Abstract
The tandem mass spectrometry, ion mobility, and normal phase HPLC of isomeric phosphatidylglycerol (PG) and bis(monoacylglycerol)phosphate (BMP) have been investigated in this study with the objective of differentiating these unique classes of lipids. Measurement of ion mobility using the traveling wave method for negative molecular and product ions from isomeric PG and BMP yielded identical results, but different ion mobilities were observed for positive product ions arising from collision-induced dissociation (CID). The fastest moving positive product ions from the ion mobility analysis of BMP(18:1/18:1) were monoglyceride-like, and the slowest moving product ions from this BMP corresponded to [M+H-2H2O]+, which were readily observed for BMP but were only at very low abundance in the CID spectra of PG. The major product ions observed from the sodium adduct of PG(18:1/18:1) were consistent with diglyceride-like ion formation, but for BMP(18:1/18:1) only monoglyceride-like product ions were formed. The usefulness of ion mobility separation was tested with the selection of positive product ions derived from the isomeric PG and BMP molecular species in the lipid extract of RAW 264.7 cells. The ion mobility spectra of monoglyceride-like ions derived from BMP species with various esterified fatty acyl groups displayed some separation in ion mobility based on fatty acyl chain length and presence of a double bond in the acyl chain. The mechanism of ion formation of the diglyceride- and monoglyceride-like ions from PG and BMP respectively was examined using deuterium-labeled species including PG(D3116:0/18:1) and PG and BMP labeled by deuterium exchange.
Keywords: bis(monoacylglycerol)phosphate (BMP), phosphatidylglycerol (PG), phospholipids, ion mobility, traveling wave, RAW 264.7 cells, tandem mass spectrometry, collision-induced dissociation
1. Introduction
Two important phospholipid classes that are similar in composition yet structurally different are phosphatidylglycerol (PG) and bis(monoacylglycerol)phosphate (BMP), both of which are found in most cells. These phospholipids are isomeric (Scheme 1) and have common biosynthetic origins [1,2], yet play completely different biochemical roles within cells [3,4]. PG is an acidic phospholipid and typically more abundant than its isomeric BMP. PG is found as a 10% component of pulmonary surfactant lipids[5] and is thought to have significant antiviral properties [6,7] essentially by inhibiting viral proliferation in the lung for viruses such as respiratory syncytial virus. BMP is also an acidic phospholipid and most typically is present in the lysosomal and endosomal compartments within cells. Data suggest that it is located on internal vesicles in the late endosome [2]. It is thought that BMP is hydrolyzed by a unique phospholipase A2 in the lysosomes and endosomes, but otherwise is resistant to common phospholipases [8]. BMP has been found to be present in high concentrations in human subjects with lysosomal lipid diseases [9,10].
Scheme 1.
Since BMP and PG are regioisomeric, appropriate measurements of these phospholipids require a means by which one can discern their unique structures. It is possible to separate these two classes of phospholipids by chromatography, including hydrophilic interaction liquid chromatography [11], as well as normal phase liquid chromatography or TLC [12]. Also, differential behavior in tandem mass spectrometry would be highly useful to define molecular species of these two phospholipids.
Negative electrospray ionization has been widely used to analyze PG [13–15], but BMP cannot be distinguished from isomeric PG in terms of negative product ion masses and abundances [9,11]. Positive electrospray ions can be generated for both PG and BMP, and their product ion spectra have been found to be quite different so as to enable specific detection of each class of phospholipid and the development of analytical methods to measure each one separately [9,11]. One positive ion species not previously investigated is the sodium adduct [M+Na]+, which can be the most abundant positive ion observed by electrospray ionization when crude biological extracts are directly analyzed.
Recent advances in mass spectrometry have integrated ion mobility capability with a tandem quadrupole time-of-flight mass spectrometer so that an additional separation strategy can now be employed that might be quite useful for lipid analysis [16,17], including challenges in the mass spectral characterization of these lipid regioisomers. We have investigated the tandem mass spectrometry and ion mobility of both positive and negative electrospray molecular ions as well as product ions of PG and BMP, and report the first results of ion mobility experiments of these unique classes of lipids. Specific experiments examine ion mobility of intact phospholipid molecular species as well as ion mobility of fragment ions of both positive and negative molecular ion species. Ion mobility has also been applied to the analysis of PG and BMP isolated from mammalian cells grown in culture in order to examine naturally occurring BMP molecular species.
2. Materials and Methods
2.1 Reagents
All solvents used for HPLC analyses were HPLC grade, purchased from ThermoFisher Scientific (Waltham MA). PG(18:1/18:1), BMP(18:1/18:1) (S,R), and PG(D3116:0/18:1) standards were from Avanti Polar Lipids (Alabaster, AL). D4-methanol (99.96 atom %D) and D4-acetic acid (99.5 atom %D used for deuterium isotope exchange experiments were purchased from Aldrich Chemicals (Milwaukee WI).
2.2 Cell culture
RAW 264.7 cells were purchased from American Type Culture Collection (Manassas, VA) and were cultured in DMEM (containing 4.5 g/L glucose and 100 μM sodium pyruvate) supplemented with 10 % heat-inactivated fetal bovine serum. Cells were grown in humidified air with 5 % CO2 at 37 °C until confluent in T-75 cell culture flasks, harvested, rinsed twice with HBSS to remove cell medium, and then extracted for lipids.
2.3 Extraction of lipids
Lipids from RAW 264.7 cells (107) were extracted using the procedure of Bligh and Dyer (18). The washed organic phase was dried under a stream of dry nitrogen gas and dissolved in 200 μL of a mixture of 75% HPLC solvent A (hexanes/isopropanol 30:40, v/v) and 25% solvent B (5 mM ammonium acetate in hexanes/isopropanol/water 30:40:7, v/v/v) for infusion studies and analysis by normal phase LC/MS. PG(18:1/18:1) or BMP(18:1/18:1) regioisomeric standards were resuspended directly in 100 μL of the HPLC mixture to a concentration of 1 ng/μL for analysis as negative ions, and 10 ng/μL with addition of 2 μL formic acid for positive ion analysis.
