Abstract
Importance
While the Pooled Cohort Equations from the recent ACC/AHA Guideline on the Assessment of Cardiovascular Risk have over-estimated cardiovascular risk in multiple external cohorts, the reasons for the discrepancy are unclear.
Objective
To determine whether increased use of statins over time, incident coronary revascularization procedures, or under-ascertainment of vascular events explain over-estimation of risk in a more contemporary population.
Design, Setting, and Participants
27,542 women aged 45-79 with complete ascertainment of plasma lipids and other risk factors from the Women's Health Study (WHS), a nationwide cohort of US women free of cardiovascular disease, cancer or other major illness at baseline in 1992-95. Women were followed for a median of 10 years.
Main Outcomes and Measures
Atherosclerotic cardiovascular disease (ASCVD), defined as any myocardial infarction, any stroke, or death due to cardiovascular cause.
Results
632 women experienced an ASCVD event over follow-up. The average predicted risk from the Pooled Cohort Equations was 3.6% over 10 years, compared to an actual observed risk of 2.2%. Predicted rates were 90% higher than the observed rates in the 0-<5% and 5-<7.5% risk groups and 40% higher in the 7.5-<10% and 10%+ risk groups. Rates of statin use and revascularizations increased over follow-up time and by risk group, and in sensitivity analyses, we estimated the hypothetical rates if no women were on statins or underwent revascularization procedures. After adjustment for intervention effects of statins and revascularization as well as hypothetical confounding by indication, predicted rates remained 80% higher than observed rates in the lower two risk groups and 30% higher in the upper two risk groups. Under-ascertainment is unlikely since follow-up rates in the WHS were 97%, and overall we would need 60% more events to match the numbers predicted using the Pooled Cohort Equations.
Conclusions and Relevance
Neither statin use, revascularization procedures, nor under-ascertainment of events explain the discrepancy between observed rates of ASCVD in the WHS and those predicted by the ACC/AHA Pooled Cohort Equations. Other explanations include changing patterns of risk within more contemporary populations.
The ACC/AHA cholesterol guidelines, released in the fall of 2013, provide new recommendations for statin therapy. The Pooled Cohort Equations upon which these are based1 now include stroke in the combined endpoint of atherosclerotic cardiovascular disease (ASCVD), rather than coronary heart disease only. This is particularly important for women, in whom rates of stroke can be as high as those for myocardial infarction (MI).2 The risk equations were developed in a pooled sample of data from five cohorts of individuals followed over at least a decade, and tested in three external validation cohorts.
Concerns have been raised, however, including how well the new prediction model works in data sets that are more contemporary than those used to derive the equations.3 In all three validation cohorts used by guideline developers, the models over-estimated risk;1 discrimination (the ability to separate cases from non-cases) was lower, and calibration (the agreement between predicted risk and actual observed risk) was poor. In both the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study and in the Multi-Ethnic Study of Atherosclerosis (MESA), external cohorts that were not used in model development, the estimated risk of ASCVD using the new Pooled Cohort Equations was too high. The same over-estimation occurred using more contemporary data from the derivation cohorts, including the Framingham and Atherosclerosis Risk in Communities (ARIC) studies, and was consistent in men and women and among blacks and whites. Calibration was also poor in four additional external cohorts.3,4 Further analyses in REGARDS improved calibration, though risk remained over-estimated overall, with better fit in a subset with additional case-finding through Medicare records.5 Because new guidelines for statin therapy are based on these equations, over-estimation of risk will have strong clinical implications, and could lead to over-prescription of medication as well as inaccurate data on risks and benefits.
Potential explanations for this discrepancy between observed rates and those predicted from the Pooled Cohort Equations include an increase in statin use over time, an increase in revascularization procedures, and failure to fully ascertain clinical events during follow-up.6-8 In this paper we explore these possibilities in the Women's Health Study, a cohort of initially healthy American women. While this analysis directly relates to the CVD risk equations, similar concerns regarding changing incidence rates over time, including increasing use of medications, may apply to other risk prediction tools.
Methods
Study Design
The Women's Health Study (WHS) is a nationwide cohort of 39,876 US women aged 45 years and older free of cardiovascular disease, cancer or other major illness at study entry from September 1992 through May 1995.9 The WHS was a randomized factorial trial of aspirin and vitamin E, and final results have been published.9,10 Neither intervention had a significant impact on major cardiovascular events, including MI, stroke, or death due to cardiovascular cause. Self-reported exposure data were collected on age, race/ethnicity, diabetes, blood pressure, blood pressure treatment, smoking status, and cholesterol lowering medication. All study participants provided written informed consent. The study protocol was approved by the institutional review board of Brigham and Women's Hospital (Boston, Mass).
