Abstract
Background
Recent studies point to the clinical utility of using peri-implant sulcular fluid (PISF) as a valuable diagnostic aid for monitoring peri-implant tissue health. The objectives of this study were to determine the levels of key biomarkers in PISF in periodontal maintenance participants and compare them to their corresponding levels in gingival crevicular fluid (GCF) obtained from the same participants.
Methods
PISF and GCF were collected from an implant and a contralateral natural tooth, after the clinical examination of 73 participants. The levels of interleukin (IL)-1α, IL-1β, IL-6, IL-8, IL-10, IL-12, IL-17A, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), osteoprotegerin (OPG), leptin, and adiponectin were determined using multiplex proteomic immunoassays. The correlation of biomarker concentrations between GCF versus PISF, within GCF or PISF, and with several covariates (age, brushing frequency, days since professional cleaning, probing depth and plaque index) were also determined.
Results
Significantly higher levels of IL-17A (p=0.02) and TNF-α (p=0.03) were noted in PISF, when compared to their levels in GCF. Significant positive correlations were noted between the concentrations of cytokines in PISF versus their levels in GCF. Among the covariates, significant positive correlation was noted between mean probing depths around implants and levels of IL-1β (p < 0.05) and IL-8 levels (p < 0.05) in PISF.
Conclusions
The results of this study point to the differential expression of specific biomarkers in GCF versus their levels in PISF in periodontal maintenance patients, which is critical information prior to establishing PISF as a diagnostic fluid to monitor peri-implant health.
Keywords: Gingival crevicular fluid, dental implants, peri-implantitis, biological markers
Introduction
Peri-implant pathologies are highly prevalent and may affect either the peri-implant mucosa alone (peri-implant mucositis) or both peri-implant soft and hard tissues (peri-implantitis).1,2 A range of complications can arise from untreated peri-implant diseases, from progressive loss of attachment and increasing peri-implant inflammation to eventual implant failure. It is widely acknowledged that the predictability of peri-implantitis therapy is generally low, with highly variable treatment outcomes.3 Therefore, early detection or prevention of peri-implant inflammation remains an utmost priority to reduce the esthetic, functional and financial complications associated with the failure of implant therapy.
None of the clinical parameters typically recorded to assess peri-implant health status (i.e. probing depth, recession, bleeding on probing, etc.) have been validated as reliable diagnostic tools to monitor early changes in peri-implant tissues4, however, recent studies point to the utility of peri-implant sulcular fluid (PISF) as a valuable diagnostic aid for detecting early stages of peri-implant pathologies.5,6 Like chronic periodontitis, peri-implantitis is a multifactorial disease with the involvement of several local and systemic risk factors.7 It is well established that chemokines, cytokines, and biological mediators all play a crucial role in regulating healthy and pathological periodontal and peri-implant conditions.7 Recent studies have demonstrated that some of these biomarkers in GCF and PISF are selectively up-regulated at sites of inflammation and tissue breakdown, as compared to healthy sites.5,6 It has been shown that some of the constituents of PISF reflect the inflammatory status of tissues around dental implants accurately.8 For example, levels of interleukin 1β (IL-1β), matrix metalloprotease-8 (MMP-8), prostaglandin E2 (PGE2) levels, myeloperoxidase and other bone turnover markers correlate well with clinical findings of peri-implantitis.4–6,9
To enable clinicians to utilize GCF and PISF biomarkers as early indicators of disease process in their daily clinical practice, it is crucial to gather knowledge regarding the biomarker levels of patients in periodontal maintenance who have implants. Deviations from established levels in patients with optimal peri-implant health may be used to detect early periodontitis and peri-implantitis. Therefore, the objectives of this study were to determine the presence and concentrations of key inflammatory and bone turnover markers in GCF and PISF from participants who are on regular periodontal maintenance and to compare the concentrations of these mediators among natural teeth sites versus implant sites. In addition, the effect of covariates such as age, brushing frequency, days since professional cleaning, mean probing depth around teeth, mean plaque levels around implants and natural teeth on the levels of mediators and the correlations among biomarkers within PISF and GCF, and between biomarkers in PISF versus GCF were also assessed.
