Abstract
MicroRNAs (miRNAs) are a class of small non-coding RNAs, whose expression levels vary in different cell types and tissues. Emerging evidence indicates that tissue-specific and -enriched miRNAs are closely associated with cellular development and stress responses in their tissues. MiR-25 has been documented to be abundant in cardiomyocytes, but its function in the heart remains unknown. Here, we report that miR-25 can protect cardiomyocytes against oxidative damage by down-regulating mitochondrial calcium uniporter (MCU). MiR-25 was markedly elevated in response to oxidative stimulation in cardiomyocytes. Further overexpression of miR-25 protected cardiomyocytes against oxidative damage by inactivating the mitochondrial apoptosis pathway. MCU was identified as a potential target of miR-25 by bioinformatical analysis. MCU mRNA level was reversely correlated with miR-25 under the exposure of H2O2, and MCU protein level was largely decreased by miR-25 overexpression. The luciferase reporter assay confirmed that miR-25 bound directly to the 3' untranslated region (UTR) of MCU mRNA. MiR-25 significantly decreased H2O2-induced elevation of mitochondrial Ca2+ concentration, which is likely to be the result of decreased activity of MCU. We conclude that miR-25 targets MCU to protect cardiomyocytes against oxidative damages. This finding provides novel insights into the involvement of miRNAs in oxidative stress in cardiomyocytes.
Keywords: cardiomyocytes, miR-25, mitochondrial calcium uniporter, oxidative stress
1. Introduction
Myocardial cell death often occurs under conditions of oxidative stress, such as ischemia reperfusion and myocardial infarction [1,2]. Hallmark events including mitochondrial permeability transition pore (mPTP) opening and dissipation of the mitochondrial inner membrane potential are usually initiated by excessive mitochondrial Ca2+ overload [3]. One important approach to cardioprotection against oxidative stress injury is to reduce oxygen consumption and inhibit apoptosis in cardiomyocytes [4].
MicroRNAs (miRNAs) are small non-coding RNAs that inhibit the translation of target mRNAs and/or cause their destabilization through imperfect sequence-specific binding to the 3'-untranslated region (UTR) of target mRNAs. Increasing evidence shows that miRNA expression is tightly regulated in a tissue-specific and a time-dependent manner [5,6]. For example, miR-l, miR-133, miR-21, miR-206 and miR-199 are highly and specifically expressed in cardiovascular tissues [7,8,9]. These cardiac-specific miRNAs are involved in the regulation of multiple physiological and pathological processes, such as cardiac morphogenesis, heart failure, and arrhythmias [10,11].
In the present study, we demonstrate that miR-25, a cardiac-enriched miRNA, is sensitive to oxidative stress. The overexpression of miR-25 can protect cardiac myocytes against oxidative injury. Mechanistically, miR-25 directly down-regulates the expression of mitochondrial MCU and inhibits the increase of the mitochondrial Ca2+ and mitochondrial apoptosis pathway.
2. Results
2.1. MiR-25 Was Remarkably Elevated under Oxidative Stress
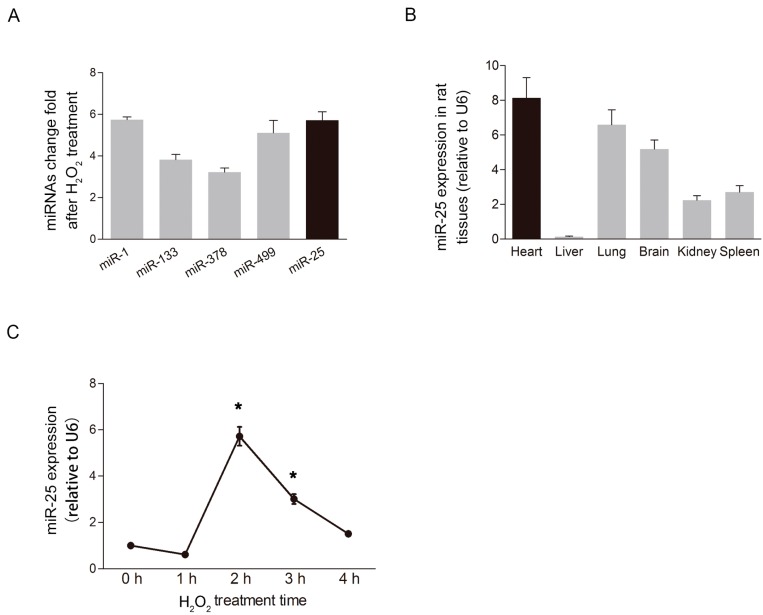
To screen out the cardiac-enriched miRNAs involved in oxidative stress, we used H9c2 cells, an embryonic rat ventricular myocyte cell line, and examined miRNA expression levels in cells exposed to oxidative stress using microarray analysis (GSE36239 and GSE55791) [12,13]. The oxidative stress was induced by 4 h of incubation with H2O2, an exogenous source of reactive oxygen species (ROS) that increases lipid peroxidation, and decreases antioxidant activity and damages cardiomyocytes [14,15,16]. The results indicated that the most significant change in response to oxidative stress was observed for miR-25 (Figure 1A). Next, we used RT-PCR to confirm the expression profile of miR-25 in different rat organs (Figure 1B). The highest expression level of miR-25 was in the heart. Finally, to verify the reaction to H2O2 stimulation, we monitored the expression of miR-25 at five different time points, and we found that the expression of miR-25 exposed to H2O2 in cardiomyoctye was time-dependent (Figure 1C). Following oxidant stimulation, the levels of miR-25 in H9c2 cells remained unchanged within 1 h, dramatically increased more than five-fold at 2 h, and then gradually returned to control levels.