Women eligible for the current analysis were aged 45-79, and had complete ascertainment of plasma lipids and information on other risk factors (N=27,542). Plasma samples were measured for total, low-density (LDL) and high-density (HDL) lipoprotein cholesterol in a core laboratory certified by the National Heart, Lung, and Blood Institute/Centers for Disease Control and Prevention Lipid Standardization Program.
Women were followed with annual questionnaires through the end of the trial in March, 2004, a median of 10.2 years (25th, 75th percentiles = 9.7, 10.6 years), for incident MI, stroke, and cardiovascular deaths, as well as coronary revascularization procedures and other study endpoints. Follow-up with respect to morbidity and mortality were 97.2% complete and 99.4% complete, respectively.9
Reported events were adjudicated by an end-points committee of physicians after medical record review. MI was confirmed if symptoms met World Health Organization criteria and if the event was associated with abnormal levels of cardiac enzymes or diagnostic electrocardiograms. A confirmed stroke was defined as a new neurologic deficit of sudden onset that persisted for at least 24 hours. Death was confirmed to be from cardiovascular causes on the basis of an examination of autopsy reports, death certificates, medical records, and information obtained from the next of kin or other family members. The use of coronary revascularization (coronary artery bypass surgery (CABG) or percutaneous coronary angioplasty (PTCA)) was confirmed by a review of the medical records. For this analysis, the endpoint was ASCVD, defined as any MI, stroke, or death due to cardiovascular cause. Due to the difficulty in distinguishing type of cardiovascular death,11 all such deaths were included rather than fatal MI and fatal stroke as used in the derivation cohorts for the pooled risk equations.
Statistical Analysis
Baseline characteristics are reported as percents or means and standard deviations both overall and within strata of predicted risk. Predicted values from the ACC/AHA Pooled Cohort Equations were obtained using published equations.1 Women were classified into four clinical risk groups based on predicted risk with cut points of 5%, 7.5%, and 10%, as previously,1 and average predicted risk was calculated.
For comparison, observed rates of ASCVD were obtained from Kaplan-Meier survival curves. Because all women were followed for a minimum of 8 years, the cumulative incidence of ASCVD was estimated as of 8 years then extrapolated to 10 years as I10=1-(1-I8)1.25, where I10 is the cumulative incidence as of 10 years and I8 is the cumulative incidence as of 8 years. Women were censored at death due to a non-cardiovascular cause or at administrative censoring on March 31, 2004.
Calibration was assessed by plotting the observed and predicted average risk within the predefined clinical risk strata defined above as well as in deciles. Expected-to-observed ratios were calculated.
Sensitivity Analyses
We examined the potential effects of statin therapy and revascularization procedures on the observed risks of ASCVD in sensitivity analyses. First, we estimated the proportion of women without prior ASCVD taking statins by follow-up time, both overall and within risk strata. Statins were not generally in use at baseline in 1992-95, but questions on use of statins were included on questionnaires at 3, 4, 9, and 10 years of follow-up. Linear interpolation was used to estimate proportions between these times. We estimated the proportions over time of women undergoing coronary revascularization procedures (CABG or PTCA), obtained from annual questionnaires, among those with no reported prior ASCVD, both overall and within risk strata. We performed five sensitivity analyses to adjust the observed risk estimates for the use of statins and/or revascularization procedures. Further details of the mathematical adjustments are in the online Appendix.
1) Adjustment for risk reduction due to statin use
We increased the rate among those using statins inversely proportional to the estimated effect of statins on ASCVD. In a meta-analysis of randomized trials,12 among those without prior vascular disease, statins reduced the risk of major vascular events by an aggregate 25% per mmol/L reduction in LDL cholesterol, similar to the effect of a typical dose. We increased the rate of ASCVD among those using statins proportionately. The rate was increased for each year of follow-up separately then aggregated in a life table analysis.
2) Adjustment for risk reduction due to statin use and confounding by indication
Because of potential confounding by indication, statin users may be at higher risk of ASCVD than those at the same estimated baseline level of risk by conventional risk factors. For example, those who were prescribed statins may have a family history of ASCVD, high CRP, or their lipids may have increased over time. We therefore increased the rate further in this group by doubling it, in addition to accounting for the statin effect as in analysis 1).
3) Adjustment for risk reduction due to statin use and revascularization and for confounding by indication for each
We adjusted for the potential effects of revascularization therapy using similar methods. First, we conservatively estimated that such procedures would reduce rates of ASCVD by 25%. This estimate is optimistic since randomized trials comparing these procedures to optimal medical therapy suggest little difference in the occurrence of ASCVD endpoints.13 Second, we allowed for confounding by indication similar to that for statins by doubling the observed risk.
4) Censoring at use of cholesterol-lowering medication
In a separate sensitivity analysis we censored women at the time of initiation of cholesterol-lowering medications.