2.4 Hydrogen/Deuterium Exchange Experiments
Exchangable hydrogen atoms on PG(18:1/18:1) and BMP(18:1/18:1) were replaced with deuterium for mechanistic studies. Briefly, 100 μg of each dioleoyl standard was taken to dryness under a stream of N2 gas after which 100 μL of D4-methanol and 5 μL D4-acetic acid were added. The solution was stirred in a capped tube under argon, kept at room temperature for 5 min and subsequently analyzed by direct infusion.
2.5 Chromatography of PG and BMP
Normal phase chromatography was performed using a silica column (Ascentis, 150 × 2.1 mm, 5 μm, Supelco, Bellefonte, PA) at a flow rate of 200 μL/min. Solvent B was maintained at 25% for 5 min, increased gradually to 60% in 10 min and then to 95% in 5 min, and was held for 20 min before re-equilibration for 15 min. Identification of phospholipids was carried out using an API 3200 triple quadrupole mass spectrometer (AB SCIEX, Concord, ON, Canada) in the negative or positive ion modes as well as on a Synapt G2-S mass spectrometer (Waters, Millford MA) with quadrupole, ion mobility, and TOF configuration. Preparative scale isolation of BMP and PG components of the RAW 264.7 cell extract was carried out using a 4.6 × 250 mm, 5 μm Luna Si column (Phenomenex, Torrance CA) with the post column effluent split 9:1 between a fraction collector and the API 3200. Fractions were collected at one-minute intervals, and the components identified by HPLC elution time based on elution of standard compounds as well as m/z values for the lipid classes of interest.
2.6 Mass Spectrometry and Ion Mobility
Analysis of the dioleoyl standards and the RAW 264.7 cell extract was carried out on the API 3200 as well as the Synapt G2-S mass spectrometers. Conditions on the API 3200 for negative ion mode included ion spray voltage of 4500 V, declustering potential of 55 V and entrance potential of 10 V.
All mobility experiments were carried out on the Synapt G2-S instrument. Mass spectra, including all full scan and CID analysis in both positive and negative ion mode were carried out with the ion mobility option invoked. Acquisition parameters for negative ion mode were as follows: capillary voltage 2 kV, sampling cone 40 V, source offset 80 V, temperature 80 °C, desolvation energy 150 V. Source gas pressures were as follows: cone gas 10 L/h, desolvation gas 500 L/h, and nebulizer gas 6 bar. Parameters were the same for positive ion mode with the exception of a higher capillary voltage (4 kV). Collision energy for CID experiments was compound-dependent and adjusted to yield informative product ions. The CID voltage employed for each phospholipid is indicated in each figure legend.
Mass spectra were evaluated using the MassLynx (Waters) software, and ion mobility data were evaluated using Driftscope (Waters) software. Ion mobilograms were generated from Driftscope by exporting the data into MassLynx with retention of the drift time information. Mobilograms acquired from HPLC data involved selection of a time slice of the mobility data at the retention time for lipid components and subsequent generation of the mobilogram.
3. Results
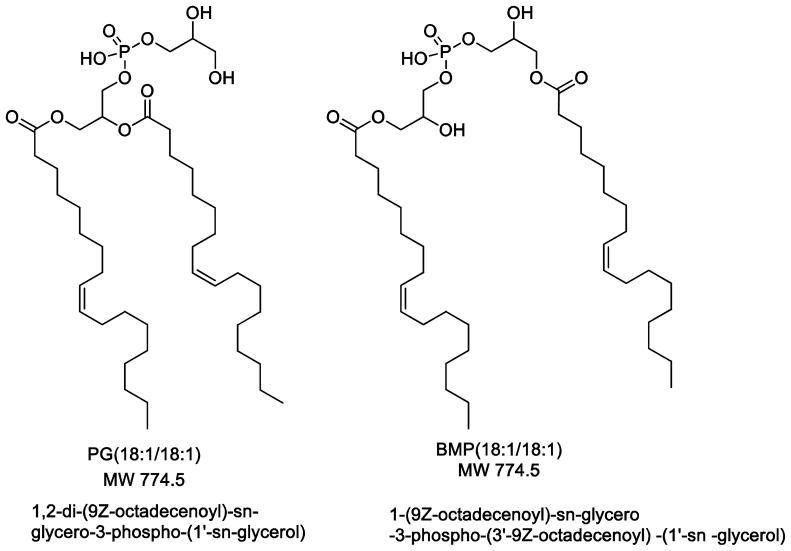
Electrospray ionization generated significantly more abundant negative molecular ions [M−H]− than positive ions [M+H]+ for both BMP and PG using the electrospray parameters and conditions that we have typically employed for lipids [19]. This is in contrast to a previous report that more abundant positive molecular ion species were observed [11]. In any event, the collisional activation of the BMP and PG [M−H]− ions yielded nearly identical product ions (Figure 1) where carboxylate anions dominate all product ions. The carboxylate anions would be derived from the fatty acyl groups of each of these phospholipids esterified to the glycerol backbone [13]. The failure to distinguish BMP from PG by negative product ion spectra has been previously noted [9,11].
Figure 1.
Ion mobility and negative-ion tandem mass spectrometry of phosphatidylglycerol (PG) and isomeric bis(monoacylglycerol)phosphate (BMP). (A) Ion mobility trace (total ion mobilogram) for [M−H]− m/z 773.5 of PG(18:1/18:1). (B) Product ion mass spectrum from CID of [M−H]− m/z 773.5 of PG(18:1/18:1). (C) Total ion mobilogram for [M−H]− m/z 773.5 of BMP(18:1/18:1). (D) Product ion mass spectrum from CID of [M−H]− m/z 773.5 of BMP(18:1/18:1). Collision energy at 30V.
Measurement of ion mobility using the traveling wave method [20] of the [M−H]− ions from isomeric dioleoyl-PG and dioleoyl-BMP yielded nearly identical results (Figure 1A and 1C). Furthermore, ion mobility of the product ions following collisional activation of both PG and BMP also yielded identical results (data not shown).
