and long-term inflammatory status has the potential to guide current stroke therapeutic interventions by reducing secondary vascular complications and decreasing overall IS risk. Two studies in this review show stronger outcome correlations with CRP beyond 24 hours, indicating a potential need for more intense anti-inflammatory therapies post-IS. Angiotensin converting enzyme (ACE) inhibitor administration within 24 hours of stroke onset is associated with decreased CRP levels and reduced 2 year cardiovascular risk (44). Additionally, healthy patients with elevated CRP levels saw a 51% reduction in IS occurrence when given rosuvastatin (45). This relationship between pharmacological intervention and lowered CRP levels may be attributed to an overall reduction in inflammation. ACE inhibitors may exude an anti-inflammatory effect through reducing angiotension-2 signaling and statins are well documented to have pleotrophic anti-inflammatory properties (46). A better understanding of the prognostic value of baseline CRP levels in IS patients could guide currently available treatments and aid in the discovery of novel therapeutic interventions for decreasing IS risk and tailoring secondary prevention while improving IS prognosis.
5. Limitations
The primary limitation of this systematic review is that strict inclusion criteria resulted in only five studies being included, which used different stroke populations that varied by age, sex and stroke severity. However, establishing very clear inclusion criteria enabled us to perform accurate comparisons of study results so that we could draw logical inferences from these five studies. A majority of excluded studies lacked either imaging criteria for defining IS patient cohorts or a scale to measure functional outcome. Since these two parameters are essential for evaluating long-term disability from IS, neither could be modified. Throughout the literature, the time point in which hs-CRP is measured widely varies. Hs-CRP measurements within 24 hours or less of symptom onset coincide with the innate inflammatory response and examining CRP outside of this time window would introduce variability for characterizing the acute inflammatory response post-IS.
6. Conclusion
The CRP Pooling Committee has previously supported the potential role of CRP as a prognostic marker for IS, but emphasized the need for further evidence before recommending measuring CRP in routine clinical practice (18). Even though the five studies meeting our inclusion criteria showed a positive relationship between elevated CRP and poor outcome, there are still significant gaps in the literature. The CRP Pooling Committee recommended that CRP be examined at different time points to more accurately assess the relationship between CRP and IS outcome and evaluating CRP in a time-series fashion would give insight to the molecular mechanisms that influence functional post-stroke recovery. However, before a recommendation could be made for clinical practice guidelines, studies that use neuroimaging for IS diagnosis need to be conducted with hs-CRP being measured over multiple time points. This would need to be established to better understand the optimum time frame on which to make clinical decisions regarding prognosis and post-stroke care. Interestingly, two of the studies included in this review reported a stronger association between CRP and outcome when CRP levels were measured beyond 24 hours (27, 29).
Current literature suggests that inflammatory status as indicated by hs-CRP levels, in conjunction with other biomarkers, has clinical utility for stratifying IS risk, diagnosing IS, predicting prognosis and monitoring response to therapeutics. Although further study is required, these findings emphasize the need for characterizing the link between imaging diagnosis of IS, inflammation and non-fatal stroke outcome.
Acknowledgements
This was supported by NINR contract Division HHS N263201100872P (to T.L.B.), WVU Foundation funding (to T.L.B.), and a WV-INBRE grant P20 RR016477 from the NIH National Center for Research Resources which supports the Appalachian Cardiovascular Research Network (to T.L.B.), NS061954 and NS061954-3S1 from NINDS (to J.D.H. and C.L.R.), 11CRP7370056 from AHA (to P.D.C.) and National Institute Of General Medical Sciences of the National Institutes of Health under Award Number U54GM104942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures
The authors declare that they have no financial or other conflicts of interest in relation to this research and its publication.