Figure 1.
MiR-25 was elevated under oxidative stress. (A) MiR-25 expression was dramatically altered in response to oxidative stress compared to other miRNAs that are highly expressed in cardiac tissue (n = 3); (B) Quantitative RT-PCR confirmed the expression profile of miR-25 in different rat organs (n = 3); (C) Time course of the relative expression of miR-25 in cardiomyocytes in response to H2O2 stimulation (500 μM). The expression of miR-25 increased significantly at 2 h, and gradually returned to baseline by 4 h. Data are means ± SD from three independent experiments. * p < 0.05 vs. control (0 h).
2.2. Over-Expression of MiR-25 Protected Cardiomyocytes against Oxidative Damage by Inactivating the Mitochondrial Apoptosis Pathway
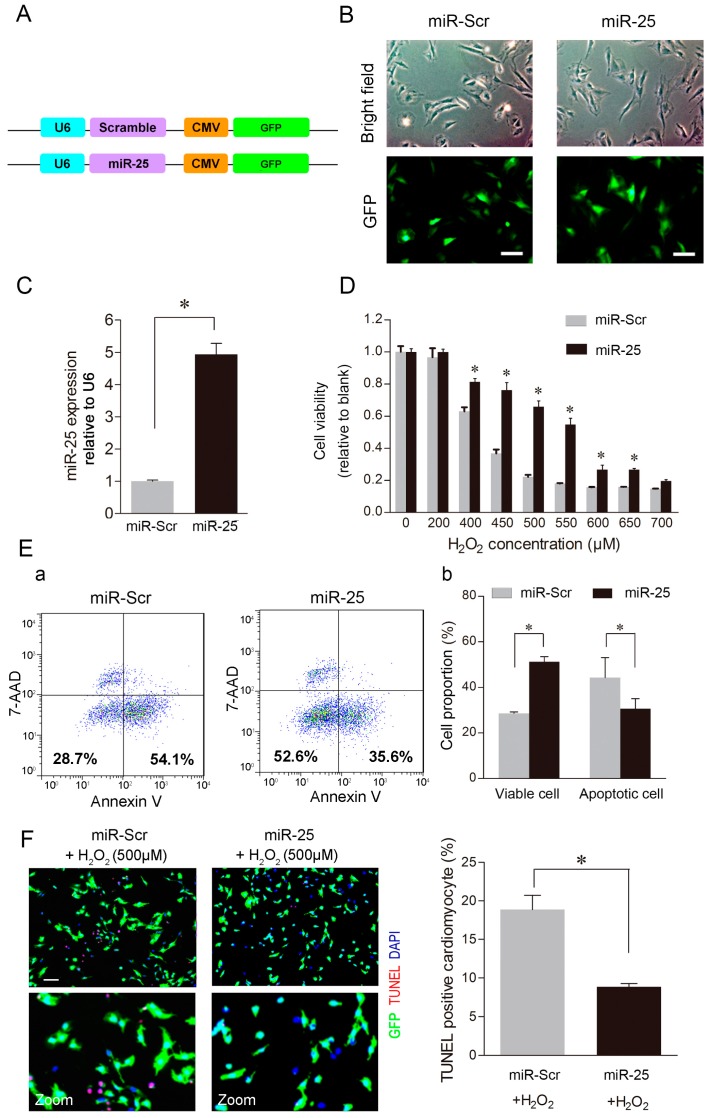
To evaluate the physiological function of miR-25, H9c2 cells were infected with a lentivirus carrying the miR-25 precursor (miR-25) or a scramble sequence (miR-Scr) (Figure 2A). The infected cardiomyocytes express green fluorescence protein (GFP), which can be observed by microscopy, as well as sorted by FACS. Under the light and fluorescence microscopy, no morphological difference was observed between cells expressing miR-25 and miR-Scr (Figure 2B). Real-time PCR analysis showed that the expression level of miR-25 was 4.94 ± 0.34-fold higher in the miR-25 group than that in the miR-Scr group (Figure 2C).
Figure 2.
Over-expression of miR-25 markedly reduced cardiomyocyte apoptosis. (A) Schematic illustration of the construction of the lentivirus vectors. H9c2 cells infected with lentiviruses containing miR-25 precursor (miR-25) or a scramble control sequence (miR-Scr) were isolated by fluorescence-activated cell sorting (FACS) based on the green fluorescence emitted from cells expressing GFP, the marker carried by the lentivirus vector, and then plated in 24-well plates for expansion; (B) The phase contrast and fluorescent images of H9c2 cells infected with lentiviruses containing miR-25 or miR-Scr. Scale bars: 100 μm; (C) Quantitative RT-PCR showed that miR-25 expression was 4.94 ± 0.34-fold higher in the miR-25 group than that in the miR-Scr group. * p < 0.05 vs. miR-Scr; (D) H9c2 cells were stimulated with H2O2 and subjected to CCK-8 assay. Serial concentrations of H2O2 were applied to examine the effects of miR-25 overexpression; (E) Panel a: representative dot-plot diagrams of FACS analysis on H9c2 cells stained with annexin V and 7-AAD. The lower left quadrants represents the viable cells (Annexin V−/7-AAD−), and the lower right quadrants represents the apoptotic cells (Annexin V+/7-AAD−); Panel b: percentages of viable and apoptotic cells; (F) Representative images and quantification of the TUNEL assay. Apoptotic cells were stained red by the TUNEL assay. Scale bars: 100 μm. Data are means ± SD from three independent experiments. * p < 0.05 vs. respective control.