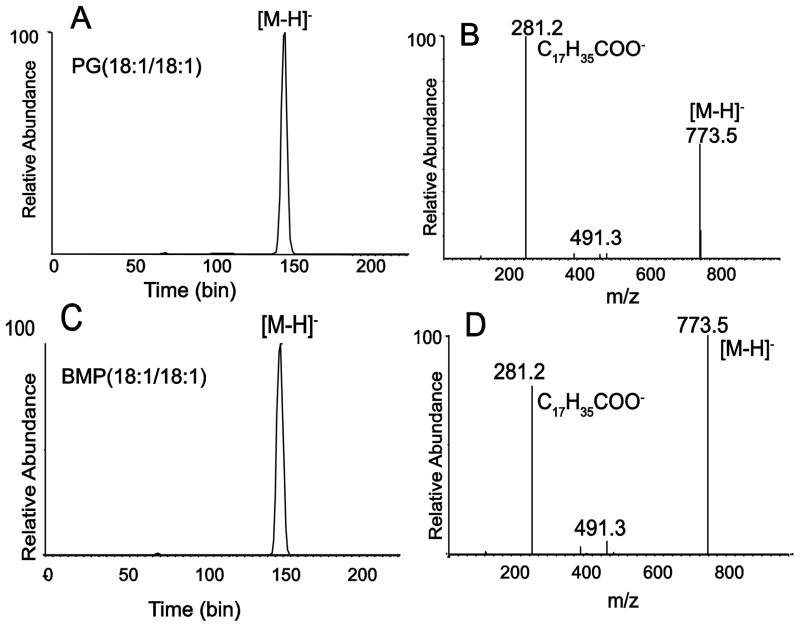
These ion mobility experiments were carried out again with the [M+H]+ ions, with the results that similar mobilities were observed for molecular ions of both PG and BMP (data not shown). However, when the ion mobility of the product ions following collisional activation was analyzed, very different drift times were observed from each phospholipid (Figure 2A and 2B). This was expected since the product ion spectra of PG and BMP as protonated molecular ions have been reported to be quite different, with PG yielding diglyceride-like ions and BMP yielding monoglyceride-like ions [9,11]. The ions that make up the fastest moving packet from the ion mobility experiment from BMP (Figure 2B) were found to be the monoglyceride-like product ions at m/z 339 (Figure 2D). The slowest moving product ions from this BMP were largely populated with m/z 739, corresponding to [M+H-2H2O]+. The ion mobility of these two sets of ions was unique to the BMP, since very little loss of either 1 or 2 molecules of water was observed for PG (18:1/18:1) (Figure 2C). The most abundant product ions with the longest drift times following collisional activation of PG (18:1/18:1) were the diglyceride-like product ions (Figure 2C).
Figure 2.
Ion mobility of product ions following collisional activation of positive ions from PG and BMP. (A) Mobilogram of all product ions from CID of [M+H]+ m/z 775.5 of PG(18:1/18:1). Predominant peak at bin 120 corresponds to the diglyceride-like fragment ion at m/z 603.5. Collision energy at 15V. (B) Mobilogram of all product ions from CID of [M+H]+ m/z 775.5 of BMP(18:1/18:1). Predominant peak at bin 77 corresponds to the monoglyceride-like fragment ion at m/z 339.3 and the broad peak at bin 140 corresponds to the cluster of ions including precursor [M+H]+ and a series of one and two losses of H2O. Collision energy at 10V. (C) Product ion mass spectrum of [M+H]+ m/z 775.5 of PG(18:1/18:1). Collision energy at 5V. (D) Product ion mass spectrum of [M+H]+ m/z 775.5 of BMP(18:1/18:1). Collision energy at 10V.
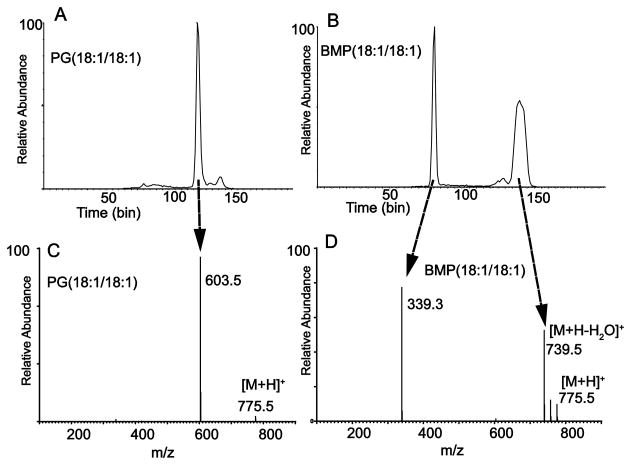
The sodium ion adduct [M+Na]+ of both PG and BMP can be observed during electrospray ionization of samples infused into the electrospray inlet, either directly as an organic solvent extract of a biological tissue or upon storage of the sample in a sodium-containing borosilicate glass vial. In these situations, sodiated ion species can be significantly more abundant than the [M+H]+. The tandem mass spectrometry and ion mobility determination of product ions of PG(18:1/18:1) and BMP(18:1/18:1) from sodiated molecular ions were carried out (Figure 3). As expected, the mobilograms of the product ions were very different since the product ions formed following collisional activation of [M+Na]+ for PG and BMP were quite different (data not shown). The intact molecular ions for each regioisomer had identical drift times just as for the [M−H]− and [M+H]+ ions, indicating similar ion mobility cross-sections for the molecular ion species. The major product ions observed from sodiated PG(18:1/18:1) appeared at m/z 603 and 195, consistent with diglyceride-like ion formation and polar head group ion formation, depending upon the charge site localized on the PG structure (Figure 3A). For these product ions, the sodium ion attachment site appeared to be localized as a salt with the phosphate anion (see Discussion), whereas the ion at m/z 625 would correspond to a sodium ion attached to the diglyceride-like ion.
Figure 3.
