Abstract
This study was carried out to characterize three aldehydes of health concern (formaldehyde, acetaldehyde, and acrolein) at a central Beijing site in the summer and early fall of 2008 (from June to October). Aldehydes in polluted atmospheres come from both primary and secondary sources, which limits the control strategies for these reactive compounds. Measurements were made before, during, and after the Beijing Olympics to examine whether the dramatic air pollution control measures implemented during the Olympics had an impact on concentrations of the three aldehydes and their underlying primary and secondary sources. Average concentrations of formaldehyde, acetaldehyde and acrolein were 29.3±15.1 μg/m3, 27.1±15.7 μg/m3 and 2.3±1.0 μg/m3, respectively, for the entire period of measurements, all being at the high end of concentration ranges measured in cities around the world in photochemical smog seasons. Formaldehyde and acrolein increased during the pollution control period compared to the pre-Olympic Games, followed the changing pattern of temperature, and were significantly correlated with ozone and with a secondary formation factor identified by principal component analysis (PCA). In contrast, acetaldehyde had a reduction in mean concentration during the Olympic air pollution control period compared to the pre-Olympic period and was significantly correlated with several pollutants emitted from local emission sources (e.g., NO2, CO, and PM2.5). Acetaldehyde was also more strongly associated with primary emission sources including vegetative burning and oil combustion factors identified through the PCA. All three aldehydes were lower during the post-Olympic sampling period compared to the before and during Olympic periods, likely due to seasonal and regional effects. Our findings point to the complexity of source control strategies for secondary pollutants.
Keywords: aldehydes, air pollution, acrolein, pollutant sources, principal component analysis, Olympics
1. Introduction
1.1 Unique opportunity to collect air pollution data
The Chinese government implemented a series of air pollution control measures to improve air quality during the 2008 Beijing Olympics and Paralympics. Control measures included the reduction of pollutant emissions from factories and industrial facilities and cutting by half the number of private cars on the road, according to an odd/even plate number rule. Additionally, all construction projects were suspended during the Olympic period (Wang et al., 2009a). These control measures resulted in significant reductions in concentrations of primarily emitted pollutants (e.g., PM2.5, SO2, NOx) (Huang et al., 2010; Li et al., 2010a; Li et al., 2010b; Wang et al., 2009a; Wang et al., 2010a; Wang et al., 2010b; Wang et al., 2010c; Wang and Xie, 2009; Wang et al., 2009b; Wang et al., 2009d; Xin et al., 2010; Zhou et al., 2010). However, it is less straightforward whether the same trend occurred for pollutants that had both primary and secondary sources, such as ozone and aldehydes. Our study utilizes a valuable data set before, during, and after the Olympic Games to verify whether, and at what extent, the change in emission source intensities resulted in the abatement of pollution.
Based on the intensity of the air pollution control measures (Wang et al., 2009a), our study used three periods defined as follows: the pre-Olympic period (June 4th – July 19th) when some light controls were implemented, the during-Olympic period (July 20th – September 19th) when the full-scale control measures were implemented, and the post-Olympic period (September 20th – October 30th) when the control measures were relaxed. Extra control measures were also adopted during the Olympic (August 8th – August 24th) and the Paralympic periods (September 6th – September 17th), which included barring an additional 20% of government-owned cars from traveling on the road, suspending outdoor construction work, and temporarily closing some gas stations. Therefore, the during-Olympic period can be further divided into the sub-period 1 with full-scale control measures (July 20th – August 7th and August 24th – September 5th), and the sub-period 2 with the extra actions described above (August 8th – August 23rd and September 6th – September 17th).
1.2 Ambient concentrations and sources of aldehydes
Aldehydes are reactive compounds that induce adverse health effects in humans and animals (Akbar-Khanzadeh and Mlynek, 1997; Benjebria et al., 1994; Cassee et al., 1996a; Cassee et al., 1996b). Although a number of papers have been published assessing the air quality impact of emission controls during the Beijing Olympics (Huang et al., 2010; Li et al., 2010a; Wang et al., 2009a; Wang and Xie, 2009; Wang et al., 2009d), only one paper dealt with formaldehyde and acetaldehyde measured at one quasi-suburban Beijing site, and none on acrolein. In fact, our overall knowledge about ambient acrolein exposure is extremely limited despite the high toxicity of this compound. Aldehydes can be directly emitted into the atmosphere from the incomplete combustion of biomass and fossil fuels (Schauer et al., 2001; Zhang and Smith, 1999), and formed in the atmosphere as a result of photochemical oxidation of reactive hydrocarbons (Altshuller, 1993; Possanzini et al., 2002). Important combustion sources of aldehydes include vehicles, power plants, and residential wood burning (Stahl 1969; Lipari 1984). Hence it is important to identify dominant sources in order to set up more effective control strategies. Compared to many other air pollutants (e.g., hydrocarbons, PM mass and certain species), the relative contributions of primary and secondary sources to aldehydes in metropolitan centers has been understudied (Altshuller, 1993; Chan and Yao, 2008; Feng et al., 2005).
To examine whether aldehyde concentrations were reduced during the air pollution control period, we measured formaldehyde, acetaldehyde, and acrolein for approximately one month during each period (pre-Olympic, during Olympic, or post-Olympic). In the during-Olympic period, aldehydes were measured in both sub-periods 1 and 2. Furthermore, in order to better understand the impact of the Beijing Olympic control measures, we also obtained data for numerous other air pollutants at the same monitoring site, and meteorological data (temperature, relative humidity, wind speed, and wind direction) from a nearby site, and analyzed their relationships to the aldehydes.
1.3 Influence of weather, meteorology, and regional sources on pollution in Beijing
Another important factor influencing the concentrations of ambient aldehydes could be meteorological conditions dominating the Beijing region during the summer months (Streets et al., 2007). Beijing is located at 39°56’N and 116°20’E on the northwest border of the Great North China Plain. It is located in a warm temperate zone and has a typical continental monsoon climate (Chan and Yao, 2008). The air quality of Beijing in the summer is largely determined by the meteorology (Streets et al., 2007), in particular, temperature and solar radiation are key factors that control the photochemistry processes (Wang et al., 2009d). The influence of wind direction is associated with the origin of air masses transported from the surrounding areas of Beijing while wind speed controls the dispersion of air pollution. In summer months, Beijing typically experiences high temperatures (mean: 27 °C) and relative humidity (mean: 64%), both favoring the photochemical reactions. In the summer, Beijing also has few windy days, which is unfavorable for atmospheric dispersion of air pollutants.
In addition, neighboring regions impact Beijing air quality (Wang et al., 2009b; Wang et al. 2010b). PM concentrations, ozone, and sulfate have all been shown to have significant regional contributions. For PM, air masses transported from the south of Beijing have been shown to increase PM concentrations in the region while air masses from the northwest have been shown to decrease PM concentrations.
Methods
2.1 Air sample collection, storage, and analysis
Sample collection, storage and analysis were performed in conjunction with the Health Effects of Air pollution Reduction Trial (HEART) study (Zhang et al. 2013). The HEART study included a comprehensive characterization of air pollution before, during, and after the games. All the air samplers and monitors were collocated at a secured spot on the Peking University 1st Hospital campus that served as the clinical base for the health outcome measurements of the HEART study. The hospital was located in the center of Beijing, within the 2nd ring road, 3 kilometers northwest of Tiananmen Square, surrounded by busy streets of local motor vehicle traffic, cyclists, and pedestrians.
2.2 Aldehyde measurement methods
A passive sampling technique was used to collect aldehydes on a 24-hour integrated basis. A C18 cartridge (LC-18, 0.5g/4.5mL, Supelco, Inc. US) coated with dansylhydrazine (DNSH) was used to collect and derivatize the aldehydes. Samples and field controls were eluted with acetonitrile and aliquots of extracts were analyzed using an HPLC system with fluorescent detection. This method was described in detail previously (Herrington et al., 2005; Weisel et al., 2005; Zhang et al., 2000). Throughout the entire sampling period, 78 aldehyde samples were collected in total, including 28, 26, and 24 samples for the pre-, during-, and post-Olympic periods, respectively. One field control and one duplicate sample were collected every 3 to 5 days for quality control purposes. Sample concentrations were corrected with the average field blank concentrations. All the samples had detectable concentrations of aldehydes.
2.3 Other pollutants and meteorological data measurement methods
Other pollutants, including O3, CO, SO2, NO, NO2, NOx, respirable particles (PM10), fine particles (PM2.5), and numerous constituents of the fine particles were measured simultaneously with the aldehydes. The constituents analyzed from PM2.5 have been reported in a previous report (Zhang et al. 2013). In brief, PM2.5 were collected on four filters (two Teflon and two quartz) simultaneously, and the constituents analyzed in the PCA included elemental carbon (EC), organic carbon (OC) and polycyclic aromatic hydrocarbons (PAHs) from the two quartz filters, and ions (Na+, NH4+, K+, Mg2+, Ca2+, F−, Cl−, NO3−, SO42−) and elements (Na, Mg, Al, K, Ca, Ti, V, Mn, Fe, Ni, Cu, Zn, Se, Pb) from the two Teflon filters. Meteorological data (temperature, relative humidity, wind speed, and wind direction) were collected at a nearby meteorological station (within 5 km). These methods are detailed in previous papers (Zhang et al. 2013).
There were 94 days of data available from the HEART study concerning the other pollutants. Among the 35 variables included in our principal component analysis, 33 had two or fewer missing days of data. The two exceptions are sulfur dioxide (SO2), which is missing 7 days of data during the middle period, and nickel, which is missing 16 days of data mostly during the middle period.
2.4 Principal component analysis and source apportionment
Principal component analysis (PCA) is a common technique to define new variables from linear combinations of initial variables (Jolliffe 2002). PCA was used for source apportionment before, during, and after the Summer Olympic Games in Beijing in August 2008. The PCA was conducted using the daily average concentrations of the PM2.5 constituents, as shown in Table 1, as well as PM10 concentrations and the daily average concentrations of several gases measured (O3, SO2, NO, NO2, and CO). Statistical analysis was completed in R. The principal() function in R from the ‘psych’ package was used. This function utilizes a correlation matrix.
Table 1.
Concentrations of aldehydes in pre-, during-, and post-Olympic periods (unit: μg/m3)*
| Pre-Olympics | During-Olympics | Post-Olympics | Whole period | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | |
|
| ||||||||||||||||
| Formaldehyde | 35.7 | 9.0 | 15.8 | 60.1 | 37.3 | 11.2 | 17.1 | 68.6 | 13.3 | 13.9 | 1.0 | 57.5 | 29.3 | 15.1 | 1.0 | 68.6 |
| Acetaldehyde | 35.2 | 15.1 | 15.7 | 67.2 | 23.8 | 14.7 | 2.0 | 63.9 | 20.6 | 13.6 | 3.3 | 62.4 | 27.1 | 15.7 | 2.0 | 67.2 |
| Acrolein | 2.4 | 0.9 | 1.0 | 4.5 | 2.9 | 0.8 | 1.8 | 4.6 | 1.4 | 0.5 | 0.6 | 2.5 | 2.3 | 1.0 | 0.6 | 4.6 |
Concentrations were field blank corrected; average concentration of aldehydes was calculated if the duplicated samples were collected.