Next, both miR-25 and miR-Scr cells were treated with H2O2, and then cell vitality assay was performed. The results showed that miR-25 protected more H9c2 cells at different H2O2 concentrations, compared to the miR-Scr control (Figure 2D). This pro-survival effect was especially prominent at concentrations of H2O2 ranging from 450–550 μM, in which miR-25 enhanced cell viability by two to three-fold as shown by the CCK-8 assay. In addition, FACS analysis using annexin V and 7-AAD staining showed that there were fewer apoptotic cells (annexin V+/7-AAD−, 30.6% ± 3.31% vs. 44.3% ± 6.53%; p < 0.05) and more live cells (annexin V−/7-AAD−, 51.3% ± 1.71% vs. 28.6% ± 0.42%; p < 0.05) in the miR-25 group than those in the miR-Scr group after 4-h exposure of H2O2 (500 μM) (Figure 2E). In line with the results of CCK-8 assay and FACS analysis, the in situ TUNEL assay showed that the number of apoptotic cells in the miR-Scr group was double of that in the miR-25 group after the treatment with H2O2 (Figure 2F). These data suggested that miR-25 reduced cardiomyocyte apoptosis.
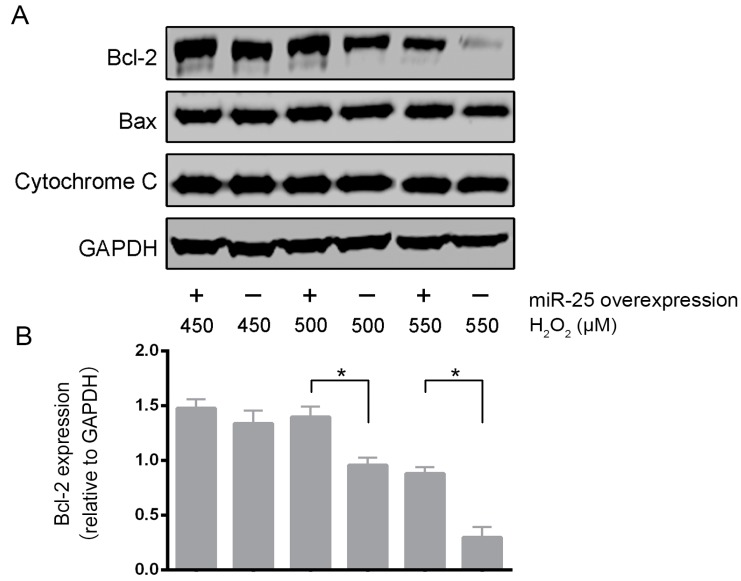
We further examined the expression of key proteins involved in the mitochondria-dependent apoptosis pathway (Figure 3). With the increment of H2O2 concentrations, the anti-apoptotic protein Bcl-2 was decreased, whereas the pro-apoptotic proteins Bax and cytochrome C remained unchanged. Compared to the control group, miR-25 overexpression preserved Bcl-2 protein levels in cells exposed to high concentrations of H2O2 (500–550 μM), but had no effects on the levels of Bax and cytochrome C. Bcl-2 is a key anti-apoptotic molecule. These results support that miR-25 protects cardiomyocytes by inhibiting apoptosis.
Figure 3.
MiR-25 protected cardiomyocytes by inhibiting the mitochondrial apoptosis pathway. (A) Representative Western blot images showing the expression levels of Bcl-2, Bax and cytochrome C in H9c2 cells infected with miR-25 lentiviruses; (B) The quantitative data of the Western blot. GAPDH served as a loading control. Data are means ± SD from three independent experiments. * p < 0.05.
2.3. MCU Was a Direct Target of MiR-25
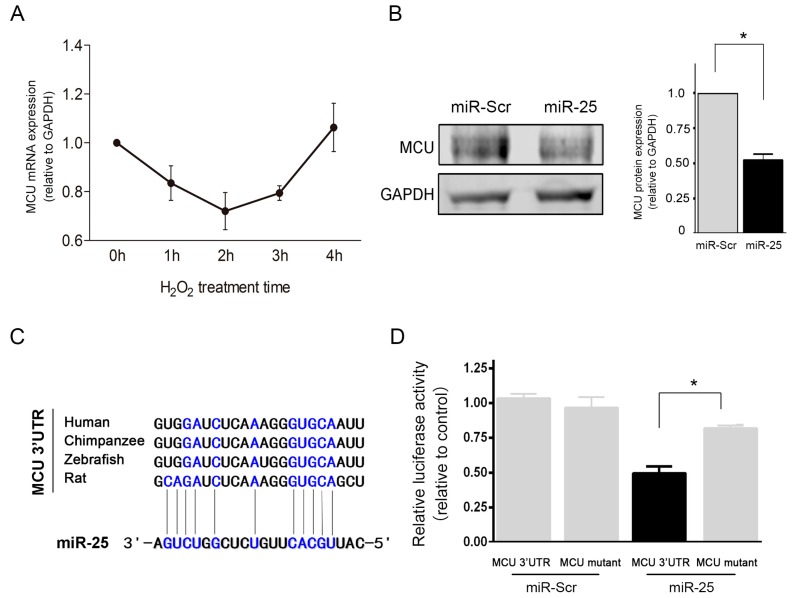
To identify the mechanism underlying miR-25-mediated anti-apoptosis, potential target genes of miR-25 were predicted using bioinformatics algorithms. Mitochondrial fission factor (MFF), mitochondrial calcium uniporter (MCU), and NADPH oxidase 4 (Nox4) were identified as putative targets of miR-25. To verify their validity, the relative expression levels of these targets were detected after H2O2 exposure. Only the mRNA level of MCU showed a significant negative correlation with miR-25 (Figure 4A). The overexpression of miR-25 also significantly reduced the protein level of MCU, which likely accounted at least in part, for its anti-apoptotic function (Figure 4B). Additionally, miR-25 significantly reduced the luciferase activity of wild-type reporters harboring MCU 3'-UTR by about 50% (p < 0.05), compared to the miR-Scr control (Figure 4C,D). In contrast, the mutant luciferase reporter with three nucleotide mutations in the potential binding area of MCU 3'-UTR to miR-25 seed sequence was rescued, demonstrating that the seed sequence of miR-25 directly mediated the repression of MCU expression.