Tandem mass spectrometry of positive ion sodium adducts of PG and BMP. (A) Product ion spectrum of [M+Na]+ m/z 797.5 for PG(18:1/18:1). (B) Product ion spectrum of [M+Na]+ m/z 797.5 for BMP(18:1/18:1). All CID at 30 V.
The major BMP product ions observed for the same sodiated regioisomer (Figure 3B) was m/z 459, consistent with the same mechanism of polar head group ion formation observed for [M+H]+ and for the PG species. In this case however, m/z 459 retained a monoglyceride portion of the molecule and not a polar head group of phosphoglycerol observed at m/z 195 for PG. These major product ions following collisional activation of the [M+Na]+ were definitive for identifying the BMP and PG structures and could be generated readily as positive ion species isolated from biological extracts.
The mechanism involved in the formation of diglyceride-like and monoglyceride-like ions from the positive-ion collision spectra from PG and BMP was examined in greater detail using deuterium-labeled variants. Previous studies with other phospholipid species such as 1,2-diacyl-PC and -PE had revealed that the formation of these diglyceride-like product ions or the corresponding phosphate-containing polar head group ions (specifically m/z 184 for PC) involved abstraction of a proton from a 2′-fatty acyl carbon atom [21]. However, when the collisional activation of PG(D3116:0/18:1), either as [M+H]+ or [M+Na]+, was carried out, the ion observed did not lose a deuterium atom and the diglyceride ion appeared at m/z 608, corresponding to a D31-diglyceride-like ion (Supplemental Figure 1). Furthermore, when the active protons on PG(18:1/18:1) were exchanged with D2O to form the D4-labeled molecular ion at m/z 779, the most abundant diglyceride-like ion at m/z 604 indicated addition of one deuterium atom (likely the charge site), but a fairly abundant ion was also observed at m/z 603, revealing no exchangeable proton on this product (Supplemental Figure 2).
3.1 BMP and PG analysis in RAW 264.7 cells
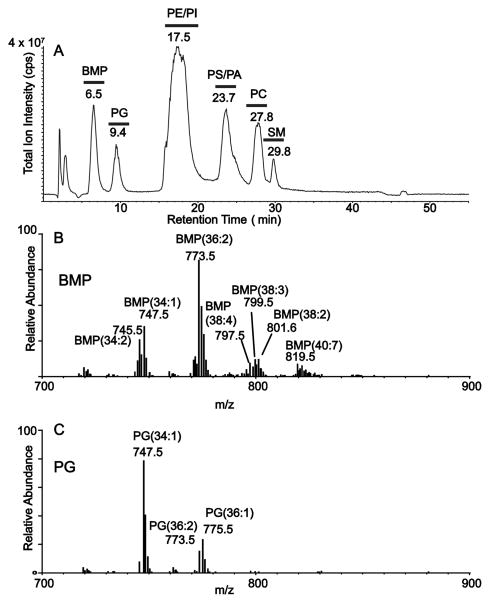
Phospholipids from the murine macrophage-like cell line termed RAW 264.7 were extracted by the method of Bligh and Dyer [18] and the extract was analyzed by normal phase LC-MS in the negative-ion mode. In this experiment the major molecular species could be identified (Figure 4). Two well-separated components of the phospholipid extract were observed eluting at 6.5 and 9.4 min from the HPLC column (Figure 4A). In separate experiments, synthetic BMP(18:1/18:1) was found to elute from this normal phase HPLC system at 6.5 min while synthetic PG(18:1/18:1) eluted at 9.4 min. Very different populations of molecular species were observed for the BMP region (Figure 4B) and the PG region (Figure 4C). Collisional activation of these negative molecular ions permitted assignment of the fatty acyl groups present in each phospholipid species. Quite surprising was the presence of BMP (18:1/20:2) and BMP (18:1/20:3) species observed at m/z 799 and 797, respectively (Supplemental Figure 3). While the 20:2 and 20:3 are unusual polyunsaturated fatty acyl groups [22], these are known to be present in the BMP molecular species [23].
Figure 4.
Separation of phospholipid extract of mammalian cells using normal phase HPLC. (A) Normal phase LC/MS (full scan, negative ion) of a lipid extract from RAW 264.7 cells showing chromatographic separation of different phospholipid classes including BMP and PG. (B) Molecular ions [M−H]− present under the BMP region of chromatographic separation in Figure 4A. (C) Molecular ions [M−H]− present under the PG region of chromatographic separation in Figure 4A.
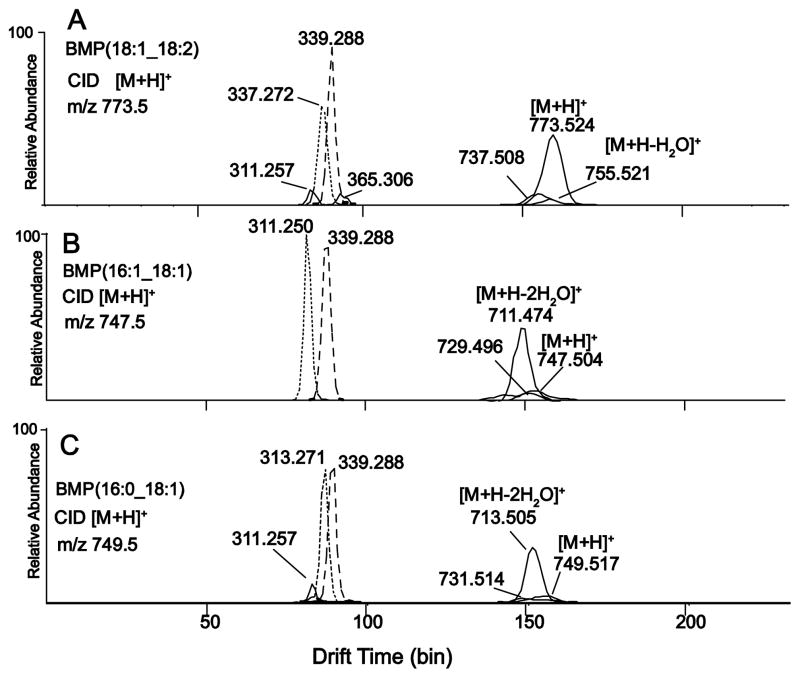
The RAW cell HPLC fractions were then analyzed by positive ion electrospray ionization and ion mobility after collisional activation of three BMP [M+H]+ ions (Figure 5). The ion mobility of BMP(18:1/18:2) monoglyceride-like ions indicated some separation (Figure 5A) of the monoglyceride-like extracted ion envelopes (extraction of the exact mass +/− 0.01Da where abundance is plotted relative to drift times). There was at least one additional isomer of BMP(36:3) which formed the monoglyceride ions at m/z 311 (16:1) and 365 (20:2) which also displayed different ion mobilities from the m/z 337 (18:2) and 339 (18:1) monoglyceride ions. The other BMP molecular species had extracted ion mobility monoglyceride ion profiles with substantial separation, including BMP(16:1/18:1) (Figure 5B), suggesting a significantly different ion mobility cross section for monoglyceride-like ions due to different structures. The molecular species BMP(16:0/18:1) (Figure 5C) had some separation of the mobility packets, suggesting that the ion mobility for 18:1 monoglyceride (m/z 339) could be differentiated from the 16:0 monoglyceride (m/z 313). These subtile differences in drift times were only observed in the extracted ion mobilograms and not in the mobilograms (total ion mobilograms) where all ion abundances are plotted relative to drift time (Supplemental figure 4).