Certain pollutant data collected was included or omitted from the PCA. Elements analyzed from PM2.5 were chosen based on two criteria – the average concentration observed and their expected utility as a tracer for particular sources. Furthermore, PAHs were chosen for inclusion in the PCA based on their molecular weight. Lower molecular weight PAHs are not as useful for source apportionment since they are converted in the atmosphere (Park et al. 2002, Schauer et al. 1996).
By inspection of the loadings for each factor, comparison to the existing literature, and consultation with co-authors on PM2.5 source compositions, assessments were made regarding the most likely source type of each factor. This analysis, along with the information provided by the PCA regarding the percent of variance in the data explained by each factor, was used to surmise the relative contribution of each major, underlying source type for air pollution in Beijing. All five factors that were included had eigenvalues greater than 1.4, and each accounted for at least 7.9% of the variability in the data. The five factors in total accounted for 85% of the variability in the data.
2.5 Regional contributions assessed with air mass back trajectories
Air mass back trajectories were calculated using the National Oceanic and Atmospheric Administration (NOAA) ARL HYSPLIT 4.0 model with meteorological data from the Global Data Assimilation System (GDAS) (Draxler and Rolph 2014). For each 24 hour sample, three trajectories were calculated (one every eight hours) and the trajectory heights were 20 m, 830 m, and 1480 m – the sample collection height, the average summer mixing height, and the average daily maximum summer mixing height for Beijing, respectively (Cheng et al., 2001).
Results
3.1 Concentrations of atmospheric aldehydes
Mean, standard deviation, minimum and maximum concentrations of aldehydes concentrations throughout the entire period and in the three specific periods are given in Table 1. The average concentrations of all three aldehydes were lowest during the post-Olympic period. Furthermore, despite the controls put in place during the Olympics, the average concentrations of both formaldehyde and acrolein peaked in this period, although the difference for formaldehyde from the pre- to the during-Olympic period was not statistically significant. In fact, formaldehyde increased by 1.6 μg/m3 (4%, p=0.576) from the pre- to the during-Olympic period and decreased by 23.4 μg/m3 (63%, p<0.0001) from the during- to the post-Olympic period. The concentration of acrolein increased by 0.5 μg/m3 (20%, p=0.038) from the pre- to the during-Olympic period and decreased by 1.5 μg/m3 (52%, p<0.0001) from the during- to the post-Olympic period. For acetaldehyde, the average concentrations were highest before the Olympics. In fact, it decreased by 11.5 μg/m3 (33%, p=0.0074) from the pre- to the during-Olympic period, and in the post-Olympic period it continued to decrease by 3.1 μg/m3 (13%, p=0.483).
Sixteen and ten samples were collected in the sub-period 1 (with full scale controls) and 2 (with full scale and extra controls), respectively. The mean concentrations of all aldehydes were higher in sub-period 1 than in the sub-period 2. The concentrations in these sub-periods were respectively, 37.7±10.7 μg/m3 (28.2±8.0 ppb) and 36.5±12.4 μg/m3 (27.2±9.3 ppb) for formaldehyde, 26.3±15.9 μg/m3 (13.4±8.1 ppb) and 19.9±12.3 μg/m3 (10.1±6.3 ppb) for acetaldehyde, and 3.0±0.8 μg/m3 (1.2±0.3 ppb) and 2.7±0.8 μg/m3 (1.1±0.3 ppb) for acrolein. The reductions in aldehyde concentrations in sub-period 2 compared to sub-period 1 were 3%, 24%, and 10% for formaldehyde, acetaldehyde and acrolein, respectively.
3.2 Meteorological conditions
The daily average temperature, relative humidity, precipitation, and wind speed and the prevailing wind direction in the three sampling periods are summarized in Rich et al, 2012. The prevailing wind direction in summer for the Beijing’s urban area was S-SSE-SE, and was similar before and during the Olympics. The change in wind speed was not statistically significant. There also was no significant change in temperature or daily average RH between the two periods. In contrast, temperature decreased by 11.7 °C (41.8%, p<0.0001) and RH decreased by 12.2% (18.9%, p=0.0560) after the Olympic Games. The daily average precipitation increased by 3.0 mm (99.4%, p=0.167) from the pre- to the during-Olympic period, and then decreased by 5.7 mm (93.3%, p=0.012).
3.3 Correlation of aldehydes with other air pollutants and meteorological conditions
Since some of the air pollutants, e.g. PM2.5, NO and NO2, did not satisfy the normality distribution assumption, the Spearman rank correlation test was used to examine the association between pollutants. The Spearman correlation coefficients among aldehydes and other air pollutants are shown in Table 2. The p-value for each coefficient was calculated using permutation test and the significance level of each correlation coefficient is indicated in Table 2 as well. Formaldehyde, acetaldehyde, and acrolein were significantly correlated with each other. The correlation coefficients were 0.59 for formaldehyde and acetaldehyde, 0.63 for formaldehyde and acrolein, and 0.43 for acetaldehyde and acrolein. Formaldehyde was significantly correlated with oxides of nitrogen (NO, NO2 and NOx) in the negative direction and the correlation coefficients ranged from −0.31 to −0.53. Formaldehyde was significantly correlated with each of daily average O3, daily maximum O3, CO and PM2.5 in the positive direction with coefficients of 0.41, 0.38, 0.26, and 0.39, respectively. Acetaldehyde was significantly and positively correlated with SO2, NO2, CO and PM2.5 with correlation coefficients of 0.51, 0.23, 0.46, and 0.47, respectively. Acrolein was significantly correlated with oxides of nitrogen in the negative direction (r=−0.53 with NO and −0.36 with NO2) and significantly correlated with daily average ozone (r=0.34), daily maximum ozone (r=0.33), and PM2.5 (r=0.24) in the positive direction. No significant correlation was found for any of the three aldehydes with daily average photooxidant, approximated as the sum of O3 and NO2. However, daily maximum photooxidant (the sum of maximum O3 and NO2) was significantly correlated with formaldehyde (r=0.26, p=0.023) and acrolein (r=0.23, p=0.053), respectively. Both formaldehyde and acrolein were significantly correlated with temperature (r=0.56 and 0.59) and RH (r=0.62 and 0.38). Acetaldehyde was significantly and positively correlated with RH (r=0.30) but not with temperature.
Table 2.
Spearman correlation coefficients among aldehydes, other air pollutants, and meteorological parameters
| Formaldehyde | Acetaldehyde | Acrolein | SO2 | NO | NO2 | NOx | 1O3 | 2O3 | 1O3+NO2 | 2O3+NO2 | CO | PM2.5 | T | RH | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formaldehyde | 1 | ||||||||||||||
| Acetaldehyde | 0.59** | 1 | |||||||||||||
| Acrolein | 0.63** | 0.43** | 1 | ||||||||||||
| SO2 | 0.13 | 0.51** | 0.005 | 1 | |||||||||||
| NO | −0.53** | −0.009 | −0.53** | 0.096 | 1 | ||||||||||
| NO2 | −0.31* | 0.23* | −0.36* | −0.40** | 0.80** | 1 | |||||||||
| NOx | −0.41** | 0.14 | −0.47** | 0.31** | 0.89** | 0.97** | 1 | ||||||||
| 1O3 | 0.41** | 0.07 | 0.34* | 0.21* | −0.73** | −0.57** | −0.65** | 1 | |||||||
| 2O3 | 0.38** | 0.04 | 0.33** | 0.27** | −0.61** | −0.40** | −0.49** | 0.92** | 1 | ||||||
| 1O3+NO2 | 0.060 | 0.14 | 0.017 | 0.58** | −0.19* | 0.11 | 0.014 | 0.68** | 0.77** | 1 | |||||
| 2O3+NO2 | 0.26* | 0.14 | 0.23 | 0.46** | −0.39** | −0.10 | −0.21 | 0.79** | 0.91** | 0.91** | 1 | ||||
| CO | 0.26* | 0.46** | 0.20 | 0.50** | 0.17* | 0.35** | 0.29** | −0.01 | 0.08 | 0.30** | 0.25** | 1 | |||
| PM2.5 | 0.39** | 0.47** | 0.24* | 0.67** | −0.15 | 0.20* | 0.09 | 0.31** | 0.34** | 0.56** | 0.49** | 0.65** | 1 | ||
| Temperature | 0.56** | 0.10 | 0.59** | 0.17* | −0.74** | −0.58** | −0.67** | 0.79** | 0.76** | 0.43** | 0.61** | 0.08 | 0.33** | 1 | |
| RH | 0.62** | 0.30* | 0.38* | −0.04 | −0.17* | −0.02 | −0.08 | −0.17* | −0.17* | −0.28** | −0.18* | 0.29** | 0.32** | −0.05 | 1 |
| Wind speed | −0.03 | −0.03 | 0.08 | −0.05 | −0.34** | −0.44** | −0.41** | 0.31** | 0.17* | 0 | 0.033 | −0.27* | −0.07 | 0.18* | −0.31** |
Significance of each coefficient is determined by the p-value,
indicates that the coefficient is significant at the significant level of 0.05;
indicates that the coefficient is significant at the significant level of 0.01;
daily average (24-hour) concentration;
1 hour maximum concentration within a day. All data is based on 24-hour average expect 2O3 and 2O3+NO2.
3.4 Principal component analysis
The principal component analysis (PCA) was performed with and without the aldehydes in the model. Inclusion of the aldehydes did not significantly change the interpretation of the source apportionment or the ordering of the factors in terms of eigenvalues or percent variance explained by each factor. By inspection of the loadings for each factor, comparison to the existing literature, and consultation with co-authors on PM2.5 source compositions, assessments were made regarding the most likely source type of each factor. This analysis is further detailed in the supplemental materials.
With the aldehdyes in the model, formaldehyde and acrolein had component loadings from 0.73 to 0.75 for the secondary formation factor, while acetaldehyde had a component loading of 0.46. Acetaldehyde had a component loading of 0.42 for the factor that represented oil combustion.
The five source types identified – vehicle and industrial combustion, natural soil/road dust, secondary formation, oil combustion, and vegetative burning- are consistent with previous source apportionment studies in Beijing (Song et al. (1) 2006, Song et al. (2) 2006, Song et al. 2007, Wang et al. 2008, Zheng et al. 2005). Motor vehicles and industrial sources were separate factors in all of these previous studies, while they were jointly included in Factor 1 (vehicle and industrial combustion) in the current study. This was due to the fact that these two sources were both targeted for control during the Olympic period, and the two sources were highly correlated during the study period.
The other major novelties of the source apportionment in this study compared to previous studies are the addition of an oil combustion source and the lesser impact of secondary formation in the variance of the data. The former in previous studies was probably merged with other combustion sources such as motor vehicles or industrial sources. The greater contribution of secondary formation in the previous studies is easily explained, since they included measurements in the winter and spring months not considered in this study; differences in secondary formation of aldehydes would therefore be more evident with the dramatic changes in atmospheric and weather conditions occurring in these seasons.
It is also notable that even though the spring Asian dust storms that impact Beijing did not occur during the time frame of this study, Factor 2 (natural soil/road dust) still accounted for a significant proportion of the variance in the principal component analysis (23.5%).
3.5 Association of aldehydes with air pollution sources
Each aldehyde was regressed with the five source types identified through PCA in order to evaluate the strength of the association between them and the identified air pollution sources. The source types determined without the aldehydes included in the PCA were used for this regression (see results in Table 3).
Table 3.