Figure 4.
MCU is directly targeted by miR-25. (A) The time-course changes in the expression of MCU mRNA in response to H2O2 exposure; (B) Western blot analysis of MCU in H9c2 cells overexpressing miR-25 or scramble control sequence (miR-Scr); (C) The potential binding site between miR-25 and the 3'-UTR of MCU mRNA. The complementary nucleotides are indicated by bolded blue letters; (D) The luciferase assay was performed using reporter plasmids harboring the wild-type and mutant MCU 3'-UTR. MiR-25 significantly inhibited the luciferase activity of the wild-type reporter, but not that of the mutant reporter. * p < 0.05.
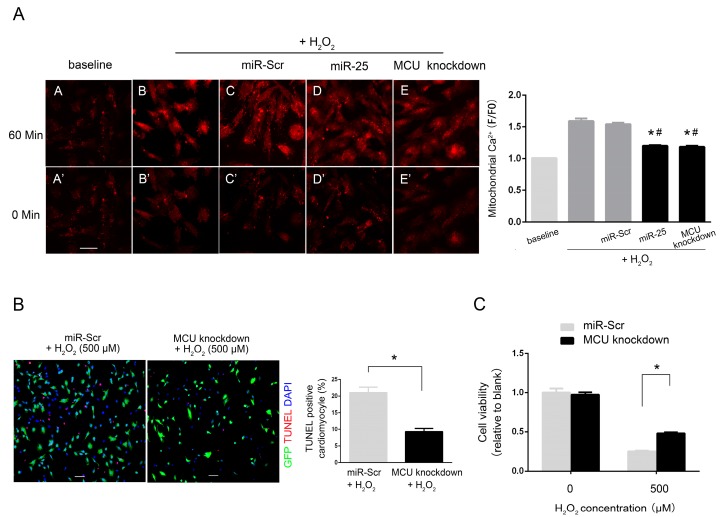
2.4. MiR-25 Significantly Decreased H2O2-Induced Elevation of Mitochondrial Ca2+ Concentration
MCU is a critical mitochondrial Ca2+ transporter that regulates mitochondrial Ca2+ concentration. Based on the above observation that MCU is a target of miR-25, we examined whether miR-25 regulates mitochondrial Ca2+ homeostasis, using a calcium imaging technique. As shown in Figure 5, mitochondrial Ca2+ in H9c2 cells was determined by Rhod-2 AM at 60 min after the treatment of H2O2 (500 μM). Increases in mitochondrial Ca2+ were observed in cells exposed to H2O2. The over-expression of miR-25 significantly attenuated such increases, demonstrating the inhibition of mitochondrial Ca2+ by miR-25 (Figure 5A). In addition, the knockdown of MCU inhibited H2O2-induced rises in mitochondrial Ca2+, which is in accordance with the basic function of MCU (Figure 5A). MCU knockdown also reduced cell apoptosis (Figure 5B) and enhanced cell viability (Figure 5C). Taken together, the inhibition of miR-25 on mitochondrial Ca2+ elevation under oxidative stress supports the role of MCU as a target for miR-25.
Figure 5.
MiR-25 over-expression and MCU knockdown individually blocked H2O2-induced mitochondrial Ca2+ elevation in H9c2 cells. Mitochondrial Ca2+ was determined by Rhod-2 AM, a mitochondrial-specific Ca2+ probe, after the treatment of 500 μM H2O2 for 60 min. Cells were infected with a lentivirus carrying the miR-25 precursor (miR-25) or a scramble control sequence (miR-Scr) or MCU shRNA (knockdown). (A) Representative images (left) and quantitative analysis (right) of cells stained with Rhod-2 AM. Scale bar: 50 μm; (B) Representative images (left) and quantitative analysis (right) of the TUNEL assay. Apoptotic cells were stained red by the TUNEL assay. Scale bar: 100 μm; (C) Cell viability of H9c2 cells stimulated with H2O2, determined by CCK-8 assay. Data are means ± SD from three independent experiments. * p < 0.05 vs. H2O2 + miR-Scr, # p < 0.05 vs. H2O2.