Figure 5.
Extracted mobilograms of product ions from the isolated BMP fraction shown in Figure 4A. (A) Product ion mobilogram of [M+H]+ m/z 773.5, BMP(18:1_18:2). Collision energy at 15V. (B) Extracted mobilograms of [M+H]+ m/z 747.5, BMP(16:1_18:1). Collision energy at 10V. (C) Extracted mobilograms of [M+H]+ m/z 749.5, BMP(16:0_18:1). Collision energy at 10V.
4. Discussion
Isomeric phospholipid molecular species typically differ only at the sn-1 and sn-2 fatty acylation positions. In such situations, tandem mass spectrometry can be used, in particular with negative ions, to distinguish isomeric lipids [14]. BMP and PG offer a unique regioisomeric pair in that the positions of esterification are quite different in these two molecules. PG has two fatty acyl groups on the same glycerol backbone while BMP has only one fatty acyl group esterified to each of the two different glycerol moieties (Scheme 1). Negative-ion tandem mass spectrometry cannot distinguish these two isomeric phospholipids from each other. This was expected based upon previous reports of the analysis of BMP and PG with this ion polarity [9,11]. It was also anticipated that a significant difference in the tandem mass spectrometry would be observed in the positive ion tandem mass spectrometry of BMP and PG due to the fact that a 1,2-diglyceride-like ion can readily form. Since BMP does not contain any 1,2-diglycerides in its structure, only monoglyceride-like ions would be expected.
4.1 Diglyceride-like and Monoglyceride-like Ion Formation
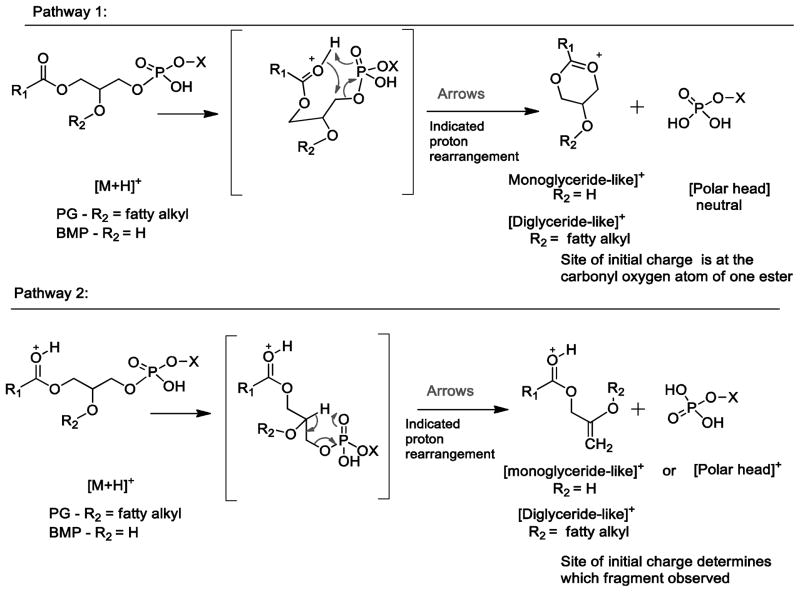
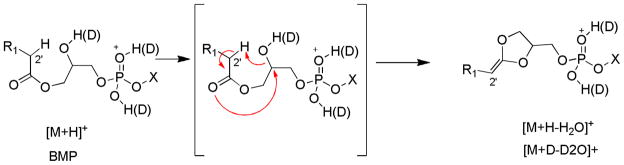
Based on the product ion mass spectra of the deuterium-labeled variants of BMP and PG, we propose two pathways to account for the mass spectral data of labeled variants and for the accurate mass measurement of their elemental composition (Supplementary Table 1). Two pathways are presented in Scheme 2. In one of the mechanisms, a product ion of PG retains all of the deuterium atoms from the fatty acyl chain from the D31-labeled PG (m/z 577+31) and no protons from an exchangeable site using deuterated methanol (Pathway 1). It is interesting to note that this mechanism differs from that previously published for other 1,2-diacyl phospholipids such as PC and PE where a proton is abstracted from the 2′-fatty acyl chain [24], which perhaps is due to the fact that the site of proton attachment as a charging site is on the ester carbonyl group and not on the polar head group as it is in PC and PE. This might make the ester-adducted proton, which is exchanged in deuterated methanol, easily abstracted by the phosphoryl oxygen atom during formation of the oxonium product ion (Pathway 1). When H/D exchange was carried out with PG and BMP (Supplemental Figure 2), the monoglyceride-like ions from BMP had a significant abundance of species with one deuterium atom in the product ion consistent with Pathway 1, but the diglyceride-like ions from PG also showed a significant abundance of a species with one deuterium atom attached, inconsistent with pathway 1 (Scheme 2). A second mechanism would involve abstraction of a proton from elsewhere in the molecule by the phosphate polar head group as the carbon-oxygen bond to the phosphate ester is cleaved (Pathway 2, Scheme 2). Based on the observation from D31-PG that this proton was not from a fatty acyl chain (Supplemental Figure 1), the most likely hydrogen atom abstracted would come from the glycerol backbone (Pathway 2). The H/D exchanged BMP species would have one deuterium atom retained in the product ion by pathway 1 (Scheme 2) and two deuterium atoms by pathway 2(Scheme 2).