Materials and Methods
Participant Identification and Recruitment
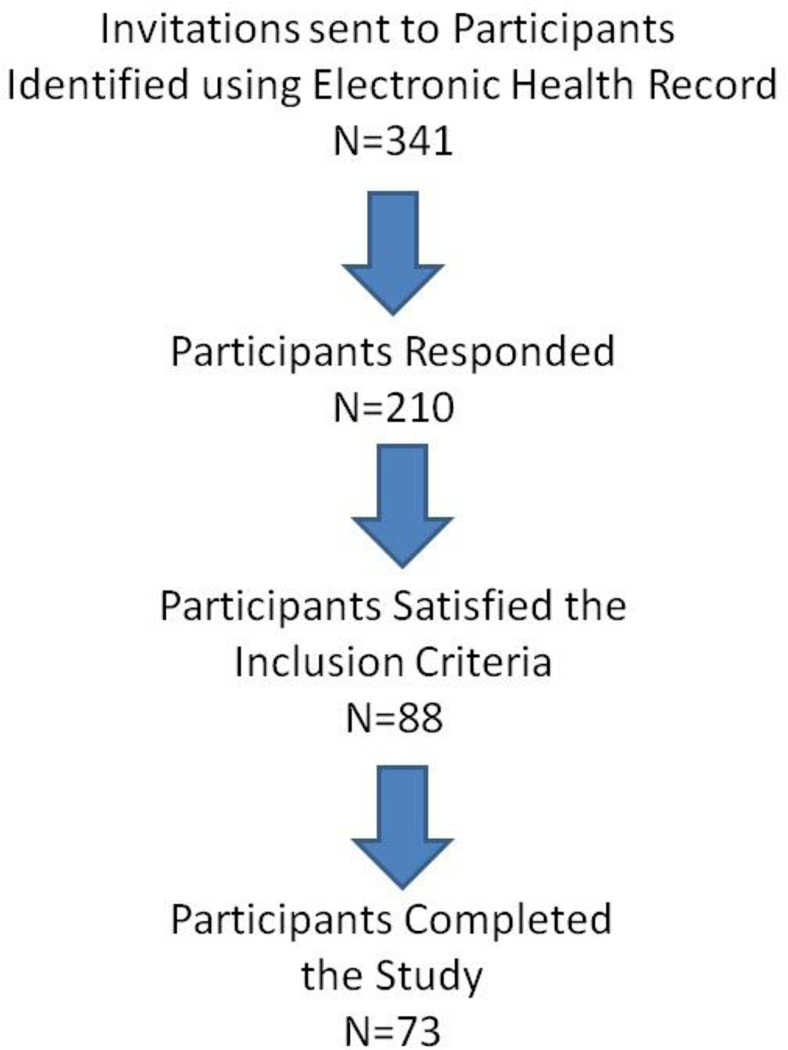
After obtaining Institutional Review Board’s approval from the University of Iowa’s Human Subjects Office (IRB # 201109878), participants enrolled in the College of Dentistry periodontal recall program and having at least one rough surface implant in function for a minimum of 6 months were identified by searching the electronic health record (EHR). Eligible participants visited the Craniofacial Clinical Research Center at the University of Iowa College of Dentistry and Dental Clinics for data and sample collection between June 2012 and April 2013. Eligible participants were 18 years of age or older, currently non-smokers and enrolled in a collegiate periodontal maintenance program. Exclusion criteria included participants with aggressive periodontitis, who were pregnant or nursing, edentulous, presenting with blade-type or smooth surface implants or participants who had taken medications such as antibiotics and anti-inflammatory agents, which are known to alter the oral inflammatory status or hormonal levels, for 3 months prior to the study visit. This study is an extension of a primary study that evaluated the effect of obesity on peri-implant health.10 Of the 341 invitations sent to potential participants, 210 responded, 88 of which fulfilled the inclusion criteria and were scheduled for the study visit. Of the 88 participants scheduled, sample collection was completed in 73 participants (Figure 1).
Figure 1.
Flowchart depicting the study participant recruitment process.
PISF Collection and Analysis
PISF was collected from the sulcus around the target dental implant using paper stripsǂ. In patients with multiple implants, preference was given to maxillary implants and the most posterior implant was preferred, as implants placed in these sites tend to exhibit more biological complications.11 With proper isolation using cotton rolls in the buccal and lingual aspects of the study site, the area was dried for 5 seconds with compressed air. The paper strip was gently introduced into the mucosal crevice around dental implants for 30 seconds per site in 4 sites (mesio-buccal, disto-buccal, mesio-lingual and disto-lingual). The strips were then removed from the crevice and the volume of fluid collected in each strip was measured using a micro-moisture metering device.Ф After confirming the adequateness of the volume, the paper strips from each implant were pooled and transferred into labeled tubes and stored at −80°C for later use. Similarly, GCF was collected from a contralateral natural tooth using the paper strips from 4 sites per tooth and stored at −80°C.
During the analysis, paper strips were suspended in 0.01M sodium phosphate buffer (pH 7.2) prepared using pyrogen free water containing protease inhibitors and the concentration (pg/30 second) of interleukin (IL)-1α, IL-1β, IL-6, IL-8, IL-10, IL-12, IL-17A, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), Osteoprotegerin (OPG), leptin, and adiponectin was analyzed using multiplexed fluorescent bead-based immunoassays* in the Luminex system±. The molecular assessments were made in triplicate and for the statistical analysis, median of the replicates was used.
Periodontal and Peri-implant Health Evaluation
Following sample collection, using the modified Quigley Hein plaque index,12 plaque levels were recorded. This was followed by a comprehensive periodontal and peri-implant examination by one calibrated examiner (G.A-O.). In order to get calibrated, partial periodontal examination (one quadrant) was performed by the examiner on two volunteers on two separate occasions with 3 days apart and Cohen’s Weighted Kappa was used to assess examiner reliability. Probing pocket depth, recession, presence or absence of bleeding on probing and suppuration on six points, as well as mobility (Miller scale), furcation involvement13 and width of facial keratinized tissue (if any) were assessed and recorded. With the exception of gingival recession, every other parameter was recorded for dental implants. In addition, information regarding the participant’s brushing and flossing frequency, time since last prophylaxis, past history of periodontal therapy, familial history of periodontal disease, any complications during or after implant placement and also if bone grafting was performed prior to or simultaneously with implant placement were all collected. Fasting blood glucose level was measured using a point-of-care glucometer.¶
Statistical Analysis
Descriptive statistics were computed for all biomarker outcomes. Differences of each outcome for the two sites within participant (i.e., the measurement at the implant site - the measurement at the control/tooth site) were calculated, and sign tests were performed to see if there was a difference in biomarker levels around dental implants versus (vs.) those around the natural tooth. The sign test was used rather than the Signed Rank test because of violations of the assumption of symmetry of the differences. Spearman correlation analysis was performed in order to evaluate the correlation between the level of each biomarker in PISF and that measure in GCF. Wilcoxon Rank Sum tests were performed to evaluate the effect of flossing and bleeding on probing on biomarker levels. Spearman rank correlations were used to assess the relationship between the biomarkers and the continuous covariates (age, brushing frequency, days since professional cleaning, mean probing depth and mean plaque index). A Bonferroni correction, in conjunction with an overall 0.05 level of Type I error, was used to adjust for multiple testing for 65 tests. In addition, the Spearman rank correlation coefficient and the associated tests were computed to evaluate the relationship among the 13 PISF biomarkers and the among the 13 GCF biomarkers. The relevant effect size pertains to the ability to detect differences in cytokine levels between implant and control (natural tooth) sites in the same individual, and calculations take into account adjustment for the multiple testing performed for 13 cytokines using the standard Bonferroni method. Based on such adjustment, two-sided testing and an overall Type I error of 0.05, a sample size of 73 would be expected to provide 80% power to detect a difference of 0.45 standard deviations or greater between the two sites.