References
- 1.Prevalence of stroke--United States, 2006-2010. MMWR Morb Mortal Wkly Rep. 2012;61(20):379–82. [PubMed] [Google Scholar]
- 2.Asplund K, Stegmayr B, Peltonen M. Ginsberg MD, Bogousslavsky J, editors. From the twentieth to the twenty-first century: a public health perspective. Cerebrovascular Disease: Pathophysiology, Diagnosis, and Management: Blackwell Science. 1998 [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121(7):948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 4.Calabro P, Golia E, Yeh ET. Role of C-reactive protein in acute myocardial infarction and stroke: possible therapeutic approaches. Curr Pharm Biotechnol. 2012;13(1):4–16. doi: 10.2174/138920112798868764. [DOI] [PubMed] [Google Scholar]
- 5.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48(4):155–70. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 6.Amemiya N, Ogawa T, Otsuka K, Ando Y, Nitta K. Comparison of serum albumin, serum C-reactive protein, and pulse wave velocity as predictors of the 4- year mortality of chronic hemodialysis patients. J Atheroscler Thromb. 2011;18(12):1071–9. doi: 10.5551/jat.10397. [DOI] [PubMed] [Google Scholar]
- 7.Idicula TT, Brogger J, Naess H, Waje-Andreassen U, Thomassen L. Admission C-reactive protein after acute ischemic stroke is associated with stroke severity and mortality: the ‘Bergen stroke study’. BMC Neurol. 2009;9:18. doi: 10.1186/1471-2377-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaptoge S, Di Angelantonio E, Lowe G, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–40. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaan BD, Pellanda LC, Maciel PT, Duarte ER, Portal VL. C-reactive protein in acute coronary syndrome: association with 3-year outcomes. Braz J Med Biol Res. 2009;42(12):1236–41. doi: 10.1590/s0100-879x2009001200019. [DOI] [PubMed] [Google Scholar]
- 10.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh SH, Kim OJ, Shin DA, et al. Alteration of immunologic responses on peripheral blood in the acute phase of ischemic stroke: blood genomic profiling study. J Neuroimmunol. 2012;249(1-2):60–5. doi: 10.1016/j.jneuroim.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Anuk T, Assayag EB, Rotstein R, et al. Prognostic implications of admission inflammatory profile in acute ischemic neurological events. Acta Neurol Scand. 2002;106(4):196–9. doi: 10.1034/j.1600-0404.2002.01224.x. [DOI] [PubMed] [Google Scholar]
- 13.Rallidis LS, Vikelis M, Panagiotakos DB, et al. Inflammatory markers and in hospital mortality in acute ischaemic stroke. Atherosclerosis. 2006;189(1):193–7. doi: 10.1016/j.atherosclerosis.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Nayak AR, Kashyap RS, Kabra D, Purohit HJ, Taori GM, Daginawala HF. Time course of inflammatory cytokines in acute ischemic stroke patients and their relation to inter-alfa trypsin inhibitor heavy chain 4 and outcome. Ann Indian Acad Neurol. 2012;15(3):181–5. doi: 10.4103/0972-2327.99707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barr TL, Latour LL, Lee KY, et al. Blood-brain barrier disruption in humans is independently associated with increased matrix metalloproteinase-9. Stroke. 2010;41(3):e123–8. doi: 10.1161/STROKEAHA.109.570515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Song TJ, Park JH, et al. Different prognostic value of white blood cell subtypes in patients with acute cerebral infarction. Atherosclerosis. 2012;222(2):464–7. doi: 10.1016/j.atherosclerosis.2012.02.042. [DOI] [PubMed] [Google Scholar]
- 17.Yan J, Read SJ, Henderson RD, et al. Frequency and function of regulatory T cells after ischaemic stroke in humans. J Neuroimmunol. 2012;243(1-2):89–94. doi: 10.1016/j.jneuroim.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Di Napoli M, Schwaninger M, Cappelli R, et al. Evaluation of C-reactive protein measurement for assessing the risk and prognosis in ischemic stroke: a statement for health care professionals from the CRP Pooling Project members. Stroke. 2005;36(6):1316–29. doi: 10.1161/01.STR.0000165929.78756.ed. [DOI] [PubMed] [Google Scholar]
- 19.Kidwell CS, Warach S. Acute ischemic cerebrovascular syndrome: diagnostic criteria. Stroke. 2003;34(12):2995–8. doi: 10.1161/01.STR.0000098902.69855.A9. [DOI] [PubMed] [Google Scholar]
- 20.Hasan N, McColgan P, Bentley P, Edwards RJ, Sharma P. Towards the identification of blood biomarkers for acute stroke in humans: a comprehensive systematic review. Br J Clin Pharmacol. 2012;74(2):230–40. doi: 10.1111/j.1365-2125.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajeshwar K, Kaul S, Al-Hazzani A, et al. C-reactive protein and nitric oxide levels in ischemic stroke and its subtypes: correlation with clinical outcome. Inflammation. 2012;35(3):978–84. doi: 10.1007/s10753-011-9401-x. [DOI] [PubMed] [Google Scholar]
- 22.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumour MARKer prognostic studies (REMARK). Br J Cancer. 2005;93(4):387–91. doi: 10.1038/sj.bjc.6602678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke. 2009;40(5):e380–9. doi: 10.1161/STROKEAHA.108.528752. [DOI] [PubMed] [Google Scholar]
- 24.Kyzas PA, Loizou KT, Ioannidis JP. Selective reporting biases in cancer prognostic factor studies. J Natl Cancer Inst. 2005;97(14):1043–55. doi: 10.1093/jnci/dji184. [DOI] [PubMed] [Google Scholar]