Scheme 2.
4.2 Ion Mobility
The difference in fundamental structure between these two phospholipids was anticipated to be made manifest by different ion mobilities due to an expected difference in their 3-dimensional shape suggested by the structures shown in Scheme 1. However, this was not observed since both PG and BMP, as the molecular ions, could not be distinguished from each other by their ion mobilities either in the positive or negative ion modes under the conditions used (Figure 1 and 2). Since the basis of separation of ions by the traveling wave ion mobility experiment is due to the cross-section of the molecule exposed to the neutral gas as the ions traverse through the drift tube region [20], it must be concluded that these two different phospholipids present a very similar ion cross-section, suggesting that the hydrophobic chains might associate with each other, even though they are on different glycerol backbones for BMP and adjacent to each other in PG. Examination of molecular models supported this suggestion. In solution, the association of these fatty acyl hydrocarbon chains of phospholipids is largely due to hydrophobic bonding [25]. This type of bonding is typically understood in the context of a increase in entropy by releasing solvent water molecules as these hydrophobic bonds form due to Van der Waals forces. Possibly such a hydrophobic interaction may occur in the gas phase, forming a stable ion containing these two hydrocarbon chains with a total of 36 carbon atoms and 74 hydrogen atoms, with sufficient energy to maintain the shape during the drift experiment. In contrast to this, the monoglyceride-like ions from the BMP phospholipids have a single fatty acyl chain with fewer atoms and may be more susceptible to alterations in shape presented to the drift gas. A very nice separation was obtained, for example, between molecular species of monoglyceride-like ions that differed by two methylene groups (Figure 5B). Although the presence of a double bond had the similar effect on ion mobility to that of a two-carbon shorter fatty acyl group, this might suggest that the double bond introduces a unique shape change that also determines ion mobility.
4.3 Water Losses from BMP Ions
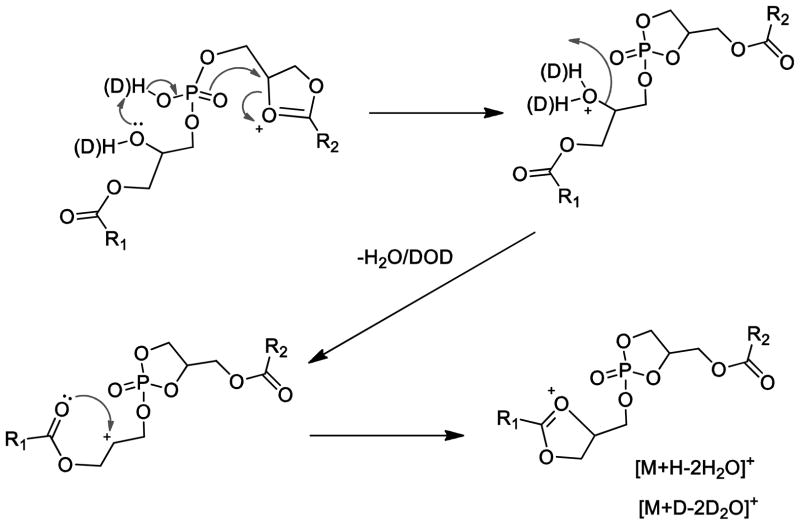
Another interesting feature of the ion mobility experiment was revealed by a late ion packet from the drift tube, which was unique to the BMP class of phospholipids. The ions that make up this population were primarily BMP positive molecular ions minus two water molecules. While it has not been previously suggested that this is a product ion that could be used to distinguish BMP from PG, it is clear after examining many different BMP and PG molecular species in RAW 264.7 cell extracts that this is a general phenomenon of collisional activation of BMP [M+H]+ ions. The [M+H-2H2O]+ ions were further investigated with H/D exchanged BMP species to examine the mechanism by which two water molecules could be lost (Supplemental Figure 5). The loss of two water molecules from PG would be much more difficult, since the hydroxyl groups on the glycerol backbone occurs as a vicinal diol where the loss of two water molecules would likely require higher collision energies. Perhaps the competitive ion decomposition pathway for PG that forms the diglyceride-like product occurs preferentially even at relatively low collision energies.
The much more facile loss of two water molecules from BMP, even at low collision energy, is likely the result of having the two free hydroxyl groups on two different glycerol portions of the BMP structure and the same mechanism operating to lose water. Several mechanisms were considered for this water loss, including charge remote mechanisms involving the phosphate residue (Scheme 3 and 4). But these mechanisms would predict loss of D2O but not HOD in the H/D exchanged BMP.
Scheme 3.
Scheme 4.