Linear regression coefficients, relative contributions of air pollution source types to aldehyde concentrations
| Linear regression coefficients | ||||||
|---|---|---|---|---|---|---|
| β0 (intercept) | β1 (mixed combustion) | β2 (natural soil/road dust) | β3 (secondary formation) | β4 (oil combustion) | β5 (vegetation burning) | |
| Formaldehyde | 28.7*** | 1.36 | −4.46*** | −8.63*** | 2.43 | 4.98*** |
| Acetaldehyde | 26.8*** | 1.61 | −3.57 | −2.89 | 6.15** | 6.22*** |
| Acrolein | 2.20*** | 0.01 | −0.17 | −0.44*** | 0.24* | 0.26** |
P-value significance: 0 ‘***’ 0.001 ‘**’ 0.01 ‘*’ 0.05 ‘.’ 0.1″ 1
The daily concentrations of both formaldehyde and acrolein were most significantly related to the secondary formation source type, in terms of both the coefficient rates and the statistical significance of those coefficients (p<0.0001). Both coefficients in relation to the secondary formation source type were negative, and this may be due to a negative and positive component loading, respectively, for ozone and nitrogen dioxide in the secondary formation source type. That is, the secondary formation source type daily score was highest when NOx concentrations were high and ozone concentrations were low. Since formaldehyde and acrolein were positively associated with ozone and negatively associated with NOx, they were negatively associated with the secondary formation source type; their concentration tended to increase when the secondary formation daily score was lower.
All three aldehydes were positively associated with the vegetative burning source type. In fact, for acetaldehyde, this was the most significant source type with regards to rate and statistical significance of the regression coefficient (p=0.0004). Formaldehyde and acrolein were also significantly associated, but less significantly than acetaldehyde (p=0.0007 and 0.009, respectively).
Acetaldehyde and acrolein also had significant associations with the oil combustion source type (p=0.005 and p=0.046, respectively), while formaldehyde well fitted with the natural soil/road dust source type (p=0.0008). Unexpectedly, none of the aldehydes had a significant association with the vehicle and industrial combustion source type, which as will be discussed may have been obscured by secondary formation and regional effects.
3.6 Regional contributions
As shown in Table 4, the air mass back trajectories show that the before- and during-Olympic periods, the air masses came predominantly from the south and east, which is consistent with other HYPLIT modelling during the summer of 2008 in Beijing (Wang et al., 2009b; Wang et al. 2010b). After the Olympics, the air masses came predominantly from the north and west.
Table 4.
Air mass back trajectory results
| Time Period | Percent of time air mass spent in each of four quadrants | |||
|---|---|---|---|---|
| North Quadrant | West Quadrant | South Quadrant | East Quadrant | |
| Before | 9% | 5% | 35% | 51% |
| During | 14% | 6% | 45% | 34% |
| After | 48% | 33% | 7% | 12% |
4. Discussion
4.1 Comparison of aldehyde concentrations in this study to other cities
As shown in Table 5, formaldehyde and acetaldehyde concentrations during the summer in Beijing were at the high-end of concentration ranges measured in other cities during seasons characterized by photochemical processes. For example, Milan and Rome in Italy, the downtown area of Savannah, Georgia in the US, Rio de Janeiro in Brazil, and Guangzhou in China, all had lower formaldehyde and acetaldehyde concentrations in the atmosphere compared to this study (Andreini et al., 2000; Baez et al., 1995; Feng et al., 2005; Feng et al., 2004; Grosjean et al., 2002; MacIntosh et al., 2000; Possanzini et al., 1996; Zhang et al., 1994).
Table 5.
Concentrations of formaldehyde and acetaldehyde in ambient air in different cities
| Locations | Study seasons | Average aldehyde concentration (μg/m3/ppb)
|
Measuring interval | References | |
|---|---|---|---|---|---|
| Formaldehyde | Acetaldehyde | ||||
| Milan, Italy | Summer (August) | 8.9/6.65 | 13.7/6.97 | Non-available | Andreini et al., 2000 |
| Rome, Italy | June – July 1994 | 22.77/17.00 | 18.27/9.30 | 1 hour | Possanzini et al., 1996 |
| Downtown Savannah, GA, USA | December 1995 through November 1996 | 2.0/1.49 | 2.3/1.17 | 12 hours | MacIntosh et al., 2000 |
| Suburban area in New Jersey, US | June – August 1992 | 15.37/11.48 | 4.75/2.42 | 2.5–3.5 hours | Zhang et al., 1994 |
| Mexico city, Mexico | March-May 1993 | 43.5/32.48 | 33.8/17.21 | 2 hours | Baez et al., 1995 |
| Rio de Janeiro, Brazil | May to November 2000 | 10.84/8.09 | 10.43/5.31 | 2–3 hours | Grosjean et al., 2002 |
| Guangzhou, China | June to September 2003 | 13.68/10.21 | 8.33/4.24 | 2–3 hours | Feng et al., 2005 |
| Guangzhou, China | August–September 2002 | 13.29/9.92 | 7.6/3.87 | 3.5 hours | Feng et al., 2004 |
| Beijing, China | June to October 2008 | 29.3/21.9 | 27.1/13.8 | 24 hours | Current study |
Acrolein concentrations recently reported are summarized in Table 6. The acrolein level observed in the summer in Beijing was in the high end of the range of acrolein compared to levels reported in the other studies. The acrolein level observed in Beijing was roughly one order of magnitude higher than those observed in most of the other studies. Only one study reported acrolein concentrations higher than those found in Beijing during this study, conducted in the urban area of Los Angeles, US, where the mean concentration of ambient acrolein was 2.58 μg/m3 close to an international airport.
Table 6.
Concentrations of acrolein in the urban air in recent studies*
| Locations | Study seasons | Acrolein concentration (μg/m3 ) | Methoda | Measuring Interval | References |
|---|---|---|---|---|---|
| Rome, Italy | June – July 1994 | 1.75 | DNPH | 1 hour | Possanzini et al., 2002 |
| Pennsylvania, US | May, 1999 | 0.102 (RSD 36%)b 0.315 (RSD 13%)c |
DNPH | 1 hour | Grosjean et al., 2001 |
| Rio de Janeiro, Brazil | May to November 2000 | 0.82 (RSD 77%) | DNPH | 2–3 hours | Grosjean et al., 2002 |
| Northern California, US | April, 2001 | 0.032–0.10 0.23–0.65 |
PFBHA PFPH/GC |
4 hours 4 hours |
Destaillats, et al., 2002 Ho et al., 2004d |
| Guangzhou, China | June to September 2003 | 1.36 | DNPH | 2–3 hours | Feng et al., 2005 |
| Northern California, US | August to September, 2005 | 0.29 (SD 0.022) | PFBHA | 3 hours | Seaman, et al., 2006d |
| CA, NJ, and TX, US | 1999–2001 | 0.46 | DNSH | 24 hours | Liu et al., 2006 |
| Zürich, Swiss | Summer, 2005 | 0.15 (IQR 0.075–0.18) | CT | 50 min | Legreid et al., 2007 |
| Roseville, CA, US | Summer, 2006 | 0.16 (range 0.073–0.62) | PFBHA | 2 hours | Spada et al., 2008 |
| Austin, TX, US | April to July, 2007 | 0.48 (range 0.36–0.60) | DNPH | 4–6 hours | Clements et al., 2009 |
| Los Angeles, CA, US | September 2005 | 2.58 (SD 0.88) | Canister | 24 hours | Zhu et al., 2011 |
| California, US | September to October 2013 | 0.17 (range 0.046–0.41) | PFBHA | 3 hours | Cahill, 2014 |
| Beijing, China | June to October 2008 | 2.3 (SD 1.0; range 0.6–4.6) | DNSH | 24 hours | Current study |
Concentrations of acrolein reported in different units (e.g. ppbv or ng/m3) were converted to μg/m3 and the results were summarized as algebraic mean, geometric mean, or mean (SD, RSD, IQR, or range) based on the data availability.
Method abbreviations are as follows: DNPH=2,4-dinitrophenylhydrazine; PFBHA: pentafluorobenzyl hydroxylamine; PFPH/GC: pentafluorophenyl hydrazine tandem gas chromatograph; CT: cryogenic trap on a gas chromatograph; DNSH: dansylhydrazine.
Tuscarora tunnel inlet sample.
Tuscarora tunnel outlet sample.
Method paper.
4.2 Changes in aldehyde concentrations before, during, and after the Olympic period
As described earlier (in section 3.1), the time trends of formaldehyde, acetaldehyde, and acrolein were different in the investigated period. The reduction in acetaldehyde concentration (−44%) from the pre-Olympics to sub-period 2 was markedly larger than from the pre-Olympics to sub-period 1 (−25%). However, the standard deviation in the two sub-periods was large (SD=14.7 μg/m3), so the change was not significant (p=0.079). Because the observation did not follow a normal distribution, the median concentration of acetaldehyde was calculated; it reached 25.1 μg/m3 and 17.7 μg/m3 in sub-periods 1 and 2, respectively. Therefore, only weak evidence was found suggesting an association between the Beijing Olympic air pollution control measures and the reduction in ambient concentration of acetaldehyde.
4.3 Sources of aldehydes in Beijing
Aldehydes in the atmosphere are generated primarily from direct emissions, e.g., industrial and/or traffic sources, and secondarily from photochemical reactions. Both direct emissions and photochemical reactions might have contributed to the high concentration of atmospheric aldehydes in Beijing in the summer of 2008. Streets with high densities of motor vehicles surrounded our monitoring site and mobile sources were expected to be important sources of aldehydes. In fact, besides primary aldehydes, they release NOx and VOCs, which are precursors of photochemical smog products including aldehydes.
According to Table 3, the three aldehydes are negatively correlated with the secondary formation source type, which in turn showed positive coefficients for NOx and a negative coefficient for ozone. Formaldehyde and acrolein in particular showed highly significant and negative regression coefficients.
For formaldehyde and acrolein, the impact of the reduction of emission rates imposed during the Olympics period was compensated by high concentrations of ozone not titrated by NOx. In fact, ozone concentrations were higher during the Olympic period, due to both high incident sunlight and decreased NOx. Ozone formation is typically either NOx limited or VOC limited (Seinfeld and Pandis, 1998). The Beijing urban area was most likely in a VOC-limited regime (Wang et al., 2009c). Therefore a reduction in NOx would be expected to result in higher ozone production, and consequently in higher concentrations of formaldehyde during the Olympics.
All three aldehydes are significantly associated in the positive direction with biomass burning sources. This relationship may be driven by wood burning for cooking (Lipari et al., 1984).
Acetaldehyde and acrolein, but not formaldehyde, are significantly associated in the positive direction with oil combustion. However, none of the aldehydes are significantly associated with the vehicle and industrial combustion source type. Although these sources, particularly motor vehicles, were expected to contribute to atmospheric aldehyde concentrations, their contribution during this study could be obscured by the strong influence of the secondary formation factor; besides, as NOx emissions from motor vehicles decreased, ozone and aldehyde concentrations tended to increase. So, although vehicle and industrial source emissions and many associated pollutants decreased during the Olympic control period, ozone, formaldehyde, and acrolein were not similarly reduced owing to these secondary formation contributions.
The only other significant relationship among the aldehydes and air pollution factors in this study is that formaldehyde was negatively correlated with the natural soil/road dust source type (p < 0.001). It may be that weather or other patterns that lead to higher natural soil and road dust, such as windy days, also caused low formaldehyde concentrations. However, as shown in Table 2, the aldehydes did not show an association with wind speed. Furthermore, the other aldehydes did not show a significant relationship with natural soil and road dust.