Results
The mean age of the 73 enrolled participants was 59.95±14.20. A large majority of the participants were Caucasians (91.78%), with a slight majority of females (61.64%). None of the participants were diabetic, but a fraction of this population (17.80%) exhibited pre-diabetic plasma glucose levels (100 to 126 mg/dl).
The results of calibration exercise suggested a moderate intra-rater agreement (K=0.41 to 0.6) for probing depth, clinical attachment level and bleeding on probing measurements and good agreement (K=0.61 to 0.8) for keratinized gingiva width, plaque index and recession measurements. On a scale of 0 to 5 with ‘0’ being no plaque and ‘5’ being >2/3 of plaque coverage, the mean full mouth plaque index was very low (0.80±0.48) and the mean plaque score around implants and natural teeth from which the samples were obtained was 0.40±0.67 and 0.61±0.60, respectively. Of the included implants, a large majority were maxillary posterior implants (45.21%), followed by mandibular posterior implants (32.88%), maxillary anterior implants (19.18%) and mandibular anterior implants (0.03%). A large majority of implants were surrounded by at least 2 mm of keratinized mucosa width (91.78%). The mean keratinized mucosa width around implants was 3.19±1.59 mm, while around natural teeth it was 3.78±1.16 mm. The mean of the deepest probing depths around implants and natural teeth, from which the samples were collected were 4.42±1.12 and 3.18±0.90, respectively. The median probing depth around the implant site tends to be greater than the median depth around natural tooth (p < 0.001).
The concentrations of IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-12, CRP, RANKL, osteoprotegerin, and adiponectin were all detected in both GCF and PISF (Table 1). Their concentrations did not differ significantly (p > 0.05) between GCF and PISF (Table 1). Only IL-17A (p=0.023) and TNF-α (p=0.032) were present in significantly higher concentrations in PISF, compared to their levels in GCF (Figures 2 and 3 and Table 1).
Table 1.
Comparison of the concentrations of biomarkers in PISF versus GCF samples
| Difference (PISF – GCF levels) |
Cytokine levels in PISF (Implant Site) |
Cytokine Levels in GCF (Tooth Site) |
|||||
|---|---|---|---|---|---|---|---|
| Biomarker* | N | Median Difference (95% CI)‡ |
p-value+ | Mean (median) | SD | Mean (median) | SD |
| IL1−α | 73 |