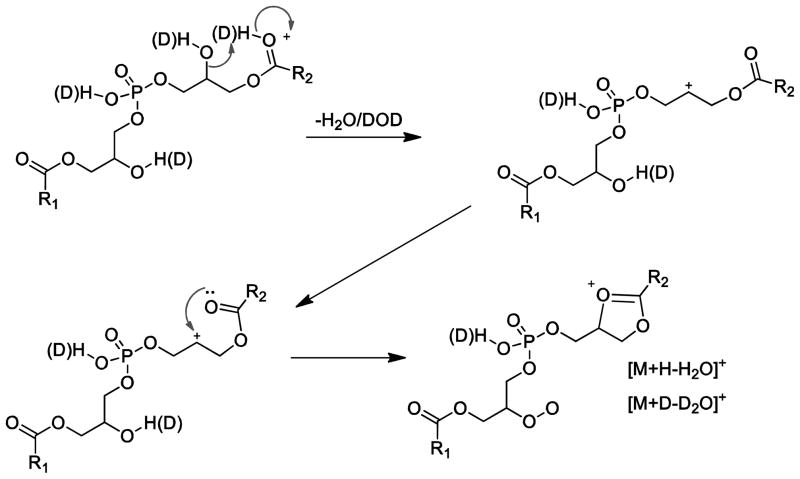
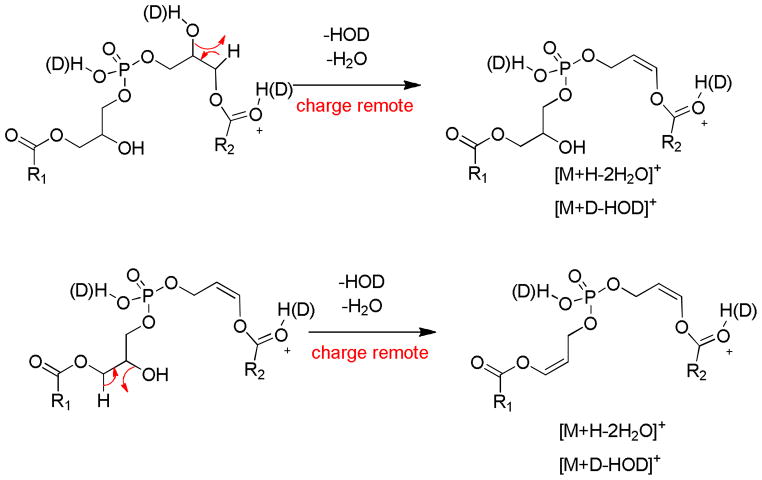
Surprisingly, the exchangeable deuterium experiment revealed losses of four exchangeable protons, three exchangeable protons and two exchangeable protons encompassing the two water losses (Supplemental Figure 4A). The isotopic distributions observed with HOD or D2O would suggest that an equal probability for HOD or D2O loss based on the similar abundance of the deuterated species after a single water loss and the 1:2:1 ratio for the losses of D2O:HOD+D2O:HOD for the ion corresponding to two losses of water (Supplemental Figure 4B). The loss of HOD was thus an important pathway. Two different mechanisms are proposed. The first is a charge remote loss of a glycerol proton in a 1,2-elimination of water which seem energetically unfavorable (Scheme 5). However the CID of PG as [M−H]−, a glycerol backbone proton is lost with the adjacent fatty acyl group in the formation of [M−H-RxCOOH]−. A second loss of HOD could arise from the loss of a fatty acyl proton which has been reported for many phospholipid decomposition reactions even from fast atom bombardment experiments [24,27]. This reaction would involve the sn-2 hydroxyl group abstracting a proton from the 2′-fatty acyl group and the intermediate then rearranging by the loss of water to form a 1,3-dioxolane structure (Scheme 6).
Scheme 5.
Scheme 6.
5. Conclusion
The collisional activation of the positive ions generated for PG and BMP, either as [M+H]+ or [M+Na]+, permits characterization of these two different phospholipid classes. These regioisomers provide an alternative perspective on the mechanism of formation of monoglyceride-like and diglyceride-like ions from phospholipid molecular species, as well as a perspective on the neutral loss of two water molecules from BMP. Finally, ion mobility of the positive product ions from BMP and PG offers another avenue to distinguish these structures. Although the different structures of an isomeric pair of [M+H]+ species of these two lipids could not be distinguished by ion mobility, the ion fragments upon CID were distinguishable by mass and mobility. Furthermore, subtle differences in acyl chain composition of the lower molecular weight fragments could be discerned by ion mobility.
Supplementary Material
Highlights.
PG and BMP regioisomers were studied by tandem mass spectrometry with ion mobility.
Precursor ions of a PG and BMP isomeric pair were indistinguishable by MS/IMS.
Product ions in positive ion mode were distinctly different by MS/MS/IMS.
Mechanisms of fragmentation were evaluated using deuterium labeling.
PG and BMP species in RAW 264.7 cells were studied using MS/MS/IMS.
Acknowledgments
This work was supported by a grant from the National Institute of Environmental Health Sciences of the National Institutes of Health (ES022172).