4.4 Effect of weather on aldehyde concentrations
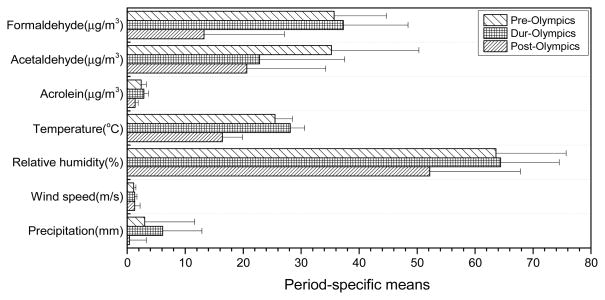
Average concentrations of aldehydes and the mean values of meteorological parameters in the three periods were plotted together pairwise in Figure 1. We observed that formaldehyde and acrolein followed the trend of temperature. The 10.1% increase of temperature was accompanied by a 4% and 20% increase in formaldehyde and in acrolein, respectively, from the pre- to the during-Olympic period. From the during to the after-Olympic period, the 41.8% decrease in temperature corresponded with a 63% reduction in formaldehyde and a 52% reduction in acrolein. Higher relative humidity favors the formation of photochemical smog (Seinfeld and Pandis, 1998); this explains our observation that concentrations of formaldehyde and acrolein tracked the RH levels (see Figure 1). In the post-Olympic period, RH decreased by 18.9% which was accompanied by large reductions in the aldehydes concentrations. These relationships between the aldehydes and temperature and RH suggest that the secondary photochemical sources were a major contributor to atmospheric formaldehyde and acrolein in Beijing. In contrast, temperature and RH had much smaller impact on acetaldehyde; and the variation in its concentration by period appears to be driven by changes in emission sources. In addition, during the Olympic period, higher precipitation was observed, and this was favorable to lower concentrations of water soluble pollutants such as aldehydes. Thus, the reduction in acetaldehyde in the during-Olympic period might be partly due to the higher rainfall intensity (Li et al., 2010a).
Figure 1.
Period-specific means of ambient aldehydes and meteorological parameters, e.g. temperature, relative humidity, wind speed, and precipitation, in three sampling periods.
4.5 Effect of VOC and regional sources on aldehyde concentrations
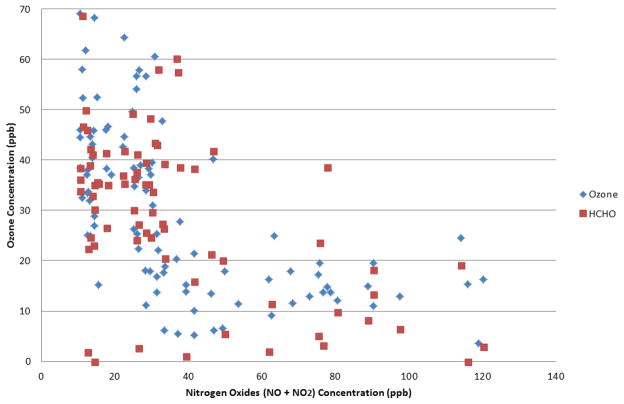
It is interesting to note that with further controls during sub-period 2 during the Olympics, formaldehyde and acrolein concentrations began to decrease, compared to sub-period 1 during the Olympics. This might suggest a transition from a VOC limited (NOx saturated) regime to a NOx limited regime, as evidenced by the fact that both NOx and ozone decreased from sub-period 1 to 2. However, as shown in Figure 2, a decrease in ozone and formaldehyde concentrations were not observed at the minimum NOx concentrations during this study. The lowest daily average NOx concentration measured during this study was 10.5 ppb. Although this may be at the cusp of the transition from a VOC-limited to a NOx-limited regime, the concentration at which this transition might occur in Beijing could not be established. The minimum concentration of NOx in this study exceeds that at which the transition to a NOx-limited regime was observed to occur in the San Joaquin Valley in California. There, the transition occurred at approximately 5 to 9 ppb NOx even at high ambient temperatures (Pusede and Cohen 2012).
Figure 2.
Ozone and formaldehyde concentrations as a function of nitrogen oxide concentration, Beijing, China, June to October 2008.
As shown in Table 4 and previously discussed, regional air mass transport to the sampling location was primarily from the south and east before and during the Olympics, but primarily from the north and west after the Olympics. The impact of this regional effect coupled with the seasonal change in sunlight, temperature, and relative humidity, along with the consequent change in atmospheric chemistry, likely explains why all three aldehydes had lower concentrations during the post-Olympic period. Not only might less aldehydes emitted by primary sources be transported into the Beijing region from the north and west compared to the south and east, but also less VOCs would be transported into the area. This lower VOC concentration along with the lower incident sunlight, temperature, and humidity would produce less aldehyde through secondary formation.
4.6 Limitations
It is important to note that our observations were made from only one monitoring site in central Beijing, and the results may not reflect the overall situation for Beijing. Furthermore, only summer and fall seasons were investigated during the study.
5. Conclusions
When Beijing hosted the 2008 Summer Olympics, concentrations of formaldehyde, acetaldehyde and acrolein were found to be at the high-end of concentration ranges measured in other cities around the globe. Although the aggressive air pollution control measures implemented during the Olympics, especially when coupled with favorable meteorological conditions, led to drastic reductions in pollutants of large primary sources (e.g., PM2.5, CO, SO2, and NOx), there was not a reduction in concentrations of formaldehyde and acrolein. Our findings point to the complexity of source control strategies for secondary pollutants, suggesting that the secondary photochemical processes may have dominated the formation of formaldehyde and acrolein. The importance of the photochemical contribution to formaldehyde and acrolein formation is evident since both had highly significant regression coefficients for the secondary formation source type identified using principal component analysis. Based on the results of this regression, it appears the high concentrations of formaldehyde and acrolein during the Beijing Olympics may be due largely to their relationship with ozone, and coincided with high incident sunlight and low NOx concentrations during the Olympic period. Concentrations of acetaldehyde, on the other hand, decreased during the Olympic period compared to the period before. Our regression for acetaldehyde indicates that the reduction in primary emissions may have contributed to the reduction in acetaldehyde concentration, since acetaldehyde was more strongly associated with primary emission sources including vegetation burning and oil combustion rather than secondary formation. Higher rainfall intensity during the Olympics may also have contributed to the lower acetaldehyde concentration. Finally, seasonal and regional effects likely led to lower concentrations of all three aldehydes during the post-Olympic period.
Supplementary Material
Highlights.
The Beijing Olympic period was unique to study sources of ambient aldehyde.
Three aldehydes were measured before, during, and after the Olympic period.
Associations of the aldehydes with other pollutants were examined.
Sources of the aldehydes were identified through principal component analysis.
The source control for aldehydes require complex strategies.
Acknowledgments
We thank the students and staff from Dr. Tong Zhu and Dr. Min Hu’s labs who assisted in sample collection and monitoring. This research was funded in part by a grant from HEI (#4760-RPFA05-3) and a grant from NIEHS (#1R01 ES0158640). Dr. Zhu was partly funded by Beijing EPA (OITC-G08026056). However, the opinions expressed in this manuscript are solely of the authors and do not necessarily reflects those of the funding agencies.
Appendix A – Potential Impact of Ozone on Aldehyde Measurements
We used the DNSH-based method instead of the DNPH-based method for the following reasons: (1) This method is not affected by ozone at concentrations up to 300 ppb, as previously reported by Rodler et al. (1993). This finding was also reproduced in a set of experiments we have conducted to test the effects of ozone on the aldehydes recovery, as shown in Appendix A. (2) This method is more reliable for acrolein. The DNSH-based method has proven to substantially improve the collection efficiency and precision for acrolein and crotonaldehyde (Herrington et al., 2005; Weisel et al, 2005). (3) This method uses passive sampling thus offering convenience in the field.
In order to evaluate the possibility of DNSH and DNSH-derivatives oxidation within the sampling cartridges, we used a dynamic dilution system. Aldehydes and ozone were introduced into the dilution system at desired concentrations. A wide range of carbonyls (1–100ppb) and ozone (0–300ppb) were achieved by adjusting the ozone generator output and regulating the total flow rates through the clean dilution air. The test conditions were as the following: (1) sampling duration was 48 hours; (2) chamber temperature was 25°C; (3) face velocity was 0.05 m/s; (4) relative humidity were 32% and 90%; (5) ozone concentrations in the chamber (ppb) were 0, 50, 100, 200, and 300.
Measured concentrations of formaldehyde, acetaldehyde, and acrolein, under different ozone concentrations are shown in Table G1. Results show that the presence of ozone from 50 ppb to 300 ppb caused <10% changes in measured concentrations of formaldehyde and that the presence of ozone from 50 ppb to 200 ppb caused <10% changes in measured concentrations of acetaldehyde and <15% changes in measured concentrations of acrolein.
Table A1.
Measured concentrations for three aldehydes with different ozone concentrations and the recovery for two different methods
| Ozone (ppb) | Aldehydes concentration: Mean±sd (ppb, n=4)
|
||||
|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 300 | |
|
| |||||
| Formaldehyde | 42.39±1.14 | 37.94±2.55 | 39.01±2.15 | 39.02±3.30 | 38.39±4.18 |
| Acetaldehyde | 22.23±0.25 | 20.12±1.30 | 20.12±1.07 | 21.01±1.66 | 17.89±0.94 |
| Acrolein | 17.03±0.39 | 14.73±0.67 | 15.31±1.22 | 14.46±1.06 | 13.02±0.81 |
The ratio of measured aldehydes concentrations, with and without the presence of ozone, ranged from 89.5%–92.0% for formaldehyde, 80.5%–94.5% for acetaldehyde, and 76.5%–89.9% for acrolein.
Samples and field controls were eluted with acetonitrile and aliquots of extracts were analyzed using an HPLC system with fluorescent detection. A Nova-Pak C18 column was used, along with a mobile phase program described as follows: mobile phase A was composed of 80% water, 10% acetonitrile, and 10% tetrahydrofuran containing 0.68 g/L of KH2PO4 and 3.48 g/L of K2HPO4; mobile phase B was composed of 30% water, 40% acetonitrile, and 30% tetrahydrofuran containing 0.68 g/L of KH2PO4 and 3.48 g/L of K2HPO4. The excitation and emission wavelengths used for detecting aldehyde–DNSH derivatives were 250 nm and 525 nm, respectively. The collection efficiencies for ambient formaldehyde, acetaldehyde and acrolein of this method were 115.5%±11.0%, 105.8%±9.1%, and 87.5%±4.7% (mean ± SD, N=30), respectively. The analytical detection limits of the method were 0.98 ng, 0.86 ng and 1.15 ng per cartridge and the analytical precision, determined as relative standard deviations (RSDs) of replicate samples, were 7.72%, 1.84% and 4.56% (N=8) for formaldehyde, acetaldehyde and acrolein, respectively.
Appendix B – Principal Component Analysis
Song et al. previously utilized PCA for source apportionment of PM2.5 in Beijing (Song et al. (1) 2006). Based on data they collected for 6 days during each season (January, April, July, and October) in 2000, the primary sources of PM2.5 in Beijing were secondary sulfate and nitrate, mixed coal/biomass burning, industrial emissions, motor vehicle exhaust, and road dust. Cao et al. also previously conducted source apportionment based on airborne particulate matter data collected just outside of Beijing (to the northwest, in an area of heavy motor vehicle traffic) from December 1998 to September 2000 (Cao et al. 2002). The four most predominant sources identified included soil and fly ash, a mixture of refuse incarnation and limestone from construction activities, motor vehicle and coal burning sources, and sea spray.