5) Censoring at cholesterol-lowering medication use or revascularization
We performed an additional sensitivity analysis censoring women when they began using cholesterol-lowering medications or underwent revascularization, by definition prior to ASCVD.
In our initial analyses, we adjusted for use of statins only because those agents have been found to reduce cardiovascular risk. In alternate analyses, we substituted use of any cholesterol-lowering agent for statins. In addition, since the risk equations are meant to be used clinically for those who are not diabetic, not on cholesterol-lowering medications at baseline, and with LDL in the range 70 to 189 mg/dL, we additionally subset to that group.
Finally, we estimated what size of effects would be necessary to reconcile any differences in event rates. In particular, we estimated the numbers of events that would have to be missed through inadequate ascertainment in order to reconcile remaining differences between observed and predicted rates.
Results
Women were of average age 54 years and primarily Caucasian (Table 1). Average total cholesterol was 212 mg/dL, LDL was 124 mg/dL, and HDL was 54 mg/dL. Mean untreated and treated blood pressures were 121 and 139 mmHg, respectively, and 13% of women were taking anti-hypertensive medications. Twelve percent were current smokers and 2% had diabetes at baseline. The average predicted 10-year risk using the ACC/AHA ASCVD risk equations was 3.6%. Most women (79%) fell into the <5% 10-year risk category (Table 1).
Table 1.
Baseline characteristics by predicted risk group in the Women's Health Study.
| 10-year Risk Group | |||||
|---|---|---|---|---|---|
| Overall | <5% | 5-<7.5% | 7.5-<10% | 10%+ | |
| N | 27,542 | 21,639 | 2,479 | 1,400 | 2,024 |
| Age (yrs) | 54.2 ± 7.0 | 51.8 ± 4.8 | 59.7 ± 6.1 | 62.6 ± 6.4 | 66.3 ± 7.2 |
| Black (%) | 1.8 | 1.4 | 2.6 | 3.4 | 4.6 |
| Total Cholesterol (mg/dl) | 211.8 ± 41.8 | 207.0 ± 40.0 | 227.6 ± 43.0 | 228.3 ± 43.3 | 231.7 ± 44.1 |
| HDL Cholesterol (mg/dL) | 53.8 ± 15.1 | 55.1 ± 15.0 | 49.3 ± 14.1 | 48.3 ± 14.1 | 48.0 ± 14.5 |
| LDL Cholesterol (mg/dL) | 124.1 ± 34.2 | 120.2 ± 32.5 | 137.8 ± 36.6 | 137.8 ± 36.7 | 139.7 ± 36.6 |
| Untreated SBP (mmHg) | 121.4 ± 12.4 | 119.6 ± 11.4 | 127.3 ± 12.8 | 130.5 ± 12.7 | 135.6 ± 13.3 |
| Treated SBP (mmHg) | 138.6 ± 12.3 | 134.6 ± 10.8 | 139.0 ± 11.7 | 140.3 ± 11.4 | 144.6 ± 12.8 |
| BP Meds (%) | 13.5 | 8.2 | 22.9 | 28.1 | 48.3 |
| Current Smoker (%) | 11.6 | 7.0 | 27.0 | 29.0 | 30.8 |
| Diabetes (%) | 2.5 | 0.8 | 4.9 | 6.1 | 15.0 |
| ASCVD events (N) | 632 | 228 | 90 | 88 | 226 |
| 10 yr KM ASCVD Rate (%) | 2.2 | 1.0 | 3.2 | 6.0 | 10.8 |
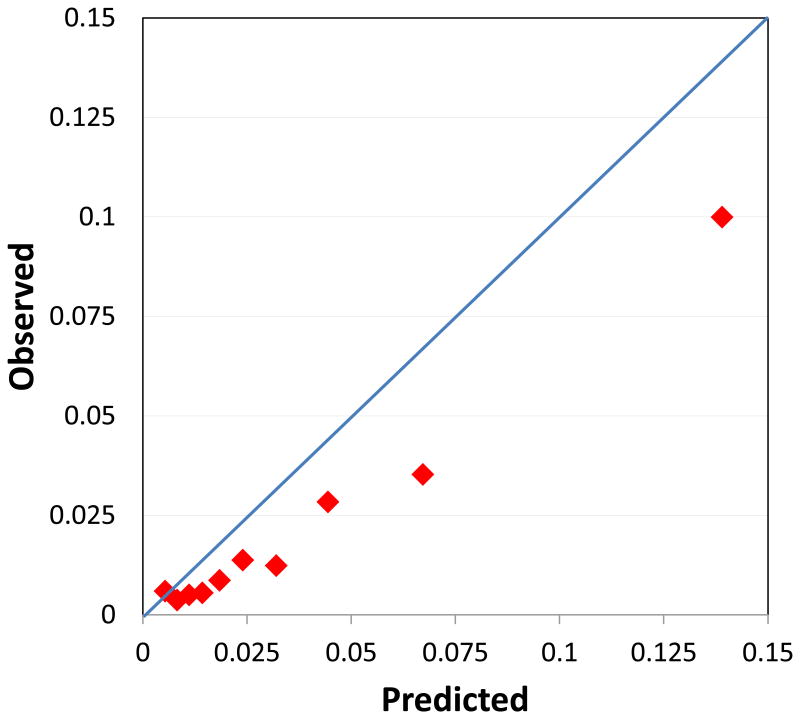
Throughout follow-up 632 women experienced an ASCVD event, 250 of which were MI, 302 stroke (3 of whom also experienced an MI), with 83 additional deaths due to cardiovascular cause. The observed 10-year ASCVD event rate based on the Kaplan-Meier curve was 2.2%, compared to the 3.6% average rate predicted by the ACC/AHA Pooled Cohort Equations. When observed and predicted rates were compared within risk categories, predicted rates were 90% higher than the actual observed rates among those with risk < 7.5%, and 40% higher among those with risk ≥ 7.5% (Table 2 and Figure 1A). Over-estimation was also apparent when considering deciles of predicted risk (Figure 2).