3. Discussion
The present study provides evidence for the anti-apoptotic effects of miR-25 by decreasing MCU expression, relieving mitochondrial Ca2+ overload and inactivating the mitochondrial apoptosis pathway in cardiomyocytes exposed to oxidative stress. First, among the cardiac-enriched miRNAs, miR-25 was identified as a sensitive responder to oxidative stress. Second, overexpression of miR-25 conferred a significant resistance to oxidative damage by inhibiting the mitochondrial apoptosis pathway. Third, among the potential target genes of miR-25, the expression of MCU in cardiomyocytes was reversely correlated with that of miR-25 after H2O2 exposure, and MCU was verified by the luciferase reporter assay to be a direct target of miR-25. Fourth, we observed that miR-25 alleviated mitochondrial Ca2+ elevation induced by H2O2, while MCU is a well-known regulator of mitochondrial Ca2+ homeostasis. These findings suggest that miR-25 in cardiomyocytes plays a protective role in oxidative stress by regulating Ca2+ homeostasis and the apoptosis pathway.
MiRNAs are emerging as critical regulators in physiological and pathogenetic processes. Physical and chemical stimuli may change miRNA expression [17,18]. The present study examined the expression levels of cardiac-enriched miRNAs in response to oxidative stimulation. Our results showed that miR-1 and miR-25 changed most dramatically. Since multiple studies have addressed the role of miR-1 in the heart [19,20], the present study made further observations on miR-25, but not miR-1. To uncover the role of miR-25 in oxidative stress, miR-25 was overexpressed using lentiviruses to observe its effect on the damages caused by the oxidative stress inducer H2O2. To address this question, we used a series of concentration of H2O2, ranging from a low dose (200 nM) to a high dose (700 nM), which undoubtedly induced necrosis and apoptosis in H9c2 cells. The most suitable and efficient concentration of H2O2 for inducing oxidative stress was established to be 500 nM in our settings, in accordance with the concentration documented to induce apoptosis in H9c2 cells [21]. By determining cell viability with CCK-8 assay and apoptotic cells with FACS and TUNEL analyses, we observed the anti-apoptotic effect of miR-25, which was most significant under the stimulation of 500 nM H2O2. The major mechanism through which miRNAs inhibit target gene expression is to induce mRNA degradation and/or mRNA translation suppression via the imperfect matching to the 3'-UTR of target mRNAs [17]. Here, we used bioinformatics analysis to search the potential targets of miR-25, with the focus on target genes involved in mitochondrial apoptosis pathway. MCU was selected as a potential target for further mechanical study. We found that MCU mRNA levels were negatively correlated with those of miR-25. Consistently, MCU protein levels were greatly reduced by miR-25 overexpression. The luciferase assay further confirmed that MCU is a direct target of miR-25. MCU is a 40-kDa protein that is sensitive to ruthenium-red-sensitive mitochondrial Ca2+ uptake [22]. It is a pivotal member of mitochondrial calcium uniporter complex, comprising of mitochondrial Ca2+ uptake1 (MICU1), MICU2, MCU and essential MCU regulator (EMRE) [23]. The MCU complex has been demonstrated to control the Ca2+ flux across the inner mitochondrial membrane [24,25]. Ca2+ homeostasis is essential for the maintenance of the physiological activity of the heart. It regulates energy production, modulates cytoplasmic Ca2+ signaling, and switches the transition from reversible to irreversible cell death [26,27]. There is evidence that an increased MCU current results in mPTP opening and myocardial cell death. Specific inhibition of MCU with Ru360 provided cardioprotective effects, while activation of MCU with spermine abolished this beneficial activity [28,29]. MCU is a target that promotes cardiomyocyte survival during mitochondrial stress [30]. The present study identified MCU as a direct target gene of miR-25. Based on the aforementioned function of MCU, it is presumable that miR-25 overexpression may facilitate mitochondrial Ca2+ homeostasis. By using a mitochondria-specific Ca2+ dye, we did observe that the increase of mitochondrial Ca2+ induced by oxidative stimulation was attenuated by miR-25 over-expression. MCU knockdown also relieved mitochondrial Ca2+ overload and reduced cell apoptosis under oxidative stress. These results are consistent with previous reports that miR-25 can inhibit mitochondrial Ca2+ uptake in Hela cells [31], and that knockdown of MCU markedly reduced mitochondrial Ca2+ uptake [22]. Taken together, it is suggested that miR-25 regulates mitochondrial Ca2+ homeostasis by targeting MCU in cardiomyocytes. This is the first time that miR-25-mediated MCU downregulation is observed to be correlated with the anti-apoptotic activity in cardiomyocytes.
SERCA was recently shown to be down-regulated by miR-25 in the failing heart, but other calcium handling proteins, including the sodium-calcium exchanger (NCX1), calmodulin kinase-2 (CaMKII), phospholamban (PLN) and Calmodulin-3 (Calm3) were unaffected. Downregulation of SERCA leads to the dysfunction of Ca2+ re-uptake and the elevation of Ca2+ in the cytoplasm, which is harmful to cardiomyocytes [32]. Meanwhile, the elevated cytosolic Ca2+ may enter mitochondria and cause mitochondrial Ca2+ overload and subsequent cell dysfunction. Our present finding that the overexpression of miR-25 conferred a significant resistance to oxidative damage in cardiomyocytes appears to be different from the above scenario. A potential reason might be that miR-25-mediated regulation of MCU protected the mitochondria from oxidative stress, which well counteracted the detrimental cytosolic Ca2+ overload by the reduced SERCA protein. Bcl-2 protein is acknowledged as one of the key regulators of programmed cell death [33]. The present study observed that miR-25 over-expression rescued Bcl-2 expression in oxidative stress. However, there is no evidence that Bcl-2 is directly regulated by MCU. We think that the increase of Bcl-2 expression might be subsequent to the inhibition of MCU by miR-25. Buffering mitochondrial Ca2+ was found to increase the expression of Bcl-2 in hepatocytes [34]. Therefore, it is possible that miR-25 facilitates Bcl-2 expression by attenuating mitochondrial Ca2+ overload in oxidative stress. Further study needs to be done to address this issue.