Liu et al. utilized the United States Environmental Protection Agency (USEPA) Community Multiscale Air Quality (CMAQ) modeling system to study seasonal variations and formation mechanisms of major air pollutants in China (Liu et al. (1) 2010, Liu et al (2) 2010). They found higher surface concentrations of sulfur dioxide, nitrogen dioxide, PM10, and carbon monoxide in winter and fall compared to spring and summer. Ozone, on the other hand, was higher in spring and summer.
In order to keep a reasonable ratio between the number of days and the number of pollutant variables, certain pollutant data was omitted from this analysis. Overall, 14 of 24 PM2.5 elements and 4 of 14 PAHs were chosen for PCA. The elements were chosen based on two criteria – the average concentration observed and their expected utility as a tracer for particular sources. PAHs were chosen for inclusion in the PCA based on their molecular weight. Lower molecular weight PAHs are not as useful for source apportionment since they are converted in the atmosphere (Park et al. 2002, Schauer et al. 1996).
Since the principal component analysis ignores rows with missing data, replacement was made for missing data with the arithmetic average value for the pollutant. Based on our sensitivity analysis with and without replacement, the impact of replacement was found to be minimal.
To evaluate how the aldehydes affected the PCA, our analysis was performed with and without the aldehydes in the models. The addition of the aldehydes did not change the interpretation of the source apportionment or the ordering of the factors in terms of eigenvalues or percent variance explained by each factor. Each aldehyde was regressed with the daily scores for the five factors in order to evaluate the strength of the associations. The factors determined without the aldehydes in the PCA were used for this regression.
Along with the information provided by the PCA regarding the percent of variance in the data explained by each factor, was used to surmise the relative contribution of each major, underlying source type for air pollution in Beijing. All five factors that were included had eigenvalues greater than 1.4, and each accounted for at least 7.9% of the variability in the data. The five factors in total accounted for 85% of the variability in the data.
The results of the PCA are shown in Table B2. By inspection of the loadings for each factor, comparison to the existing literature, and consultation with co-authors on PM2.5 source compositions, assessments were made regarding the most likely source type of each factor. This analysis, along with the information provided by the PCA regarding the percent of variance in the data explained by each factor, was used to surmise the relative contribution of each major, underlying source type for air pollution in Beijing. All five factors that were included had eigenvalues greater than 1.4, and each accounted for at least 7.9% of the variability in the data. The five factors in total accounted for 85% of the variability in the data.
Table B2.
Correlation matrix for principal component analysis
| SO2HR24 | NOHR24 | NO2HR24 | O3HR24 | COHR24 | PM25 | OC | EC | Na | Mg | Al | K | Ca | Ti | Mn | Fe | Cu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO2HR24 | 1.000 | 0.118 | 0.497 | 0.020 | 0.545 | 0.620 | 0.528 | 0.526 | 0.640 | 0.357 | 0.320 | 0.754 | 0.234 | 0.236 | 0.562 | 0.497 | 0.448 |
| NOHR24 | 0.118 | 1.000 | 0.706 | −0.550 | 0.184 | 0.031 | 0.509 | 0.545 | 0.312 | 0.264 | 0.427 | 0.122 | 0.379 | 0.368 | 0.387 | 0.384 | 0.086 |
| NO2HR24 | 0.497 | 0.706 | 1.000 | −0.528 | 0.532 | 0.534 | 0.869 | 0.848 | 0.694 | 0.381 | 0.579 | 0.550 | 0.456 | 0.470 | 0.698 | 0.610 | 0.523 |
| O3HR24 | 0.020 | −0.550 | −0.528 | 1.000 | −0.184 | 0.043 | −0.247 | −0.299 | −0.125 | −0.001 | −0.255 | −0.143 | −0.145 | −0.123 | −0.258 | −0.242 | 0.035 |
| COHR24 | 0.545 | 0.184 | 0.532 | −0.184 | 1.000 | 0.677 | 0.523 | 0.519 | 0.618 | 0.277 | 0.306 | 0.740 | 0.214 | 0.237 | 0.629 | 0.537 | 0.448 |
| PM25 | 0.620 | 0.031 | 0.534 | 0.043 | 0.677 | 1.000 | 0.726 | 0.693 | 0.852 | 0.390 | 0.396 | 0.757 | 0.368 | 0.421 | 0.633 | 0.566 | 0.763 |
| OC | 0.528 | 0.509 | 0.869 | −0.247 | 0.523 | 0.726 | 1.000 | 0.897 | 0.803 | 0.389 | 0.516 | 0.601 | 0.446 | 0.499 | 0.687 | 0.595 | 0.694 |
| EC | 0.526 | 0.545 | 0.848 | −0.299 | 0.519 | 0.693 | 0.897 | 1.000 | 0.771 | 0.432 | 0.570 | 0.581 | 0.481 | 0.511 | 0.678 | 0.631 | 0.612 |
| Na | 0.640 | 0.312 | 0.694 | −0.125 | 0.618 | 0.852 | 0.803 | 0.771 | 1.000 | 0.633 | 0.647 | 0.768 | 0.621 | 0.687 | 0.803 | 0.772 | 0.745 |
| Mg | 0.357 | 0.264 | 0.381 | −0.001 | 0.277 | 0.390 | 0.389 | 0.432 | 0.633 | 1.000 | 0.902 | 0.446 | 0.944 | 0.901 | 0.628 | 0.802 | 0.288 |
| Al | 0.320 | 0.427 | 0.579 | −0.255 | 0.306 | 0.396 | 0.516 | 0.570 | 0.647 | 0.902 | 1.000 | 0.436 | 0.936 | 0.912 | 0.669 | 0.867 | 0.324 |
| K | 0.754 | 0.122 | 0.550 | −0.143 | 0.740 | 0.757 | 0.601 | 0.581 | 0.768 | 0.446 | 0.436 | 1.000 | 0.380 | 0.371 | 0.669 | 0.655 | 0.497 |
| Ca | 0.234 | 0.379 | 0.456 | −0.145 | 0.214 | 0.368 | 0.446 | 0.481 | 0.621 | 0.944 | 0.936 | 0.380 | 1.000 | 0.952 | 0.637 | 0.808 | 0.291 |
| Ti | 0.236 | 0.368 | 0.470 | −0.123 | 0.237 | 0.421 | 0.499 | 0.511 | 0.687 | 0.901 | 0.912 | 0.371 | 0.952 | 1.000 | 0.732 | 0.801 | 0.375 |
| Mn | 0.562 | 0.387 | 0.698 | −0.258 | 0.629 | 0.633 | 0.687 | 0.678 | 0.803 | 0.628 | 0.669 | 0.669 | 0.637 | 0.732 | 1.000 | 0.727 | 0.555 |
| Fe | 0.497 | 0.384 | 0.610 | −0.242 | 0.537 | 0.566 | 0.595 | 0.631 | 0.772 | 0.802 | 0.867 | 0.655 | 0.808 | 0.801 | 0.727 | 1.000 | 0.391 |
| Cu | 0.448 | 0.086 | 0.523 | 0.035 | 0.448 | 0.763 | 0.694 | 0.612 | 0.745 | 0.288 | 0.324 | 0.497 | 0.291 | 0.375 | 0.555 | 0.391 | 1.000 |
| Zn | Pb | Ni | V | Se | Na1 | NH41 | K1 | Mg2 | Ca2 | F_ | Cl_ | NO3_ | SO42_ | BbF | BeP | IcP | BghiP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO2HR24 | 0.626 | 0.740 | 0.330 | 0.374 | 0.679 | 0.680 | 0.477 | 0.740 | 3.352 | 0.221 | 0.241 | 0.381 | 0.507 | 0.491 | 0.356 | 0.252 | 0.406 | 0.351 |
| NOHR24 | 0.198 | 0.045 | 0.222 | −0.131 | 0.236 | 0.146 | −0.257 | −0.035 | 3.356 | 0.322 | 0.415 | 0.035 | 0.051 | −0.299 | −0.255 | −0.385 | −0.098 | −0.180 |
| NO2HR24 | 0.643 | 0.525 | 0.427 | 0.072 | 0.730 | 0.684 | 0.179 | 0.419 | 0.503 | 0.402 | 0.458 | 0.358 | 0.572 | 0.097 | 0.029 | −0.166 | 0.224 | 0.115 |
| O3HR24 | −0.269 | −0.075 | −0.220 | −0.034 | −0.291 | −0.135 | 0.186 | −0.085 | −0.167 | −0.154 | −0.246 | −0.282 | −0.207 | 0.317 | 0.158 | 0.244 | 0.076 | 0.124 |
| COHR24 | 0.704 | 0.755 | 0.420 | 0.455 | 0.724 | 0.593 | 0.530 | 0.667 | 0.164 | 0.099 | 0.064 | 0.494 | 0.568 | 0.451 | 0.577 | 0.480 | 0.632 | 0.595 |
| PM25 | 0.652 | 0.823 | 0.327 | 0.310 | 0.757 | 0.775 | 0.852 | 0.718 | 0.392 | 0.323 | 0.276 | 0.607 | 0.838 | 0.819 | 0.599 | 0.484 | 0.701 | 0.639 |
| OC | 0.602 | 0.621 | 0.360 | 0.035 | 0.713 | 0.614 | 0.358 | 0.450 | 0.439 | 0.351 | 0.401 | 0.341 | 0.630 | 0.303 | 0.142 | −0.047 | 0.353 | 0.239 |
| EC | 0.627 | 0.655 | 0.346 | 0.065 | 0.750 | 0.669 | 0.400 | 0.489 | 0.497 | 0.408 | 0.446 | 0.365 | 0.672 | 0.345 | 0.194 | 0.013 | 0.372 | 0.276 |
| Na | 0.669 | 0.796 | 0.516 | 0.355 | 0.730 | 0.866 | 0.572 | 0.653 | 0.613 | 0.524 | 0.513 | 0.547 | 0.728 | 0.566 | 0.408 | 0.265 | 0.566 | 0.471 |
| Mg | 0.383 | 0.332 | 0.567 | 0.328 | 0.338 | 0.525 | 0.091 | 0.302 | 0.799 | 0.814 | 0.801 | 0.200 | 0.254 | 0.146 | 0.114 | 0.048 | 0.175 | 0.113 |