−120.83 (−193.75 to 27.91) |
0.24 | 897.29 (581) |
927.36 | 847.37 (734.44) | 584.30 |
| IL1−β | 73 |
0.28 (−0.99 to 2.61) |
0.72 | 25.79 (10.25) |
43.77 | 15.71 (11.82) |
17.83 |
| IL−4 | 73 |
0 (0.00 to 0.88) |
0.25 | 14.70 (13) |
12.09 | 14.45 (12.9) |
12.27 |
| IL−6 | 73 |
0 (−0.10 to 0.08) |
0.54 | 1.04 (0.4) |
1.59 | 1.20 (0.48) |
1.92 |
| IL−8 | 73 |
47.90 (−18.57 to 100.40) |
0.10 | 782.21 (448.75) | 899.31 | 608.81 (433.69) | 632.24 |
| IL−10 | 73 |
0.23 (−0.39 to 0.83) |
0.64 | 7.14 (6.2) |
5.17 | 5.98 (5.49) |
2.94 |
| IL−12 | 73 |
0.05 (−0.48 to 0.63) |
0.81 | 6.87 (6.03) |
3.48 | 6.63 (6.51) |
3.00 |
| IL−17 | 73 |
0.10 (0.00 to 0.18) |
<0.05 | 1.75 (1.75) |
0.77 | 1.63 (1.62) |
0.70 |
| TNF−α | 73 |
0.52 (0.00 to 0.87) |
<0.05 | 4.16 (2.84) |
5.45 | 3.01 (2.29) |
2.61 |
| CRP+ | 60 |
0.27 (−0.53 to 1.19) |
0.70 | 11.90 (3.61) |
19.59 | 11.35 (2.31) |
19.14 |
| OPG | 57 |
1 (−2.02 to 6.11) |
0.34 | 26.37 (19.22) |
20.31 | 22.06 (19.67) |
14.66 |
| Leptin | 57 |
2.17 (−3.20 to 4.79) |
0.43 | 45.14 (39) |
21.91 | 39.70 (38) |
14.18 |
| Adiponectin | 39 |
1522 (−723.3 to 4500.00) |
0.11 | 11332 (6638) |
13151 | 6969 (4410) |
6532 |
All biomarker levels except CRP are in pg/30seconds; for CRP alone the unit is ng/30sec
Significance probability associated with the sign test, assessing whether biomarker measurements at the implant site differed from those at the tooth site
Rank−based confidence interval with a minimum of 95% confidence on the median difference between cytokine level measured in the PISF at the implant site versus that measured in the GCF at the control (natural tooth) site.27
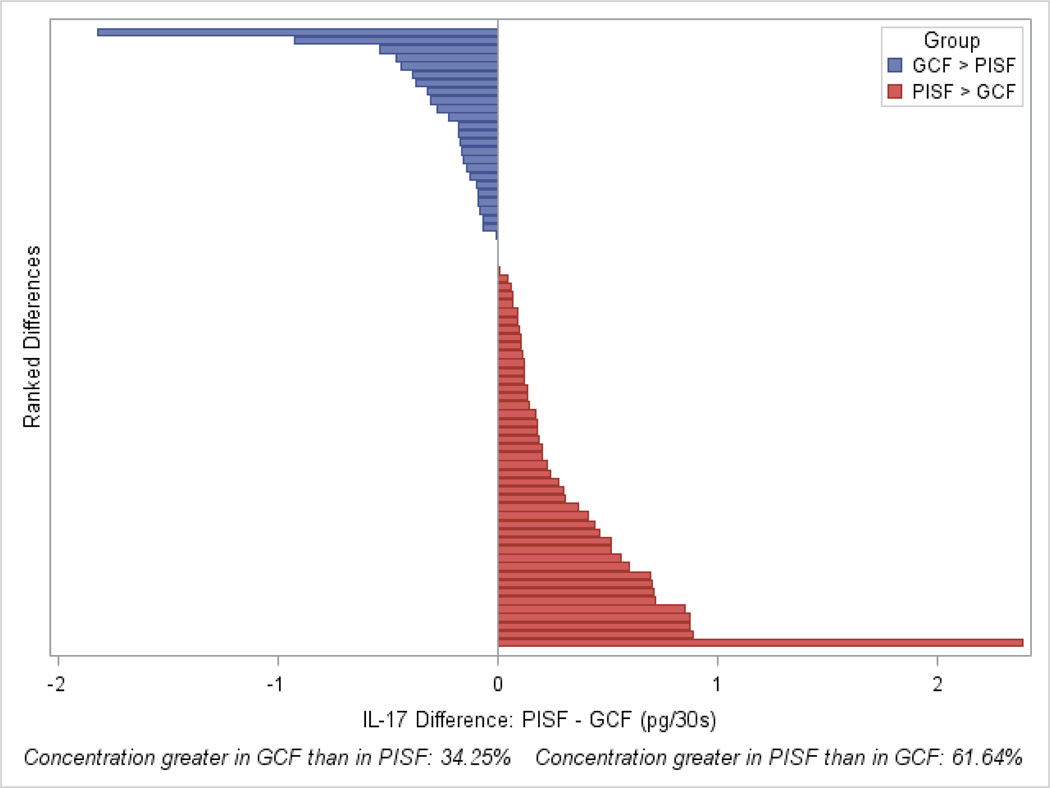
Figure 2.
Representation of ranked differences in levels of IL-17A between implant and tooth sites for 73 participants, indicating that IL-17A levels at the implant site tended to be greater than those at the tooth site. Higher PISF levels of IL-17A relative to GCF (red bars) were found in 61.6% of participants (sign test, p=0.023).
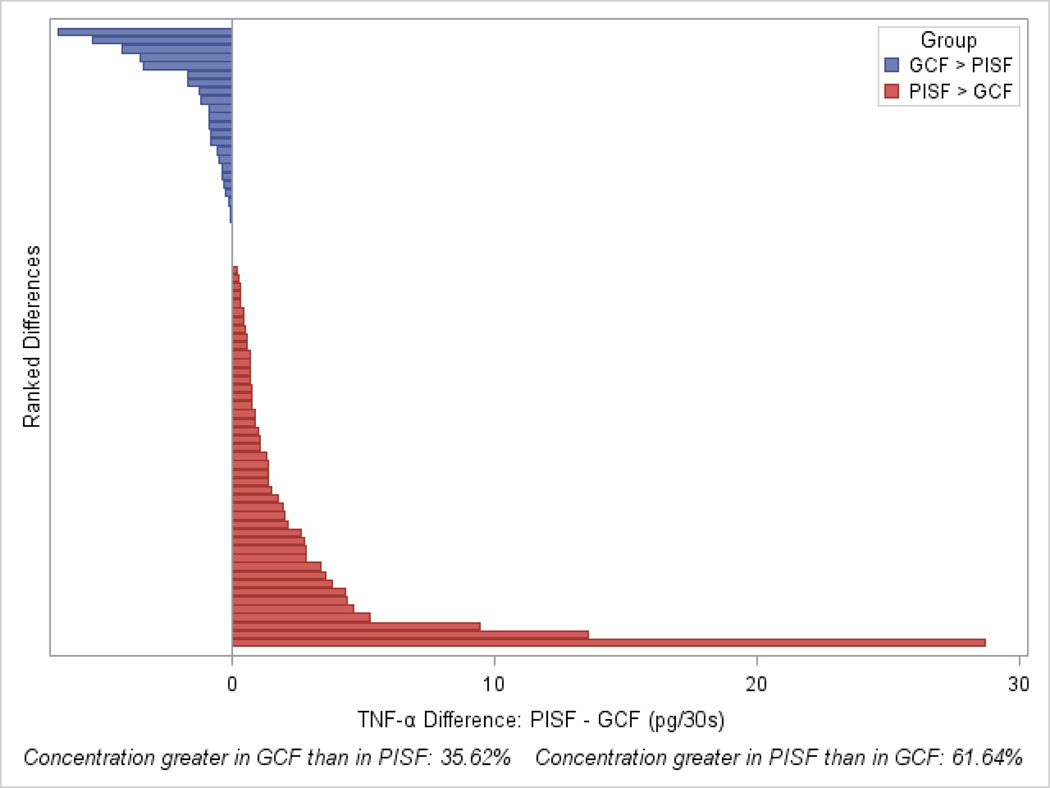
Figure 3.
Representation of ranked differences in levels of TNF-α between implant and tooth sites for 73 participants, indicating that TNF-α levels at the implant site tended to be greater than those at the tooth site. Higher PISF levels of TNF-α relative to GCF levels (red bars) were found in 61.6% of participants (sign test, p=0.032).