4. Methods and Materials
4.1. Cell Culture and Viral Infection
The rat cardiac myoblast H9c2 cell line was cultured in DMEM supplemented with 10% fetal bovine serum (Hyclone Laboratories, Logan, UT, USA), 2 mM l-glutamine, and penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) in a humidified 5% CO2 incubator at 37 °C. The plv-miR vector was provided by Jian-hua Wang (Shanghai Jiaotong University School of Medicine, Shanghai, China). The construction of lentiviral vectors containing miR-25 precursor, MCU interference sequence (GCCAGAGACAGACAATACT) and scramble sequence (TTCTCCGAACGTGTCACGT) were generated by insertion of corresponding sequences. Replication-incompetent lentiviral particles were packaged in 293T cells with helper plasmids (gag-pol and VSV-G) and used to infect H9c2 cells.
4.2. Quantitative Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated using the mirVana kit (for miRNA; Ambion, Austin, TX, USA) or with TRIzol (mRNA; Invitrogen, Carlsbad, CA, USA). MiRNA was quantified by reverse transcription-polymerase chain reaction (qRT-PCR; Applied Biosystems, Foster City, CA, USA). Cardiac-enriched miRNAs with RNU-6B were quantified by SYBR Green detection and miScript primer assays (Qiagen, Valencia, CA, USA). The mRNA quantification was conducted using SYBR Green-based detection technology. The primers were as follows: rat MCU: forward 5'-AGATGGTGTTCGAGTTGCTG-3'; reverse 5'-AGGGTCTCTGCGTTTTCATG-3'. GAPDH: forward 5'-CTGGTGCTGAGTATGTCGTGGA-3'; reverse 5'-AGTTGGTGGTGCAG-GATGCATT-3'. The expression of each miRNA under the baseline and the exposure of H2O2 was first normalized to the internal reference (∆Ct) and then, the fold change of miRNAs in H2O2 group was compared to that of the baseline (∆∆Ct) and expressed as 2−(∆∆Ct).
4.3. Fluorescence-Activated Cell Sorting (FACS)
The apoptosis was determined by FACS assay. The H9c2 cells infected with recombinant lentivirus were digested with 0.25% trypsin-EDTA solution (Invitrogen, Carlsbad, CA, USA) for 5 min at 37 °C and then resuspended in the culture medium to obtain a single cell suspension. The detector settings were calibrated using uninfected H9c2 cells that were likewise digested. The sorted cells were plated in gelatin-coated 24-well plates at a density of 105 cells/well. For the apoptosis analysis, H2O2 (Sigma, St. Louis, MO, USA)-treated H9c2 cells were washed with calcium buffer and incubated with annexin V and 7-AAD for 10 min and then analyzed using a BD FACSAria II (BD Biosciences, San Jose, CA, USA).
4.4. Cell Vitality Analysis
Cell vitality was determined using CCK-8 dye (Beyotime, Zhejiang, China) according to the manufacturer’s instructions. Briefly, 5 × 103 cells/well were seeded in a 96-well flat-bottomed plate and then cultured at 37 °C for 24 h. Subsequently, the cells were treated with H2O2 at increasing concentrations (200, 400, 450, 500, 550, 600, 650, 700 μM) in the presence of 10% FBS for 4 h. After the addition of 10 μL CCK-8 dye to each well, the cells were incubated at 37 °C for further 2 h and then the absorbance was determined at 450 nm using a microplate reader.
4.5. TUNEL Assay
Apoptosis was induced with H2O2 from Sigma (Sigma, St. Louis, MO, USA). After exposure to 500 μM H2O2 for 4 h, cells cultured on coverslips (1 × 105 cells/well of a 24-well plate) were used for apoptotic TUNEL detection using the in situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN, USA) and analyzed by fluorescence microscopy.
4.6. Immunoblotting
Protein lysates for immunoblotting were prepared in RIPA buffer (Beyotime, Zhejiang, China) containing protease inhibitor cocktail tablets (Roche Applied Science, Mannheim, Germany), and the protein concentrations were quantified using the BCA protein assay kit (Pierce, Rockford, IL, USA). Western blot analysis was performed using standard techniques. The primary antibodies used were MCU (1:200; Santa Cruz, CA, USA), Bcl-2, Bax, Cytochrome (1:1000; Cell Signaling, Danvers, MA, USA) and GAPDH (1:5000; Beyotime, Zhejiang, China). The appropriate IRDye 800-conjugated secondary antibodies were used (1:10,000; LI-COR Biosciences, Lincoln, NE, USA) to visualize the blotting. Images were recorded using the Odyssey infrared imaging system and analyzed with Odyssey Application Software v2 (LI-COR Biosciences).
4.7. Luciferase Reporter Assay
TargetScan and Pictar were used to predict the miR-25 binding sites within MCU. The MCU 3'-UTR was amplified from rat genomic DNA using the following primers: forward 5'-CCGTTGTTTAAACTCAGAGATGAGCGTGAATCCCG-3'; reverse 5'-CGGAAGCGGCCGCCCCTGCAAAGATGCACGAAGC-3'.