Table 2.
Analysis of predicted and observed risks in the Women's Health Study.
| 0-<5% | 5-<7.5% | 7.5-<10% | 10%+ | |
|---|---|---|---|---|
| N | 21,639 | 2,479 | 1,400 | 2,024 |
| Predicted risk (%) | 1.92 | 6.13 | 8.62 | 15.60 |
| Observed risk (%) | 1.01 | 3.20 | 6.04 | 10.79 |
| E/O Ratio | 1.90 | 1.91 | 1.43 | 1.45 |
| Sensitivity analyses | ||||
| Statin RR=0.75 | ||||
| Adjusted observed risk (%) | 1.03 | 3.32 | 6.28 | 11.23 |
| E/O Ratio | 1.87 | 1.85 | 1.37 | 1.39 |
| Statin RR=0.75, Indication=2.0 | ||||
| Adjusted observed risk (%) | 1.04 | 3.40 | 6.44 | 11.54 |
| E/O Ratio | 1.84 | 1.80 | 1.34 | 1.35 |
| Statin RR = Revasc RR =0.75, Indication for each =2.0 | ||||
| Adjusted observed risk (%) | 1.04 | 3.41 | 6.47 | 11.60 |
| E/O Ratio | 1.84 | 1.80 | 1.33 | 1.35 |
| Chol Med RR=0.75, Indication=2.0 | ||||
| Adjusted observed risk (%) | 1.04 | 3.43 | 6.50 | 11.67 |
| E/O Ratio | 1.83 | 1.79 | 1.33 | 1.34 |
| Chol Med RR = Revasc RR =0.75, Indication for each =2.0 | ||||
| Adjusted observed risk (%) | 1.05 | 3.44 | 6.53 | 11.72 |
| E/O Ratio | 1.83 | 1.78 | 1.32 | 1.33 |
| Among Non-Diabetics, not on on Chol Meds at Baseline, LDL 70-189 | ||||
| Predicted risk (%) | 1.89 | 6.12 | 8.61 | 14.96 |
| Observed risk (%) | 0.94 | 2.70 | 5.82 | 9.53 |
| E/O Ratio | 2.02 | 2.26 | 1.48 | 1.57 |
| Chol Med RR = Revasc RR =0.75, Indication for each =2.0 | ||||
| Adjusted observed risk (%) | 0.96 | 2.85 | 6.14 | 10.02 |
| E/O Ratio | 1.97 | 2.15 | 1.40 | 1.49 |
| Censoring at Chol Meds | ||||
| Predicted risk (%) | 1.90 | 6.12 | 8.62 | 15.56 |
| Observed risk (%) | 0.96 | 3.05 | 6.50 | 11.13 |
| E/O Ratio | 1.99 | 2.01 | 1.33 | 1.40 |
| Censoring at Chol Meds or Revasc | ||||
| Observed risk (%) | 0.95 | 2.94 | 6.33 | 10.91 |
| E/O Ratio | 2.00 | 2.08 | 1.36 | 1.43 |
Abbreviations: E/O Ratio, Expected-to-observed ratio; Chol Meds, use of cholesterol-lowering medication; Revasc, revascularization procedure; RR, relative risk due to intervention; Indication, relative risk associated with confounding by indication; LDL, low-density lipoprotein cholesterol.
Figure 1.