Spearman rank correlation analysis revealed statistically significant correlations between the level of biomarkers in GCF and that in PISF for each of the 13 biomarkers, with coefficients ranging from 0.45 pg/30 for adiponectin to 0.87 pg/30 for IL-4 (Table 2). The median level of cytokines in PISF and GCF among individuals who flossed was lower than the median level of individuals who did not floss. Among cytokines measured in the PISF, there was a difference in IL-1α (p=0.0357; median of 571.53 pg/30 seconds for those flossing vs. median of 1101.88 pg/30 seconds for those not flossing), IL-4 (p=0.0067; median of 12.90 pg/30 seconds for those flossing vs. 16.29 pg/30 seconds for those not flossing) and IL-12 (p=0.0374; median of 5.88 pg/30 seconds for those flossing vs. 8.06 pg/30 seconds for those not flossing). For the GCF cytokines, there was a difference in IL-4 (p=0.0031; medians of 12 pg/30 seconds for those flossing vs. 18.25 pg/30 seconds for those not flossing). In several instances, the median level of cytokines in GCF was greater when there was bleeding on probing than when bleeding on probing was absent: significant differences were found in IL1-α (p=0.0003; median of 848.89 pg/30 seconds when there was bleeding vs. 323.13 pg/30 seconds when bleeding was absent), IL-1β (p=0.0019; median of 15 pg/30 seconds when there was bleeding vs. 4.13 pg/30 seconds when bleeding was absent) and IL-4 (p=0.0266; median of 13 pg/30 seconds when there was bleeding vs. 10.95 pg/30 seconds when bleeding was absent). Since most of the individuals had bleeding on probing at the implant site, formal statistical evaluation of the impact of this covariate on biomarker levels in the PISF was not feasible.
Table 2.
Spearman rank correlations between 13 biomarker concentrations in PISF versus in GCF
| Biomarker | Spearman Rank Test | ||
|---|---|---|---|
| r | n | p | |
| IL-1α | 0.61 | 73 | <0.0001 |
| IL-1β | 0.62 | 73 | <0.0001 |
| IL-4 | 0.87 | 73 | <0.0001 |
| IL-6 | 0.48 | 73 | <0.0001 |
| IL-8 | 0.68 | 73 | <0.0001 |
| IL-10 | 0.70 | 73 | <0.0001 |
| IL-12 | 0.76 | 73 | <0.0001 |
| IL-17 | 0.84 | 73 | <0.0001 |
| TNF-α | 0.53 | 73 | <0.0001 |
| CRP | 0.67 | 60 | <0.0001 |
| OPG | 0.75 | 57 | <0.0001 |
| Leptin | 0.80 | 57 | <0.0001 |
| Adiponectin | 0.45 | 39 | 0.0039 |
The levels of cytokines in PISF and GCF were found to be associated with multiple quantitative covariates (Table 3). Of particular interest among these are the significant correlations noted between mean probing depths around implants and levels of IL-1β and IL-8 levels in PISF. Statistically significant correlations were noted between mean plaque index values and the levels of IL-1α and IL-12 measured in the GCF (Table 3). These correlations remained significant after adjusting for multiple testing.
Table 3.
Spearman rank correlations between measurements taken at the implant and at the control tooth site for each of 13 biomarkers, and the covariates age, brushing frequency, days since professional cleaning, mean probing depth and mean plaque index.
| Biomarker | N | Age | Brushing Frequency | Days Since Professional Cleaning |
Mean Probing Depth |
Mean Plaque Index | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PISF | GCF | PISF | GCF | PISF | GCF | PISF | GCF | PISF | GCF | |||
| IL1−α | 73 | r | 0.10 | 0.03 | −0.33 | −0.36 | 0.24 | 0.10 | 0.29 | 0.34 | 0.28 | 0.40 |
| p | 0.40 | 0.78 | <0.01* | <0.01* | <0.05* | 0.38 | <0.05* | <0.01* | <0.05* | <0.01** | ||
| IL1−β | 73 | r | 0.13 | 0.12 | −0.30 | −0.32 | 0.33 | 0.15 | 0.39 | 0.38 | 0.22 | 0.34 |
| p | 0.27 | 0.33 | <0.01* | <0.05* | <0.05* | 0.19 | <0.01** | <0.01* | 0.06 | <0.01* | ||
| IL−4 | 73 | r | 0.14 | 0.07 | −0.17 | −0.15 | 0.20 | 0.19 | 0.16 | 0.24 | 0.13 | 0.27 |
| p | 0.23 | 0.56 | 0.14 | 0.20 | 0.09 | 0.12 | 0.17 | <0.05* | 0.26 | <0.05* | ||
| IL−6 | 73 | r | 0.18 | −0.10 | 0.05 | −0.04 | 0.09 | 0.13 | 0.29 | 0.18 | 0.08 | 0.07 |
| p | 0.13 | 0.39 | 0.68 | 0.74 | 0.44 | 0.28 | <0.05* | 0.12 | 0.52 | 0.55 | ||
| IL−8 | 73 | r | −0.07 | −0.21 | −0.22 | −0.20 | 0.11 | −0.04 | 0.40 | 0.08 | −0.03 | 0.04 |
| p | 0.55 | 0.07 | 0.06 | 0.09 | 0.35 | 0.71 | <0.01** | 0.49 | 0.81 | 0.73 | ||
| IL−10 | 73 | r | 0.24 | 0.09 | −0.20 | −0.11 | 0.32 | 0.31 | 0.30 | 0.23 | 0.15 | 0.25 |
| p | <0.05* | 0.44 | 0.08 | 0.34 | <0.01* | <0.01* | <0.01* | 0.05 | 0.19 | <0.05* | ||
| IL−12 | 73 | r | 0.30 | 0.32 | −0.28 | −0.19 | 0.16 | 0.23 | 0.23 | 0.23 | 0.23 | 0.47 |
| p | <0.01* | <0.01* | <0.05* | 0.10 | 0.17 | <0.05* | 0.05 | <0.05* | <0.05* | <0.01** | ||
| IL−17 | 73 | r | 0.14 | 0.10 | −0.17 | −0.09 | 0.24 | 0.29 | 0.16 | 0.11 | 0.17 | 0.19 |
| p | 0.22 | 0.40 | 0.14 | 0.47 | <0.05* | <0.05* | 0.19 | 0.37 | 0.15 | 0.11 | ||
| TNF−α | 73 | r | 0.11 | 0.03 | −0.16 | −0.15 | 0.09 | 0.06 | 0.33 | 0.18 | 0.11 | 0.23 |
| p | 0.35 | 0.78 | 0.17 | 0.19 | 0.47 | 0.60 | <0.01* | 0.13 | 0.34 | 0.05 | ||
| CRP | 60 | r | 0.11 | 0.02 | 0.06 | −0.04 | 0.18 | 0.03 | 0.18 | 0.11 | 0.13 | 0.01 |
| p | 0.42 | 0.86 | 0.65 | 0.76 | 0.17 | 0.80 | 0.16 | 0.42 | 0.33 | 0.92 | ||
| OPG | 57 | r | −0.03 | −0.01 | −0.05 | 0.06 | 0.06 | 0.04 | 0.29 | 0.19 | −0.07 | −0.05 |
| p | 0.81 | 0.96 | 0.74 | 0.68 | 0.66 | 0.77 | <0.05* | 0.16 | 0.62 | 0.74 | ||
| Leptin | 57 | r | 0.16 | 0.24 | −0.09 | −0.09 | 0.00 | 0.00 | 0.10 | 0.05 | 0.10 | 0.11 |
| p | 0.23 | 0.07 | 0.52 | 0.51 | 0.99 | 0.98 | 0.48 | 0.73 | 0.47 | 0.43 | ||
| Adiponectin | 39 | r | 0.11 | 0.03 | −0.16 | −0.09 | 0.20 | 0.09 | 0.43 | 0.08 | −0.01 | −0.03 |
| p | 0.52 | 0.84 | 0.33 | 0.57 | 0.21 | 0.59 | <0.01* | 0.62 | 0.94 | 0.87 | ||
Spearman correlation coefficient is denoted by ‘r’ and ‘p’ refers to p−value for that correlation.