The 301-base-pair product was cloned and inserted into the PmeI/NotI sites of psiCheck2 (Promega, Madison, WI, USA). The resultant p3'UTR-MCU plasmids were mutated by PCR using primers designed to incorporate a three-base substitution into the miR-25 seed sequence (5'-GAATCCAAGTGCAA converted to 5'-GAATCCAACACCAA). The resulting pMut-3'UTR-MCU plasmids were co-transfected with miR-25. Forty-eight hours later, the firefly and Renilla luciferase activities were analyzed using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
4.8. Mitochondrial Calcium Recordings and Analyses
Mitochondrial calcium concentrations in H9c2 cells were measured by the mitochondria-selective Ca2+ indicator Rhod-2 AM. H9c2 cells over-expressed with miR-25, MCU-shRNA or scramble miRNA were grown on glass slides and loaded with 5 μM Rhod-2 AM, followed by H2O2 treatment (500 μM) for 60 min at room temperature. The fluorescence intensities were immediately analyzed with a Nikon inverted fluorescence microscope. Ca2+ transients are presented as the fluorescence ratio, normalized to the background intensity (F/F0).
4.9. Statistical Analysis
All of the data are presented as the means ± SD. Differences between multiple groups were analyzed with one-way ANOVA followed by the Fisher’s least significant difference (LSD) test. Differences between two groups were analyzed with Student’s t-test. A p < 0.05 was considered statistically significant.
5. Conclusions
In summary, our observations provide evidence that miR-25 protects cardiomyocytes against oxidative damage by reducing the expression of MCU, and subsequently attenuating mitochondrial Ca2+ overload and apoptosis. We first addressed the role of miR-25 in the cardiomyocytes. Our findings provide novel insights for miRNA in defending against oxidative stress, which might represent a novel target for the treatment of heart diseases associated with oxidative stress.
Acknowledgments
This work was supported by the Fund for National Innovative Research Groups of the National Natural Science Foundation of China (81221001, to Yi-Han Chen), the Youth Program of National Natural Science Foundation of China (81100124, to Liang Xu), the National Key Basic Research Program of China (2013CB531100, to Yi-Han Chen), the Major International Joint Research Program Fund of China (81120108004, to Yi-Han Chen), and the General Program of National Natural Science Foundation of China (81170224 and 81270313, to Jun Li; 31271214, to Yi-Han Chen).
Author Contributions
Yi-Han Chen conceived and designed this study. Lei Pan, Bi-Jun Huang, Xiu-E Ma, Shi-Yi Wang, Jing Feng, Fei Lv, Yuan Liu, Yi Liu, Chang-Ming Li, Dan-Dan Liang and Jun Li performed the experiments. Lei Pan, Liang Xu and Yi-Han Chen analyzed the data. Lei Pan, Jun Li, Liang Xu and Yi-Han Chen provided discussions and advice. Liang Xu and Yi-Han Chen wrote the manuscript. Yi-Han Chen supervised the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tamargo J., Lopez-Sendon J. Novel therapeutic targets for the treatment of heart failure. Nat. Rev. Drug Discov. 2011;10:536–555. doi: 10.1038/nrd3431. [DOI] [PubMed] [Google Scholar]
- 2.Ansley D.M., Wang B. Oxidative stress and myocardial injury in the diabetic heart. J. Pathol. 2013;229:232–241. doi: 10.1002/path.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulda S., Galluzzi L., Kroemer G. Targeting mitochondria for cancer therapy. Nat. Rev. Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 4.Walters A.M., Porter G.A., Jr., Brookes P.S. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ. Res. 2012;111:1222–1236. doi: 10.1161/CIRCRESAHA.112.265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu N., Olson E.N. MicroRNA regulatory networks in cardiovascular development. Dev. Cell. 2010;18:510–525. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordes K.R., Srivastava D. MicroRNA regulation of cardiovascular development. Circ. Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu N., Bezprozvannaya S., Williams A.H., Qi X., Richardson J.A., Bassel-Duby R., Olson E.N. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M., Galuppo P., Just S., Rottbauer W., Frantz S., et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 9.Yang B., Lin H., Xiao J., Lu Y., Luo X., Li B., Zhang Y., Xu C., Bai Y., Wang H., et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 10.Ivey K.N., Muth A., Arnold J., King F.W., Yeh R.F., Fish J.E., Hsiao E.C., Schwartz R.J., Conklin B.R., Bernstein H.S., et al. MicroRNA regulation of cell lineages in mouse and human embryonic stem cells. Cell Stem Cell. 2008;2:219–229. doi: 10.1016/j.stem.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y., Ransom J.F., Li A., Vedantham V., Von D.M., Muth A.N., Tsuchihashi T., McManus M.T., Schwartz R.J., Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Hu Y., Matkovich S.J., Hecker P.A., Zhang Y., Edwards J.R., Dorn G.W., 2nd. Epitranscriptional orchestration of genetic reprogramming is an emergent property of stress-regulated cardiac microRNAs. Proc. Natl. Acad. Sci. USA. 2012;109:19864–19869. doi: 10.1073/pnas.1214996109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y., Fuscoe J.C., Zhao C., Guo C., Jia M., Qing T., Bannon D.I., Lancashire L., Bao W., Du T., et al. A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat. Commun. 2014;5:3230. doi: 10.1038/ncomms4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ku H.C., Chen W.P., Su M.J. DPP4 deficiency exerts protective effect against H2O2 induced oxidative stress in isolated cardiomyocytes. PLOS One. 2013;8:e54518. doi: 10.1371/journal.pone.0054518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dulchavsky S.A., Davidson S.B., Cullen W.J., Devasagayam T.P., Diebel L.N., Dutta S. Effects of deferoxamine on H2O2-induced oxidative stress in isolated rat heart. Basic Res. Cardiol. 1996;91:418–424. doi: 10.1007/BF00788722. [DOI] [PubMed] [Google Scholar]