- 25.Mallett S, Timmer A, Sauerbrei W, Altman DG. Reporting of prognostic studies of tumour markers: a review of published articles in relation to REMARK guidelines. Br J Cancer. 2010;102(1):173–80. doi: 10.1038/sj.bjc.6605462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquardt L, Ruf A, Mansmann U, et al. Inflammatory response after acute ischemic stroke. J Neurol Sci. 2005;236(1-2):65–71. doi: 10.1016/j.jns.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Winbeck K, Poppert H, Etgen T, Conrad B, Sander D. Prognostic relevance of early serial C-reactive protein measurements after first ischemic stroke. Stroke. 2002;33(10):2459–64. doi: 10.1161/01.str.0000029828.51413.82. [DOI] [PubMed] [Google Scholar]
- 28.Montaner J, Fernandez-Cadenas I, Molina CA, et al. Poststroke C-reactive protein is a powerful prognostic tool among candidates for thrombolysis. Stroke. 2006;37(5):1205–10. doi: 10.1161/01.STR.0000217744.89208.4e. [DOI] [PubMed] [Google Scholar]
- 29.Song IU, Kim YD, Kim JS, Lee KS, Chung SW. Can high-sensitivity C-reactive protein and plasma homocysteine levels independently predict the prognosis of patients with functional disability after first-ever ischemic stroke? Eur Neurol. 2010;64(5):304–10. doi: 10.1159/000321415. [DOI] [PubMed] [Google Scholar]
- 30.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA. 2004;292(15):1823–30. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 31.Prabhakaran S, Silver AJ, Warrior L, McClenathan B, Lee VH. Misdiagnosis of transient ischemic attacks in the emergency room. Cerebrovasc Dis. 2008;26(6):630–5. doi: 10.1159/000166839. [DOI] [PubMed] [Google Scholar]
- 32.Worthmann H, Tryc AB, Goldbecker A, et al. The temporal profile of inflammatory markers and mediators in blood after acute ischemic stroke differs depending on stroke outcome. Cerebrovasc Dis. 2010;30(1):85–92. doi: 10.1159/000314624. [DOI] [PubMed] [Google Scholar]
- 33.Montaner J, Mendioroz M, Delgado P, et al. Differentiating ischemic from hemorrhagic stroke using plasma biomarkers: The S100B/RAGE pathway. J Proteomics. 2012;75(15):4758–65. doi: 10.1016/j.jprot.2012.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Roudbary SA, Saadat F, Forghanparast K, Sohrabnejad R. Serum C-reactive protein level as a biomarker for differentiation of ischemic from hemorrhagic stroke. Acta Med Iran. 2011;49(3):149–52. [PubMed] [Google Scholar]
- 35.Gaudinski MR, Henning EC, Miracle A, Luby M, Warach S, Latour LL. Establishing final infarct volume: stroke lesion evolution past 30 days is insignificant. Stroke. 2008;39(10):2765–8. doi: 10.1161/STROKEAHA.107.512269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulter G, Steen C, De Keyser J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke. 1999;30(8):1538–41. doi: 10.1161/01.str.30.8.1538. [DOI] [PubMed] [Google Scholar]
- 37.Adams HP, Jr., Woolson RF, Biller J, Clarke W. Studies of Org 10172 in patients with acute ischemic stroke. TOAST Study Group. Haemostasis. 1992;22(2):99–103. doi: 10.1159/000216301. [DOI] [PubMed] [Google Scholar]
- 38.Govan L, Langhorne P, Weir CJ. Categorizing stroke prognosis using different stroke scales. Stroke. 2009;40(10):3396–9. doi: 10.1161/STROKEAHA.109.557645. [DOI] [PubMed] [Google Scholar]
- 39.Bang OY, Park HY, Yoon JH, et al. Predicting the Long-Term Outcome after Subacute Stroke within the Middle Cerebral Artery Territory. J Clin Neurol. 2005;1(2):148–58. doi: 10.3988/jcn.2005.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seo WK, Seok HY, Kim JH, et al. C-Reactive protein is a predictor of early neurologic deterioration in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2012;21(3):181–6. doi: 10.1016/j.jstrokecerebrovasdis.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Adams H, Adams R, Del Zoppo G, Goldstein LB. Guidelines for the early management of patients with ischemic stroke: 2005 guidelines update a scientific statement from the Stroke Council of the American Heart Association/American Stroke Association. Stroke. 2005;36(4):916–23. doi: 10.1161/01.STR.0000163257.66207.2d. [DOI] [PubMed] [Google Scholar]
- 42.Barr TL, Conley Y, Ding J, et al. Genomic biomarkers and cellular pathways of ischemic stroke by RNA gene expression profiling. Neurology. 2010;75(11):1009–14. doi: 10.1212/WNL.0b013e3181f2b37f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jickling GC, Sharp FR. Blood biomarkers of ischemic stroke. Neurotherapeutics. 2011;8(3):349–60. doi: 10.1007/s13311-011-0050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Napoli M, Papa F. Angiotensin-converting enzyme inhibitor use is associated with reduced plasma concentration of C-reactive protein in patients with first-ever ischemic stroke. Stroke. 2003;34(12):2922–9. doi: 10.1161/01.STR.0000099124.84425.BB. [DOI] [PubMed] [Google Scholar]
- 45.Everett BM, Glynn RJ, MacFadyen JG, Ridker PM. Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). Circulation. 2010;121(1):143–50. doi: 10.1161/CIRCULATIONAHA.109.874834. [DOI] [PubMed] [Google Scholar]
- 46.Prasad K. C-reactive protein (CRP)-lowering agents. Cardiovasc Drug Rev. 2006;24(1):33–50. doi: 10.1111/j.1527-3466.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 47.Liberati A, Altman DG, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]