p<0.05
Significant after adjusting for multiple comparisons, i.e., unadjusted p−value < 0.0008. The significant correlations are highlighted.
Additionally, among biomarkers present in PISF, we noticed that the majority of biomarkers analyzed were mutually correlated. Notable were the correlations between IL-1α and IL-1β (r=0.86; p < 0.05) and between IL-12 and IL-17A (r=0.73; p < 0.05). With the exception of IL-12 and IL-17A, the remainder of the biomarkers assessed in the PISF correlated significantly with IL-6. Of the assessed biomarkers, leptin did not correlate with IL-1α, IL-12, IL-17A, TNF-α, CRP and adiponectin. Fewer significant correlations were noted among the biomarkers measured in the GCF. Of importance are correlations between IL-1α and IL-1β (r= 0.88; p < 0.05) and between IL-10 and IL-17A (r=0.75; p < 0.05). CRP was correlated only with IL-6 and TNF-α. TNF-α was correlated will all biomarkers but adiponectin. Similarly, IL-1β and IL-10 were correlated with all biomarkers except CRP.
Discussion
To our knowledge, this is among the largest cross-sectional assessments exploring the differential expression of several key molecular markers in PISF vs. GCF samples in participants enrolled in a periodontal maintenance program. This study showed that the levels of several biomarkers in PISF correlated well with their levels in GCF. However, we found that IL-17A and TNF-α levels were significantly higher (p < 0.05) in PISF samples.
Both GCF and PISF are osmotically mediated inflammatory exudates that originate from the vessels of the mucosal plexus around natural teeth or implants. These fluids are considered particularly attractive in the diagnostic realm due the non-invasive collection methods and the fact that they contain molecules whose levels may reflect both local and systemic inflammation.14 Several clinical studies in the past assessed the correlation between clinical parameters of periodontal disease and markers of inflammation in GCF samples.15,16 These past studies clearly point to the positive correlation that exists between select biomarkers and the severity of periodontal inflammation. It is also evident from these studies that molecular markers of inflammation clearly correlate not just with the existing clinical inflammatory conditions, but may also predict future periodontal breakdown.17
Like GCF, PISF is known to contain host derived enzymes and their inhibitors, inflammatory mediators, and tissue breakdown products.18 A recent review nicely summarized human clinical trials showing that sites affected with peri-implantitis exhibited higher levels of IL-1β, TNF-α, IL-6 and IL-8, compared to health controls.19 In addition, healthy sites exhibited higher levels of IL-4, an anti-inflammatory cytokine and OPG, an anti-resorptive mediator, compared to diseased sites.20 Additionally, it was demonstrated in a case series study that a reduction in TNF-α level followed anti-infective therapy in sites with peri-implantitis.21 Apart from peri-implantitis, PISF samples taken from implant sites with peri-implant mucositis exhibited significantly higher IL-6 levels, compared to healthy sites.22
Though the literature supporting the diagnostic value of select markers in PISF for detecting existing peri-implantitis is accumulating, studies that assess the diagnostic utility of these biomarkers to detect early changes is lacking.19 To explore the effect of early changes on the levels of molecular marker of inflammation, the basal levels of these markers in PISF must be established. It is equally important to know how these basal levels in PISF compare with that of GCF levels. There are only a handful of studies in the dental literature that examined the levels of key biological mediators between GCF and PISF samples.4,23,24 . Two of these past studies were cross-sectional in nature and they analyzed only a handful of biomarkers in a small population sample.4,21 Nowzari and coworkers assessed the measures of 6 inflammatory mediators (IL-1β, IL-8, IL-6, IL-10, 1L-12 and TNF-α) in PISF versus GCF samples in 24 participants. In addition they also assessed the microbial composition in plaque samples obtained from implants and natural teeth and they found them to be similar. They concluded that the profile of inflammatory cytokines in PISF was distinctive of an innate immune response and in higher concentration than in GCF.23 Of the cytokines assessed, the difference in concentration between PISF and GCF samples reached statistical significance only for TNF-α.23 Our results concur with this previous study with regard to the differential expression of TNF-α in PISF versus GCF samples. In addition, we show for the first time that IL-17A is significantly higher in PISF, compared to GCF samples. IL-17A is one of the key effector cytokines of Th17 cells and it is a pro-inflammatory cytokine with both pathological and protective roles during inflammation.25 Other previous study evaluated the levels of nitrite and myeloperoxidase in GCF and PISF samples and reported a correlation between the levels of these markers and clinical signs of inflammation.4 A very recent prospective study evaluated the levels of select biomarkers in 21 subjects in GCF versus PISF using multiplex ELISA and they reported no significant difference in the cytokine levels between the two diagnostic fluids.24 Differences in study design, number of participants, selected biomarkers panel and the inclusion criteria between our study and theirs could have influenced the outcomes. Moreover, the previous study did not include IL-17A in their panel.