- 16.Stadlmann S., Rieger G., Amberger A., Kuznetsov A.V., Margreiter R., Gnaiger E. H2O2-mediated oxidative stress versus cold ischemia-reperfusion: Mitochondrial respiratory defects in cultured human endothelial cells. Transplantation. 2002;74:1800–1803. doi: 10.1097/00007890-200212270-00029. [DOI] [PubMed] [Google Scholar]
- 17.Leung A.K., Sharp P.A. MicroRNA functions in stress responses. Mol. Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendell J.T., Olson E.N. MicroRNAs in stress signaling and human disease. Cell. 2012;148:1172–1187. doi: 10.1016/j.cell.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan Z., Sun X., Ren J., Li X., Gao X., Lu C., Zhang Y., Sun H., Wang Y., Wang H., et al. miR-1 exacerbates cardiac ischemia-reperfusion injury in mouse models. PLoS One. 2012;7:e50515. doi: 10.1371/journal.pone.0050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shan Z.X., Lin Q.X., Fu Y.H., Deng C.Y., Zhou Z.L., Zhu J.N., Liu X.Y., Zhang Y.Y., Li Y., Lin S.G., et al. Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction. Biochem. Biophys. Res. Commun. 2009;381:597–601. doi: 10.1016/j.bbrc.2009.02.097. [DOI] [PubMed] [Google Scholar]
- 21.Jiang L., Wang H., Shi C., Liu K., Liu M., Wang N., Wang K., Zhang H., Wang G., Xiao X. ZNF667/Mipu1 is a novel anti-apoptotic factor that directly regulates the expression of the rat Bax gene in H9c2 cells. PLoS One. 2014;9:e111653. doi: 10.1371/journal.pone.0111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sancak Y., Markhard A.L., Kitami T., Kovacs-Bogdan E., Kamer K.J., Udeshi N.D., Carr S.A, Chaudhuri D., Clapham D.E., Li A.A., et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallilankaraman K., Cardenas C., Doonan P.J., Chandramoorthy H.C., Irrinki K.M., Golenar T., Csordas G., Madireddi P., Yang J., Muller M., et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat. Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alam M.R., Groschner L.N., Parichatikanond W., Kuo L., Bondarenko A.I., Rost R., Waldeck-Weiermair M., Malli R., Graier W.F. Mitochondrial Ca2+ uptake 1 (MICU1) and mitochondrial Ca2+ uniporter (MCU) contribute to metabolism-secretion coupling in clonal pancreatic β-cells. J. Biol. Chem. 2012;287:34445–34454. doi: 10.1074/jbc.M112.392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorn G.W., 2nd, Maack C. SR and mitochondria: Calcium cross-talk between kissing cousins. J. Mol. Cell. Cardiol. 2013;55:42–49. doi: 10.1016/j.yjmcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Csordas G., Varnai P., Golenar T., Sheu S.S., Hajnoczky G. Calcium transport across the inner mitochondrial membrane: Molecular mechanisms and pharmacology. Mol. Cell. Endocrinol. 2012;353:109–113. doi: 10.1016/j.mce.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffiths EJ. Mitochondrial calcium transport in the heart: Physiological and pathological roles. J. Mol. Cell. Cardiol. 2009;46:789–803. doi: 10.1016/j.yjmcc.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Drago I., de Stefani D., Rizzuto R., Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc. Natl. Acad. Sci. USA. 2012;109:12986–12991. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santo-Domingo J., Vay L., Hernandez-Sanmiguel E., Lobaton C.D., Moreno A., Montero M., Alvarez J. The plasma membrane Na+/Ca2+ exchange inhibitor KB-R7943 is also a potent inhibitor of the mitochondrial Ca2+ uniporter. Br. J. Pharmacol. 2007;151:647–654. doi: 10.1038/sj.bjp.0707260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchi S., Lupini L., Patergnani S., Rimessi A., Missiroli S., Bonora M., Bononi A., Corra F., Giorgi C., de Marchi E., et al. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr. Biol. 2013;23:58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wahlquist C., Jeong D., Rojas-Munoz A., Kho C., Lee A., Mitsuyama S., van Mil A., Park W.J., Sluijter J.P., Doevendans P.A., et al. Inhibition of miR-25 improves cardiac contractility in the failing heart. Nature. 2014;508:531–535. doi: 10.1038/nature13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirsch D.G., Doseff A., Chau B.N., Lim D.S., de Souza-Pinto N.C., Hansford R., Kastan M.B., Lazebnik Y.A., Hardwick J.M. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J. Biol. Chem. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- 34.Guerra M.T., Fonseca E.A., Melo F.M., Andrade V.A., Aguiar C.J., Andrade L.M., Pinheiro A.C., Casteluber M.C., Resende R.R., Pinto M.C., et al. Mitochondrial calcium regulates rat liver regeneration through the modulation of apoptosis. Hepatology. 2011;54:296–306. doi: 10.1002/hep.24367. [DOI] [PMC free article] [PubMed] [Google Scholar]