| Al | 0.460 | 0.349 | 0.580 | 0.242 | 0.474 | 0.557 | 0.031 | 0.294 | 0.855 | 0.875 | 0.879 | 0.252 | 0.347 | 0.052 | −0.018 | −0.121 | 0.087 | 0.004 |
| K | 0.747 | 0.852 | 0.480 | 0.488 | 0.804 | 0.777 | 0.582 | 0.936 | 0.419 | 0.320 | 0.314 | 0.630 | 0.671 | 0.537 | 0.542 | 0.428 | 0.596 | 0.535 |
| Ca | 0.346 | 0.259 | 0.533 | 0.264 | 0.314 | 0.507 | 0.033 | 0.236 | 0.862 | 0.901 | 0.899 | 0.207 | 0.268 | 0.077 | 0.018 | −0.063 | 0.114 | 0.043 |
| Ti | 0.395 | 0.309 | 0.593 | 0.337 | 0.316 | 0.557 | 0.081 | 0.229 | 0.837 | 0.858 | 0.853 | 0.183 | 0.298 | 0.136 | 0.032 | −0.049 | 0.143 | 0.069 |
| Mn | 0.839 | 0.702 | 0.755 | 0.627 | 0.703 | 0.728 | 0.315 | 0.539 | 0.595 | 0.526 | 0.537 | 0.369 | 0.554 | 0.296 | 0.292 | 0.156 | 0.403 | 0.328 |
| Fe | 0.553 | 0.597 | 0.556 | 0.308 | 0.600 | 0.694 | 0.235 | 0.532 | 0.757 | 0.740 | 0.737 | 0.381 | 0.480 | 0.231 | 0.209 | 0.092 | 0.316 | 0.233 |
| Cu | 0.602 | 0.664 | 0.282 | 0.195 | 0.614 | 0.577 | 0.546 | 0.418 | 0.269 | 0.198 | 0.170 | 0.412 | 0.637 | 0.530 | 0.349 | 0.228 | 0.480 | 0.411 |
| SO2HR24 | NOHR24 | NO2HR24 | O3HR24 | COHR24 | PM25 | OC | EC | Na | Mg | Al | K | Ca | Ti | Mn | Fe | Cu | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | 0.626 | 0.198 | 0.643 | −0.269 | 0.704 | 0.652 | 0.602 | 0.627 | 0.669 | 0.383 | 0.460 | 0.747 | 0.346 | 0.395 | 0.839 | 0.553 | 0.602 |
| Pb | 0.740 | 0.045 | 0.525 | −0.075 | 0.755 | 0.823 | 0.621 | 0.655 | 0.796 | 0.332 | 0.349 | 0.852 | 0.259 | 0.309 | 0.702 | 0.597 | 0.664 |
| Ni | 0.330 | 0.222 | 0.427 | −0.220 | 0.420 | 0.327 | 0.360 | 0.346 | 0.516 | 0.567 | 0.580 | 0.480 | 0.533 | 0.593 | 0.755 | 0.556 | 0.282 |
| V | 0.374 | −0.131 | 0.072 | −0.034 | 0.455 | 0.310 | 0.035 | 0.065 | 0.355 | 0.328 | 0.242 | 0.488 | 0.264 | 0.337 | 0.627 | 0.308 | 0.195 |
| Se | 0.679 | 0.236 | 0.730 | −0.291 | 0.724 | 0.757 | 0.713 | 0.750 | 0.730 | 0.338 | 0.474 | 0.804 | 0.314 | 0.319 | 0.703 | 0.600 | 0.614 |
| Na1 | 0.680 | 0.146 | 0.584 | −0.135 | 0.593 | 0.775 | 0.614 | 0.669 | 0.866 | 0.525 | 0.557 | 0.777 | 0.507 | 0.557 | 0.728 | 0.694 | 0.577 |
| NH41 | 0.477 | −0.257 | 0.179 | 0.186 | 0.530 | 0.852 | 0.358 | 0.400 | 0.572 | 0.091 | 0.031 | 0.582 | 0.033 | 0.081 | 0.315 | 0.235 | 0.546 |
| K1 | 0.740 | −0.035 | 0.419 | −0.085 | 0.667 | 0.718 | 0.450 | 0.489 | 0.653 | 0.302 | 0.294 | 0.936 | 0.236 | 0.229 | 0.539 | 0.532 | 0.418 |
| Mg2 | 0.352 | 0.356 | 0.503 | −0.167 | 0.164 | 0.392 | 0.439 | 0.497 | 0.613 | 0.799 | 0.855 | 0.419 | 0.862 | 0.837 | 0.595 | 0.757 | 0.269 |
| Ca2 | 0.221 | 0.322 | 0.402 | −0.154 | 0.099 | 0.323 | 0.351 | 0.408 | 0.524 | 0.814 | 0.875 | 0.320 | 0.901 | 0.858 | 0.526 | 0.740 | 0.198 |
| F_ | 0.241 | 0.415 | 0.458 | −0.246 | 0.064 | 0.276 | 0.401 | 0.446 | 0.513 | 0.801 | 0.879 | 0.314 | 0.899 | 0.853 | 0.537 | 0.737 | 0.170 |
| Cl_ | 0.381 | 0.035 | 0.358 | −0.282 | 0.494 | 0.607 | 0.341 | 0.365 | 0.547 | 0.200 | 0.252 | 0.630 | 0.207 | 0.183 | 0.369 | 0.381 | 0.412 |
| NO3_ | 0.507 | 0.051 | 0.572 | −0.207 | 0.568 | 0.838 | 0.630 | 0.672 | 0.728 | 0.254 | 0.347 | 0.671 | 0.268 | 0.298 | 0.554 | 0.480 | 0.637 |
| SO42_ | 0.491 | −0.299 | 0.097 | 0.317 | 0.451 | 0.819 | 0.303 | 0.345 | 0.566 | 0.146 | 0.052 | 0.537 | 0.077 | 0.136 | 0.296 | 0.231 | 0.530 |
| BbF | 0.356 | −0.255 | 0.029 | 0.158 | 0.577 | 0.599 | 0.142 | 0.194 | 0.408 | 0.114 | −0.018 | 0.542 | 0.018 | 0.032 | 0.292 | 0.209 | 0.349 |
| BeP | 0.252 | −0.385 | −0.166 | 0.244 | 0.480 | 0.484 | −0.047 | 0.013 | 0.265 | 0.048 | −0.121 | 0.428 | −0.063 | −0.049 | 0.156 | 0.092 | 0.228 |
| IcP | 0.406 | −0.098 | 0.224 | 0.076 | 0.632 | 0.701 | 0.353 | 0.372 | 0.566 | 0.175 | 0.087 | 0.596 | 0.114 | 0.143 | 0.403 | 0.319 | 0.480 |
| BghiP | 0.351 | −0.180 | 0.115 | 0.124 | 0.595 | 0.639 | 0.239 | 0.276 | 0.471 | 0.113 | 0.004 | 0.535 | 0.043 | 0.069 | 0.328 | 0.233 | 0.411 |
| Zn | Pb | Ni | V | Se | Na1 | NH41 | K1 | Mg2 | Ca2 | F_ | Cl_ | NO3_ | SO42_ | BbF | BeP | IcP | BghiP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | 1.000 | 0.804 | 0.694 | 0.638 | 0.869 | 0.657 | 0.436 | 0.663 | 0.323 | 0.245 | 0.253 | 0.506 | 0.637 | 0.364 | 0.418 | 0.280 | 0.479 | 0.422 |
| Pb | 0.804 | 1.000 | 0.436 | 0.444 | 0.849 | 0.809 | 0.681 | 0.823 | 0.309 | 0.203 | 0.182 | 0.681 | 0.766 | 0.633 | 0.625 | 0.498 | 0.691 | 0.634 |
| Ni | 0.694 | 0.436 | 1.000 | 0.653 | 0.493 | 0.463 | 0.074 | 0.355 | 0.497 | 0.454 | 0.422 | 0.277 | 0.358 | 0.050 | 0.067 | −0.006 | 0.121 | 0.073 |
| V | 0.638 | 0.444 | 0.653 | 1.000 | 0.352 | 0.404 | 0.289 | 0.466 | 0.194 | 0.181 | 0.135 | 0.285 | 0.243 | 0.310 | 0.339 | 0.343 | 0.278 | 0.289 |
| Se | 0.869 | 0.849 | 0.493 | 0.352 | 1.000 | 0.734 | 0.534 | 0.742 | 0.368 | 0.270 | 0.277 | 0.570 | 0.768 | 0.455 | 0.381 | 0.226 | 0.481 | 0.410 |
| Na1 | 0.657 | 0.809 | 0.463 | 0.404 | 0.734 | 1.000 | 0.628 | 0.797 | 0.623 | 0.506 | 0.505 | 0.593 | 0.810 | 0.603 | 0.477 | 0.364 | 0.573 | 0.514 |
| NH41 | 0.436 | 0.681 | 0.074 | 0.289 | 0.534 | 0.628 | 1.000 | 0.694 | 0.091 | 0.026 | −0.042 | 0.609 | 0.783 | 0.965 | 0.712 | 0.677 | 0.718 | 0.716 |
| K1 | 0.663 | 0.823 | 0.355 | 0.466 | 0.742 | 0.797 | 0.694 | 1.000 | 0.346 | 0.236 | 0.218 | 0.670 | 0.730 | 0.645 | 0.603 | 0.520 | 0.617 | 0.587 |
| Mg2 | 0.323 | 0.309 | 0.497 | 0.194 | 0.368 | 0.623 | 0.091 | 0.346 | 1.000 | 0.966 | 0.923 | 0.242 | 0.396 | 0.138 | −0.023 | −0.107 | 0.085 | 0.018 |
| Ca2 | 0.245 | 0.203 | 0.454 | 0.181 | 0.270 | 0.506 | 0.026 | 0.236 | 0.966 | 1.000 | 0.954 | 0.201 | 0.304 | 0.079 | −0.055 | −0.120 | 0.034 | −0.028 |
| F_ | 0.253 | 0.182 | 0.422 | 0.135 | 0.277 | 0.505 | −0.042 | 0.218 | 0.923 | 0.954 | 1.000 | 0.208 | 0.267 | −0.009 | −0.084 | −0.166 | 0.013 | −0.062 |
| Cl_ | 0.506 | 0.581 | 0.277 | 0.285 | 0.570 | 0.593 | 0.609 | 0.670 | 0.242 | 0.201 | 0.208 | 1.000 | 0.716 | 0.471 | 0.564 | 0.485 | 0.618 | 0.581 |
| NO3_ | 0.637 | 0.766 | 0.358 | 0.243 | 0.768 | 0.810 | 0.783 | 0.730 | 0.396 | 0.304 | 0.267 | 0.716 | 1.000 | 0.673 | 0.467 | 0.349 | 0.557 | 0.505 |
| SO42_ | 0.364 | 0.633 | 0.050 | 0.310 | 0.455 | 0.603 | 0.965 | 0.645 | 0.138 | 0.079 | −0.009 | 0.471 | 0.673 | 1.000 | 0.661 | 0.647 | 0.655 | 0.658 |
| BbF | 0.418 | 0.625 | 0.067 | 0.339 | 0.381 | 0.477 | 0.712 | 0.603 | −0.023 | −0.055 | −0.084 | 0.564 | 0.467 | 0.661 | 1.000 | 0.976 | 0.957 | 0.975 |
| BeP | 0.280 | 0.498 | −0.006 | 0.343 | 0.226 | 0.364 | 0.677 | 0.520 | −0.107 | −0.120 | −0.166 | 0.485 | 0.349 | 0.647 | 0.976 | 1.000 | 0.889 | 0.932 |
| IcP | 0.479 | 0.691 | 0.121 | 0.278 | 0.481 | 0.573 | 0.718 | 0.617 | 0.085 | 0.034 | 0.013 | 0.618 | 0.557 | 0.655 | 0.957 | 0.889 | 1.000 | 0.989 |
| BghiP | 0.422 | 0.634 | 0.073 | 0.289 | 0.410 | 0.514 | 0.716 | 0.587 | 0.018 | −0.028 | −0.062 | 0.581 | 0.505 | 0.658 | 0.975 | 0.932 | 0.989 | 1.000 |
The five factors identified all have meaningful interpretations with respect to sources contributing to the air pollution in Beijing during this study. Factor 1 appears to be a mixed vehicle and industrial combustion source, based on the contribution of OC, EC, Cu, NO2−, Se, Na, PM2.5, Zn, Pb, NO3−, and Mn to the factor. Factor 2 is predominated by elements including Ca, Ti, Al, Mg, Fe, Na, Mn, and Ni, as well as the ions Ca2+, F−, Mg2+, and Na+. This source is consistent with natural soil and road dust. Factor 4 is primarily V, Ni, Zn, and Mn, which is indicative of an oil combustion source.