Though not confirmed in this cross-sectional assessment, differences in the anatomy, histology and function between periodontal and peri-implant tissues are cited as plausible reasons for the differential expression of certain cytokines.23 Deeper probing depths are noted frequently around dental implants even in healthy populations, due to the positioning of implant platform relative to the crestal bone. This may favor the colonization of anaerobic gram-negative species, which may be involved in triggering a pro-inflammatory response.23 Even in our study, we noticed a deeper average deep probing depth (4.42±1.12) surrounding implants versus natural tooth (3.18±0.90). Selective reaction to titanium molecules and selective adherence of plaque to implant surface has been also proposed as a possible reason for these differential expressions.23
Of the covariates assessed, some of them, specifically plaque levels and mean probing depths, had a significant correlation on the levels of some key inflammatory mediators. Of the correlations assessed between these covariates and levels of measures biomarkers, we found it interesting that levels of IL-1β correlated positively with increasing mean probing depth around implants. There are previous studies that pointed out the importance of this key pro-inflammatory cytokine as a possible biomarker for peri-implantitis.6,26
Given the cross-sectional nature of the study, causality cannot be established nor capturing the true baseline levels of these markers as the participants received implants at different time points and information on how long they are in maintenance is lacking. Though this study has the above limitations, as mentioned earlier, the biggest strengths of this study include adequate sample size and the inclusion of several key inflammatory and bone turnover markers. The reported concentrations of the measured biomarkers may be of use in long-term prospective studies with shorter intervals that assess the correlation between changes in the levels of cytokines from reported levels and the clinical parameters of peri-implant pathologies.
Conclusion
The results of this study point to the differential expression of specific biomarkers in GCF versus their levels in PISF in periodontal maintenance patients, which is critical information prior to establishing PISF as a diagnostic fluid to monitor peri-implant health. Statistically significant higher concentrations of IL-17A and TNF-α were noted in PISF as compared to GCF and a number of recorded clinical parameters had a significant effect on the levels of key measured biomarkers.
Acknowledgements
This study was supported by Osseointegration Foundation grant, USA and the ICTS, USA. This work was also supported by funds from National Institutes of Health, USA -National Institute of Dental and Craniofacial Research grants RO1 DEO14390. The Institute for Clinical and Translational Science (ICTS) at the University of Iowa is supported by the National Institutes of Health (NIH) Clinical and Translational Science Award (CTSA) program, grant 2 UL1 TR000442-06. The CTSA program is led by the NIH’s National Center for Advancing Translational Sciences (NCATS). This publication's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Authors would also like to thank Ms. Lauren Hughes (Craniofacial Clinical Research Center at the University of Iowa College of Dentistry), the research coordinator in this study. Authors report no conflict of interest with regard to this study.
Footnotes
Disclaimer: A portion of this research was presented at the Johnson & Johnson Hatton Competition, 43rd American Association for Dental Research Annual Meeting and Exhibition, Charlotte, NC. March 19, 2014.
Periopaper®, Oralflow, Plainview, NY, USA
Periotron®, Oraflow, Plainview, NY, USA
Millipore, Billerica, MA, USA
Luminex 100 IS Instrument , Austin, TX, USA
One Touch Ultra 2 Blood Glucose Meter, Milpitas, CA, USA
References
- 1.Atieh MA, Alsabeeha NH, Faggion CM, Jr, Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol. 2013;84:1586–1598. doi: 10.1902/jop.2012.120592. [DOI] [PubMed] [Google Scholar]
- 2.Mombelli A, Muller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012;23(Suppl 6):67–76. doi: 10.1111/j.1600-0501.2012.02541.x. [DOI] [PubMed] [Google Scholar]
- 3.Khoshkam V, Chan HL, Lin GH, et al. Reconstructive procedures for treating peri-implantitis: a systematic review. J Dent Res. 2013;92:131S–138S. doi: 10.1177/0022034513509279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tozum TF, Akman AC, Yamalik N, et al. Analysis of the inflammatory process around endosseous dental implants and natural teeth: myeloperoxidase level and nitric oxide metabolism. Int J Oral Maxillofac Implants. 2007;22:969–979. [PubMed] [Google Scholar]
- 5.Basegmez C, Yalcin S, Yalcin F, Ersanli S, Mijiritsky E. Evaluation of periimplant crevicular fluid prostaglandin E2 and matrix metalloproteinase-8 levels from health to periimplant disease status: a prospective study. Implant Dent. 2012;21:306–310. doi: 10.1097/ID.0b013e3182588408. [DOI] [PubMed] [Google Scholar]