Abbreviations
- BMP
bis(monoacylglycerol)phosphate
- PG
phosphatidylglycerol
- NP HPLC
normal phase HPLC
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horvath SE, Daum G. Lipids of mitochondria. Prog Lipid Res. 2013;52:590–614. doi: 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Scherer M, Schmitz G. Metabolism, function and mass spectrometric analysis of bis(monoacylglycero)phosphate and cardiolipin. Chem Phys Lipids. 2011;164:556–562. doi: 10.1016/j.chemphyslip.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Veldhuizen R, Nag K, Orgeig S, Possmayer F. The role of lipids in pulmonary surfactant. Biochim Biophys Acta. 1998;1408:90–108. doi: 10.1016/s0925-4439(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 4.Gallala HD, Sandhoff K. Biological function of the cellular lipid BMP-BMP as a key activator for cholesterol sorting and membrane digestion. Neurochem Res. 2011;36:1594–1600. doi: 10.1007/s11064-010-0337-6. [DOI] [PubMed] [Google Scholar]
- 5.Agassandian M, Mallampalli RK. Surfactant phospholipid metabolism. Biochim Biophys Acta. 2013;1831:612–625. doi: 10.1016/j.bbalip.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Numata M, Nagashima Y, Moore ML, Berry KZ, Chan M, Kandasamy P, Peebles RS, Jr, Murphy RC, Voelker DR. Phosphatidylglycerol provides short-term prophylaxis against respiratory syncytial virus infection. J Lipid Res. 2013;54:2133–2143. doi: 10.1194/jlr.M037077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Numata M, Chu HW, Dakhama A, Voelker DR. Pulmonary surfactant phosphatidylglycerol inhibits respiratory syncytial virus-induced inflammation and infection. Proc Natl Acad Sci U S A. 2010;107:320–325. doi: 10.1073/pnas.0909361107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito M, Tchoua U, Okamoto M, Tojo HJ. Purification and properties of a phospholipase A2/lipase preferring phosphatidic acid, bis(monoacylglycerol) phosphate, and monoacylglycerol from rat testis. J Biol Chem. 2002;277:43674–43681. doi: 10.1074/jbc.M202817200. [DOI] [PubMed] [Google Scholar]
- 9.Meikle PJ, Duplock S, Blacklock D, Whitfield PD, Macintosh G, Hopwood JJ, Fuller M. Effect of lysosomal storage on bis(monoacylglycero)phosphate. Biochem J. 2008;411:71–78. doi: 10.1042/BJ20071043. [DOI] [PubMed] [Google Scholar]
- 10.Flis VV, Daum G. Lipid transport between the endoplasmic reticulum and mitochondria. Cold Spring Harb Perspect Biol. 2013;5:a013235. doi: 10.1101/cshperspect.a013235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherer M, Schmitz G, Liebisch G. Simultaneous quantification of cardiolipin, bis(monoacylglycero)phosphate and their precursors by hydrophilic interaction LC-MS/MS including correction of isotopic overlap. Anal Chem. 2010;82:8794–8799. doi: 10.1021/ac1021826. [DOI] [PubMed] [Google Scholar]
- 12.Holbrook PG, Pannell LK, Murata Y, Daly JW. Bis(monoacylglycero)phosphate from PC12 cells, a phospholipid that can comigrate with phosphatidic acid: molecular species analysis by fast atom bombardment mass spectrometry. Biochim Biophys Acta. 1992;1125:330–334. doi: 10.1016/0005-2760(92)90063-2. [DOI] [PubMed] [Google Scholar]
- 13.Hsu FF, Turk J, Williams TD, Welti R. Electrospray ionization multiple stage quadrupole ion-trap and tandem quadrupole mass spectrometric studies on phosphatidylglycerol from Arabidopsis leaves. J Am Soc Mass Spectrom. 2007;18:783–790. doi: 10.1016/j.jasms.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy RC, Axelsen PH. Mass spectrometric analysis of long-chain lipids. Mass Spectrom Rev. 2011;30:579–599. doi: 10.1002/mas.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duffin K, Obukowicz M, Raz A, Shieh JJ. Electrospray/tandem mass spectrometry for quantitative analysis of lipid remodeling in essential fatty acid deficient mice. Anal Biochem. 2000;279:179–188. doi: 10.1006/abio.1999.4452. [DOI] [PubMed] [Google Scholar]
- 16.Shah V, Castro-Perez JM, McLaren DG, Herath KB, Previs SF, Roddy TP. Enhanced data-independent analysis of lipids using ion mobility-TOFMSE to unravel quantitative and qualitative information in human plasma. Rapid Commun Mass Spectrom. 2013;27:2195–2200. doi: 10.1002/rcm.6675. [DOI] [PubMed] [Google Scholar]
- 17.May JC, Goodwin CR, Lareau NM, Leaptrot KL, Morris CB, Kurulugama RT, Mordehai A, Klein C, Barry W, Darland E, Overney G, Imatani K, Stafford GC, Fjeldsted JC, McLean JA. Conformational ordering of biomolecules in the gas phase: nitrogen collision cross sections measured on a prototype high resolution drift tube ion mobility-mass spectrometer. Anal Chem. 2014;86:2107–2116. doi: 10.1021/ac4038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 19.Berry KA, Henson PM, Murphy RC. Effects of acrolein on leukotriene biosynthesis in human neutrophils. Chem Res Toxicol. 2008;21:2424–2432. doi: 10.1021/tx800333u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapthorn C, Pullen F, Chowdhry BZ. Ion mobility spectrometry-mass spectrometry (IMS-MS) of small molecules: separating and assigning structures to ions. Mass Spectrom Rev. 2013;32:43–71. doi: 10.1002/mas.21349. [DOI] [PubMed] [Google Scholar]
- 21.Hsu FF, Turk J. Electrospray ionization with low-energy collisionally activated dissociation tandem mass spectrometry of glycerophospholipids: mechanisms of fragmentation and structural characterization. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2673–2695. doi: 10.1016/j.jchromb.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cook HW, Emken EA. Geometric and positional fatty acid isomers interact differently with desaturation and elongation of linoleic and linolenic acids in cultered gliomal cells. Biochem Cell Biol. 1990;68:653–660. doi: 10.1139/o90-094. [DOI] [PubMed] [Google Scholar]
- 23.Bouvier J, Zemski Berry KA, Hullin-Matsuda F, Makino A, Michaud S, Geloën A, Murphy RC, Kobayashi T, Lagarde M, Delton-Vandenbroucke I. Selective decrease of bis(monoacylglycerol)phosphate content in macrophages by high supplementation with docosahexaenoic acid. J Lipid Res. 2009;50:243–255. doi: 10.1194/jlr.M800300-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Hsu FF, Turk J. Electrospray ionization/tandem quadrupole mass spectrometric studies on phosphatidylcholines: the fragmentation processes. J Am Soc Mass Spectrom. 2003;14:352–363. doi: 10.1016/S1044-0305(03)00064-3. [DOI] [PubMed] [Google Scholar]
- 25.Kyte J. The basis of the hydrophobic effect. Biophys Chem. 2003;100:193–203. doi: 10.1016/s0301-4622(02)00281-8. [DOI] [PubMed] [Google Scholar]
- 26.Haroldsen PE, Clay KL, Murphy RC. Quantitation of lyso-platelet activating factor molecular species from human neutrophils by mass spectrometry. J Lipid Res. 1987;28:42–49. [PubMed] [Google Scholar]
- 27.Hsu FF, Turk J. Studies on phosphatidylglycerol with triple quadrupole tandem mass spectrometry with electrospray ionization: Fragmentation processes and structural characterization. J Am Soc Mass Spectrom. 2001;12:1036–1043. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.