Factor 3 has a strong positive correlation to NO and NO2, and a strong negative correlation to ozone, that is, it is strongly related to the titration reaction between ozone and the nitrogen oxides. Factor 5 is primarily associated with the four PAHs included in the model, although NH4+, SO42-, Cl-, CO, Pb, PM2.5, K+, and K are also significant components. This factor appears to be associated with vegetative burning, based on the presence of the PAHs along with CO and K. The reason for the heavy loading for the PAHs with the vegetative burning source type and negligible loadings for the other combustion source types does not necessarily indicate that PAHs were not emitted by the other sources, but does imply that the variation of PAHs during this study followed the trend for vegetative burning sources more closely than the other combustion sources.
Table B1.
Acrolein levels of the field blanks in three Olympic period and the whole period
| Pre-Olympics | During-Olympics | Post-Olympics | Whole period | |
|---|---|---|---|---|
| N | 7 | 8 | 3 | 24 |
| Mean | 0.6 | 0.7 | 0.8 | 0.7 |
| Standard deviation | 0.3 | 0.6 | 0.7 | 0.5 |
Table B3.
VARIMAX rotated factor loading matrix for Beijing air pollution data (without aldehydes)
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | |
|---|---|---|---|---|---|
| Vehicle/industrial combustion | Natural soil/road dust | Secondary formation | Oil Combustion | Vegetative burning | |
| SO2 | 0.655 | 0.143 | −0.060 | 0.372 | 0.181 |
| NO | 0.195 | 0.315 | 0.756 | −0.092 | −0.222 |
| NO2 | 0.679 | 0.319 | 0.605 | 0.091 | −0.047 |
| O3 | −0.026 | −0.070 | −0.850 | −0.122 | 0.033 |
| CO | 0.490 | 0.050 | 0.255 | 0.432 | 0.492 |
| PM2.5 | 0.794 | 0.254 | −0.114 | 0.085 | 0.474 |
| OC | 0.827 | 0.304 | 0.330 | −0.021 | 0.013 |
| EC | 0.780 | 0.352 | 0.363 | −0.023 | 0.084 |
| Na | 0.720 | 0.512 | 0.077 | 0.173 | 0.301 |
| Mg | 0.138 | 0.903 | −0.042 | 0.218 | 0.076 |
| Al | 0.239 | 0.897 | 0.195 | 0.173 | −0.031 |
| K | 0.600 | 0.245 | 0.095 | 0.441 | 0.439 |
| Ca | 0.131 | 0.957 | 0.085 | 0.115 | 0.022 |
| Ti | 0.196 | 0.924 | 0.046 | 0.165 | 0.006 |
| Mn | 0.503 | 0.504 | 0.221 | 0.542 | 0.150 |
| Fe | 0.376 | 0.749 | 0.206 | 0.239 | 0.180 |
| Cu | 0.772 | 0.161 | −0.075 | 0.038 | 0.179 |
| Zn | 0.584 | 0.164 | 0.225 | 0.648 | 0.241 |
| Pb | 0.717 | 0.127 | 0.030 | 0.382 | 0.467 |
| Ni | 0.200 | 0.438 | 0.137 | 0.732 | −0.036 |
| V | 0.055 | 0.162 | −0.152 | 0.866 | 0.232 |
| Se | 0.761 | 0.151 | 0.258 | 0.374 | 0.245 |
| Na+ | 0.641 | 0.445 | 0.017 | 0.238 | 0.398 |
| NH4+ | 0.617 | −0.040 | −0.318 | 0.014 | 0.635 |
| K+ | 0.568 | 0.136 | −0.015 | 0.385 | 0.525 |
| Mg2+ | 0.239 | 0.904 | 0.058 | 0.063 | −0.013 |
| Ca2+ | 0.109 | 0.950 | 0.039 | 0.027 | −0.017 |
| F−_ | 0.105 | 0.936 | 0.157 | 0.004 | −0.043 |
| Cl−_ | 0.359 | 0.126 | 0.211 | 0.137 | 0.628 |
| NO3− | 0.752 | 0.174 | 0.061 | 0.089 | 0.405 |
| SO42−_ | 0.584 | 0.028 | −0.468 | 0.008 | 0.563 |
| BbF | 0.181 | −0.040 | −0.107 | 0.129 | 0.946 |
| BeP | 0.033 | −0.091 | −0.225 | 0.124 | 0.945 |
| IcP | 0.326 | 0.040 | 0.011 | 0.056 | 0.906 |
| BghiP | 0.245 | −0.019 | −0.046 | 0.064 | 0.934 |
|
| |||||
| Eigenvalue | 16.79 | 6.92 | 1.41 | 1.87 | 2.84 |
| % Var. | 26% | 23% | 8% | 10% | 19% |
| Cum % var. | 26% | 49% | 57% | 66% | 85% |
References for Appendices
- Cao L, Tian W, Ni B, Zhang Y, Wang P. Preliminary study of airborne particulate matter in a Beijing sampling station by instrumental neutron activation analysis. Atmospheric Environment. 2002;36:1951–1956. [Google Scholar]
- Liu XH, Zhang Y, Cheng SH, Xing J, Zhang Q, Streets DG, Jang C, Wang WX, Hao JM. Understanding of regional air pollution over China using CMAQ, part I performance evaluation and seasonal variation. Atmospheric Environment. 2010;44(20):2415–2426. [Google Scholar]
- Liu XH, Zhang Y, Xing J, Zhang Q, Streets DG, Jang C, Wang WX, Hao JM. Understanding of regional air pollution over China using CMAQ, part II. Process analysis and sensitivity of ozone and particulate matter to precursor emissions. Atmospheric Environment. 2010;44(30):3719–3727. [Google Scholar]
- Park S, Kim Y, Kang C. Atmospheric polycyclic aromatic hydrocarbons in Seoul, Korea. Atmospheric Environment. 2002;36(17):2917–2924. [Google Scholar]
- Rodier DR, Nondek L, Birks JW. Evaluation of ozone and water vapor interferences in the derivatization of atmospheric aldehydes with dansylhydrazine. Environ Sci Technol. 1993;27 (13):2814–2820. [Google Scholar]
- Schauer J, Rogge W, Hildemann L, Mazurek M, Cass G, Simoneit B. Source apportionment of airborne particulate matter using organic compounds as tracers. Atmospheric Environment. 1996;30(22):3837–3855. [Google Scholar]
- Weisel CP, Zhang J, Turpin BJ, Morandi MT, Colome S, Stock TH, et al. Research Report 130. Health Effects Institute (HEI) and the Mickey Leland National Urban Air Toxics Center; Boston, MA: 2005. Investigators’ report: Relationships of Indoor, Outdoor, and Personal Air (RIOPA). Part I. Collection methods and descriptive analyses. [PubMed] [Google Scholar]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akbar-Khanzadeh F, Mlynek JS. Changes in respiratory function after one and three hours of exposure to formaldehyde in non-smoking subjects. Occupational and Environmental Medicine. 1997;54:296–300. doi: 10.1136/oem.54.5.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuller AP. Production of aldehydes as primary emissions and from secondary atmospheric reactions of alkenes and alkanes during the night and early morning hours. Atmospheric Environment Part a-General Topics. 1993;27:21–32. [Google Scholar]
- Andreini BP, Baroni R, Galimberti E, Sesana G. Aldehydes in the atmospheric environment: Evaluation of human exposure in the north-west area of Milan. Microchemical Journal. 2000;67:11–9. [Google Scholar]
- Baez AP, Belmont R, Padilla H. Measurements of formaldehyde and acetaldehyde in the atmosphere of Mexico City. Environmental Pollution. 1995;89:163–7. doi: 10.1016/0269-7491(94)00059-m. [DOI] [PubMed] [Google Scholar]
- Benjebria A, Marthan R, Rossetti M, Savineau JP, Ultman JS. Human bronchial smooth-muscle responsiveness after in-vitro exposure to acrolein. American Journal of Respiratory and Critical Care Medicine. 1994;149:382–6. doi: 10.1164/ajrccm.149.2.8306034. [DOI] [PubMed] [Google Scholar]
- Cahill TM. Ambient Acrolein Concentrations in Coastal, Remote, and Urban Regions in California. Environmental Science & Technology. 2014;48(15):8507–8513. doi: 10.1021/es5014533. [DOI] [PubMed] [Google Scholar]
- Cassee FR, Arts JHE, Groten JP, Feron VJ. Sensory irritation to mixtures of formaldehyde, acrolein, and acetaldehyde in rats. Archives of Toxicology. 1996a;70:329–37. doi: 10.1007/s002040050282. [DOI] [PubMed] [Google Scholar]
- Cassee FR, Groten JP, Feron VJ. Changes in the nasal epithelium of rats exposed by inhalation to mixtures of formaldehyde, acetaldehyde, and acrolein. Fundamental and Applied Toxicology. 1996b;29:208–18. doi: 10.1006/faat.1996.0024. [DOI] [PubMed] [Google Scholar]
- Chan CK, Yao X. Air pollution in mega cities in China. Atmospheric Environment. 2008;42:1–42. [Google Scholar]
- Chenga SY, Huanga GH, Chakmaa A, Haob RX, Liua L, Zhang XH. Estimation of atmospheric mixing heights using data from airport meteorological stations. Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering. 2001;36(4):521–532. doi: 10.1081/ese-100103481. [DOI] [PubMed] [Google Scholar]
- Clements AL, Jia YL, Denbleyker A, McDonald-Buller E, Fraser MP, Allen DT, Collins DR, Michel E, Pudota J, Sullivan D, Zhu YF. Air pollutant concentrations near three Texas roadways, part II: Chemical characterization and transformation of pollutants. Atmospheric Environment. 2009;43(30):4523–4534. [Google Scholar]
- Destaillats H, Spaulding RS, Charles MJ. Ambient air measurement of acrolein and other carbonyls at the Oakland-San Francisco Bay Bridge toll plaza. Environmental Science & Technology. 2002;36(10):2227–2235. doi: 10.1021/es011394c. [DOI] [PubMed] [Google Scholar]
- Draxler RR, Rolph GD. HYSPLIT (HYbrid Single-Particle Lagrangian Integrated Trajectory) Model access via NOAA ARL READY Website. NOAA Air Resources Laboratory; College Park, MD: 2004. ( http://www.arl.noaa.gov/HYSPLIT.php) [Google Scholar]
- Feng YL, Wen S, Chen YJ, Wang XM, Lu HX, Bi XH, et al. Ambient levels of carbonyl compounds and their sources in Guangzhou, China. Atmospheric Environment. 2005;39:1789–800. [Google Scholar]
- Feng YL, Wen S, Wang XM, Sheng GY, He QS, Tang JH, et al. Indoor and outdoor carbonyl compounds in the hotel ballrooms in Guangzhou, China. Atmospheric Environment. 2004;38:103–12. [Google Scholar]
- Grosjean D, Grosjean E, Gertler AW. On-road emissions of carbonyls from light-duty and heavy-duty vehicles. Environmental Science & Technology. 2001;35:45–53. doi: 10.1021/es001326a. [DOI] [PubMed] [Google Scholar]
- Grosjean D, Grosjean E, Moreira LFR. Speciated ambient carbonyls in Rio de Janeiro, Brazil. Environmental Science & Technology. 2002;36:1389–95. doi: 10.1021/es0111232. [DOI] [PubMed] [Google Scholar]
- Herrington J, Zhang L, Whitaker D, Sheldon L, Zhang J. Optimizing a dansylhydrazine (DNSH) based method for measuring airborne acrolein and other unsaturated carbonyls. Journal of Environmental Monitoring. 2005;7:969–76. doi: 10.1039/b502063h. [DOI] [PubMed] [Google Scholar]