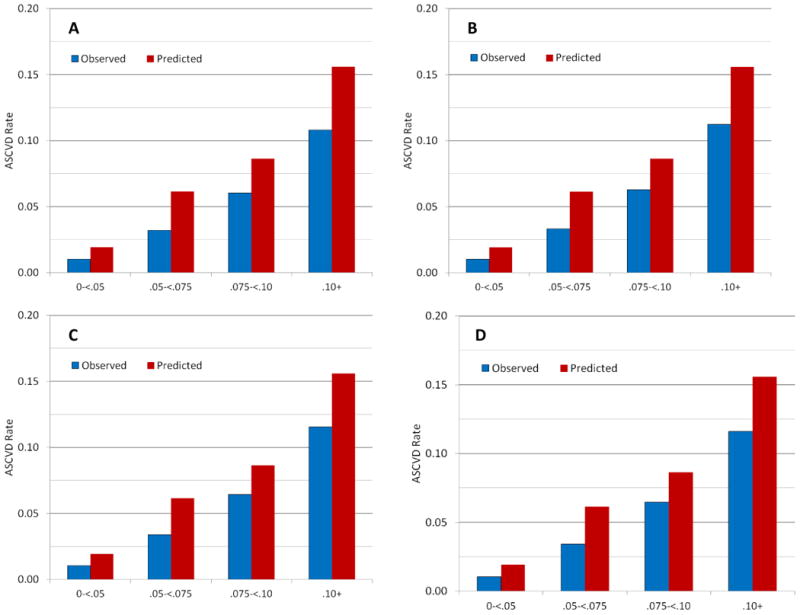
Observed 10-year risk of ASCVD in the Women's Health Study compared to that predicted by the ACC/AHA pooled cohort equations in clinical risk groups: A) Unadjusted; B) Accounting for a 25% reduction in ASCVD due to statin use; C) Accounting for the intervention effect (25% reduction) as well as a doubling of risk in statin users due to confounding by indication; D) Accounting for the intervention effects (25% reduction) and confounding by indication (doubling of risk) due to both statin use and revascularization procedures.
Figure 2.
Calibration plot comparing observed 10-year risk of ASCVD in the Women's Health Study to that predicted by the ACC/AHA pooled cohort equations within deciles.
Sensitivity Analyses
The proportions using statins increased over time, reaching approximately 20% at ten years among women without a previous ASCVD event. Use was higher among women at higher risk, reaching 35% in high-risk women by ten years (Figure 3A).
Figure 3.
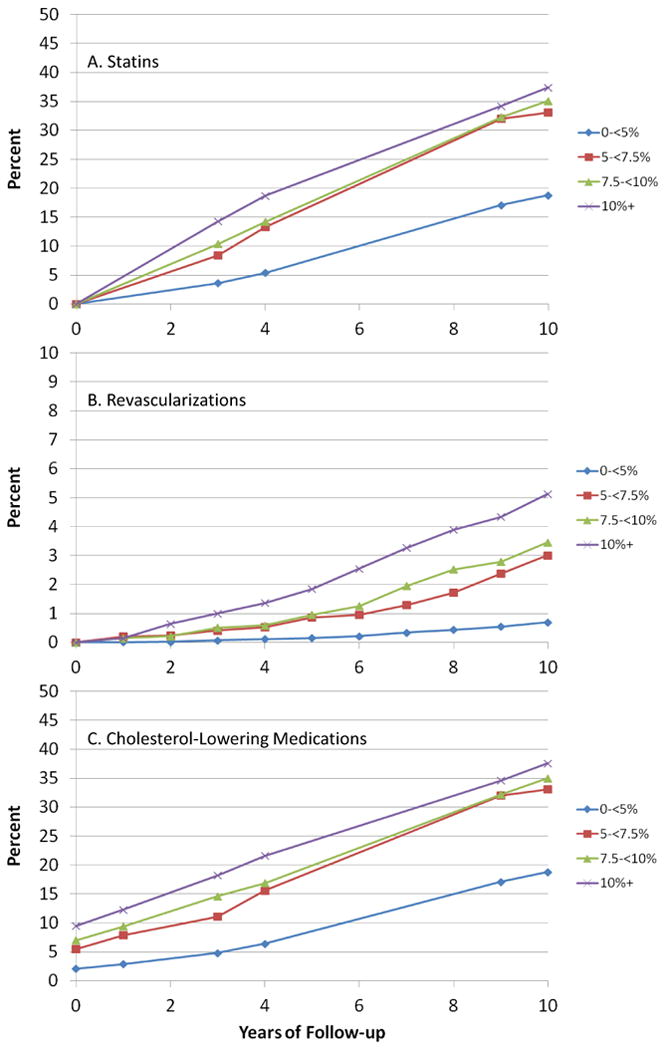
Use of medications and revascularization procedures over time in the Women's Health Study: A) Proportions using statins; B) Cumulative incidence of revascularization procedures; C) Proportions using any cholesterol-lowering medications.