- 6.Fonseca FJ, Moraes Junior M, Lourenco EJ, Teles Dde M, Figueredo CM. Cytokines expression in saliva and peri-implant crevicular fluid of patients with peri-implant disease. Clin Oral Implants Res. 2014;25:e68–e72. doi: 10.1111/clr.12052. [DOI] [PubMed] [Google Scholar]
- 7.Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35:292–304. doi: 10.1111/j.1600-051X.2008.01275.x. [DOI] [PubMed] [Google Scholar]
- 8.Kaklamanos EG, Tsalikis L. A review on peri-implant crevicular fluid assays potential in monitoring and predicting peri-implant tissue responses. J Int Acad Periodontol. 2002;4:49–59. [PubMed] [Google Scholar]
- 9.Rakic M, Lekovic V, Nikolic-Jakoba N, Vojvodic D, Petkovic-Curcin A, Sanz M. Bone loss biomarkers associated with peri-implantitis. A cross-sectional study. Clin Oral Implants Res. 2013;24:1110–1116. doi: 10.1111/j.1600-0501.2012.02518.x. [DOI] [PubMed] [Google Scholar]
- 10.Elangovan S, Brogden K, Dawson DV, Blanchette D, Pagan-Rivera K, Stanford CM, Johnson GK, Recker E, Bowers R, Haynes WG, Gustavo Avila-Ortiz. Body Fat Indices and Biomarkers of Inflammation. A Cross-sectional Study with Implications for Obesity and Peri-implant Oral Health. Int J Oral Maxillofac Implants (In Press) 2014 doi: 10.11607/jomi.3758. [DOI] [PubMed] [Google Scholar]
- 11.Rodoni LR, Glauser R, Feloutzis A, Hammerle CH. Implants in the posterior maxilla: a comparative clinical and radiologic study. Int J Oral Maxillofac Implants. 2005;20:231–237. [PubMed] [Google Scholar]
- 12.Turesky S, Gilmore ND, Glickman I. Reduced plaque formation by the chloromethyl analogue of victamine C. J Periodontol. 1970;41:41–43. doi: 10.1902/jop.1970.41.41.41. [DOI] [PubMed] [Google Scholar]
- 13.Hamp SE, Nyman S, Lindhe J. Periodontal treatment of multirooted teeth. Results after 5 years. J Clin Periodontol. 1975;2:126–135. doi: 10.1111/j.1600-051x.1975.tb01734.x. [DOI] [PubMed] [Google Scholar]
- 14.Buduneli N, Kinane DF. Host-derived diagnostic markers related to soft tissue destruction and bone degradation in periodontitis. J Clin Periodontol. 2011;38(Suppl 11):85–105. doi: 10.1111/j.1600-051X.2010.01670.x. [DOI] [PubMed] [Google Scholar]
- 15.Lamster IB. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann Periodontol. 1997;2:123–137. doi: 10.1902/annals.1997.2.1.123. [DOI] [PubMed] [Google Scholar]
- 16.Lamster IB, Ahlo JK. Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann N Y Acad Sci. 2007;1098:216–229. doi: 10.1196/annals.1384.027. [DOI] [PubMed] [Google Scholar]
- 17.Reinhardt RA, Stoner JA, Golub LM, et al. Association of gingival crevicular fluid biomarkers during periodontal maintenance with subsequent progressive periodontitis. J Periodontol. 2010;81:251–259. doi: 10.1902/jop.2009.090374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorsa T, Tervahartiala T, Stenman M, Suomalainen K, Mäntylä P. Nordic dentistry yearbook. Lyngby, Denmark: Quintessence Publishing Co.; 2004. Chair-side diagnostic point-of-care MMP-tools in periodontitis and peri-implantitis; pp. 79–95. [Google Scholar]
- 19.Javed F, Al-Hezaimi K, Salameh Z, Almas K, Romanos GE. Proinflammatory cytokines in the crevicular fluid of patients with peri-implantitis. Cytokine. 2011;53:8–12. doi: 10.1016/j.cyto.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Duarte PM, de Mendonca AC, Maximo MB, Santos VR, Bastos MF, Nociti Junior FH. Differential cytokine expressions affect the severity of peri-implant disease. Clin Oral Implants Res. 2009;20:514–520. doi: 10.1111/j.1600-0501.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 21.de Mendonca AC, Santos VR, Cesar-Neto JB, Duarte PM. Tumor necrosis factor-alpha levels after surgical anti-infective mechanical therapy for peri-implantitis: a 12-month follow-up. J Periodontol. 2009;80:693–699. doi: 10.1902/jop.2009.080521. [DOI] [PubMed] [Google Scholar]
- 22.Ata-Ali J, Flichy-Fernandez AJ, Ata-Ali F, Penarrocha-Diago M, Penarrocha-Diago M. Clinical, microbiologic, and host response characteristics in patients with peri-implant mucositis. Int J Oral Maxillofac Implants. 2013;28(3):883–890. doi: 10.11607/jomi.2654. [DOI] [PubMed] [Google Scholar]
- 23.Nowzari H, Phamduong S, Botero JE, Villacres MC, Rich SK. The profile of inflammatory cytokines in gingival crevicular fluid around healthy osseointegrated implants. Clin Implant Dent Relat Res. 2012;14:546–552. doi: 10.1111/j.1708-8208.2010.00299.x. [DOI] [PubMed] [Google Scholar]
- 24.Nogueira-Filho G1, Pesun I, Ploegman C, Wijegunasinghe M, McCulloch CA. J Longitudinal Comparison of Cytokines in Peri-Implant Fluid and Gingival Crevicular Fluid in Healthy Mouths. J Periodontol. 2014 May 16;:1–9. doi: 10.1902/jop.2014.130642. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 25.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casado PL, Canullo L, de Almeida Filardy A, Granjeiro JM, Barboza EP, Leite Duarte ME. Interleukins 1beta and 10 expressions in the periimplant crevicular fluid from patients with untreated periimplant disease. Implant Dent. 2013;22:143–150. doi: 10.1097/ID.0b013e3182818792. [DOI] [PubMed] [Google Scholar]
- 27.Nair KR. Table of confidence interval for the median in samples from any continuous population. Sankhya. 1940;4:551–558. [Google Scholar]