- Ho SSH, Yu JZ. Determination of airborne carbonyls: Comparison of a thermal desorption/GC method with the standard DNPH/HPLC method. Environmental Science & Technology. 2004;38(3):862–870. doi: 10.1021/es034795w. [DOI] [PubMed] [Google Scholar]
- Huang XF, He LY, Hu M, Canagaratna MR, Sun Y, Zhang Q, et al. Highly time-resolved chemical characterization of atmospheric submicron particles during 2008 Beijing Olympic Games using an Aerodyne High-Resolution Aerosol Mass Spectrometer. Atmospheric Chemistry and Physics. 2010;10:8933–45. [Google Scholar]
- Jolliffe IT. Principal Component Analysis. 2. New York: Springer; 2002. [Google Scholar]
- Legreid G, Loov JB, Staehelin J, Hueglin C, Hill M, Buchmann B, Prevot ASH, Reimann S. Oxygenated volatile organic compounds (OVOCs) at an urban background site in Zurich (Europe): Seasonal variation and source allocation. Atmospheric Environment. 2007;41(38):8409–8423. [Google Scholar]
- Li Y, Shao M, Lu SH, Chang CC, Dasgupta PK. Variations and sources of ambient formaldehyde for the 2008 Beijing Olympic Games. Atmospheric Environment. 2010a;44:2632–9. [Google Scholar]
- Li Y, Wang W, Kan HD, Xu XH, Chen BH. Air quality and outpatient visits for asthma in adults during the 2008 Summer Olympic Games in Beijing. Science of the Total Environment. 2010b;408:1226–7. doi: 10.1016/j.scitotenv.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Lipari F, Dasch JM, Scruggs WF. Aldehyde emissions from wood-burning fireplaces. Environmental Science and Technology. 1984;18(5):326–30. doi: 10.1021/es00123a007. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhang J, Zhang L, Turpin BJ, Welsel CP, Morandi CT, Stock TH, Colome S, Korn LR. Estimating contributions of indoor and outdoor sources to indoor carbonyl concentrations in three urban areas of the United States. Atmospheric Environment. 2006;40(12):2202–2214. [Google Scholar]
- MacIntosh DL, Zimmer-Dauphinee SA, Manning RO, Williams PL. Aldehyde concentrations in ambient air of coastal Georgia, USA. Environmental Monitoring and Assessment. 2000;63:409–29. [Google Scholar]
- Possanzini M, Di Palo V, Cecinato A. Sources and photodecomposition of formaldehyde and acetaldehyde in Rome ambient air. Atmospheric Environment. 2002;36:3195–201. [Google Scholar]
- Pusede SE, Cohen RC. On the observed response of ozone to NOx and VOC reactivity reductions in San Joaquin Valley California 1995-present. Atmospheric Chemistry and Physics. 2012;12:8323–8339. [Google Scholar]
- Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR, Lu SE, Tong J, Gong J, Thomas D, Zhu T, Zhang JF. Association between changes in air pollution levels during the beijing olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA. 2012;307(19):2068–2078. doi: 10.1001/jama.2012.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 3. C-1-C-29 organic compounds from fireplace combustion of wood. Environmental Science & Technology. 2001;35:1716–28. doi: 10.1021/es001331e. [DOI] [PubMed] [Google Scholar]
- Seaman VY, Charles MJ, Cahill TM. A sensitive method for the quantification of acrolein and other volatile carbonyls in ambient air. Analytical Chemistry. 2006;78(7):2405–2412. doi: 10.1021/ac051947s. [DOI] [PubMed] [Google Scholar]
- Seinfeld JH, Pandis SN. Atmospheric chemistry and physics: from air pollution to climate change. New York: John Wiley and Sons; 1998. Chemistry of the atmospheric aqueous phase; pp. 357–8. [Google Scholar]
- Song Y, Tang X, Xie S, Zhang Y, Wei Y, Zhang M, Zeng L, Lu S. Source apportionment of PM2.5 in Beijing in 2004. Journal of Hazardous Materials. 2007;146(1–2):124–130. doi: 10.1016/j.jhazmat.2006.11.058. [DOI] [PubMed] [Google Scholar]
- Song Y, Xie S, Zhang Y, Zeng L, Salmon L, Zheng M. (1) Source apportionment of PM2.5 in Beijing using principal component analysis/absolute principal component scores and UNMIX. Science of the Total Environment. 2006;372(1):278–286. doi: 10.1016/j.scitotenv.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Song Y, Zhang Y, Xie S, Zeng L, Zheng M, Salmon L, Shao M, Stanina S. (2) Source apportionment of PM2.5 in Beijing by positive matrix factorization. Atmospheric Environment. 2006;40(8):1526–1537. [Google Scholar]
- Spada N, Fujii E, Cahill TM. Diurnal cycles of acrolein and other small aldehydes in regions impacted by vehicle emissions. Environmental Science & Technology. 2008;42(19):7084–7090. doi: 10.1021/es801656e. [DOI] [PubMed] [Google Scholar]
- Stahl QR. Prepared for the National Air Pollution Control Administration Consumer Protection & Environmental Health Service. Department of Health, Education, and Welfare; 1969. Air pollution aspects of aldehydes. (Contract No. PH-22-68-25) [Google Scholar]
- Streets DG, Fu JS, Jang CJ, Hao JM, He KB, Tang XY, et al. Air quality during the 2008 Beijing Olympic Games. Atmospheric Environment. 2007;41:480–92. 18. [Google Scholar]
- Tang XY. Urbanization, energy, and air pollution in china. Washington DC: The National Academies Press; 2004. pp. 47–54. [Google Scholar]
- Wang H, Zhuang Y, Wang Y, Sun Y, Yuan H, Zhuang G, Hao Z. Long-term monitoring and source apportionment of PM2.5/PM10 in Beijing, China. Journal of Environmental Sciences. 2008;20(11):1323–1327. doi: 10.1016/s1001-0742(08)62228-7. [DOI] [PubMed] [Google Scholar]
- Wang M, Zhu T, Zheng J, Zhang RY, Zhang SQ, Xie XX, et al. Use of a mobile laboratory to evaluate changes in on-road air pollutants during the Beijing 2008 Summer Olympics. Atmospheric Chemistry and Physics. 2009a;9:8247–63. [Google Scholar]
- Wang W, Primbs T, Tao S, Massey Simonich SL. Atmospheric particulate matter pollution during the 2008 Beijing Olympics. Envrion Sci Technol. 2009b;43:5314–5320. doi: 10.1021/es9007504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SX, Zhao M, Xing J, Wu Y, Zhou Y, Lei Y, et al. Quantifying the air pollutants emission reduction during the 2008 Olympic Games in Beijing. Environmental Science & Technology. 2010a;44:2490–6. doi: 10.1021/es9028167. [DOI] [PubMed] [Google Scholar]
- Wang T, Nie W, Gao J, Xue LK, Gao XM, Wang XF, et al. Air quality during the 2008 Beijing Olympics: secondary pollutants and regional impact. Atmospheric Chemistry and Physics. 2010c;10:7603–15. [Google Scholar]
- Wang T, Xie SD. Assessment of traffic-related air pollution in the urban streets before and during the 2008 Beijing Olympic Games traffic control period. Atmospheric Environment. 2009;43:5682–90. [Google Scholar]
- Wang X, Westerdahl D, Chen LC, Wu Y, Hao JM, Pan XC, et al. Evaluating the air quality impacts of the 2008 Beijing Olympic Games: On-road emission factors and black carbon profiles. Atmospheric Environment. 2009b;43:4535–43. [Google Scholar]
- Wang XS, Li JL, Zhang YH, Xie SD, Tang XY. Ozone source attribution during a severe photochemical smog episode in Beijing, China. Science in China Series B-Chemistry. 2009c;52:1270–80. [Google Scholar]
- Wang Y, Hao J, McElroy MB, Munger JW, Ma H, Chen D, et al. Ozone air quality during the 2008 Beijing Olympics: effectiveness of emission restrictions. Atmospheric Chemistry and Physics. 2009d;9:5237–51. [Google Scholar]
- Weisel CP, Zhang J, Turpin BJ, Morandi MT, Colome S, Stock TH, Spektor DM, Korn L, Winer AM, Kwon J, Meng QY, Zhang L, Harrington R, Liu W, Reff A, Lee JH, Alimokhtari S, Mohan K, Shendell D, Jones J, Farrar L, Maberti S, Fan T. HEI (Health Effects Institute) Research Report 130-I. Boston, MA: 2005. Relationships of indoor, outdoor, and personal air (RIOPA). Part I. Collection methods and descriptive analysis. [PubMed] [Google Scholar]
- Xin JY, Wang YS, Tang GQ, Wang LL, Sun Y, Wang YH, et al. Variability and reduction of atmospheric pollutants in Beijing and its surrounding area during the Beijing 2008 Olympic Games. Chinese Science Bulletin. 2010;55:1937–44. [Google Scholar]
- Zhang J, Zhang L, Fan Z, Ilacqua V. Development of the personal aldehydes and ketones sampler based upon DNSH derivatization on solid sorbent. Environmental Science & Technology. 2000;34:2601–7. [Google Scholar]
- Zhang JF, He QC, Lioy PJ. Characteristics of aldehydes - concentrations, sources, and exposures for indoor and outdoor residential microenvironments. Environmental Science & Technology. 1994;28:146–52. doi: 10.1021/es00050a020. [DOI] [PubMed] [Google Scholar]
- Zhang JF, Smith KR. Emissions of carbonyl compounds from various cookstoves in China. Environmental Science & Technology. 1999;33:2311–20. [Google Scholar]
- Zhang JF, Zhu T, Kipen H, Wang G, Huang W, Rich D, Zhu P, Wang Y, Lu S, Ohman-Strickland P, Diehl S, Hu M, Tong J, Gong J, Thomas D. Cardiorespiratory biomarker responses in healthy young adults to drastic air quality changes surrounding the 2008 Beijing Olympics. Health Effects Institute Report #. 2013;174:2013-03-11. [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Salmon L, Schauer J, Zeng L, Kiang C, Zhang Y, Cass G. Seasonal trends in PM2.5 source contributions in Beijing, China. Atmospheric Environment. 2005;39:3967–3976. [Google Scholar]
- Zhou Y, Wu Y, Yang L, Fu LX, He KB, Wang SX, et al. The impact of transportation control measures on emission reductions during the 2008 Olympic Games in Beijing, China. Atmospheric Environment. 2010;44:285–93. [Google Scholar]
- Zhu YF, Fanning E, Yu RC, Zhang QF, Froines JR. Aircraft emissions and local air quality impacts from takeoff activities at a large International Airport. Atmospheric Environment. 2011;45(36):6526–6533. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.