To account for use of statins, we adjusted for a 25% decrease in CVD risk with statin use, increasing the observed rates slightly, from 10.8% to 11.2% in the highest risk group, versus 15.6% predicted by the Pooled Cohort Equations (Table 2 and Figure 1B). Additional adjustment for indication bias, including a doubling of risk among those taking statins, had little impact on the discrepancy between observed and predicted event rates (Table 2 and Figure 1C).
Revascularization procedures occurred in 523 women, of which 343 had no prior ASCVD event. Cumulative incidence over ten years was less than 1% overall, but reached 5% among those with ≥10% predicted risk (Figure 3B). Similar adjustments for a sizeable 25% reduction with intervention as well as confounding by indication for both statins and revascularization procedures increased adjusted rates slightly (Table 2 and Figure 1D). A larger five-fold increase in risk of ASCVD among those with a revascularization procedure increased the rate in those in the highest risk group to 11.7%, versus the 15.6% predicted.
Use of all cholesterol-lowering medications, including non-statins, was about 3% at baseline, and increased to about 22% overall at ten years (Figure 3C), when they likely comprised mostly statins. Similar adjustments had little impact (Table 2). Subsetting to 24,084 women who were non-diabetic, not using medications at baseline, with LDL in the range 70-189 mg/dL, led to similar results (Table 2). Additional analyses censoring at initiation of cholesterol-lowering medication use led to little change in the adjusted risk (Table 2). The same was true when also censoring at revascularization. In these analyses the difference between observed and predicted rates in the lower risk groups worsened.
To understand the extent of bias necessary to reconcile observed and predicted rates, we used the equations provided in the appendix. If the average annual proportion using statins was 20%, and the effect of statins was a 25% reduction in risk, then using equation (1) the rate in the absence of statin use would be 5% higher, versus the 40-100% higher rates predicted by the Pooled Cohort Equations. If in addition the risk in those taking statins was two times higher due to indication, then the rate in absence of statin use would be 9% higher than that observed using equation (2). Considering the treatment effect only and using equation (1), with a 50% reduction with statin treatment, the average annual proportion using statins would need to be 57% to equalize observed and predicted rates. Additionally allowing for a doubling of risk by confounding by indication, the average annual proportion using statins would need to be 40% to explain the discrepancy.
The relatively low rates of revascularization have little additional impact. Assuming an average proportion with a procedure (and going on statins) of 5%, with another 15% going on statins, and the same treatment and indication effects, the rate in the absence of statins or revascularization using equation (3) would be 11% higher than observed. Only if all those having a revascularization procedure would otherwise have an ASCVD event would the numbers be high enough to match those predicted.
Under-ascertainment
We can assess how large any potential ascertainment bias would need to be to account for these differences in observed and predicted rates. After adjustment for intervention effects of statins and revascularization as well as hypothetical confounding by indication, predicted rates remained 80% higher in the lower two risk groups and 30% higher in the upper two risk groups. Overall, we would need 60% more events to match the numbers predicted by the Pooled Cohort Equations.
Discussion
Since the initial publication of the new ACC/AHA guidelines, there has been concern about the new risk equations. First, in the three external validation cohorts examined by the committee, the model over-predicted risk.1 When examined in three additional cohorts,3 as well as in Rotterdam,4 the over-estimation of risk was repeated. Several possible reasons for the discrepancies have been suggested.6,7 One is that both the WHS and the Physicians' Health Study were trials of health professionals; 75% of WHS participants were RNs, with another 15% LPNs.14 These two cohorts thus may be at lower risk than other more contemporary cohorts. However, much of the lower risk should be reflected in their lower risk factors, which are included in the risk equations. In addition, MESA, REGARDS, the Women's Health Initiative Observational Study, and the Rotterdam Study were observational cohorts. Over-estimation was seen in these, as well as in the “contemporary cohorts” of the original Framingham, Framingham Offspring, and ARIC studies used in guideline validation.1
The WHS has been criticized because of self-reports.6 While women self-reported their ASCVD endpoints, these were adjudicated using medical records. Lipids were directly measured. Blood pressure was self-reported, which has been shown to correlate well with measured blood pressure in a similar cohort of female health professionals.15 Diabetes was self-reported, but in a validation of incident reports in the WHS, 91% were confirmed.16 Under-reports of either hypertension or diabetes, however, should lead to under- rather than over-estimation of risk.
Concerns have been raised about follow-up rates in some cohorts.5,7 Updated results from REGARDS using Medicare claims suggest that incomplete ascertainment could explain the previous lack of fit.5 Follow-up in the WHS, however, was excellent, reaching 97.2% for morbidity and 99.4% for mortality.9 Because the WHS was a trial, follow-up efforts were extensive, and participants may have had a greater commitment to the study. We would need an additional 60% of observed events to reach the number predicted by the Pooled Cohort Equations. It is unlikely that the WHS follow-up missed that many events.
Increasing use of statins is a plausible explanation for discrepancies in more contemporary populations. Use of statins greatly expanded over the last two decades, although these were recommended only for women at high risk or with high LDL.2 Adjustment using strong assumptions, however, could not explain the discrepancy between observed and predicted rates. For example, assuming a 25% intervention effect and a doubling of risk due to confounding by indication, rates predicted by the Pooled Cohort Equations remained 80% higher in the two lower risk groups and 30-40% higher in the two higher risk groups than the actual observed rates.
Revascularization procedures are another possible explanation for lower event rates in more contemporary cohorts. Rates were too low in the WHS to have much impact, however. Further, more recent randomized trials of revascularization versus optimal medical therapy have suggested little difference in events, despite relief of angina.13 As revascularizations are part of the endpoint used in most statin trials, incorporation of these into prediction models as in the Reynolds Risk Scores17,18 might provide patients with a better overall perspective on vascular risk.
Censoring at the time of use of cholesterol-lowering medications or revascularization actually increased the discrepancy between observed and predicted rates. Such censoring is non-ignorable and biased, meaning that those who go on statins are likely at higher risk due to factors not included in the risk equations. Such factors could include a family history of ASCVD, elevated C-reactive protein, or an increase in lipids over time. While censoring can adjust for the intervention effect, it does not take into account such confounding by indication.
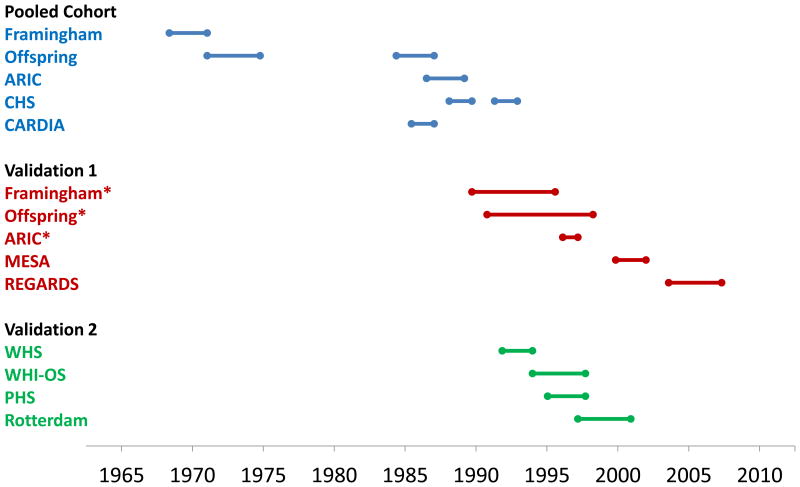
Other explanations should be considered. The baseline measurements in the various cohorts took place over different time periods, with some of the earliest cohorts in the development set collecting data as far back as 1968 (Figure 4). All of the validation cohorts, including the WHS, started much later, beginning baseline data collection in the late 1980's to early 2000's. Whether equations developed using earlier data apply to more contemporary cohorts is a substantive issue as cardiovascular event rates have declined in a consistent manner over the past 40 years, particularly among diabetics.19,20 Other changes over time may involve additional medical therapies, such as improved treatment of diabetes or hypertension, or even aspirin use, particularly in men in whom the greatest reductions have been seen.21 Changes in lifestyle such as amount smoked or passive smoking may not be completely controlled. Changes over time also undoubtedly took place in the development cohorts used for the risk equations. If these equations are to be used to recommend statin therapy, the appropriate background risk needs to be clarified. A simple increase in the risk cutoff for statin use from 5% to 7.5%,21 instead of model recalibration, is not optimal since the risk-benefit discussion remains muddied.
Figure 4.
Approximate timeline of study baseline data collection, including the derivation samples used for the pooled cohort equations (Pooled Cohort), the external validation cohorts used in the guideline report (Validation 1), and additional external cohort samples (Validation 2). * “Contemporary” cohort.
Limitations to the current analyses should be noted. First, we did not assess statin use at every follow-up visit, making finer adjustment for individual statin use difficult. Instead we interpolated rates over time and used life table methods for adjustment. Second, while risk factors were updated on annual questionnaires, we did not collect repeat blood samples, so could not examine the impact of changing cholesterol levels on statin use. Third, estimates of intervention effects are available from meta-analyses of randomized trials, but our estimates of confounding by indication are hypothetical. Fourth, the WHS excluded those with a history of angina at baseline, which eliminated a small group of women who were included in the pooled cohorts. These would be at high risk, but would already have clinical ASCVD, and should be recommended for statin therapy.22 Fifth, because of difficulty assigning accurate cause of death, the outcome here included all cardiovascular deaths. Restriction to deaths due to MI or stroke would only increase the observed discrepancies. Finally, there have been differences in the diagnosis of MI over time, particularly with the increasing use of troponin levels.23 These, however, would make the observed rates higher in more contemporary cohorts and thus not explain any over-estimation.
The pooled risk equations have now been found to over-predict the rate of CVD in at least seven external validation cohorts. While alternative explanations may exist, we found that the use of statins, revascularizations, or under-ascertainment could not explain the extent of over-estimation in the WHS. Recalibration of the Pooled Cohort equations using available contemporary data sets might provide a solution to this problem.
Supplementary Material
Acknowledgments
The WHS was supported by grants from the National Heart, Lung, and Blood Institute (HL043851) and the National Cancer Institute (CA047988), Bethesda, Maryland, who had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Cook had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Dr. Ridker is listed as a co-inventor on patents held by the Brigham and Women's Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to AstraZeneca and Seimens, and has received research grant support from AstraZeneca and Pfizer, manufacturers of statin therapy.
References
- 1.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 2.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women: 2011 update: A guideline from the American Heart Association. Circ. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762–1765. doi: 10.1016/S0140-6736(13)62388-0. [DOI] [PubMed] [Google Scholar]
- 4.Kavousi M, Leening MJG, Nanchen D, et al. Comparison of application of the ACC/AHA guidelines, Adult Treatment Panel III guidelines, and European Society of Cardiology guidelines for cardiovascular disease prevention in a European cohort. JAMA. 2014;311(14):1416–1423. doi: 10.1001/jama.2014.2632. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P, Colantonio LD, Cushman M, et al. Validation of the atherosclerotic cardiovascular disease pooled cohort risk equations. JAMA. 2014;311(14):1406–1415. doi: 10.1001/jama.2014.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Goff DCJ, Stone NJ. Statins, risk assessment, and the new American prevention guidelines. Lancet. 2013;383:600–602. doi: 10.1016/S0140-6736(13)62348-X. [DOI] [PubMed] [Google Scholar]
- 7.Muntner P, Safford MM, Cushman M, Howard G. Comment on the reports of over-estimation of ASCVD risk using the 2013 AHA/ACC risk equation. Circulation. 2014;129:266–267. doi: 10.1161/CIRCULATIONAHA.113.007648. [DOI] [PubMed] [Google Scholar]
- 8.Cook NR, Ridker PM. Response to comment on the reports of overestimation of ASCVD risk using the 2013 AHA/ACC risk equation. Circ. 2014;129:268–269. doi: 10.1161/CIRCULATIONAHA.113.007680. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 10.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women's Health Study. J Amer Med Assoc. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 11.Sesso HD, Gaziano JM, Glynn RJ, Buring JE. Value of an Endpoints Committee versus the use of nosologists for validating cause of death. Contemp Clin Trials. 2006;27:333–339. doi: 10.1016/j.cct.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Cholesterol Treatment Trialists' (CTT) Collaborators. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380:581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pursnani S, Korley F, Gopaul R, et al. Percutaneous coronary intervention versus optimal medical therapy in stable coronary artery disease: A systematic review and meta-analysis of randomized clinical trials. Circ Cardiovasc Interv. 2012;5:476–490. doi: 10.1161/CIRCINTERVENTIONS.112.970954. [DOI] [PubMed] [Google Scholar]
- 14.Rexrode KM, Lee IM, Cook NR, Hennekens CH, Buring JE. Baseline characteristics of participants in the Women's Health Study. J Womens Health Gend Based Med. 2000 Jan-Feb;9(1):19–27. doi: 10.1089/152460900318911. [DOI] [PubMed] [Google Scholar]
- 15.Colditz GA, Martin P, Stampfer MJ, et al. Validation of questionnaire information on risk factors and disease outcomes in a prospecitve cohort study of women. Am J Epidemiol. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Lee IM, Song Y, et al. Vitamin E and risk of type 2 diabetes in the Women's Health Study Randomized Controlled Trial. Diabetes. 2006;55:2856–2862. doi: 10.2337/db06-0456. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women. JAMA. 2007;297:611–619. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980–2000. N Engl J Med. 2007;356:2388–2398. doi: 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 20.Gregg EW, Li Y, Wang J, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 21.D'Agostino RB., Sr Understanding the cardiovascular disease risk functions - Aim, development, and evaluation. N Engl J Med. 2014;370(17):1652–1653. [Google Scholar]
- 22.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 23.Myerson M, Coady S, Taylor H, Rosamond WD, Goff DCJ. Declining severity of myocardial infarction from 1987 to 2002: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2009;119:503–514. doi: 10.1161/CIRCULATIONAHA.107.693879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.